Structural design and mechanism analysis of hierarchical porous carbon fibers for advanced energy and environmental applications
Chao
Liu†
a,
Quanxiang
Li†
 *a,
Weimin
Kang
b,
Weiwei
Lei
*a,
Weimin
Kang
b,
Weiwei
Lei
 a,
Xungai
Wang
a,
Chunxiang
Lu
a,
Xungai
Wang
a,
Chunxiang
Lu
 c and
Minoo
Naebe
c and
Minoo
Naebe
 *a
*a
aInstitute for Frontier Materials, Deakin University, Waurn Ponds Campus, Locked Bag 20000, Victoria 3220, Australia. E-mail: quanxiang.li@deakin.edu.au; minoo.naebe@deakin.edu.au
bState Key Laboratory of Separation Membranes and Membrane Processes/National Center for International Joint Research on Separation Membranes, School of Textile Science and Engineering, Tiangong University, Tianjin 300387, China
cNational Engineering Laboratory for Carbon Fiber Technology, Institute of Coal Chemistry, Chinese Academy of Sciences, Taiyuan 030001, PR China
First published on 15th November 2021
Abstract
Hierarchical porous carbon fibers (PCFs) combining the structural and functional features of commercial carbon fibers and porous carbonaceous materials have attracted extensive interest in energy conversion/storage, catalysis, adsorption/separation, sensing and other applications. The structures, morphologies and compositions of PCFs and the incorporation of active materials are considered crucial to boost their performance in the energy and environmental fields. However, for PCFs, as relatively new materials, their synthetic routes and characteristic development are still limited. This review focuses on the structural design and mechanism analysis of PCFs with five major porous structures and their superior application profiles. Firstly, we summarize the primary strategies to access and control the porosities and morphologies of PCFs. Subsequently, the improvement mechanisms of the performance of PCFs in various applications are comprehensively discussed, and emerging strategies to further enhance their properties by utilizing the synergistic effect with heteroatom doping and guest active material hybridization are highlighted. Finally, this review demonstrates the major challenges in the structural design and mechanism exploration of PCFs for different applications and provides an overall perspective for the future development of advanced PCF-based devices.
1. Introduction
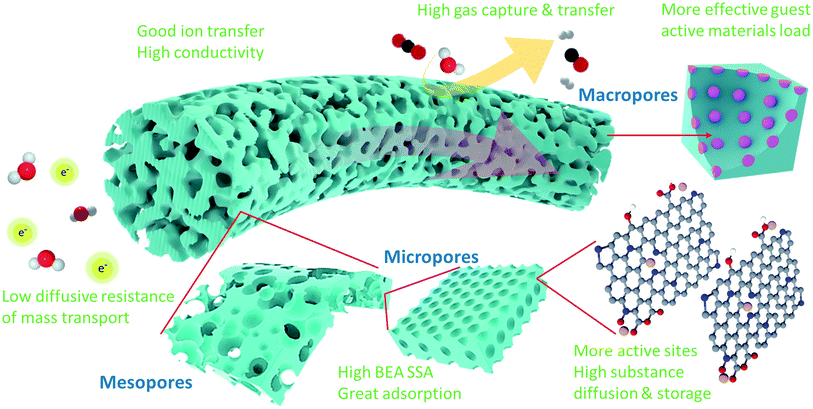
Severe issues regarding energy security uncertainty and environmental concerns have driven the world to seriously consider sustainable and clean solutions and energy sources for society. Accordingly, carbon fibers (CFs) are one of the most exceptional candidates for energy and environmental applications given that they offer a combination of high mechanical strength, low density, freestanding nature, flexibility, good chemical stability, and excellent thermal and electrical conductivity.1–4 Carbon fibers have been adopted as supporting scaffolds and self-supporting active components over the last decade. However, due to the inert nature of carbon, CFs synthesized via traditional methods usually have a limited specific surface area (SSA), which renders them incapable of providing a large amount of active sites.5 Nevertheless, with effective activation and surface treatment approaches, their critical properties for applications in which high surface areas are desirable can be improved. Compared to conventional activated carbon fibers (ACFs) with uniform pore dimensions on their surface (usually less than 2 nm), strategies to improve the porosity and permeability of carbon fibers are more innovative and beneficial to expand their potential as active materials.6–8 To boost their advanced technological applications, ideal porous carbon fibers (PCFs) should possess highly uniform micropores (<2 nm) and hierarchically porous structures and topologies (mesoporous, 2 nm < pore size < 50 nm, macroporous, pore size >50 nm).9 This is mainly because micro and small mesopores can provide high surface area for the dispersion of active sites, and larger mesopores and macropores have the ability to minimize the diffusive resistance of mass transport and permit easy access to the micropores.10,11 Of course, the improved porosity can further reduce the weight of the material and increase the space for more extensive surface modification efforts, such as elemental doping, surface functionalization, and hybridization.12 Nevertheless, creating novel PCFs possessing a well-defined morphology and structure, particularly carbon frameworks with hierarchical porosity, is not easy. The major challenges in the fabrication of PCFs arise from the complexity of the mechanism for the formation of hierarchical micro-/meso-/macroporous structures and the difficulty in preserving the fiber integrity.In recent years, significant progress and strategies have been developed for the selection of CF precursors and carbon nanomaterials, structural design and modification, and innovations in the configuration of PCFs. In this review, we categorize PCFs into five major porous structures (Fig. 1), including (1) PCFs with rich meso/macro-pores, which contain a large amount of mixed meso-and macropores on their surface and inside of the carbon fibers besides micropores; (2) PCFs with a highly interconnected pore structure, in which there are continuous channels cross-linked by a high percentage of micro-, meso- and macropores; (3) PCFs with defect-rich surface pore structure, possessing numerous micro-, meso- and macropores primarily existing on the carbon fiber surface; (4) PCFs with a carbonaceous surface pore structure, which mean the carbon fiber is coated or grafted by porous carbonaceous materials, and (5) PCFs with graphene assemblies, which refer to porous graphene fibers synthesized via spinning or self-assembly. Unlike most hierarchically porous carbon powder materials, which require the help of insulating binders to realize different forms, PCF is an ideal binder-free, self-standing and strong material with a fiber shape/morphology and well-defined porous structure, thereby inspiring its applications in light, portable, flexible and wearable devices.13 In this review, particular attention is paid to the selection of carbon-rich precursors and carbon nanomaterials, the pore forming mechanisms, and principal characters of the resulting PCFs. Subsequently, a perspective on the advancements of emerging PCFs for energy storage and environmental applications including supercapacitors, batteries, catalysis, adsorption and absorption, sensors and other potentials are highlighted. More importantly, the mechanisms for promoting the performance of PCFs in different applications are discussed in detail. The effect of varying the porous morphology on the efficiency of PCFs for specific applications and options to enhance their performance are emphasized. Finally, the current issues and future developmental directions in the area of PCFs are discussed.
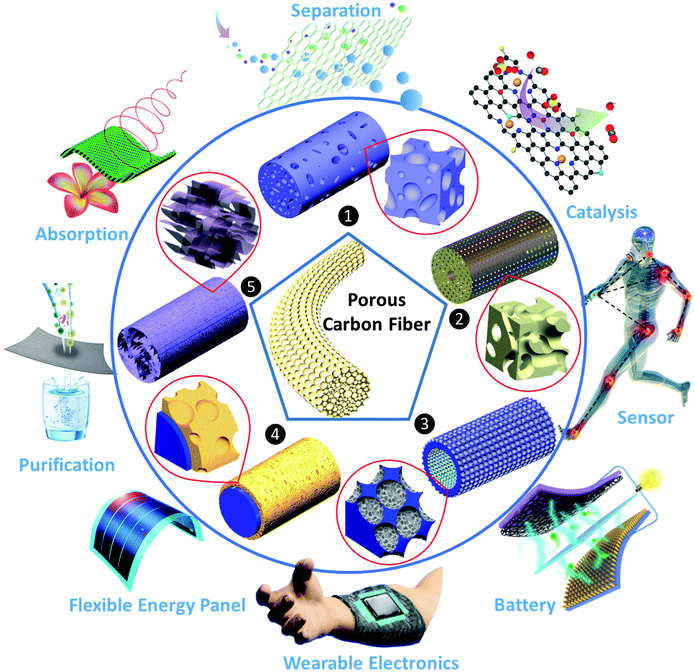 | ||
| Fig. 1 Overview of porous carbon fibers with different structures and their prospective applications. | ||
2. Fabrication of PCFs with different porous structures
CFs can be manufactured via two major routes, the carbonization of precursor fibers (polymer, pitch, biomass, etc.) and the assembly of carbon-based nanomaterials. For the carbonization of precursor fibers, the biomass-based precursor can be converted into CFs directly via high-temperature pyrolysis. In the case of polymer-based precursors, various spinning methods such as solution spinning and melt spinning are usually selected to synthesize continuous precursor fibers with a well-controlled fibrous morphology and structure. The spinning or synthesis methods for carbon fiber precursors are presented as follows.Electrospinning of the polymer solution or melt can simply fabricate precursor fibers on the nanoscale level using electrostatic force to draw the charged thread of polymers. This technique combines the advantages of both electrospraying and traditional dry spinning, making it particularly suitable for the synthesis of nanofibers using large and complex molecules on a multi-scale.14 The morphology of the electrospun nanofibers is strongly determined by some critical factors, including polymer solution features, processing conditions and atmosphere conditions.
Sol–gel process has also been developed for the synthesis of polymer nanofibers, which involves the transformation of monomers into a colloidal solution (sol) acting as the precursor for a gel-like polymer network containing both a liquid phase and solid phase.15,16 The hydrolysis and polycondensation of the precursor are the critical reaction steps in this process, which can impact the structure and morphology of the polymer nanofibers and the following carbon nanofibers.
Melt spinning, wet spinning and dry spinning are common processes to achieve precursor fibers on a larger scale, in which the polymer in the fluid state passing through the spinneret is solidified into a fiber. The polymer characteristics, solidification approach, and process/ambient conditions are significant in the formation of the structure of the carbon fiber precursors.17 Under the carbonization process, except for carbon, most elements, such as nitrogen, oxygen and hydrogen are removed, and the carbon chains join together. In addition, some emerging carbonization techniques, such as template-directed hydrothermal carbonization at low temperature, can produce carbonaceous nanofibers directly from some precursors in conjunction with molecular, supramolecular, and colloidal crystal templates, and sometimes no additional spinning and pyrolysis steps are required.18 Besides the carbonization of precursor fibers, carbon-based nanomaterials, including carbon nanotubes (CNT), graphene, graphene oxide (GO), and reduced graphene oxide (rGO) can be directly assembled into macroscopic CFs without pyrolysis through self-assembly, phase assembly or spinning assembly. In terms of the fabrication of PCFs, additional additives and more complex steps are required during the spinning and/or carbonization process to generate a high pore volume, while preserving the fiber integrity. In this review, various strategies and mechanisms for the introduction of pores, such as template addition, activation, surface modification, self-assembly and hydrothermal methods for the synthesis of hierarchical PCFs with five different porous structure are presented and discussed. The differences among the various types of PCFs are distinguished based on their porous structure and synthetic methods. Briefly, in addition to their inherent nature, carbon nanofibers are an ideal option to possess a high pore volume due to their submicron diameter. Also, the synthetic methods for their precursors, such as electrospinning and sol–gel method, facilitate the implementation of pore-formation.16,19 Therefore Section 2.1 presents PCFs with rich meso/macro-pores, where porous nanocarbon fibers with a substantial proportion of internal meso/macro-pores constitute the majority. With the improvement of pore-forming technology and deeper understanding of the mechanism, a more adequate number of pores can be achieved in carbon nanofibers, which realize PCFs with a highly interconnected pore structure, as presented in Section 2.2. Of course, there are some drawbacks, such as a sharp decline in mechanical properties, and thus at the end of Section 2.2, one of the latest carbon micron-fiber containing interconnected pore structure, which was developed by Toray, is also highlighted. In Section 2.3, PCFs with a defect-rich surface pore structure refer to the use of highly effective surface treatment technology on the existing carbon fiber surface to achieve a large number of pores and large pore volume. In this type of PCF, micron-scale carbon fibers such as biomass fibers and commercial carbon fibers as basic materials represent the majority. In Section 2.4, PCFs with a carbonaceous surface pore structure, opposite to that in Section 2.3, are presented, where rather than carbon consumption, this type of PCF is mainly synthesized through the strategy of adding/producing porous carbon materials on the surface of existing carbon fibers. In Section 2.5, PCFs with graphene assemblies specifically refer to porous graphene fibers. The porous structure of this type of PCF is mainly built based on the characteristics of the assembled graphene, which has little correlation with the pore-forming mechanism mentioned in the previous sections. The detailed structure design and synthesis methods of these five different PCFs will be systematically discussed in the following subsections.
2.1 PCFs with rich meso/macro-pores
Intensive research has been conducted on mesoporous inorganic materials in the past decade,20–22 and consequently well-controlled mesoporous carbon with narrow pore-size distributions has been prepared successfully using several synthetic methods including hard and soft template approaches.10,23 PCFs with rich meso/macro-pores can be also obtained by these methods, and these novel structures can provide a high specific surface area and large pore volume, offering more active sites for improved performances in different applications. Compared to the conventional activation methods, which can only generate micropores on the surface of CFs, the fabrication process of PCFs via template approaches can produce mixed macro- and mesopores on the surface and/or inside the fiber. The main mechanism for the formation of an internal porous structure is by eliminating the involved template material in the precursor fiber before or after carbonization, and hence PCFs with varying pore structures can be molded using template materials with different shapes and sizes.17Casting nanoparticles in the carbon fiber precursor is a straightforward route to generate PCFs with a controlled pore structure, in which the mesostructure is the major constituent. Silica (SiO2) nanoparticles are a common hard-template to fabricate porous carbon materials.24,25 For instance, Ji et al. prepared PCFs by adding SiO2 nanoparticles to the polyacrylonitrile (PAN) precursor.26 It was found that both the micropore and mesopore volumes could be amplified by enhancing the SiO2 ratio in the PAN/SiO2 precursors. Other metal oxide nanoparticles, such as ZnO,27 can also function as a pore template. However, it is still challenging to simply add inorganic nanoparticles to realize a homogeneous distribution in organic polymers due to the complex spinning process and much larger aspect ratios of fibers than other forms, which means that protruding and aggregated nanoparticles from the surface of the PCFs can be irregularly formed.28 To obtain homogeneously confined nanoparticles in the precursor, combining metal oxide precursors, which can be converted into metal oxide nanoparticles in situ via phase separation, with carbon fiber precursors is an elegant approach.29,30 For example, Wang et al. successfully designed double-capillary PCFs with micropores in the inner capillary and mesopores in the outer capillary by encapsulating tetraethoxysilane as a precursor of SiO2 in the PAN precursor followed by spinning, calcination and acid treatment (Fig. 2A).30 The resultant PCFs demonstrated a high specific surface area of 870 m2 g−1 together with the coexistence of micropores (64%) and mesopores (36%).
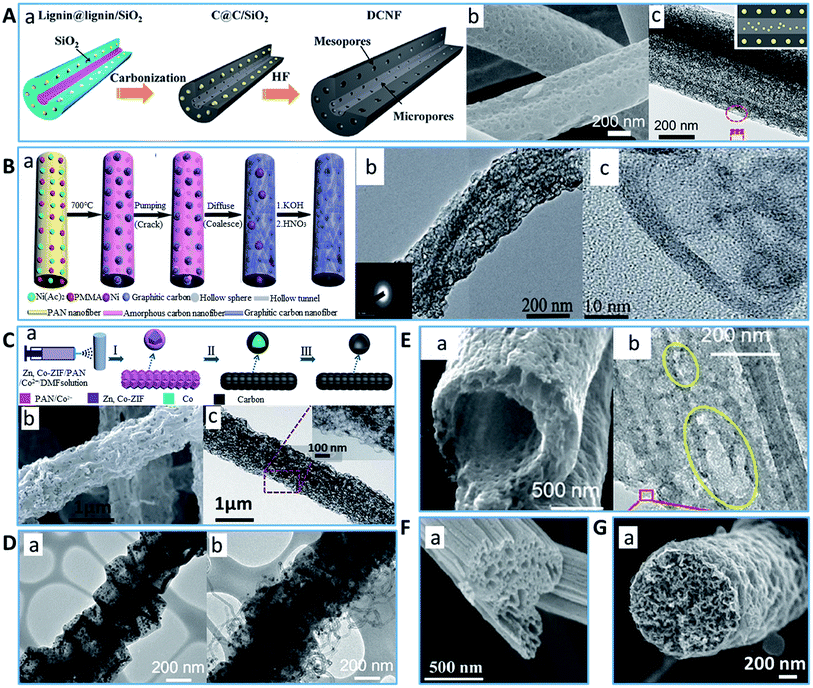 | ||
| Fig. 2 Typical examples of PCFs with rich meso/macro pore structure. (A) PCF synthesized using SiO2 precursor, (a) illustration of the fabrication process, (b) SEM image and (c) TEM image.30 Reproduced with permission from ref. 30. Copyright 2016, Elsevier. (B) N-Doped hollow-tunneled PCF, (a) schematic of material processing, (b) and (c) TEM images.45 Reproduced with permission from ref. 45. Copyright 2014, The Royal Society of Chemistry. (C) PCF prepared using Zn, Co-ZIF as a template, (a) illustration of process, (b) SEM image and (c) TEM image.51 Reproduced with permission from ref. 51. Copyright 2017, The Royal Society of Chemistry. (D) TEM of (a) PCF and (b) PCF with “tube on cube” nanohybrid structure obtained using ZIF as a template.52 Reproduced with permission from ref. 52. Copyright 2018, The Royal Society of Chemistry. (E) (a) SEM and (b) TEM images of PCF fabricated using PMMA as a pore generating agent.55 Reproduced with permission from ref. 55. Copyright 2019, Wiley. (F) (a) SEM image of PCF with churro-like morphology through the kinetically different pore formation of PS and PAN polymer.66 Reproduced with permission from ref. 66. Copyright 2013, Elsevier. (G) (a) SEM image of PCF from PS precursor.74 Reproduced with permission from ref. 74. Copyright 2015, The Royal Society of Chemistry. | ||
Alternatively, inorganic metal salt nanoparticles, such as CaCO3,31 Mg(NO3)2 (ref. 32) and NaHCO3 (ref. 33) have been explored for their potential to generate pores in CFs. Considering the application of PCFs, the salts or precursors of the metal catalyst, including Co,34–36 Ni,37 Ru,38 Fe,39–41 Sn,42 Mn (ref. 43) and their mixtures8,44 have been added to the CF precursor, which not only can help to harvest pores but also can be reduced to a catalyst directly. For example, Kim's group successfully synthesized CoO nanoparticles embedded in PCFs by electrospinning a cobalt acetate anhydrous and PAN mixture and subsequent calcination.35 Xu et al. prepared PCFs comprising hollow graphitic carbon spheres from PAN and iron acetylacetonate with different mass ratios.40 With the assistance of KOH activation, the optimized PCFs showed an extraordinary specific surface area of 1277 m2 g−1 and pore volume 0.86 cm3 g−1 due to the increase in the generation of micropores and small to mid-size mesopores. More interestingly, after the synthesis of PCFs from PAN as an N-doped carbon precursor, nickel acetate as a normal catalyst and PMMA as a sacrificial template to form the pores, Zhou's group further controlled the Ni-induced graphitization by diffusing Ni nanoparticles within the PCFs under vacuum, which turned amorphous carbon into graphitic carbon and produced a hollow-tunnel structure (Fig. 2B).45 The as-obtained activated PCFs had a specific surface area of 538 m2 g−1 and total pore volume of 0.77 cm3 g−1 with a hierarchical porous structure ranging from <1 nm to >120 nm and CNT nanochannels.
In recent years, metal–organic frameworks (MOFs), which possess a large number of carbon atoms in their skeletons, have been employed as sacrificial templates to synthesize PCFs due to their highly functional features such as manageable architectures, adjustable pore sizes and large surface areas.46,47 MOFs may retain the previous porous structure, while metallic ions are reduced to the corresponding metal or metallic, which can generate hierarchical pores in CFs.47–50 For example, Wang et al. developed a novel N, Co-containing MOF-based hierarchical PCF by combining Zn, Co-ZIF with PAN as precursor (Fig. 2C).51 The resultant PCF possessed a large surface area (515.2 m2 g−1) and high porosity (0.54 cm3 g−1) with the distribution of the dominant pore size of 2–12 nm. Li et al. developed a novel PCF by carbonizing a ZIF-67/PAN composite fiber followed by a chemical vapor deposition (CVD) process (Fig. 2D).52 The resulting PCF was composed of hierarchical pores, porous hollow carbon cube fillers and CNTs. It exhibited a large surface area of 665.6 m2 g−1 and a pore volume of 1.45 cm3 g−1 with a remarkable increase in the ratio of meso/macro-pores. Similarly, Lu's group synthesized FeNi alloy-encapsulated N-doped CNTS-tangled PCFs via electrospinning and thermal process by blending PAN, ZIF-8, Fe-salt and Ni-salt.53 The novel porous structure was created because the evaporation of metallic Zn and some CNTs grew on the surface during the carbonization process. Consequently, the PCF exhibited a large surface area of 402 m2 g−1 and pore volume of 0.528 cm3 g−1 with a high content of micropores/mesopores.
Although the introduction of a nanoparticle template is a facile approach to control the pore size, it has the distribution issue of additives in the precursor. Additionally, the procedure for template removal usually needs corrosive and hazardous chemical agents, which is complicated, time consuming and not environment-friendly. Conversely, the soft-templating method can be a good approach to obtain PCFs. Different from the hard-templating approach, which usually produces a uniform distribution of pore sizes, the soft-templating method is more likely to generate a hierarchically porous structure and more suitable for the preparation of thicker carbon forms with no need for additional template removal steps.10,54 Various sacrificial polymers as pore-generating agents have been utilized in the PAN precursor, e.g., poly(methyl methacrylate) (PMMA),6,55–58 polystyrene (PS),59,60 polyvinyl pyrrolidone (PVP),61,62 dimethyl sulfone63 and polytetrafluoroethylene (PTFE),64 which can decompose or evaporate during preoxidation or carbonization without collapse of the pore structure. For example, Zhang et al. synthesized PCFs with abundant internal pores by carbonizing PAN/PMMA blend nanofibers. The obtained PCFs exhibited a free-standing hierarchical structure (micro/meso (2–10 nm)/macropores (50–80 nm) and nanochannels).6 Similarly, to further improve the porosity and surface area of PCFs, Naraghi's group activated PCFs fabricated from PAN/PMMA emulsion and coaxial electrospinning by KOH under various conditions (Fig. 2E).55 It was shown that the specific surface area and pore volume of the activated PCFs improved from 52.3 m2 g−1 to 1753.9 m2 g−1, and from 0.31 to 2.15 cm3 g−1, respectively. Ahn's group used a different strategy to produce PCFs from a PAN/PVP blend polymer. The water etching process to remove PVP on the as-spun nanofiber surface was beneficial for the formation of a mesoporous structure before carbonization.65 The obtained PCFs possessed 43.9% mesopores and 56.1% micropores, showing a specific surface area of up to 692 m2 g−1 and pore volume of 0.45 cm3 g−1.
Besides the standard PCFs with a meso/macro pore structure both in and outside the fibers, even more interesting morphologies can be prepared by adjusting the type and ratio of precursor and sacrificial polymer. Cho's group produced PCFs with churro-like morphology by utilizing the kinetically different pore formation of PS and PAN polymer during high temperature fabrication (Fig. 2F).66 The resulting PCFs showed a high surface area (1271.2 m2 g−1) contributed by the increase in the mixed meso- and macropores. Later, blending more PAN with PS as a precursor was used to prepare PCFs with a lotus-root like porous structure.59,60,67,68 Interestingly, it was found that not only the PS phase but also other polymers, such as PMMA57 and PVA,69 can be easily stretched along the longitudinal direction within the PAN fibers during the electrospinning process. Consequently, in many studies using the electrospinning process, the PCFs formed with the aid of a sacrificial polymer showed a similar morphology, channel-like inner pores with different pore sizes along the fiber axis and a great deal of micro–mesoporous on the surface of the fibers.
Obviously, PAN is currently the best precursor for the fabrication of CFs due to its ability to grow into a well-tuned carbon network after carbonization.70 For example, Qiu's group and Xie et al. took advantage of the principle that thick polymer fibers have more structural flaws and irregular molecular chains, successfully preparing PCF containing a lot of finger-like macro-porous structures through the thermal decomposition of pure PAN fibers.71,72 By using carbon dioxide (CO2) as an active agent to etch more micropores, the final PCFs demonstrated a specific surface area of 387 m2 g−1.71 However, some polymers with a medium carbon content are also feasible for the construction of porous carbon structures when mechanical properties are not highly required.73–76 Yan et al. electrospun porous PS fibers as templates and fabricated nitrogen-doped mesoporous composite PCFs via a mussel-inspired biomimetic polydopamine-coating process and subsequent high-temperature carbonization (Fig. 2G).74 The obtained PCFs had a specific surface area of 356 m2 g−1 and a pore size mainly distributed in the range of 20–70 nm. Rutledge's group employed phase separation and electrospinning to produce PVDF-based PCFs using PEO as the sacrificial agent and water as the non-solvent.75 The obtained PCFs exhibited a high surface area of greater than 380 m2 g−1 and hierarchical pore structure. Guo et al. synthesized coal-derived PCFs with tunable internal channels.76 In their work, the pre-treated coal contributed to the carbon component and PVA acted as a sacrificial template for creating pores ranging from 1.7 to 300 nm in the fibers. By optimizing the mass ratio of PVA and coal, a specific surface area and pore volume as high as 728 m2 g−1 and 0.526 cm3 g−1, respectively, were obtained.
A summary of the different methods to produce PCFs with rich meso/macro-pores is shown in Table 1. Generally, the nanocasting method is a simple approach that can generate a more uniform distribution of mesopore sizes. It is extremely suitable for the fabrication porous carbon nanofibers because fibers with an ultra-thin diameter can facilitate the entire removal of the hard template. However, the dispersion and subsequent removal of the nanoparticles in the PCFs are complex, costly and time-consuming. The soft-templating approach is more feasible for fiber spinning with less consideration of diameter and can lead to a dramatic increase in hierarchical pores both in and outside the fiber. However, through simply blending the sacrificial polymer, the pores are generally disordered and randomly distributed. In addition, the formation of meso-structures is highly sensitive to the reaction conditions.
| Porous structure type | Precursor for porous structure | Pore formation mechanism | SSA (m2 g−1) | V T (cm3 g−1) | Pore types | V Micro | V Meso | Ref. |
|---|---|---|---|---|---|---|---|---|
| a V T: total pore volume; VMicro: micropore volume; and VMeso: mesopore volume. Indicated the presence of well-controlled mesopores, with a size of ∼9.3 nm on average. | ||||||||
| Rich meso/macro-pores | Ppy | KOH activation | 1923 | 0.86 | Micro-/meso- | 0.82 | 0.04 | 103 |
| PAN | NaHCO3 template | 724 | 0.71 | Micro-/meso- | 0.44 | 0.27 | 33 | |
| PAN | Poly(m-aminophenol) template | 1031 | 1.34 | Micro-/meso- | 0.7077 | 0.635 | 104 | |
| PAN | PMMA template + steam activation | 1426 | 1.20 | Micro-/meso- | 0.60 | 0.60 | 38 | |
| Coal | PVA template | 691 | 0.53 | Micro-/meso- | 0.226 | 0.27 | 76 | |
| Phenolic | KOH activation | 1317 | 0.70 | Micro-/meso-/macro- | — | 0.540 | 105 | |
| PAN | Porous carbonaceous guest | 478 | 1.067 | Micro-/meso-/macro | — | 0.926 | 106 | |
| Highly interconnected pore structure | PVA | PTFE template | 591 | 0.58 | Micro-/meso-/macro- | — | 0.457 | 95 |
| Cu-MOF | KOH activation + acid etching | 1906 | 1.35 | Micro-/meso-/macro- | — | — | 99 | |
| PAN/SIO2 | SiO2 template + KOH activation | 1796 | 1.45 | Micro-/meso-/macro- | 0.86 | 0.59 | 82 | |
| PAN | ZIF-8 template | 560 | 0.79 | Micro-/meso-/macro- | 0.137 | 0.264 | 90 | |
| PAN | Fe(acac)3 template + KOH activation | 509 | 0.78 | Micro-/meso-/macro- | — | — | 40 | |
| PAN | Zn(ac)2 template | 588 | — | Micro-/meso-/macro- | — | — | 85 | |
| PAN | Phase separation + KOH activation | 2176 | 1.27 | Micro-/meso-/macro- | — | 0.74 | 107 | |
| PI | Humidity-induced phase separation | 456 | — | Meso-/macro- | — | — | 7 | |
| Defect-rich surface pore structure | Cotton | KOH activation | 1120 | 0.49 | Micro-/meso-/macro- | — | — | 108 |
| Collagen | Zr(SO4)2 template | 1212 | 0.68 | Micro-/meso- | — | — | 109 | |
| Cotton | KOH activation | 584 | 0.38 | Micro-/meso-/macro- | — | — | 110 | |
| Wood sawdust | KOH activation | 2294 | 1.41 | Micro-/meso-/macro- | 0.721 | 0.610 | 111 | |
| Bacterial cellulose | NaOH hydrothermal | 111 | 0.21 | Micro-/meso-/macro- | — | — | 112 | |
| Wood fiber | CO2 and KOH dual activation | 1529 | Micro-/meso-/macro | 113 | ||||
| Carbonaceous surface pore structure | Cellulose/graphene | KOH activation | 831 | 0.42 | Micro-/meso-/macro- | — | — | 114 |
| Glucose | K3[Fe(C2O4)3] activation | 1516 | 0.56 | Micro-/meso-/macro- | 0.53 | — | 115 | |
| Polyaniline | Polyaniline degradation | 1487 | — | Micro-/meso- | — | — | 116 | |
| Amylum | KOH activation | 493 | — | Meso-/macro- | — | — | 117 | |
| PANI | PANI degradation | 485 | 0.29 | Micro-/meso- | — | — | 118 | |
| Platanus fiber | Catalytic self-deposition technology | 219 | 0.30 | Micro-/meso- | — | — | 119 | |
| Graphene assemblies | GO/CNT | Graphene assembly | 404 | — | Meso-/macro- | — | — | 120 |
| GO | Graphene assembly | 389 | 0.31 | Meso-/macro- | — | — | 121 | |
| GO gel | Phase separation | 884 | 5.85 | Meso-/macro- | — | — | 122 | |
| GO/SWNT | Graphene assembly | 396 | — | Micro-/meso- | — | — | 1 | |
| GO/phenol formaldehyde | Graphene assembly | 416 | — | Micro-/meso-/macro- | 0–8.3% | 88–97% | 123 | |
2.2 PCFs with highly interconnected pore structure
With the constant pursuit of broader applications and higher performances, a novel type of PCF containing interconnected micro and meso/macro-pores is currently attracting considerable attention. Pores interconnecting each other within a fiber can provide sufficient pathways/channels and accommodation for the transport and storage of core materials and significantly elevate the utilization of active sites, which will be beneficial for applications such as electrodes in electrochemical energy storage devices,77 catalysts78 and adsorption and separation.79 In fact, it is difficult to draw a strict distinction between PCFs with isolated pores and PCFs with interconnected pores given that some synthetic methods or post treatment processes can produce two types of structures within one fiber. Hence, this section will mainly discuss the PCFs with a very high percentage of interconnected pores. Although the traditional nanocasting method is usually difficult to directly produce crisscrossing channels between the pores for sufficient interconnection, there has been some success where additional nanoparticles are used, leading to PCFs with an interconnected pore structure.80–85 For example, Fan81 and Lee et al.82 obtained PCFs with interconnected macropores by simply electrospinning the precursor polymer containing an SiO2 nanoparticle template (66 wt% and 50 wt%, respectively) followed by carbonization and SiO2 removal process (Fig. 3A). With an increase in the amount of SiO2, the connected mesopores and macropores were developed. Consequently, the N2 adsorption/desorption isotherms of the resultant PCF exhibited type-IV isotherm behaviors along with H2 hysteresis, implying the presence of mesopores because of the spherical chambers and the connecting passages. Sun et al. proposed a bamboo-like PCF with well-balanced micro-, meso-, and macro-porosity using tetraethyl orthosilicate as an SiO2 precursor.83 The PCFs containing hollow interiors showed a hierarchical and interconnected porous structure, namely, micro- and small mesopores in the external surface and large mesopores in the internal area of the shell.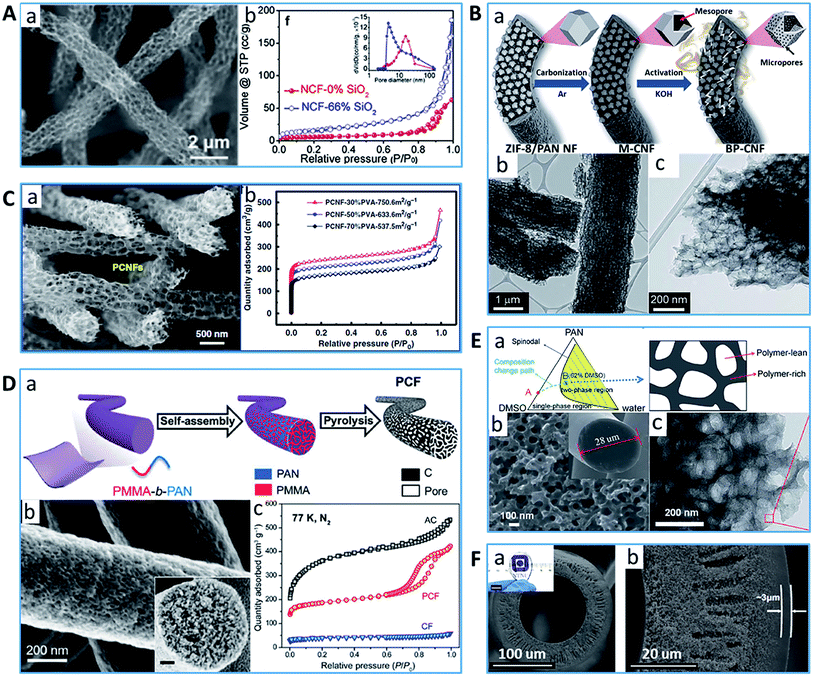 | ||
| Fig. 3 Typical examples of PCFs with highly interconnected meso/macro-pore structure. (A) (a) SEM image of PCF synthesized using SiO2 precursor and (b) N2 sorption isotherms.81 Reproduced with permission from ref. 81. Copyright 2016, Elsevier. (B) Illustration of the fabrication process of PCF using ZIF as a template, (b) and (c) TEM images.86 Reproduced with permission from ref. 86. Copyright 2018, Royal Society of Chemistry. (C) (a) SEM image of the PCFs with continuous macropores, (b) N2 sorption isotherms.97 Reproduced with permission from ref. 97. Copyright 2019, Nature. (D) (a) Schematic of material processing, and (b) SEM image and (c) N2 sorption isotherms.79 Reproduced with permission from ref. 79. Copyright 2020, Science. (E) (a) Phase diagram of the PAN/DMSO/water ternary system (left) and schematic diagram of the separation phase, (b) SEM image and (c) TEM image.107 Reproduced with permission from ref. 107. Copyright 2015, Royal Society of Chemistry. (F) (a) and (b) Cross-sectional SEM images of cellulose-based asymmetric carbon hollow fiber.127Reproduced with permission from ref. 127. Copyright 2021, Nature. | ||
More interestingly, Kang's group synthetized PCFs comprising interconnected bimodal pores by adding 50 wt% of ZIF-8 to the PAN precursor (Fig. 3B).86 The internal structure of the obtained PCFs presented numerous hollow carbon nanocages derived from the ZIF-8 nanoparticles with a good distribution. The volatilization and acid etching of the massive zinc within the CF and partial deterioration of the mesoporous structure by KOH activation may contribute to the increased pore interconnection. Lou's group prepared a PCF precursor by mixing PAN (0.175 g) with an even higher ratio of ZIF-8 (0.263 g).87 After heat treatment, the large mass loss within the interconnected ZIF-8 particles resulted in the formation of hierarchically PCFs with a specific surface area of 417.9 m2 g−1. More importantly, the porous architecture composed of abundant interconnected carbon hollow nanoparticles ensures ample channels and active sites. Similar works to produce PCFs with interconnected porous morphologies by encapsulating a large amount of MOF have been reported by Yang,88 Lin89 and Yao et al.90
From a polymer perspective, high dosages of sacrificial component will lead to a highly porous structure in CFs.91,92 Kang's group successfully fabricated PCFs with well-distributed interconnected meso- and macro-pores derived via electro-blown spinning of PVA/PTFE blends.93–96 The obtained highly porous structure offered more active sites for catalyst doping compared with normal CFs, which efficiently avoided the accumulation of the catalyst. Ding's group developed a new strategy, i.e., cross linking PVA and PTFE with boric acid to form water-sol webs, which were electrospun into nanofibers (Fig. 3C).97 Then continuous porous carbon nanofibers with interconnected well-controlled micro–meso–macro pores were synthesized, showing an ultrahigh porosity of >80% and outstanding surface area of ∼750 m2 g−1. The sharp N2 adsorption–desorption isotherms at P/P0 > 0.9 indicated that there were large amounts of macropores in the PCFs. Based on a humidity-induced phase separation mechanism, Zhang et al. reported a new template-free approach to produce different PCFs with a rich topology and interconnected cavities by well-regulating the structure of the polymer precursor.7 The fabricated PCFs exhibited a high specific surface area and wide distribution (form micro to macro pore size). Moreover, some new strategies to produce interconnected pores were reported, such as novel structure design98 and new precursor exploration.92,99,100
However, unlike the nanoparticle templating method, it is still challenging for the sacrificial polymer approach to control the size and uniformity of the interconnected mesopore structure. Recently Liu's group demonstrated a disruptive method for synthesizing PCFs with uniform interconnected mesopores with a narrow pore size distribution from a PAN-b-PMMA block copolymer prepared via metal-free reversible addition–fragmentation chain transfer polymerization (Fig. 3D).11,79,101,102 Different from polymer blends during thermal treatment, the crosslinking in the block copolymer prevented the precursor from forming long range-ordered nanostructures, resulting in the formation of an interconnected porous structure. The as-prepared PCFs had a high surface area of 503 m2 g−1 and total pore volume of 0.45 cm3 g−1. More importantly, the type-IV isotherm behaviours and H1 hysteresis from the N2 adsorption–desorption measurements.
Notably, the electrospinning approach has been used for the preparation of precursor nanofibers for PCFs with a high specific surface area, and moreover the ultrafine diameter of the fibers obtained via a simple process makes it easier to remove the templates and generate pores. Especially for the requirement of PCFs with well-arranged porous morphologies and high pore volume, a precursor with a nanoscale diameter is the optimum choice at the current stage. However, the fibers obtained from electrospinning are commonly in a randomly oriented form and relatively limited in quantity, which the at current stage cannot meet the requirements of practical applications when a large amount of long continuous fibers is required. Traditional spinning methods, such as solution spinning and melt spinning, are the most established industrial processes to produce polymer fibers with a micron-size diameter. Nevertheless, there are limited works reported on PCFs, especially with a highly porous structure, from polymer precursors produced through these conventional spinning processes. Thus, it remains a great challenge to produce PCFs owning the hierarchical pore size and high pore volume of the precursor fibers with a micron diameter through traditional pore-making approaches such as templating method, activation/oxidation method and coating/grafting method. This is because not only the complex spinning and carbonization technologies of the thick fiber hybridize the guest materials but also the difficulties in removing the templates, preventing the collapse of the ordered pores/channels and loss of the surface area and pore volume and retaining the fiber formation and mechanical/flexible properties of PCFs. In 2019, the world's leading manufacturer of PAN-based CFs, Toray Industries, Inc., announced the world's first porous CF with a nanosized continuous pore structure.124 It is hard to find the exact preparation scheme of this PCF innovated by combining Toray's outstanding polymer technology and its market share-leading CF technologies. However, by reviewing the patent published by Toray, the PCF having a continuous porous structure could be produced by wet-spinning of a mixture of PAN (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 molecular weight) and eliminable resin, such as PVP in DMSO followed by phase separation in a pure water coagulating bath and pyrolysis process with a maximum temperature of 1500 °C.125 The obtained PCF presented more than 40% porosity and controllable structural period (0.002–1 μm) of continuous bimodal porous structure (meso: ∼50 nm and macro: ∼200 nm), which is quite important for a fluid to be filled and/or passed into or through. In another study, Lu's group took advantage of their developed understanding in the effects of stretching temperature and ratio on the porous architecture of wet-spun PAN fibers and produced PCFs with an interpenetrating 3D meso- and macro-porous network successfully (Fig. 3E).107,126 It was found that the 3D porous network can be formed due to the phase-separation during coagulation, and the variation in the pore structure within the fibers can be adjusted by changing the PAN molecular chain or microfibril arrangements during the water stretching process. With the aid of KOH activation, the obtained activated PCFs showed a total specific surface area of 2176.6 m2 g−1 and the total pore volume of 1.272 cm3 g−1. Recently, Lei et al. reported the fabrication of cellulose-based porous carbon hollow fibers.127 By tuning the coagulation temperature of the microcrystalline cellulose/ionic liquid/water system, the obtained carbon fibers exhibited an asymmetric structural morphology throughout their body, including a porous inner layer, a middle layer rich in macrovoids and a dense outer layer. By controlling the conversion of sp3 to sp2 hybridized carbon via different the carbonisation temperature, finely tuned ultramicropores were achieved in the dense outer layer of the PCF (Fig. 3F).
000 molecular weight) and eliminable resin, such as PVP in DMSO followed by phase separation in a pure water coagulating bath and pyrolysis process with a maximum temperature of 1500 °C.125 The obtained PCF presented more than 40% porosity and controllable structural period (0.002–1 μm) of continuous bimodal porous structure (meso: ∼50 nm and macro: ∼200 nm), which is quite important for a fluid to be filled and/or passed into or through. In another study, Lu's group took advantage of their developed understanding in the effects of stretching temperature and ratio on the porous architecture of wet-spun PAN fibers and produced PCFs with an interpenetrating 3D meso- and macro-porous network successfully (Fig. 3E).107,126 It was found that the 3D porous network can be formed due to the phase-separation during coagulation, and the variation in the pore structure within the fibers can be adjusted by changing the PAN molecular chain or microfibril arrangements during the water stretching process. With the aid of KOH activation, the obtained activated PCFs showed a total specific surface area of 2176.6 m2 g−1 and the total pore volume of 1.272 cm3 g−1. Recently, Lei et al. reported the fabrication of cellulose-based porous carbon hollow fibers.127 By tuning the coagulation temperature of the microcrystalline cellulose/ionic liquid/water system, the obtained carbon fibers exhibited an asymmetric structural morphology throughout their body, including a porous inner layer, a middle layer rich in macrovoids and a dense outer layer. By controlling the conversion of sp3 to sp2 hybridized carbon via different the carbonisation temperature, finely tuned ultramicropores were achieved in the dense outer layer of the PCF (Fig. 3F).
The different formation mechanisms to produce PCFs with interconnected porous structures are shown in Table 1. The templating approach by adding additional nanoparticles or sacrificial polymer is a relatively facile approach to obtain interconnected pores. However, highly uniform interconnected mesopores and a narrow pore size distribution are still challenging, and thus the use of block copolymers as PCF precursors may be a good option. In addition to the widely used electrospun fibers, wet-spun fibers have been also successfully converted into PCFs with a continuous porous structure by researchers as well as world-leading CF manufacturers in recent years.
2.3 PCFs with defect-rich surface pore structure
Besides innovative synthetic approaches, the world is paying increasing attention to the development of renewable, sustainable and cost-effective technologies, where the exploration of PCFs is no exception. Thus, compared to the methods discussed in the previous sections, the direct processing of the existing inexpensive and abundant fibers to form PCFs may be more worthwhile from an economic perspective. In addition, due to the inherent pores of the fiber precursor, post-treatment of CFs via activation and chemical exfoliation is the most common method to create abundant surface defects and generate pores. One option as the PCF precursor is biomass due to its abundance and additional value derived from its renewable, eco-friendly and biodegradable character. Theoretically, almost all natural biomass can be carbonized into carbon with unique structures and natural porosity; however, only natural fibers with well-organized structures lead to PCFs. These fibers include cotton,110,128–132 sisal,133 jute,134 silk,135 bamboo,136 fungi,137 collagen,109,138 and bacterial cellulose.139 For example, Yang et al. produced activated PCFs with a large amount of surface defects through the carbonization of cotton fibers followed by activation with KOH.130 The resultant PCFs presented a large number of disordered micro- (∼2.5 nm pore diameter) and meso- (∼20 nm pore diameter) porous structures. Gao and co-workers investigated the effect of NaOH/urea swelling of cellulose on the formation of porosity in PCFs.110 The resultant PCFs possessed a defective nature and hierarchical porous characteristic. Rangel-Mendez et al. explored two physical activation methods and one chemical activation method to produce PCFs from jute and henequen fibers, respectively.134 It was found that the PCFs made by ZnCl2 activation had a narrow microporosity and high specific surface area of up to 1210 m2 g−1, while the steam and CO2 activation methods resulted in PCFs with a wide pore range, from micropores to mesopores. Shi's group synthesized PCFs by carbonizing collagen fibers containing metal salt under vacuum.109 The obtained PCFs with a great number of micropores showed a surface area of up to 1212 m2 g−1, pore volume of 0.68 cm3 g−1 and some amount of mesopores (<10 nm). Cao's group developed carbon nanofiber webs with a micro- and meso- (<15 nm) porous structure, a surface area of 1235.58 m2 g−1 and abundant surface defects via KOH activation of the pyrolyzed bacterial cellulose.139 Notably, most natural fibers cannot be over-exfoliated or activated to get larger meso- or even macro pores because their relative weak molecular structure can be destroyed and collapse into a powder or short fibers with a size on the mm scale by the harsh activation treatment process.140,141 Our group proposed an inventive “dynamic template (KOH, SiO2, and Al2O3) calcination” strategy to convert cotton fabric into a hierarchical micro-/meso-/macro-porous and hollow CF textile material without diminishing its mechanical robustness (Fig. 4A).142 It was found that the water generated by the reaction between KOH and SiO2 or Al2O3 at high temperature was beneficial in enlarging the pores up to 200 nm by reacting with metallic potassium particles. However, although a great deal of cavities and surface defect sites were introduced, the resulting PCFs retained a long fibrous structure and presented a rougher surface, which effectively prevented the single fiber from sliding and rendered the porous carbon fiber fabric even more rigid than the carbon textile achieved from neat cotton.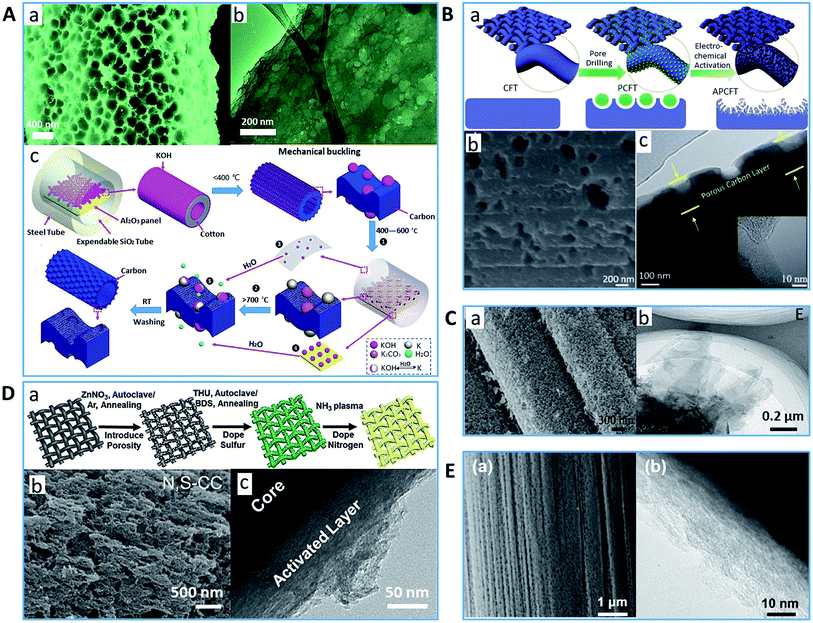 | ||
| Fig. 4 Typical examples of PCFs with defect-rich surface pore structure (A) (a) SEM image and (b) TEM image of PCF synthesized using cotton and (c) illustration of its fabrication process.142 Reproduced with permission from ref. 142. Copyright 2019, Wiley. (B) (a) Schematic diagram of PCF with more hierarchical porous structures from commercial CF, (b) SEM image and (c) TEM image.146 Reproduced with permission from ref. 146. Copyright 2019, Wiley. (C) (a) SEM image and (b) TEM image of PCFs with edge/defect-rich graphene by plasma treatment.3 Reproduced with permission from ref. 3. Copyright 2017, Wiley. (D) (a) Schematic of material processing, (b) SEM image and (c) TEM image of PCF synthesized by ZnO etching and plasma treatment.149 Reproduced with permission from ref. 149. Copyright 2018, Wiley. (E) (a) SEM image and (b) TEM image of PCF from commercial CF via H2-etching process.150 Reproduced with permission from ref. 150. Copyright 2018, Elsevier. | ||
Besides biomass, the activation plus carbonization process can also create a porous surface on CFs derived from synthetic polymer fibers. Zhang et al. obtained PCFs with uniform micropores (mainly <1 nm) and micron-scale etched texture using KOH as an activating agent with a KOH/melt-spun pitch-based CF ratio of 4/1.143 The as-prepared PCFs possessing 83.56% micropores exhibited a high disorder carbon ratio, a specific surface area of 1753 m2 g−1 and pore volume of 0.73 cm3 g−1. Qian's group produced PCFs with a large mesopore volume (2.3 cm3 g−1) by carbonizing pre-oxidized PAN fibers in an atmosphere of CO2.144 The optimal operating condition (950 °C for 12 h) gave a product with a specific surface area of up to 2404 m2 g−1 and high mesopore (∼6.6 nm) ratio (up to 92.8%).
With the growing popularity of flexible devices, the demand for corresponding flexible and continuous PCFs is significantly increasing. Commercial CFs have been widely used in high-performance applications due to their excellent mechanical strength and high conductivity. Thus, endowing CFs with a large accessible surface area and high porosity would be an elegant approach for synthesizing PCFs with good mechanical properties. For example, Lou et al. demonstrated the facile preparation of PCFs with micropores and small-size mesopores via a freeze-drying-assisted KOH activation method.145 The produced PCFs displayed a specific surface area of 2109 m2 g−1 and pore volume of 0.713 cm3 g−1. However, it is still very challenging for conventional activation and exfoliation approaches to realize more mid- and large-size mesopores or even macropores. This is due to the super-organized graphite structure of commercial CFs, which makes it difficult to get well-controlled porosity and pore size, and to preserve the fiber integrity. Hence, more advanced strategies are being developed to directly tune CFs into a high-performance PCFs with more hierarchical porous structures. Han et al. produced PCFs by synthesizing NiOOH nanoflakes on the surface of CFs via the hydrothermal method followed by annealing in an N2 atmosphere (Fig. 4B).146 At high temperature, the nickel oxide hydroxide nanoflakes were converted into Ni nanoparticles, and plentiful pores were developed on the surface and inside of the CFs. The porosity could be controlled by changing the NiOOH concentration or the calcination temperature. The obtained PCFs exhibited an increase in defective sites on the carbon surface, a high specific surface area of 201.3 m2 g−1 and wide pore size distribution ranging from 0.5 to 32 nm. Zhou's group demonstrated a new activation method to produce PCFs via a two-step procedure, namely, homogeneous growth of MOF on CFs and subsequent activation catalyzed by the MOF derivatives at high temperature.147 The achieved PCFs showed the coexistence of micropores, mesopores, and macropores. Liu et al. successfully realized the generation of micro/nanopores and in situ exfoliation of edge/defect-rich graphene on the surface of CFs with Ar plasma treatment (Fig. 4C).3 The obtained PCFs possessed an enhanced specific surface area, good intrinsic electron conductivity, efficient mass transport, and abundant active sites. Similarly, Duan's group reported a PCF paper prepared via thermal annealing and subsequent O2 plasma etching of the commercial pristine carbon fiber paper.148 The resulting PCFs had a highly porous, roughened surface with many open mesopores. Zhao et al. successfully produced PCFs by utilizing the mechanism that the uniformly deposited ZnO on the surface of CFs via hydrothermal treatment can be reduced into Zn and the metal Zn can evaporate by carbothermic reaction at a desirable temperature (Fig. 4D).149 The obtained PCFs demonstrated a super rough surface with a pore network. With the assistance of NH3 plasma treatment, more defect sites were generated. The final product showed a hierarchical micro–meso–macroporous morphology with a specific surface area of 146.2 m2 g−1 and a pore volume of 0.24 cm3 g−1. Zhang's group synthesized PCFs with a defect-rich, surface porous, coaxial cable-like structure though a high-temperature H2 etching process on commercial carbon fibers oxidized by H2SO4 and H2O2 (Fig. 4E).150 They found that the exfoliated graphene layer rendered by the pre-oxidation procedure played an important role in facilitating the H2-etching process and resulted in the formation of an outer nanostructured porous carbon shell and uniformly distributed macropores with an average diameter of ∼100 nm.
Some typical formation mechanisms to produce PCFs with defect-rich surface pore structures are shown in Table 1. In general, post-treatment of carbon fibers via activation and chemical exfoliation is an effective and facile method to improve the porosity, particularly for fibers with a relatively thick diameter, such as well-known synthetic fibers and natural fibers. However, it is difficult to use this method to control the pore size distribution and porosity, and sometimes corrosive chemicals are needed, which are not environmentally friendly. Converting commercial CFs to PCFs with a large accessible surface area and high porosity presents significant potential for industrial production even though there are still challenges with regards to destroying the super-organized graphite structure, obtaining well-controlled porosity and preserving the fiber integrity.
2.4 PCFs with carbonaceous surface pore structure
The smooth surface, low specific area and poor electrochemical properties of commercial CFs significantly limit their application, particularly in the energy and environmental fields. In contrast, porous carbon materials display a high specific surface area and surface roughness, but their unique porous structure could limit, and even sacrifice the mechanical properties. Therefore, coating or grafting using porous carbon materials can be a facile strategy to combine the advantages of CFs and porous carbon materials. Some porous carbon materials such as graphene and CNT were coated on CFs as novel surface treatments for polymeric composites to improve the interfacial bonding between the fiber and polymer matrix,151,152 but in this review, only carbon–carbon composites for energy and environmental applications are discussed.Physical coating methods such as dip-coating117,133,153 and brush coating114 are facile methods to coat porous carbon materials or their precursor on carbon fibers. For example, Fan's group coated a thin film of homogeneous precursor of metal salts (Ni, Fe, and Mo) and PVP onto carbon fiber surface through the dipping method, and then CFs with a metal nanoparticle/porous carbon hybrid film were obtained through anneal treatment (Fig. 5A).153 The approximately 200 nm-thick porous layer effectively improved the specific surface area of the CFs and provided intensive active sites at a specific area; moreover, the presence of metal catalyzed the etching of the carbon fibers and generated more pores. Zhang's group synthesized a 3D network porous carbon matrix covering the entire surface of each CF by soaking CFs in a sol–gel solution of amylum and KOH followed by drying, carbonization and washing (Fig. 5B).117 It was found that the carbon layer on the CF surface was composed of interconnected and intersected carbon nanoflakes with a thickness <100 nm, and the 3D networked carbon matrix contained irregular pores with a size ranging from 30 to 80 nm, as well as a specific surface area of 493 m2 g−1. Zhang's group brushed a graphene–cellulose–KOH slurry on the surface of a spread CF/graphene hybrid film to fabricate a hierarchical porous carbon-concrete/CF hybrid (G-ac/CF) film by thermal treatment.114 The G-ac/CF hybrid film exhibited a high surface area of 831 m2 g−1 and total pore volume of 0.42 cm3 g−1 with a mixed micro-, meso- and macro-porous structure. More interestingly, the as-obtained G-ac/CF hybrid film exhibited good flexibility and favorable tensile strength of 5.3 GPa.
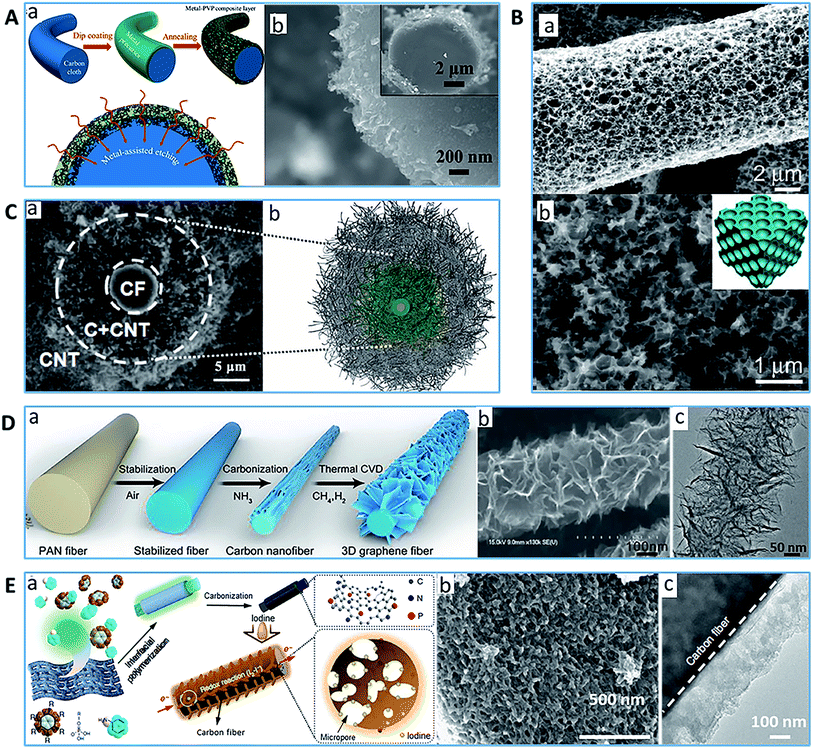 | ||
| Fig. 5 Typical examples of PCFs with a carbonaceous surface pore structure (A) (a) Schematic of metal-based nanoparticles/N-doped PCF and (b) SEM images of PCF.153 Reproduced with permission from ref. 153. Copyright 2017, Wiley. (B) (a) and (b) SEM images of CF decorated by 3D networked porous carbon matrix.117 Reproduced with permission from ref. 117. Copyright 2017, Wiley. (C) (a) SEM cross-sectional image and (b) illustration of the CNT@CF fiber consisting of a CF at the center.62 Reproduced with permission from ref. 62. Copyright 2018, Wiley. (D) (a) Schematic diagram of PCF decorated with interconnected graphene, (b) SEM image and (c) TEM image of PCF.154 Reproduced with permission from ref. 154. Copyright 2018, Wiley. (E) (a) Illustration of the fabrication process of PCF via PANI deposition, (b) SEM image and (c) TEM image of PCF.116 Reproduced with permission from ref. 116. Copyright 2017, Nature. | ||
Alternatively, compared with physical coating, chemical coating or deposition methods (such as CVD,62,154,155 polymerization116,118,156 and electrochemical deposition6,157) usually involve chemical bonding between the carbon fibers and coated materials, which facilitates further robust bonding. Cao's group fabricated a single CF with a 3D highly porous CNT sponge layer and macroscopic-thickness via the CVD approach (Fig. 5C).62 The resulting PCF presented a high porosity of 87.1% with mesopores (2–25 nm) and a significant portion of macropores (20–50 μm). Yu's group prepared densely arranged and interconnected 3D graphene sheets on the surface of carbon nanofibers using NH3 during carbonization followed by thermal CVD (Fig. 5D).154 Compared to the traditional carbonization process with a protective atmosphere of Ar or N2, NH3 can facilitate the radial growth of the graphene sheet on the CF surface. The finally produced vertical graphene sheet with edges exposed on the CNF surface exhibited a bending and crumpling morphology, which interconnected to form a porous interspace ranging from 20 to 100 nm. In addition, it was found that PANI during carbonization can create considerable micro-/mesopores and also ample nitrogen-containing functionalities. For example, Zhang and Li et al. coated carbon nanofibers with PANI via in situ polymerization to obtain nitrogen-doped hierarchical porous carbon ensembles on CFs followed by carbonization.118,156 The as-modified carbon nanofibers showed the coexistence of micro- and mesoporous structures and a remarkable improvement in surface area. Lu et al. produced a hierarchically porous carbon matrix via the interfacial polymerization of aniline on a cellulose wiper in the presence of phytic acid, followed by carbonization (Fig. 5E).116 The as-prepared PCFs covered uniformly with a hierarchical porous carbon network were reported to exhibit a high specific surface area of 1487 m2 g−1 and porous structure with a narrow size distribution of micro- and mesopores. In addition, Wang et al. fabricated 3D porous graphene scaffold-wrapped ACFs via the electrodeposition of GO nanosheets on CFs using ionic liquids as electrolytes, and found that this nanohybrid structure dramatically increased the surface area and roughness of the CFs, and provided abundant nanoscale pores and/or channels.157
Examples of coating porous carbon materials on the surface of CFs are provided in Table 1. Overall, physical coating is an effective and facile method to improve the porosity of CFs with less consideration of fiber diameter. However, the evenness of the porous carbon coating may not be well controlled. Furthermore, the relatively weak bonding between the coating layer and carbon fiber can affect their properties and hinder their future applications. Although chemical coating can generate stronger chemical bonding and more uniform distribution of pores, there are still challenges, such as complex process as well as being costly and time-consuming, which limit the production scale.
2.5 PCFs with graphene assemblies
Owing to its outstanding properties and special structure, graphene has gained considerable attention from scientists and engineers since its discovery. Since its initial development in 2011, graphene-based fibers have become one of the most promising carbonaceous fibers with high expectations in science and technology.158 Graphene-based fibers not only inherit the extraordinary mechanical robustness, electrical and thermal conductivities of graphene, but also exhibit admirable features of fibers such as mechanical flexibility, light weight, high aspect ratio and ease of functionalization.159–163 More importantly, special porous structures can be introduced in graphene fibers by controlling the coagulation conditions and nozzle type.122,164 For example, Gao's group exploited liquid crystalline graphene oxide gels to fabricate a neat “core–shell” structure graphene aerogel through spinning using liquid nitrogen as the coagulation bath followed by freeze-drying, chemical reduction with hydroiodic acid and further thermal treatment (Fig. 6A).122 This ice-templating strategy caused the as-obtained reduced GO fiber to own a relatively high specific surface area (up to 884 m2 g−1) and rich hierarchical porous structure with a wide pore distribution from several to 100 nm. Cheng's group tailored porous RGO fibers via a scalable non-liquid-crystal wet-spinning route from a basified GO dispersion using acetic acid as the coagulation bath and subsequent chemical reduction (Fig. 6B).165 Given that the basified GO sheets were more negatively charged, and therefore tended to have a greater mutual repulsion after extrusion to generate pores, particularly with a low rotator speed during spinning, the lack of stretching could help graphene fibers with a separate pore structure rather than a lamellar structure, achieving a high specific surface area (up to 419 m2 g−1) with a tunable, locally aligned pore structure. Aboutalebi et al. designed a novel wet-spinning approach using acetone as the coagulation bath and subsequent thermal reduction to synthesize continuous porous graphene fibers from the acidic GO dispersion (Fig. 6C).166 The thermal reduction process of GO, the fiber formation/solidification rate, and fast dehydration of GO due to the remarkable ability of acetone to expel water were beneficial to form a highly porous structure of graphene-based PCFs with a high surface area (up to 2210 m2 g−1). Jiang et al. tailored hierarchical 3D and porous RGO fiber fabrics by wet spinning from a GO solution using ethyl acetate as the coagulation bath.167 The generation of gas by the decomposition of oxygen-containing functional groups during hydrazine hydrate vapor reduction created a porous and defective structure in the graphene fibers, with a pore size of dozens of micrometers. Moreover, for the wet-spinning, the spinning parameters are also significant factors that affect the porosity of graphene-based fibers. For example, Ye's group prepared graphene fibers with a nanoscale porous surface using a metal needle from a medical syringe as the spinneret (Fig. 6D).168 The concave–convex-shaped internal wall of the needle caused the as-formed graphene to possess a rich-defect surface and a remarkable specific surface area up to 839 m2 g−1.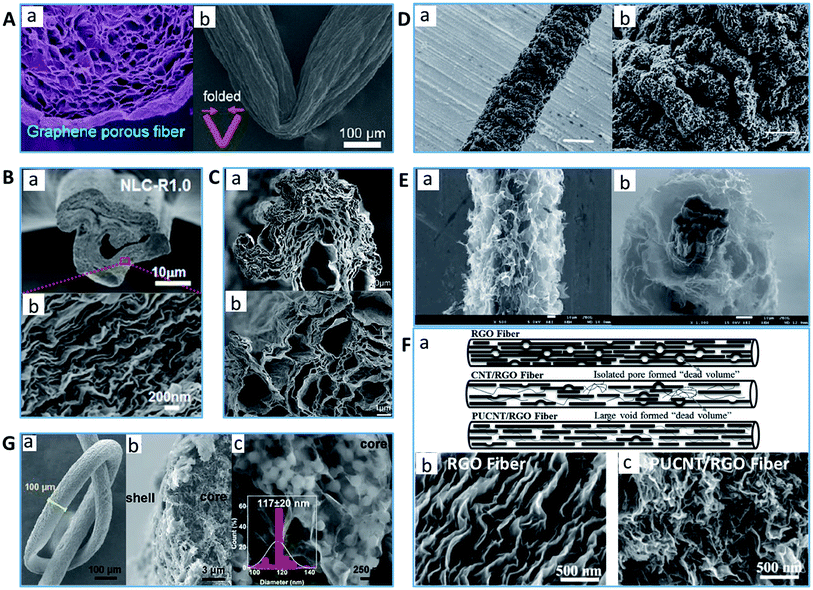 | ||
| Fig. 6 Typical examples of PCFs with graphene assemblies (A) SEM images of fracture morphology of PCF and folded graphene-based PCF.122 Reproduced with permission from ref. 122. Copyright 2012, American Chemical Society. (B) SEM images of porous RGO fiber by a scalable non-liquid-crystal wet-spinning route.165 Reproduced with permission from ref. 165. Copyright 2015, Elsevier. (C) SEM images of highly porous graphene fiber through control of the coagulation conditions.166 Reproduced with permission from ref. 166. Copyright 2014, American Chemical Society. (D) SEM images of graphene-based PCF with rich-defect surface.168 Reproduced with permission from ref. 168. Copyright 2015, Royal Society of Chemistry. (E) SEM images of 3D porous network-like graphene framework on neat graphene-based PCF.169 Reproduced with permission from ref. 169. Copyright 2013, Wiley. (F) (a) Schematic illustration of the porous structures of CNT/RGO fiber, and cross-sectional SEM images of (b) RGO fiber and (c) partially unzipped CNT (PUCNT)/RGO.174 Reproduced with permission from ref. 174. Copyright 2021, Wiley. (G) SEM images of PCF composed of polymer nanoball-decorated graphene.175 Reproduced with permission from ref. 175. Copyright 2019, Wiley. | ||
In addition to the adjustment of the coagulation conditions and spinning parameters, further techniques for enhancing the porosity of graphene fibers have been developed. For example, by adopting a dimensionally-confined hydrothermal strategy, Qu's group fabricated neat graphene fibers by injecting GO suspensions into a closed glass pipeline followed by thermal treatment.160 The obtained graphene-based PCFs showed a highly 3D cross-linking porous structure. To further improve the porosity, they designed a sheath of 3D porous network-like graphene framework on that neat graphene-based PCFs through directly electrochemically electrolyzing a GO suspension (Fig. 6E).169 The uniformly distributed highly-exposed graphene layers contributed a pore-rich structure with pore sizes in the range of several micrometers to larger than ten micrometers. Similarly, Chen's group assessed hydrothermal and thermal treatments to obtain nitrogen-doped porous graphene fibers by injecting a mixture of GO and urea solution into a microreactor.121 The homogeneous diffusion and reaction of the precursors under a uniform vapor pressure and the decomposition of the amino- and oxygen-functional groups under high-temperature annealing were critical to create abundant pores with a wide size distribution ranging from 2.4 to 190.5 nm along the whole fiber. The as-obtained PCFs delivered a relatively high specific surface area of 388.6 m2 g−1 and total pore volume of 0.31 cm3 g−1. Zheng et al. obtained hierarchically porous sheath–core graphene-based fibers by dip-coating a slurry of GO and phenol formaldehyde (PF) on the graphene fiber followed by thermal annealing.123 In addition to its own porous structure of graphene fiber, the carbonized PF containing a small size graphene contributed ultrahigh porosity (88–97% micropores, 0–8.3% mesopores and 1.9–4.2% macropores) and high specific surface area (up to 416.4 m2 g−1).
However, for wet-spun graphene fibers, the pores generated by the phase separation mechanism are often isolated and not interconnected, which may limit their application in the energy field. The introduction of some other nanofiller layers between the graphene sheets to inhibit their restacking is another strategy to improve the porosity. As a promising nanomaterial, carbon nanotubes can be used as a spacer to increase the distance between graphene layers and augmentation of porosity to increase the specific surface area.1,120,170–173 For example, Chen's group fabricated hierarchical PCFs containing a single-walled carbon nanotube (SWNT) and nitrogen-doped rGO sheet interconnected network architecture by injecting SWNT/rGO/ethylenediamine aqueous dispersions into a fused-silica capillary column as a hydrothermal microreactor.1 The vertically aligned SWNTs interposed between the rGO layers not only reduced the stacking of the rGO and built a 3D architecture, but also provided well developed porosity in the hybrid fiber. Therefore, the hybrid SWNT/rGO fiber revealed the presence of highly micro-/mesoporous structure with a pore size distribution of around 1.5 to 18 nm and a high specific surface area of 396 m2 g−1. Remarkably, Zhang's group mixed a partially unzipped oxidized CNT with GO and achieved a hybrid fiber with a well-ordered porous structure (Fig. 6F).174 Compared with the neat RGO fiber and unmodified CNT/RGO fiber, which both formed isolated and not interconnected pores and “dead volume”, the unzipped oxidized CNT displayed better water solubility and higher SSA due to its structural defects and abundant oxygen functional groups, which is effective to inhibit the restacking of graphene sheets to endow the hybrid fiber with high porosity, SSA and a good interconnected network structure. Ding's group designed a porous graphene fiber inserted with polyvinylidene fluoride (PVDF) nanoballs between graphene sheets by injecting a mixed slurry of graphene/PVDF/PU into water for phase separation followed by drying (Fig. 6G).175 The novel porous core–shell with polymer nanoball structure allowed the as-obtained graphene-based PCFs to possess a larger special surface area, porosity, flexibility and stretchability than the unmodified graphene fiber. Hou's group fabricated hybrid fibers from GO and nano clay through non-liquid-crystal spinning followed by chemical reduction.176 It was proven that the unfirm dispersion of clay on the GO sheets is helpful to prevent π–π stacking within graphene. The obtained graphene-based PCFs possessed a dramatically increased inner surface area due to the clay spacer effect causing loose restacking of the rGO sheets.
Due to the features of the synthetic process and precursors, graphene fibers have demonstrated more porous structures, especially macro-pores, and impressive rich functional uses but a lower tensile strength than conventional PCF.177,178 In addition to the facile wet spinning method, the hydrothermal strategy, post treatment and introduction of nanomaterials can further improve the porosity. However, some critical factors of the synthetic process still need to be addressed, and the scalable fabrication of graphene fiber still remains a challenge.174
3. Mechanism analysis of PCFs for advanced energy and environmental applications
Activated carbon, as a versatile carbon material with a large SSA and high microporosity, can be easily produced on the industrial scale via the combination of direct pyrolysis with different activation processes. However, the conventional fabrication procedures only lead to simple compositions of mainly micropores and enclosed porous structures in activated carbon.179 For energy storage applications, these numerous dead-end micropores and bulk architecture of activated carbon neither provide efficient surface area for ion storage nor supply fast transport pathways for ion diffusion, resulting in the low utilization of the inner active sites and limited electrochemical performance, particular under a high current loading.179–181 Although many researchers have created mesoporous to activated carbon successfully, the controllability of the porous structure in terms of size, volume and location of pores still needs to be optimized. In contrast, even though the synthesis of PCFs is more complicated due to the involvement of various fiber spinning procedures and pore-forming processes, it is facile to achieve carbon materials with a well-controlled fiber diameter and porous structure, as well as the introduction of various guest active materials or heteroatom dopants, which promote PCFs to be more desirable for many energy and environment applications. There are three main advantages of the hierarchically porous structure of PCFs in practical applications, as follows: (1) besides demonstrating robust mechanical properties and conductivity, PCFs can reduce the weight and increase the utilization of materials, and more importantly, they can promise a high SSA and simultaneously offer more active sites and greater access for mass transport. (2) Macro/mesopores help to improve the equilibrium coverage of micropores and reduce the length of the diffusion path, and interconnected pores can decrease the diffusive resistance of mass transport. (3) The pore-forming process of PCFs can also generate structural defects in the graphitic layers and expansion between graphitic interlayers, which can effectively facilitate substance storage. Alternatively, the fibrous structure is another main benefit of PCFs in many applications. For example, compared with granular activated carbon or other carbon-based materials, PCFs and their fabrics, as freestanding materials, can be used as electrodes directly to not only avoid the complex multistep of powder electrode preparation to save cost and time, but also avoid many possible challenges, such as the agglomeration issue of powder-based materials, the decrease in electrical conductivity and blockage of active sites due to the use of a non-conductive polymeric binder, the decomposition issue of organic binders and the serious safety, environmental and health hazards with the use of organic solvents.182 As binder-free materials, PCFs display admirable mechanical strength, flexibility, electrical conductivity and continuity and ease of functionalization, and thus are promising in designing supercapacitors and batteries.17 The above-mentioned merits of PCFs are schematically illustrated in Fig. 7. The structure-related merits of PCF are often universally referenced by researchers to understand the performance improvement mechanism in energy and environmental applications. Nevertheless some specific mechanisms between the properties and different porous structures should not be neglected. The five different structure designs discussed in Section 2 also present unique and novel characteristics. For instance, compared with carbon nanofibers, (2.1) PCFs with rich meso/macro-pores and (2.2) PCFs with highly interconnected pore structures exhibit an excellent aspect ratio and relatively higher pore capacity, which will provide more space for guest material and element loading. Moreover the non-woven fabric structure forms a novel network, which not only guarantees high conductivity but also offers more transmission channels. In addition, the interconnected pores can further decrease the diffusive resistance of mass transport due to the large number of macropores.11 The (2.3) PCFs with defect-rich surface pore structures prepared using efficient surface treatment methods usually have an even higher surface area and more micropores, which are more favorable for applications with high adsorption performance requirements. Some biomass precursors also offer more distinctive structures, such as honeycombs, hollow structure, wrinkles and cracks.142,183 Compared to the carbon consumption approaches, (2.4) PCFs with a carbonaceous surface pore structure can maximize the inherent properties of pristine carbon fibers including very high strength to weight ratio, superior electrical and thermal conductivity, and excellent chemical resistance.153 Regarding (2.5) PCFs with graphene assemblies, the effective prevention of graphene agglomeration allows the greater exposure of structural defects on the graphene surface and micropores, which can effectively facilitate substance storage. Furthermore, the large mesopores and macropores generated from self-assembly are very beneficial to host guest materials and element loading. Besides the porous structure, the unique properties of graphene itself will give PCFs more functions.177 In practical large-scale applications, such as the treatment of wastewater, PCFs are still uncompetitive compared with the lower cost activated carbon. PCFs are still a relatively new materials, and their applications are still mainly focused on the energy area. More advanced applications related to catalysis, sensing, and fine chemical reactions are expected to obtain significant benefits from their well-controlled porous structures. In this section, a number of emerging applications of PCFs are introduced, and the mechanisms for promoting the performance of PCFs in different applications are discussed in detail.3.1 PCF-based supercapacitors
Supercapacitors are considered promising electronic energy storage devices due to their high-power capacity, superior charge/discharge rate, and exceptional cyclability. Among the electrode materials, carbon materials are the preferred material and used in commercial supercapacitors owing to their good electrical conductivity, low cost, physical and chemical stability, and large specific surface area.133,184–186 PCFs are usually regarded as a rising family of carbon materials and ideal supercapacitor electrodes, especially when flexible/wearable/portable characteristics are desirable.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 cycles. The mechanism proposed was that the abundant hierarchical mesoporous carbon monolayers are helpful for more effective ion transport. Initially, the capacitance increased mainly owing to the opening of the pores and functional groups on the outer surfaces of the PCF. As the charge/discharge process continues, more mesopore surface in the interior regions can be wetted and used, which can promote fast ion diffusion and improve the capacitance and retention.
900 cycles. The mechanism proposed was that the abundant hierarchical mesoporous carbon monolayers are helpful for more effective ion transport. Initially, the capacitance increased mainly owing to the opening of the pores and functional groups on the outer surfaces of the PCF. As the charge/discharge process continues, more mesopore surface in the interior regions can be wetted and used, which can promote fast ion diffusion and improve the capacitance and retention.
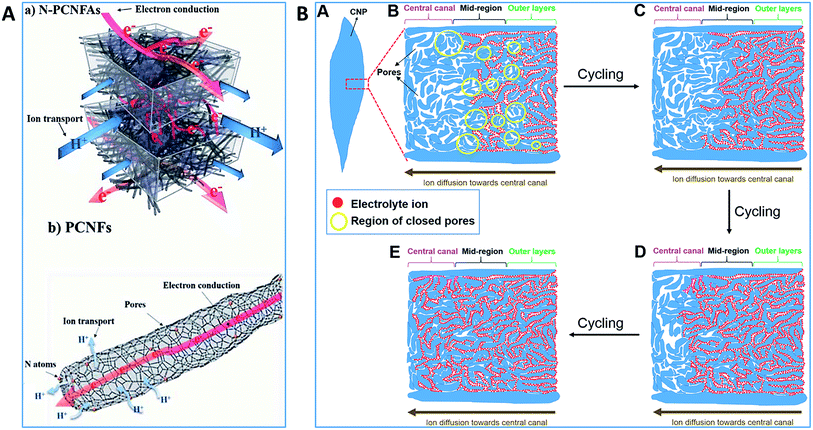 | ||
| Fig. 8 Schematic of (A) electron conduction and ion transport in hierarchical porous structures of PCF aerogel.188 Reproduced with permission from ref. 188. Copyright 2020, Royal Society of Chemistry. (B) Ion diffusion mechanisms through the hierarchical mesoporous carbon nanopetals upon prolonged galvanostatic charge/discharge cycling of a supercapacitor.189 Reproduced with permission from ref. 189. Copyright 2015, Royal Society of Chemistry. | ||
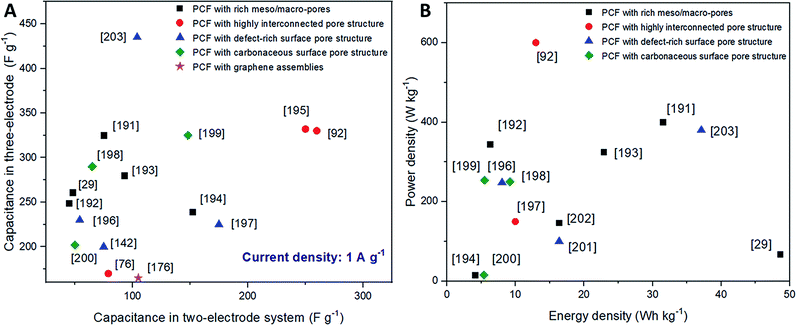
In addition, to strengthen the inherent advantages of carbon fibers, PCFs with a highly interconnected meso/macro-pore structure seem to have more potential to introduce additional surface area via microphase separation and improve the ion diffusion kinetics. Liu's group demonstrated the use of PMMA-b-PAN to form PCFs with highly uniform mesopores interconnected with micropores.11 Without further chemical activation or post-synthesis treatment, the obtained PCF was directly used as an SC electrode and showed an extraordinary gravimetric capacitance of 360 F g−1 at a high current density of 1 A g−1 in a 6 M KOH aqueous electrolyte.29 Huang et al. proposed a bamboo-like PCF with well-balanced micro-, meso-, and macro-porosity. By utilizing the unique porous architecture, which can offer a high ion-accessible surface area and low ion transport resistance, the bamboo-like PCF with an interconnected network significantly improved the volumetric energy and power densities of the related supercapacitor.83 As previously mentioned, with proper strategies to prevent the hydrophobicity and π–π stacking of graphene sheets, PCFs with graphene assemblies can be produced with an improvement in porosity and electrolyte affinity.122,176,190 Aboutalebi et al. fabricated PCFs with graphene assemblies possessing an exceptionally large specific surface area of 2210 m2 g−1. Owing to their highly porous nature, the as-prepared PCFs presented a continuous ion transport network, which contributed excellent charge storage capacity (409 F g−1 at 1 A g−1) and rate capability (56 F g−1 at 100 A g−1) in 1 M H2SO4 electrolyte without scarifying their strong flexible features.166 A summary of the performance of PCFs with different porous structures in some typical supercapacitors is listed in Fig. 9.29,76,92,142,176,191–203
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles.
000 cycles.
In addition to heteroatoms, PCFs show high potential to form hybrids with various active materials and improve the performance of supercapacitors by implementing short diffusion paths, large ion adsorption and fast electrolyte access to redox-active sites, such as metal compounds (e.g., MnO2,77,213 RuO2,206 CoS2,214 Co3O4,215 MoS2,216 Na2Ti3O7,217 ZnFe2O4,130 and NiCo2O4 (ref. 218)), specific polymers (e.g., PANi,219,220 PPy,221–223 PEDOT,224,225 and anthraquinone (AQ)226) and their mixtures.64 For example, Liu's group synthesized PCFs with uniform interconnected mesopores of ∼11.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nm, which were filled with MnO2 of <2 nm in thickness.77 Due to their highly interconnected porous structure, the mass loading of MnO2 approached 7 mg cm−2. Consequently, the obtained PCF@MnO2 presented an extremely large specific capacitance of 462 F g−1 at 10 mV s−1 in 6 M KOH aqueous electrolyte. Cao's group prepared cotton-derived hierarchically PCFs as a template/scaffold, in which ZnFe2O4 nanoparticles were confined to mesopores uniformly, while the micropores were preserved.130 By utilizing the well-dispersed nanoparticles in the porous structure and continuous electron pathway of carbon fibers, the obtained supercapacitor electrode with an aqueous electrolyte of 2 M KOH achieved a high specific capacitance with enhanced capacitance retention. Through the use of the porous structure, Wang et al. successfully functionalized the surface and inside of PCFs by loading active AQ molecules.226 The asymmetric supercapacitor using the PCF as the positive electrode and AQ-PCF as the negative electrode presented a large energy density of 19.3 W h kg−1 with an operating potential of 1.2 V and ultrahigh power capacity in 1 M H2SO4 electrolyte, up to 120 A g−1.
nm, which were filled with MnO2 of <2 nm in thickness.77 Due to their highly interconnected porous structure, the mass loading of MnO2 approached 7 mg cm−2. Consequently, the obtained PCF@MnO2 presented an extremely large specific capacitance of 462 F g−1 at 10 mV s−1 in 6 M KOH aqueous electrolyte. Cao's group prepared cotton-derived hierarchically PCFs as a template/scaffold, in which ZnFe2O4 nanoparticles were confined to mesopores uniformly, while the micropores were preserved.130 By utilizing the well-dispersed nanoparticles in the porous structure and continuous electron pathway of carbon fibers, the obtained supercapacitor electrode with an aqueous electrolyte of 2 M KOH achieved a high specific capacitance with enhanced capacitance retention. Through the use of the porous structure, Wang et al. successfully functionalized the surface and inside of PCFs by loading active AQ molecules.226 The asymmetric supercapacitor using the PCF as the positive electrode and AQ-PCF as the negative electrode presented a large energy density of 19.3 W h kg−1 with an operating potential of 1.2 V and ultrahigh power capacity in 1 M H2SO4 electrolyte, up to 120 A g−1.
In summary, the PCFs described above have successfully demonstrated capability to promote the electrochemical performance when used as electrode materials in supercapacitors; however, some challenges still need to be faced before realizing large-scale commercial applications. The unique porous and fibrous structure endows PCFs with a high SSA and capacity for supercapacitors, but leads to limited electrical conductivity and poor mechanical strength. The design of PCFs with a tunable pore structure to balance their electrochemical properties, electrical conductivity and mechanical strength is still critical. Accordingly, the introduction of highly conductive or/and high-capacity guest materials in PCFs can be an ideal solution to achieve the combination of good electrical conductivity and high electrochemical performance for PCF-based supercapacitors. However, the strong interaction between the guest active materials and PCF should be further investigated. Moreover, as-synthesized PCFs using high-temperature carbonization are hydrophobic, which significantly affect their wettability with aqueous and other polar electrolytes, and thus further surface treatment strategies should be considered in-depth.227 In addition, PCF-based supercapacitors usually have a relatively low volumetric energy and power density due to the high volume of pores inside or on the fibers, which may limit their practical application for different types of supercapacitors. Thus, the following recommendations for future research directions can be considered: (a) optimizing hierarchically porous structures for the diffusion and storage of ions, (b) regulating and controlling the interaction of PCFs with the electrolyte, (c) creating more effective redox sites within PCFs and (d) improving the interaction between PCFs and the guest active materials.
3.2 PCF-based rechargeable battery
The increasing global energy crisis has urged researchers to intensively study high efficiency energy technology. Among the existing energy conversion and storage technologies, rechargeable batteries, such as lithium-ion batteries (LIBs), lithium–sulfur batteries, potassium-ion batteries (PIBs) and sodium-ion batteries (SIBs), have been considered as efficient methods for the storage and delivery of electrical energy with high relatively energy densities.228 Recently, hierarchical PCFs, which inherit the excellent properties of carbon fibers and combine the competitive advantages of porous materials, have been successfully utilized as electrode materials for rechargeable batteries.18,229–232 Thanks to the freestanding and mechanically flexible properties of CFs, PCFs are also a great option for the design of binder-free electrodes to improve the overall weight energy density of batteries with a simplified cell preparation process.219,233 | ||
| Fig. 10 (A) Schematic illustration of the advantages of PCFs in enhancing LIBs.249 Reproduced with permission from ref. 249. Copyright 2015, American Chemical Society. (B) Schematic diagram of the benefit of PCF anode for Si nanoparticle expansion, electron transmission and Li+ storage.267 Reproduced with permission from ref. 267. Copyright 2016, American Chemical Society. (C) Schematic illustration of the advantage of porous structure during the potassiation process.283 Reproduced with permission from ref. 283. Copyright 2020, Wiley. (D) Schematic illustration of the continuous electron pathways, highly efficient ion pathways and good protection of the reference sample provided by hierarchical PCF cathode.242 Reproduced with permission from ref. 242. Copyright 2012, Elsevier. (E) Schematic diagram of the benefits of the porous carbon matrix decorated PCF cathode.117 Reproduced with permission from ref. 117. Copyright 2017, Wiley. (F) Schematic illustration of the charge-storage mechanisms for Li-ion capacitors.293 Reproduced with permission from ref. 293. Copyright 2017, Wiley. | ||
Although PCFs show fantastic Li/Na/K storage capacity, many of PCFs as anodes cannot balance the high capacity with good plateau, initial coulombic efficiency and satisfactory cycling stability. Due to this issue, much effect has been devoted to the fabrication of hybrid PCFs with other types of anode materials, which can create a high capacity and a good charge–discharge plateau simultaneously, including elements,229,251 alloys,252,253 metal oxides,49,254–259 metal fluorides260 and metal sulfides,245,261–266 hence improving the energy density of Li/Na/K-ion batteries. For example, to overcome the inferior and inadequate cyclability of elements even though they display an overwhelming advantage in theoretical capacity for Li/Na/K storage, Lu's group took advantage of PCFs with precise control of the expansion space for Si nanoparticles as a flexible anode for high-performance LIBs (Fig. 10B).267 Besides the well-known properties of PCFs for battery application, their hierarchically porous structure not only can allow Si nanoparticles to expand without rupturing the overall morphology of the fiber matrix to improve the cycle stability of the electrode during deep electrochemical cycles, but also limit the formation of a solid electrolyte interphase (SEI) and prevent fracture of the matrix. Yu and Yang et al. utilized different precursor-based PCFs to encapsulate red phosphorus, which has the issue of dramatic capacity decay and poor cycle stability for metal ion batteries.268 With the help of PCFs, the composite materials were proven to successfully reduce the diffusion barrier for both ions and electrons and relieve the volume expansion, leading to an outstanding rate capability and cycling performance of SIBs and LIBs, respectively. Xia et al. prepared anodes by compositing Fe2O3 with peapod-like PCFs for LIBs and SIBs, which can significantly alleviate the aggregation of Fe2O3 nanoparticles, relieve the volume expansion/shrinkage during repeated insertion/extraction of Li+ or Na+, and enhance the electrical conductivity of the electrode. Similarly, various forms of PCFs exhibited an excellent collaborative effect with metal sulfides as an anode, in which the PCFs can act as a stress buffer to accommodate the volume change of metal sulfides, such as NiS2, MoS2, SnS and SnS2 during cycling and improve the cycling stability.245,261–266 Moreover, the porous surface and graphitic carbon layers of PCFs are beneficial for the formation of a stable SEI layer and reducing the continuous consumption of electrolyte during the cycling process. A summary of the PCF-based electrodes for different batteries is listed in Table 2.
| Porous structure type of PCF | Battery type and electrode | Guest materials | Cc, Cd and CE at the first cycle (mA h g−1) | Capacity at high rate (mA h g−1) | Reversible capacity at nth cycle (mA h g−1) | Ref. |
|---|---|---|---|---|---|---|
| a C c: charge capacity; Cd: discharge capacity; and CE: coulombic efficiency. | ||||||
| Rich meso/macro-pores | LIB-anode | — | 875/495/56.6 (1 A g−1) | 172 (20 A g−1) | 625@300th (1 A g−1) | 249 |
| LIB-anode | MoS2 | ∼1115.34/1946.5/57.3% (1 A g−1) | 647.3 (5 Ag−1) | ∼1116.2@450th (1 A g−1) | 262 | |
| LIB-anode | ZnO | 1369/1923/71% (0.1 A g−1) | ∼618 (3 A g−1) | ∼610@500th (3 A g−1) | 256 | |
| LIB-anode | Si | 1565/2143/78.6% (0.7 A g−1) | 582 (28 A g−1) | 2002@1050th (0.7A g−1) | 267 | |
| SIB-cathode | NaFePO4 | 142/116/81.7% (0.0124 A g−1) | 46.4 (2.48 A g−1) | ∼52.7@2000th (0.62 A g−1) | 272 | |
| LIB-anode | SnS | 1286.4/1037.9/80.6% (0.5 A g−1) | 312.2 (10 A g−1) | 529@1000th (5 A g−1) | 192 | |
| Interconnected pore structure | SIB-anode | P | 1580.12/2260.6/69.9% (0.1 A g−1) | 500 (10 A g−1) | 700@920th (2 A g−1) | 268 |
| SIB-anode | — | 563.4/766.8/73.5% (0.1 A g−1) | 333.4 (5 A g−1) | 335.8@800th (5 A g−1) | 286 | |
| LIB-anode | CoO | 984/1850/53.2% (1 A g−1) | 802.4 (1.2 A g−1) | 983.5@400th (1 A g−1) | 287 | |
| Li–S cathode | CeF3 | —/∼1395/— (0.84 A g−1) | ∼819.3 (3.35 A g−1) | ∼901.2@500th (0.84 A g−1) | 96 | |
| Li–S cathode | S | —/1351/— (0.335 A g−1) | 847 (8.375A g−1) | 920@300th (0.335 A g−1) | 285 | |
| K–Se cathode | Se | 631/1045/60.4% (0.1 A g−1) | 209 (2 A g−1) | 367@1670th (0.5 A g−1) | 283 | |
| Defect-rich pore structure | SIB-anode | NiS2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
679/1106/61.4% (0.087 A g−1) | 245 (8.7 A g−1) | 275@500th (4.35 A g−1) | 264 |
| Li–S cathode | CoO/Co | —/∼1214.2/— (0.168 A g−1) | ∼684.3 (3.3 A g−1) | ∼684.1@100th (0.838 A g−1) | 288 | |
| Carbonaceous surface pore structure | Li–S | — | —/977/— (0.8375 A g−1) | 433 (8.375A g−1) | 605.74@500th (0.8375 A g−1) | 289 |
| SIB-anode | MoS2 | 659/1288/52% (0.2 A g−1) | 235 (2 A g−1) | 265@1000th (1 A g−1) | 263 | |
| LIB-anode | — | 1343/2214/60.6% (0.1 A g−1) | 750 (1 A g−1) | 659@1000th (2 A g−1) | 290 | |
| LIB-anode | — | 965.3/705.6/73% (0.2 A g−1) | 309.8 (5 A g−1) | 475.2@1000th (1 A g−1) | 291 | |
| LIB-cathode | V2O5 | —/360.6/— (0.0294A g−1) | 115 (14.7 A g−1) | 183@5000th (0.294 A g−1) | 117 | |
| Graphene assemblies | LIB-anode | SnO2 | 827.2/1036.3/79.8% (0.1 A g−1) | 500 (4 A g−1) | 846@100th (0.1 A g−1) | 233 |
| L–S cathode | — | —/1270/— (0.335 A g−1) | 859 (8.375 A g−1) | 917@300th (0.335 A g−1) | 271 | |
| LIB-anode | Carbon-coated CoO | ∼988/1520/65% (1 A g−1) | 222 (100 A g−1) | 823@500th (1 A g−1) | 292 | |
Recently, significant progress has been made in PCFs for new applications, such as current collectors and interlayers for rechargeable batteries and cathodes/anodes for metal-ion capacitors.293–295 For example, Yu's group fabricated B and N dual-doped 3D PCF networks derived from commercial bacterial cellulose as both self-standing anodes and cathodes for Li-ion capacitors with a large specific capacity, outstanding rate capability and superior durability (Fig. 10F).293 The obtained excellent capacitor performance could be attributed to the porous and interconnected conductive structure, which not only can improve the interface between the electrolyte and electrode to achieve a large pseudocapacitance, but also accelerate the electron transfer. Meanwhile, B, N co-doping could remarkably increase the carbon interlayer space, the number of active sites and electrode kinetics, which also contributed to the great electrochemical performance.
With the development of new anode/cathode materials, given their unique and appealing advantages, PCF composites would be highly desirable as high-performance electrode materials for electrochemical energy conversion and storage devices. Of course, it is undeniable that some critical challenges of current PCF-based electrode materials still exist. For example, some research has reported that porous carbon-based electrode materials with a high SSA may consume more electrolyte to form a stable SEI layer.296 Generally, the SEI is inhomogeneous and not fully passivate, which can induce some side reactions because of the high contact area between the electrolyte and electrode, resulting in a decrease in battery reversibility and lower initial coulombic efficiency.297 The superior heteroatom doping amount in PCFs than other carbon materials can provide more active sites for Li/Na/K storage and higher pore volume for ion diffusion to enhance the storage of ion batteries; however, heteroatom doping can also decrease the initial coulombic efficiency and average delithiation/desodiation/depotassiation potential of PCFs.17 Moreover, the high volume of pores inside the fiber not only hinders the mechanical properties and electrical conductivity, but can also cause a low volumetric energy and power density, which may significantly limit the practical applications of PCFs in energy store devices.298 Thus, careful structural design with good mechanism understanding of PCFs is essential to build a balanced relationship between porous structure and electrochemical performance in various types of rechargeable batteries.
3.3 PCFs for catalysis
Although the classic carbon materials, such as activated carbon, carbon black, carbon fibers, and carbon aerogels, have been used in various reactions as catalysts or catalyst supports, their relatively low activity significantly limits the performance of the catalyst.299,300 These classic carbon-based catalysts have nonporous or micropore-dominant structures that significantly weaken the reaction kinetics by limiting the mass transport and the use of inner active sites.181 Conversely, the unique nature of the porous architectures of PCFs can promise a large surface for fast mass transport, and highly accessible active sites for efficient catalytic reactions.301,302Additionally, co-doping of heteroatoms with different electronegativities in PCFs can create more catalytically active sites than their single-doped counterparts to boost the electrocatalytic activity because of the synergistic effect, such as N/S, N/P, N/F, N/B, N/O.78,318–323 For example, Deng's group reported the hybridization of an N, S-doped PCF network coated with N, P-doped carbon nanoparticles as bifunctional electrocatalysts toward the ORR and hydrogen evolution reaction (HER), which exhibited excellent properties.320 The synergistic integration between the N, S-doped PCF architecture and N, P-doped surface-active carbon layers resulted in more effective electrocatalytic activity. Moreover, an appropriate integrated amount of N, P-doped carbon layers on N, S-doped PCFs can be beneficial for efficient interface reactions and fast electrode kinetics. In addition to electrochemical catalysts, N-doped PCFs as photocatalysts are attracting increasing attention thanks to their advantage of rich hierarchical porous structure, inherent excellent electrical conductivity and high nitrogen content. C–N structures have been proven to be useful to create semiconducting materials for photocatalytic applications,324 and graphitic carbon can decrease the recombination of photogenerated electrons and holes,325 which can enhance the performance of photocatalysts. For example, Wang's group prepared N-doped graphene on silk cocoon-based CFs to achieve a PCF-based photocatalyst with a well-tailored meso-/macroporous architecture for H2 production.326 The interconnected CFs can create multiple paths to improve the electron transport, and the growth of graphene can allow rapid transport of charge carriers and effective separation of electron–hole pairs to accelerate solar photocatalytic H2 production activity.
Carbon materials have also attracted considerable interest as heterogeneous metal-free catalysts for the efficient degradation of environmental waste in water. Compared to other carbonaceous materials, PCFs have more notable advantages to promote the catalytic activities.327 Wang et al. synthesised a PCF from ZIF-8, which demonstrated a much greater total organic carbon (TOC) removal rate during advanced oxidation processes than pure PAN-based carbon fiber and porous carbon power from ZIF-8 (Fig. 11A).328 Besides, the highly porous and hollow structure of PCFs can provide more active sites and contact area for the catalytic reaction. The mechanism of the excellent catalytic performance for peroxymonosulfate (PMS) activation was further revealed to be that the hollow pores interconnected with each other by fibers can not only serve as a highway for rapid electron transport but also enhance the pollutant transfer and ion diffusion. Furthermore, the high content of graphitic-N and pyrrolic-N in the carbon frameworks of PCF further improved the adsorption capability and catalytic oxidative degradation of organic pollutants.
 | ||
| Fig. 11 (A) Schematic illustration of the advantages of PCFs in catalytic performance for PMS activation. (a) Proposed mechanism of tetracycline decomposition using a PCF system. (b) Schematic advantages of mass and ion transfer in PCFs.328 Reproduced with permission from ref. 328. Copyright 2019, Royal Society of Chemistry. (B) Mechanism of the ORR and OER for synergistic effect in FeNi/N-doped PCF.53 Reproduced with permission from ref. 53. Copyright 2020, Elsevier. | ||
During the past decade, single-metal-atom catalysts have attracted significant attention due to the potential of 100% metal atom utilization, superior activity, and outstanding selectivity.358,359 Compared with the conventional PCF-supported guest metal NP catalysts, not only can the method for the synthesis of PCFs suppress the agglomeration and migration of metal atoms in nanoparticles, but also the resulting substantial pores and topological defects in PCFs are suitable for preparing high metal loading single-metal-atom catalysts and exposing the catalytic atoms, contributing excellent catalytic behavior. For example, Haag's group designed atomic Fe–Nx site-coupled N-doped PCFs with interconnected structures to achieve outstanding oxygen electrocatalytic performances for both alkaline and neutral electrolytes.360 The united advantages of the hierarchical porous structure and coupled atomic Fe–Nx sites enhanced the ORR catalytic activity by boosting the oxygen diffusion and dynamic reaction kinetics. Wu's group prepared an atomically dispersed cobalt/nitrogen-doped PCF catalyst with well-controlled hierarchical porous structure as the ORR cathode in a fuel cell.361 The abundant micropores of the as-obtained catalyst provided ample active sites to host single atomic CoN4 and showed high catalytic activity. The intensive meso- and macropores within and between fibers not only allowed sufficient gas flow to diffuse in the catalyst layers to form a freeway network for electron transfer, but also promoted the mass transfer of oxygen and hydrogen ions, as well as the timely removal of water, which can importantly enhance the stability and durability of catalysts.
Recently, owing to the advantage of porous structures, which can offer considerable channels for CO2 diffusion and electron transport, PCFs as low-cost electrocatalysts for the CO2 reduction reaction (CO2RR) also have received attention from the academic and industrial communities.330,362–365 The micropores are mainly used to capture CO2 molecules, while the meso- and macropores can allow the fast diffusion and adsorption of CO2 in the micropores, resulting in CO2 enrichment around the active sites.366 For example, He and co-workers created a self-supported Ni single-atom decorated hierarchical PCF membrane catalyst from electrospun ZIF-8 for high-efficiency CO2RR.363 This is because the hierarchical porous structures can speed up the diffusion and adsorption of reactants and generate more effective Ni single atoms to participate in the CO2RR, leading to an extraordinary CO2 adsorption capacity and high electrochemical active surface area.330
Water pollution is posing a significant challenge globally. Although traditional ACFs exhibit potential as metal-free catalysts or catalyst supports, the structural limitation of interior blockage and lack of active sites for metallic nanoparticle loading have largely impeded the application of ACFs in wastewater treatment. Accordingly, hierarchically PCFs, which are superior to ACFs, can be used together with semiconductor metal oxides as photocatalysts due to their higher specific surface area, pore volume and better gas permeability.364 For example, Yang et al. produced a PCF-based photocatalyst for the oxidation of phenol and the reduction of Cr(VI).367 The porous structure not only can play a significant role in effective electron capture, transfer and storage, but also provide an increased surface area for adsorption, improved charge transfer and spatial charge separation, which further boost the photocatalytic activities. In the case of the reactive PMS activation for the degradation of a diversity of organic contaminants in water, Zhu et al. hybridized ferro-cobalt alloyed crystals during the preparation of PCFs.8 By utilizing the synergistic adsorption of porous structures and activated PMS, the prepared hierarchical PCFs exhibited highly efficient activity for the removal of various refractory organic pollutants and robust recyclability. Hwang et al. fabricated novel cobalt manganese oxide-embedded hollow activated carbon nanofibers with micro- and mesoporous structures, which improved the adsorption of aqueous contaminants and offered triple phase (solid–liquid–gas) regions required for effective catalytic reactions.368 Benefiting from the synergistic effect between the spinel oxide and hybridized hollow-porous-carbonic framework, the prepared materials exhibited an exceptional adsorption and catalytic performance in PMS activation and rhodamine B degradation. A compact overview of PCF-based catalysts for different catalytic applications is given in Table 3.
| Porous structure type of PCF | Element doping | Guest material | Catalytic application | Catalytic efficiency | Catalytic stability | Ref. |
|---|---|---|---|---|---|---|
| a ZAB: Zn–air battery; FE: faradaic efficiency; and ΔE = E10 − E1/2 (ΔE: potential gap; E10: potential for 10 mA cm−2 OER current density; and E1/2: ORR half-wave potential). | ||||||
| Rich meso/macro-pores | N | — | ORR | ≈Pt/C | 16% decrease in current after 15 h | 249 |
| N, S, P | — | ORR/HER | Slightly lower than Pt/C | Onset potential slightly shifted negatively (HER) and relative stable ORR performance after 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 s 000 s |
320 | |
| N | — | PMS | 80% TOC remove rate | Removal rate decreases to 38.3% in the third run | 328 | |
| N | FeNi | OER/ORR | >Pt/C–RuO2 | 86.5% current retention after 12 h (OER); no obvious discharge/charge voltage fading after 640 h (ZAB) | 53 | |
| N | Ni2P | HER | Slightly lower than Pt/C | Well-maintained potential after 30 h | 357 | |
| N | Cu | CO2RR | C2H4@62% FE | Over 56% faradaic efficiency after 10 h | 330 | |
| Highly interconnected pore structure | N | Ni | CO2RR | CO@96% FE | More than 95% faradaic efficiency after 120 h | 363 |
| N | CoFe | OER/ORR | >Pt/C–IrO2 | 85% performance retention after 20 h (OER); more than 60% energy efficiency retention over 330 h (ZAB) | 384 | |
| N | Fe–Nx | ORR | >Pt/C | No obvious voltage drops after 16.7 h | 360 | |
| N | Co | ORR | >Pt/C | 88% current retention after 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 s 000 s |
331 | |
| N, S | Co | OER/ORR | ≈Pt/C–RuO2 | 91.9% current retention after 24 h (ORR). Better stability than RuO2 after 2000 cycles (OER) | 333 | |
| Carbonaceous surface pore structure | O | — | OER/ORR | Potential gap (ΔE): 1.01 V | No obvious change after 9 h | 150 |
| N | H2O2 | 85% phenol removal | Phenol removal rate decreases to 42% in the 3rd run | 312 | ||
| Defect-rich surface pore structure | N, B | — | ORR | ≈Pt/C | 97.2% current retention after 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 s 000 s |
319 |
| O | — | OER/ORR | ≈Pt-based and RuO2 | Good durability over 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 s 000 s |
3 | |
| N | — | PMS | 100% carbamazepine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) degradation degradation |
Carbamazepine degradation rate decreased to 72.41% within 60 min in the 7th run | 311 | |
Although PCF-based catalysts have achieved considerable progress owing to the availability of porous structures with abundant accessible active sites, some obstacles still need future investigation. For example, almost all the reported PCF-based electrocatalysts have shown outstanding ORR/OER performances under alkaline conditions; unfortunately, very few studies have presented efficient catalysts for the ORR/OER in acidic electrolyte, which is more useful for real practical application. Furthermore, many researchers have observed low stability and durability of carbon-based electrocatalysts, where similarly for PCFs, this is the primary challenge compared with traditional platinum-group metal catalysts, which may be caused by various reasons, including carbon corrosion/oxidation, leaching/demetallation, protonation of active sites, and micropore flooding.369–371 Due to the easy oxidation of carbon materials, active carbon atoms tend to be electrochemically oxidized to CO2 and CO, particularly under increasing temperatures and applied potentials, directly causing the destruction of the carbon surface and indirectly leading to a negative influence on the amount of active sites.372 It can be inferred that the oxidation of carbon not only can enhance the hydrophilicity of PCFs but also block their micropores to reduce the accessible amount of active sites because of the greater interaction between the micropores and flowing water.373–375 More importantly, in terms of PCF-supported metal catalysts for the ORR and OER, the surface oxidation of carbon can affect the turnover frequency of the metal active sites by weakening the O2-binding and lead to the leaching/demetalation of the elements from the active moieties of M–N–C.376 Some possible methods have been suggested based on the current research, for example, adding cocatalysts to scavenge the generation of reactive oxygen species and H2O2,377 but this is still in its infancy and requires continuous efforts, particularly for metal-free PCF-based catalysts. Moreover, a great deal of effort by researchers has been focused on the enhancement of the catalytic performance of PCFs in different electrochemical systems; however, the reaction mechanisms and active sites of PCF-based catalysts are still under debate. It has been noticed that the catalytically active species may be changed during the actual reactions, and thus to better understand the mechanism of the PCF catalytic performance, in situ or operando measurement is highly beneficial to confirm the actual active phase/intermediates by monitoring and tracing the dynamic morphologies and structures of the active sites in the process of electrocatalysis.377,378 Specifically, valuable information over the catalytic process, including coordination number, structure change, valence state, crystal face, chemical configuration, and elemental content can be verified in detail; however, these types of measurements on PCF-based electrocatalysts are rarely reported.
Similarly, the poor stability and durability of PCF-based catalysts are also common issues for their application in water purification, which is probably because of the destruction of the catalytically active sites on the PCF surface by the reactants generated from the degradation of pollutants via adsorption. Thus, additional investigations on low-cost but highly active and stable PCF-based materials as catalysts for energy and environmental applications are essential research goals to accelerate their practical application on the industrial scale.
3.4 Adsorption and absorption
Air and water pollution and dwindling freshwater supplies are often cited as worldwide problems for the eco-environment and society. Although tremendous efforts, such as catalysis oxidation, condensation, biological degradation, and adsorption, have been devoted to the removal of various pollutants including inorganic and organic compounds from the environment, this still is a big challenge.379 The adsorption technique using carbon materials is usually recognized as one of the most promising control strategies for the elimination of pollution due to its low lost and high efficiency. However, the adsorption capacities of carbonaceous materials rely on their porous structure and surface properties. Among the carbon materials, activated carbon, ACFs, biochar, CNT/graphene and their derivatives have shown excellent potential for adsorption applications owing to their large surface area, plentiful pore structure and high stability. The last few years have witnessed rapid progress in the development of porous carbon fibers, which combine the properties of porous carbon materials in a single material.As porous adsorbents, the morphology and structure of porous carbon fibers especially the distribution of pore size are responsible for their adsorption ability. It has been proven that micropores, which contribute a greater surface area, can provide principal adsorption sites, while mesopores/macropores are beneficial to improve the intra-particle diffusion and shorten the adsorption time.76,380–382 Hierarchical PCFs have been proven to have excellent adsorption performances in the removal of dyes and organic compounds.61,76,97,383 Thus, to further explore the potential adsorption capability of PCFs, Liu's group employed hierarchical PCFs with controlled porosity for the removal of an organic dye through electrosorptive treatment, which exhibited an extremely high adsorption capacity and fast infiltration rates for both cationic and anionic dyes.385 It was proved that the hierarchical porous structure formed by the coexistence of micropores, mesopores, and interfiber voids provided the dye ions with easy accessibility to the surface sites in the fibers, and the pore interconnectivity could further facilitate the diffusion of the dye ions. More importantly, the hierarchical pores allowed rapid ion desorption, which was advantageous to regenerate the PCF easily.
Besides large organic molecules, PCFs have also shown a promising effect on the sorption of smaller compounds, such as metal ions, sulfide and gas.62,386–393 Although it is well-known that ultramicropores less than 1 nm are proposed to have a greater adsorption capacity of the above-mentioned ions, the micropore-dominant structure restricts solution infiltration, which consequently hinders ion diffusion. Accordingly, PCFs composed of micro- and mesopores can provide a good solution to overcome the above-mentioned issue and further improve the selectivity and efficiency of adsorption through surface functionalization or heteroatom doping. It has been reported that a great deal of active sites and pyrrolic-N existing in PCFs can facilitate the accessibility of functional groups to enhance the chemical interaction between the PCF and meal ions in water, such as lead and hexavalent chromium.388,390 Moreover, a highly porous structure within the fiber can efficiently facilitate better water permeation, and hence a higher efficiency of ion removal can be realized. In addition to the removal of heavy metals, Liu et al. utilized PCFs as a capacitive deionization material and achieved a high desalination capacity of 30.4 mgNaCl gPCF−1 and an ultrafast desalination rate of 38.0 mgNaCl gPCF−1 min−1, surpassing the benchmark activated carbon, conventional PAN-based CFs, and other advanced carbon electrodes under similar experimental conditions (Fig. 12A).79 The mechanism was deeply understood to be that the abundant mesopores interconnected with micropores offer a large ion-accessible surface area, and the continuous fibrous network enable fast electron/ion transport. In addition, the plentiful oxygen and nitrogen heteroatoms prevailing on PCFs can further improve the wettability and local charge redistribution. Cheng's group tailored an N-doped PCF film reinforced by halloysite nanotubes (HNTs) with prominent mechanical properties for adsorptive desulfurization.393 The unique hierarchically porous structure not only shortened the mass transfer paths and facilitated the movement of the thiophene (TH) molecule to the absorption active sites in the PCF, and then be adsorbed, but also improved the van der Waals' interaction with the TH molecule and further improved the adsorption performance. The considerable hydroxyl groups on the surface of the PCF and its porous structure resulted in a synergistic effect on both chemical and physical adsorption to achieve a good desulfurization effect. Li et al. synthesized PCFs with micro-/meso-/macropores, which showed great CO2 capture capability and excellent CO2 reusability.419 It was found that the nitrogen functionalities and porous structure of the PCFs played an equivalently important role in the adsorption of CO2. Ma et al. revealed that PCFs with a hierarchically multilevel gradient porous structure showed a better performance than that with a uniform pore structure for CO2 adsorption.418 The mechanism was explained to be that the connection between the pores of different sizes acts as a bottleneck for the diffusion process. The impediment of the bottleneck is less obvious at low pressure or for the adsorption of smaller molecules. However, for a high pressure or a high electric driving force, the multilevel gradient porous structure is beneficial for the diffusion of ions or gas through the bottleneck. Attia et al. developed a high porosity flexible nanoporous carbon textile by depositing polypyrrole nanoparticles on viscose rayon textile followed by carbonization and activation.394 By utilizing the porous structure with a high specific surface area, the obtained PCF achieved superior H2 and CH4 storage capacities, CO2 capture and selective CO2/CH4 separation compared with the conventional ACF.
 | ||
| Fig. 12 (A) Schematic illustration of the advantages of PCF in a capacitive deionization cell, where abundant interconnected mesopores in PCF provide large ion-accessible surface areas and fast ion diffusion, which promote the desalination capacity and desalination rate.79 Reproduced with permission from ref. 79. Copyright 2020, Science. (B) Scheme of primary EM wave attenuation models in CoFe/PCF absorbent. (a) Interfacial polarization, (b) dipole polarization, (c) magnetic loss and conductive loss, and (d) multi-scattering.396 Reproduced with permission from ref. 396. Copyright 2019, Elsevier. | ||
Due to their highly hydrophobic and oleophilic properties, PCFs with no functional groups or CF-based porous aerogels also exhibit brilliant oil absorption capacity.76,395 Comparing to activated carbon microporous materials, the numerous hierarchical porous channels and large pore volume of PCFs can more effectively accelerate viscose oil transportation and the interaction between carbon and oil absorbate, thus leading to a high absorption capacity with quick response. More importantly, the abundant pore structure of PCFs is also beneficial to sustain prominent oil retention and absorption recyclability, allowing PCFs to be reused many times.76 In recent years, electromagnetic pollution has become a serious problem affecting human health, and electromagnetic (EM) wave absorption materials are playing an important role in daily life. PCFs, as relative new carbon nanostructure materials, stand out due to their unique structures and properties compared with the other absorption materials. Compared with traditional carbon fiber EM wave absorbers, the unique porous structure on the surface of PCFs is not only beneficial for the reduction of weight density but also significantly changes the EM wave absorption performance.119,396–398 For example, Song et al. fabricated CoFe alloy-decorated hierarchically PCFs as an EM wave absorber, which presented an extremely strong reflection loss and excellent comprehensive EM wave absorption performances (Fig. 12B).396 Under an alternating EM field, the heterogeneous interfaces including CoFe alloy−CF interface and solid-air interface around the pores resulted in strong interfacial polarization relaxation. Also, the oxygen functional groups and surface defect of PCFs can act as dipoles and generate dipole polarization loss. Moreover, the interconnected conductive CF network can facilitate electron hopping and transfer. The macropores can provide more opportunities for EM wave multiple scattering. Ding's group fabricated a hybrid PCF membrane combined with rGO and magnetic nanoparticles, which demonstrated superior absorption abilities in a wide frequency range.397 They concluded that the existing porous structure and rGO nanosheets located on the surface of the PCF were very useful in reducing the transmitted waves by multi-reflections. Therefore, the construction of hierarchical pores in CFs will be a promising strategy for the improvement of EM wave absorption.
In summary, the hierarchical porous structure of PCFs combining micropores with a system of meso/macro-pores can be advantageous to balance the adsorption enthalpy, which is neither too strong due to their ultra-small pore sizes nor too weak in case of increased pore sizes. However, there are still knowledge gaps about the adsorption mechanism of PCFs that need to be filled. Similar to other carbonaceous adsorbents, pure PCFs are inherently nonpolar adsorbents, which would inevitably limit the adsorption toward hydrophilic adsorbates. Also, the selectivity and adsorption capacity of PCFs towards various adsorbates vary significantly. Chemical modification and functionalization can be influential approaches to not only change the polarity of the pore walls in PCFs but also improve the electron density and electron density distribution, and thus their properties as an adsorbent. However, simultaneous control of the defined atomic composition and/or functional groups with the porosity of the hierarchical pore architecture in PCFs remains a great challenge.399 The profound understanding of the adsorption mechanisms involving electrostatic interaction, covalent bonding, hydrogen bridges, π–π interaction and hydrophobic interaction will be constructive for the practical application of PCFs. In addition, due to their complex porous structure design, the desorption capacity for target adsorbates and the regeneration of exhausted PCFs for recycling are costly and energy intensive. The hierarchical pore structure of PCFs has made significant progress in EM wave absorption; however, the deep absorption mechanism is still unclear. Simply using the influence of pore size and distribution on the interfacial polarization and electromagnetic properties to describe the attenuation or absorption of EM is far from sufficient. The investigation on the PCFs as high-performance microwave absorption materials is still in the early stage.
3.5 PCFs for sensing
Nowadays, sensors, as one of the most important devices in daily life, are used to detect and respond to physical stimuli (such as gas, pressure, chemical, and particular ingredients or motion) to ensure the safe and healthy life of human beings. Accordingly, PCFs can be an outstanding material for electrochemical sensors or gas detectors due to their high porosity with sufficient active sites, high specific surface area, good conductivity and stability.50,319,400,401 For example, Zhou's group used a PANI-based N-doped PCF to modify a pencil graphene electrode for detecting the hydroxyl radical (–OH).50 Compared with the unmodified graphene electrode, the PCF-modified sensor presented a better electrochemical behavior for –OH capture, indicating a fast electron transfer rate due to its higher specific surface area and better catalytic activity. Hu's group fabricated highly interconnected 3D N, S co-doped PCFs for the determination of Cd(II).400 Owing to the high N and S content and interconnected 3D porous structure, which resulted in better surface chemistry and electrochemical sensitivity, higher surface area and more active sites, respectively, the as-prepared PCF modified electrode exhibited an enhanced electrochemical sensing performance towards the detection pf Cd(II) with outstanding selectivity, low detection limits, wide linear range, high reproducibility and repeatability. Lee's group fabricated PCFs as a gas sensor from PAN precursor and carbon black mixture.402 This high porosity and subsequent fluorination treatment created more gas adsorption sites and functional groups on the surface of the CF, respectively, further promoting the gas capture. The rich micropores and the carbon black additive enhanced the resistance response and improved the sensitivity of the prepared sensor for NO and CO gases to around 5-times that of the pristine CF-based sensor.To further develop the chemical sensing characteristics of PCFs, surface functionalization of PCFs through the loading of a guest catalyst or creation of heterogeneous interfaces has been employed. For example, Li's group fabricated bacterial cellulose-based PCFs to build porous electrodes for the immobilization of glucose oxidase (GOx) as a glucose sensor (Fig. 13A).401 The highly meso-porous structure resulted in the immobilization of much more GOx to achieve a large current and highly negative redox potential, further improving the electrochemical catalytic activity and strong anti-interference ability. In addition, the increasing amount of mesopores also can realize the fast direct electrochemistry of GOx for the highly sensitive detection of glucose due to the intimate contacts between the immobilized GOx and the conductive PCF, enabling fast direct electrochemistry. Zhang's group manufactured a hemoglobin-graphene-modified carbon fiber microelectrode as an H2O2 electrochemical microsensor.403 The prepared CF with a 3D porous structure graphene layer coating possessed a large specific surface area and interconnected channels, where these structural characteristics can greatly assist the diffusion and adsorption of hemoglobin in the network, which further improved the electrocatalytic activity and sensitivity of the microelectrode towards H2O2. Metal-based nanoparticles decorated in/on porous carbon scaffolds have attracted great interest as sensors due to their high electrocatalytic activity, fast detection and high sensitivity.392,404–410 For example, Cha et al. proposed multitubular PCFs functionalized by monolayered tungsten disulfide (WS2) for the effective detection of NO2 (Fig. 13B).404 It was found that the prepared PCF composite exhibited highly enhanced sensing behavior due to its improved specific surface area and the exceedingly strong NO2 interaction energies of edge sites provided by WS2. The multiple tubular and porous structure inside the carbon matrix extraordinarily increase the number of reaction sites for NO2 molecules, and the uniformly dispersed WS2 in the PCF allowed more NO2 molecules to be absorbed on its surface. Furthermore, the variation in resistance of the PCF-based composite via electron transfer between WS2 and the PCF contributed significantly to its great sensing characteristics. Zhang's group tailored PtNi-embedded N-doped porous PCFs for the detection of hydrogen peroxide.405 The porous structure with a high surface area and homogeneously dispersed metal nanoparticles not only provided enough space and routes for mass and electron transport, but also prevented the aggregation and detachment of nanoparticles, which further improved its electrocatalytic ability and performance for H2O2 reduction as a detection sensor, such as a wide linear range, high sensitivity, low detection limit, high selectivity and anti-interference, as well as excellent reproducibility and excellent stability. Sharma's group prepared a highly sensitive PCF decorated with Ag nanoparticles as an enzymatic biosensor for the detection of triglyceride.406 The porous structure of the CF and its high surface-to-volume resulted in the growth of Ag nanoparticles on the fiber surface with uniform dispersion, which contributed the enhancement in electrical conductivity. Moreover, the hierarchical porous structure gave rise to improved stability and loading capacity for enzyme absorption, which led to higher sensitivity with a wide detection range as well as a low detection limit.
 | ||
| Fig. 13 (A) Schematic illustration of PCF electrode materials realizing the direct electrochemistry of GOx.401 Reproduced with permission from ref. 401. Copyright 2019, Wiley. (B) Schematic illustration of the sensing mechanism for WS2@PCF toward NO2. Crossed SEM images of WS2@PCFs.404 Reproduced with permission from ref. 404. Copyright 2017, Royal Society of Chemistry. (C) Shrink effect-enabled enzyme glucose oxidase immobilization in graphene-based PCF.414 Reproduced with permission from ref. 414. Copyright 2019, Elsevier. (D) Suggested sensing mechanism of porous WO3 NR-RGO.415 Reproduced with permission from ref. 415. Copyright 2019, American Chemical Society. | ||
Many studies have highlighted graphene or graphene oxide as a very promising candidate for sensing applications due to its unique characteristics such as excellent electrical properties, mechanical strength, and flexibility.365,411–413 Accordingly, hierarchical porous graphene fibers with a high special surface area have been investigated for wearable and fordable sensing applications. For example, Qu's group presented an electrochemical enzyme sensor based on a PCF with assembled 3D graphene by means of a shrink effect-enabled glucose oxidase (GOD) immobilization method (Fig. 13C).414 The porous structure of the 3D graphene hollow fiber delivered abundant binding sites for the adsorption of GOD and increased electron transmission and well-immobilized, robust enzyme with high reactivity, which promoted the detection of glucose with high sensitivity, selectivity and long-term stability. Kim's group prepared a sensitive nitrogen dioxide detector from a freestanding and ultra-porous reduced graphene oxide fiber functionalized with WO3 nanorods (Fig. 13D).415 The highly porous structure with high surface area could create numerous gas adsorption sites and high gas accessibility to obtain better sensitivity. Also, this well-maintained wrinkled surface and highly porous structure helped to achieve a uniform and non-agglomeration distribution of WO3 nanorods on the entire surface of the PCF, and it significantly enhanced the NO2 sensing characteristics because numerous heterogeneous junctions in the novel PCF were formed between the p-type rGO fibers and n-type WO3 NRs. Thus, this as-prepared composite PCF delivered reversible NO2 sensing kinetics and excellent compatibility with various objects even at 1 ppm NO2. Ding's group175 fabricated wearable and highly sensitive strain sensors derived from porous graphene fibers inserted with PVDF polymer nanoballs between graphene sheets to detect various data. The novel porous nanoball-decorated structure provided a much higher mechanical compliance of composites than the graphene fiber without the pore and nanoball structure due to its larger structure deformation for the same strain and improved specific area from the small space between nanoballs, which allowed a higher sensitivity of the PCF. In addition, the richer porous structure endowed the graphene fiber with not only higher resistivity due to the smaller contact area in the porous graphene frame, but also larger stretchability than the layer-by-layer stacking structure because the porous structure can theoretically provide a bigger space via bending of the network skeletons or rotation of the pore walls toward the stretching direction. Thus, by combing the porous and polymer nanoball structures, the prepared PCF exhibited high gauge factors (51 within 0–5% and 87 within 5–8% strain), a stable cycling performance (for 6000 cycles), fast response time (<100 ms), and low detection limit (0.01% strain).
Owing to their hierarchical porous structure, the PCF-based flexible materials devised in the laboratory show outstanding sensing performances including high sensitivity, wide sensing range, quick response, long-term stability and durability. Nevertheless, before PCF-based sensors can be used in practical applications, a number of limitations and challenges should be considered in-depth as further research aims. For wearable and foldable sensing or strain and pressure sensing applications, the negative effects of the porous structure on the mechanical properties and electrical conductivity of PCF-based sensors should be addressed. In addition, the sensing mechanisms of varying fields also have marked differences, and PCF-based sensors are still an emerging area. A more comprehensive understanding of the relationship and mechanism between the design of PCFs (porous structure, fiber diameter, pore volume, etc.) and performance feedback of sensors should be further investigated. Moreover, to meet the needs of various sensing requirements and overcome the excessive impact of external parameters such as temperature and humidity on sensitivity, more strategies including the introduction of functional guest materials, and surface and chemical modification should be further explored.
4. Conclusions and prospects
PCFs have received considerable attention in recent years due to their outstanding properties. This comprehensive review discussed five different structure design strategies of PCFs. Both template-based and template-free strategies are very adept at controlling the porosity and pore shape of carbons, especially in carbon nanofibers to get (1) PCFs with rich meso/macro-pores and (2) PCFs with highly interconnected pore structures. However, the challenges include their scalable fabrication with robust mechanical stability and flexibility. Therefore top-down routes using pristine carbon fibers as a resource to produce PCFs can be more practical. Post-treatment of carbon fibers via severe activation and chemical exfoliation is an effective and facile method to get (3) PCFs with defect-rich surface pore structures. These PCFs possess a higher surface area and ratio of micropores and small mesopores, but usually lack of a well-controlled pore size distribution. (4) Decorating porous carbon materials on the surface of pristine carbon fibers via physical or chemical approaches is preferred for the large-scale production of PCFs, which combines the advantages of porous carbon materials and carbon fibers. However, the evenness and relatively weak bonding force of porous carbonaceous materials with carbon fibers may limit the ultimate performance of the resulting PCFs. (5) PCFs with graphene assemblies demonstrate more porous structures, especially macro-pores, and inspiring graphene related features but a lower tensile strength than conventional PCFs.Owing to their exceptional characteristics such as large specific surface area, great pore volume, hierarchical porosity, numerous space for storage and 1D configuration, PCFs have found extensive applications in energy storage/conversion, catalysis, adsorption/absorption and sensing. More importantly, the hierarchically porous structure offers exciting opportunities for functionalizing materials, accessing more active sites, and facilitating mass transport/diffusion with a spectrum of interesting and remarkable performances. However, some disadvantages of hierarchically porous carbon fibers are also inevitable, such as relatively low volumetric energy and power density in supercapacitors, low initial coulombic efficiency in batteries, inadequate stability and durability in catalysis, variable selectivity and adsorption capacity, and insufficient mechanical properties and electrical conductivity for freestanding sensing. Besides, the five different types of PCFs with unique characteristics can also reveal some specific mechanisms between properties and their distinctive porous structure; however, there are still relatively few relevant targeted studies and comparative experiments in this field.
With recent works on the synthesis and application of PCFs, understanding the mechanism of their porous structure on their properties, and thus controllably tuning the performances of PCFs have progressively gone deeper from the macroscopic level to the molecular or even atomic level. However, to realize the full potential of PCFs in energy and environmental applications, several challenges need to be overcome, as follows.
(a) The mass and controlled production of PCFs with satisfactory properties still limits their wide applications. Currently, the most popular spinning method for PCF precursor fibers is the electrospinning technique, which has realized conversion from the laboratory to industrial scale. However, the corresponding approach for properly carbonizing electrospun PCF precursors on a large scale and continuous production of the relevant porous carbon nanofiber product are rarely reported. Alternatively, the pore-forming techniques to fabricate PCFs with good performances from wet-spun precursor fibers are very restricted. Despite the significant advances in the fabrication process of graphene-based PCFs in recent years, reliable and convenient methods to prepare continuous graphene-based PCFs are still lacking. The introduction of defects and porous carbonaceous decoration approach can be indispensable steps to produce PCFs on a large scale, but their relatively high cost and energy consumption are still important obstacles. Some newly emerging techniques can provide more options for the production of PCFs. For instance, 3D printing can quickly produce PCF precursor fibers or preforms containing porous carbon additives or sacrificial polymers, which can exhibit high porosity and surface area after pyrolysis.416,417 Plasma modification not only can directly synthesize PCFs by efficiently producing abundant defects on the surface of carbon fibers but also induce the reaction of carbon atoms with gas molecules or generated ions, leading to various functional groups and increased SSA, thus improving the affinity of PCFs with surrounding materials.3
(b) Several approaches for the production of porous structures have been developed; however, the regulation of the ratio among micro-, meso- and macro-pores is not fully optimized, and their interconnection is still hard to control. Although the structures with a large SSA, high porosity and pore volume are desirable for improving various activities, these features also lead to some undesirable side reactions, such as uncontrollable interphase reactions, low energy and power conversion efficiency, and low thermal stability. Considering this, the development of technology to accurately control the volume of different pores in the carbon matrix would be a meaningful direction for the future. For example, the templating method is still the most suitable way to generate a uniform pore size distribution. The dual-templating or multi-templating approach and templating and combined activation method can be beneficial to adjust the pore volume and distribution in hierarchical PCFs more precisely. Moreover, designing new templating agents or templates polymerized with precursors, block copolymers and more effective template-removal approaches may provide low-cost and scalable pathways for the production of PCFs with well-controlled porous structures under less consideration of the influence of fiber diameter.
(c) Improvement of the performance and realization of the multi-functionality of PCFs in various applications are still in their infancy. Due to their porous features, which can provide more active sites and space, a great number of heteroatoms or active materials have been loaded into PCFs and hybridized. However, not enough attention has been paid to understand the mechanism of the interaction of PCFs with complex loading materials and the surrounding media, such as electrolyte, water, and gas. Fundamental research on both the optimum use of pores and the communication adjustment of carbon pores with other materials is highly desired. For example, advanced characterization techniques should be further exploited to precisely reveal the structure of the pores, defects and active sites. In situ/operando characterization techniques to monitor the dynamic morphologies and structural change in the active site and interaction between PCFs and other components during performance testing will be highly beneficial. Comprehensive thermodynamic and kinetics studies, density-functional theory calculations and theoretical simulations of the pore formation and mechanism of the porous structure on energy and environmental applications will provide more valuable knowledge and guidance in the synthesis of PCFs with desirable structures and properties.
(d) The applications of PCFs are mainly focused on electrochemical energy applications, and thus it is necessary to broaden their application for environmental protection. In addition, many potential applications of PCFs, such as drug delivery, gas diffusion layers, solar energy conversion, and EMI shielding should be explored in the future.
Author contributions
Chao Liu: conceptualization, writing – original draft. Quanxiang Li: conceptualization, supervision, writing – review & editing. Weimin Kang: writing – review & editing. Weiwei Lei: writing – review & editing. Xungai Wang: writing – review & editing. Chunxiang Lu: writing – review & editing. Minoo Naebe: supervision, writing – review & editing, project administration, funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Chao Liu and Quanxiang Li contributed equally to this work. Quanxiang Li acknowledges support from Alfred Deakin Postdoctoral Fellowship from Deakin University.Notes and references
- D. S. Yu, K. Goh, H. Wang, L. Wei, W. C. Jiang, Q. Zhang, L. M. Dai and Y. Chen, Nat. Nanotechnol., 2014, 9, 555–562 CrossRef CAS.
- T. T. Zuo, X. W. Wu, C. P. Yang, Y. X. Yin, H. Ye, N. W. Li and Y. G. Guo, Adv. Mater., 2017, 29, 1700389 CrossRef PubMed.
- Z. J. Liu, Z. H. Zhao, Y. Y. Wang, S. Dou, D. F. Yan, D. D. Liu, Z. H. Xia and S. Y. Wang, Adv. Mater., 2017, 29, 1606207 CrossRef PubMed.
- N. Behabtu, C. C. Young, D. E. Tsentalovich, O. Kleinerman, X. Wang, A. W. K. Ma, E. A. Bengio, R. F. ter Waarbeek, J. J. de Jong, R. E. Hoogerwerf, S. B. Fairchild, J. B. Ferguson, B. Maruyama, J. Kono, Y. Talmon, Y. Cohen, M. J. Otto and M. Pasquali, Science, 2013, 339, 182–186 CrossRef CAS PubMed.
- X. Cai, C. Q. Zhang, S. S. Zhang, Y. P. Fang and D. C. Zou, J. Mater. Chem. A, 2017, 5, 2444–2459 RSC.
- M. Zhang, M. Shoaib, H. L. Fei, T. Wang, J. Zhong, L. Fan, L. Wang, H. Y. Luo, S. Tan, Y. Y. Wang, J. Zhu, J. W. Hu and B. G. Lu, Adv. Energy Mater., 2019, 9, 1901663 CrossRef CAS.
- L. D. Tian, D. X. Ji, S. Zhang, X. W. He, S. Ramakrishna and Q. Y. Zhang, Small, 2020, 16, 2001743 CrossRef CAS.
- Z. G. Zhu, C. H. Ji, L. L. Zhong, S. Liu, F. Y. Cui, H. L. Sun and W. Wang, J. Mater. Chem. A, 2017, 5, 18071–18080 RSC.
- L. Borchardt, M. Oschatz and S. Kaskel, Mater. Horiz., 2014, 1, 157–168 RSC.
- S. Dutta, A. Bhaumik and K. C. W. Wu, Energy Environ. Sci., 2014, 7, 3574–3592 RSC.
- Z. P. Zhou, T. Y. Liu, A. U. Khan and G. L. Liu, Sci. Adv., 2019, 5, eaau6852 CrossRef.
- H. Wang, Y. Shao, S. L. Mei, Y. Lu, M. Zhang, J. K. Sun, K. Matyjaszewski, M. Antonietti and J. Y. Yuan, Chem. Rev., 2020, 120, 9363–9419 CrossRef CAS PubMed.
- W. Zeng, L. Shu, Q. Li, S. Chen, F. Wang and X. M. Tao, Adv. Mater., 2014, 26, 5310–5336 CrossRef CAS PubMed.
- J. J. Xue, T. Wu, Y. Q. Dai and Y. N. Xia, Chem. Rev., 2019, 119, 5298–5415 CrossRef CAS.
- Z. Y. Wu, H. W. Liang, B. C. Hu and S. H. Yu, Angew. Chem., Int. Ed., 2018, 57, 15646–15662 CrossRef CAS PubMed.
- Q. Yu, J. S. Lv, Z. H. Liu, M. Xu, W. Yang, K. A. Owusu, L. Q. Mai, D. Y. Zhao and L. Zhou, Sci. Bull., 2019, 64, 1617–1624 CrossRef CAS.
- S. H. Chen, L. Qiu and H. M. Cheng, Chem. Rev., 2020, 120, 2811–2878 CrossRef CAS PubMed.
- L. F. Chen, Y. Feng, H. W. Liang, Z. Y. Wu and S. H. Yu, Adv. Energy Mater., 2017, 7, 1700826 CrossRef.
- C. Huang and N. L. Thomas, Polym. Rev., 2020, 60, 595–647 CrossRef CAS.
- F. Hoffmann, M. Cornelius, J. Morell and M. Froba, Angew. Chem., Int. Ed., 2006, 45, 3216–3251 CrossRef CAS.
- M. Kruk and M. Jaroniec, Chem. Mater., 2001, 13, 3169–3183 CrossRef CAS.
- G. J. D. Soler-illia, C. Sanchez, B. Lebeau and J. Patarin, Chem. Rev., 2002, 102, 4093–4138 CrossRef PubMed.
- J. Lee, J. Kim and T. Hyeon, Adv. Mater., 2006, 18, 2073–2094 CrossRef CAS.
- S. H. Joo, S. J. Choi, I. Oh, J. Kwak, Z. Liu, O. Terasaki and R. Ryoo, Nature, 2001, 412, 169–172 CrossRef CAS.
- J. Liang, Y. Zheng, J. Chen, J. Liu, D. Hulicova-Jurcakova, M. Jaroniec and S. Z. Qiao, Angew. Chem., Int. Ed., 2012, 51, 3892–3896 CrossRef CAS PubMed.
- L. W. Ji, Z. Lin, A. J. Medford and X. W. Zhang, Carbon, 2009, 47, 3346–3354 CrossRef CAS.
- G. H. An, D. Y. Lee and H. J. Ahn, ACS Appl. Mater. Interfaces, 2017, 9, 12478–12485 CrossRef CAS PubMed.
- J. W. Jung, C. L. Lee, S. Yu and I. D. Kim, J. Mater. Chem. A, 2016, 4, 703–750 RSC.
- Y. B. Xiao, Y. Z. Xu, K. Y. Zhang, X. N. Tang, J. Huang, K. Yuan and Y. W. Chen, Carbon, 2020, 160, 80–87 CrossRef CAS.
- J. Wang, J. Tang, Y. L. Xu, B. Ding, Z. Chang, Y. Wang, X. D. Hao, H. Dou, J. H. Kim, X. G. Zhang and Y. Yamauchi, Nano Energy, 2016, 28, 232–240 CrossRef CAS.
- L. J. Zhang, Y. Z. Jiang, L. W. Wang, C. Zhang and S. X. Liu, Electrochim. Acta, 2016, 196, 189–196 CrossRef CAS.
- C. Ma, Z. Y. Li, J. J. Li, Q. C. Fan, L. Q. Wu, J. L. Shi and Y. Song, Appl. Surf. Sci., 2018, 456, 568–576 CrossRef CAS.
- K. K. Karthikeyan and P. Biji, Microporous Mesoporous Mater., 2016, 224, 372–383 CrossRef CAS.
- Y. Cao, H. M. Lu, Q. S. Hong, J. J. Bai, J. R. Wang and X. D. Li, J. Power Sources, 2017, 368, 78–87 CrossRef CAS.
- W. H. Ryu, J. Shin, J. W. Jung and I. D. Kim, J. Mater. Chem. A, 2013, 1, 3239–3243 RSC.
- Y. Liu, J. Y. Zhou, L. L. Chen, P. Zhang, W. B. Fu, H. Zhao, Y. F. Ma, X. J. Pan, Z. X. Zhang, W. H. Han and E. Q. Xie, ACS Appl. Mater. Interfaces, 2015, 7, 23515–23520 CrossRef CAS.
- J. L. Xu, L. Zhang, G. C. Xu, Z. P. Sun, C. Zhang, X. Ma, C. L. Qi, L. Zhang and D. Z. Jia, Appl. Surf. Sci., 2018, 434, 112–119 CrossRef CAS.
- K. S. Yang and B. H. Kim, Electrochim. Acta, 2015, 186, 337–344 CrossRef CAS.
- J. S. Cho, Y. J. Hong and Y. C. Kang, ACS Nano, 2015, 9, 4026–4035 CrossRef CAS PubMed.
- Z. L. Xu, J. Q. Huang, W. G. Chong, X. Y. Qin, X. Y. Wang, L. M. Zhou and J. K. Kim, Adv. Energy Mater., 2017, 7, 1602078 CrossRef.
- X. Y. Qin, H. R. Zhang, J. X. Wu, X. D. Chu, Y. B. He, C. P. Han, C. Miao, S. A. Wang, B. H. Li and F. Y. Kang, Carbon, 2015, 87, 347–356 CrossRef CAS.
- Z. Shen, Y. Hu, Y. L. Chen, X. W. Zhang, K. H. Wang and R. Z. Chen, J. Power Sources, 2015, 278, 660–667 CrossRef CAS.
- D. G. Lee, J. H. Kim and B. H. Kim, Electrochim. Acta, 2016, 200, 174–181 CrossRef CAS.
- H. K. Wang, Q. Z. Wu, D. X. Cao, X. Lu, J. K. Wang, M. K. H. Leung, S. D. Cheng, L. Lu and C. M. Niu, Mater. Today Energy, 2016, 1–2, 24–32 Search PubMed.
- Y. M. Chen, X. Y. Li, X. Y. Zhou, H. M. Yao, H. T. Huang, Y. W. Mai and L. M. Zhou, Energy Environ. Sci., 2014, 7, 2689–2696 RSC.
- C. H. Wang, Y. V. Kaneti, Y. Bando, J. J. Lin, C. Liu, J. S. Li and Y. Yamauchi, Mater. Horiz., 2018, 5, 394–407 RSC.
- Y. B. Dou, W. J. Zhang and A. Kaiser, Adv. Sci., 2020, 7, 1902590 CrossRef CAS PubMed.
- C. L. Zhang, B. R. Lu, F. H. Cao, Z. L. Yu, H. P. Cong and S. H. Yu, J. Mater. Chem. A, 2018, 6, 12962–12968 RSC.
- Z. Li and B. H. J. Tang, Green Chem., 2017, 19, 5862–5873 RSC.
- J. Ouyang, Z. Q. Li, J. Zhang, C. Wang, J. Wang, X. H. Xia and G. J. Zhou, Analyst, 2014, 139, 3416–3422 RSC.
- C. Liu, J. Wang, J. S. Li, J. Z. Liu, C. H. Wang, X. Y. Sun, J. Y. Shen, W. Q. Han and L. J. Wang, J. Mater. Chem. A, 2017, 5, 1211–1220 RSC.
- G. R. Li, W. Lei, D. Luo, Y. P. Deng, Z. P. Deng, D. L. Wang, A. P. Yu and Z. W. Chen, Energy Environ. Sci., 2018, 11, 2372–2381 RSC.
- Z. Wang, J. M. Ang, J. Liu, X. Y. D. Ma, J. H. Kong, Y. F. Zhang, T. Yan and X. H. Lu, Appl. Catal., B, 2020, 263, 118344 CrossRef CAS.
- W. Li, J. Liu and D. Y. Zhao, Nat. Rev. Mater., 2016, 1, 16023 CrossRef CAS.
- J. Chen, A. Amiri, J. C. Boyd and M. Naraghi, Adv. Funct. Mater., 2019, 29, 1901425 CrossRef.
- M. Dirican, Y. Lu, Y. Q. Ge, O. Yildiz and X. W. Zhang, ACS Appl. Mater. Interfaces, 2015, 7, 18387–18396 CrossRef CAS PubMed.
- L. W. Ji, M. M. Rao, S. Aloni, L. Wang, E. J. Cairns and Y. G. Zhang, Energy Environ. Sci., 2011, 4, 5053–5059 RSC.
- J. Ramachandran, M. X. Lu, P. J. Arias-Monje, M. H. Kirmani, N. Shirolkar and S. Kumar, Carbon, 2021, 173, 724–735 CrossRef CAS.
- H. Y. Lu, W. Fan, Y. P. Huang and T. X. Liu, Nano Res., 2018, 11, 1274–1284 CrossRef CAS.
- W. M. Kang, L. L. Fan, N. P. Deng, H. J. Zhao, Q. X. Li, N. Minoo, J. Yan and B. W. Cheng, Chem. Eng. J., 2018, 333, 185–190 CrossRef CAS.
- G. Zainab, A. A. Babar, N. Ali, A. A. Aboalhassan, X. F. Wang, J. Y. Yu and B. Ding, J. Colloid Interface Sci., 2020, 561, 659–667 CrossRef CAS PubMed.
- M. C. Zou, W. Q. Zhao, H. S. Wu, H. Zhang, W. J. Xu, L. S. Yang, S. T. Wu, Y. S. Wang, Y. J. Chen, L. Xu and A. Y. Cao, Adv. Mater., 2018, 30, 1704419 CrossRef PubMed.
- H. J. Pan, J. M. Yang, S. P. Wang, Z. B. Xiong, W. S. Cai and J. Y. Liu, J. Mater. Chem. A, 2015, 3, 13827–13834 RSC.
- M. Abdah, N. H. N. Azman, S. Kulandaivalu, N. A. Rahman, A. H. Abdullah and Y. Sulaiman, J. Power Sources, 2019, 444, 227324 CrossRef.
- G. H. An, B. R. Koo and H. J. Ahn, Phys. Chem. Chem. Phys., 2016, 18, 6587–6594 RSC.
- G. S. Park, J. S. Lee, S. T. Kim, S. Park and J. Cho, J. Power Sources, 2013, 243, 267–273 CrossRef CAS.
- Y. E. Miao, Y. P. Huang, L. S. Zhang, W. Fan, F. L. Lai and T. X. Liu, Nanoscale, 2015, 7, 11093–11101 RSC.
- J. Sun, L. Zeng, H. R. Jiang, C. Y. H. Chao and T. S. Zhao, J. Power Sources, 2018, 405, 106–113 CrossRef CAS.
- S. J. Zhang, H. Q. Yu and H. M. Feng, Carbon, 2006, 44, 2059–2068 CrossRef CAS.
- H. Khayyam, R. N. Jazar, S. Nunna, G. Golkarnarenji, K. Badii, S. M. Fakhrhoseini, S. Kumar and M. Naebe, Prog. Mater. Sci., 2020, 107, 100575 CrossRef CAS.
- X. H. Gao, X. Y. Wu and J. Qiu, Adv. Electron. Mater., 2018, 4, 1700565 CrossRef.
- W. Xie, H. F. Cheng, Z. Y. Chu and Z. H. Chen, Ceram. Int., 2009, 35, 2705–2710 CrossRef CAS.
- X. L. Wu, L. L. Chen, S. Xin, Y. X. Yin, Y. G. Guo, Q. S. Kong and Y. Z. Xia, ChemSusChem, 2010, 3, 703–707 CrossRef CAS PubMed.
- J. J. Yan, H. Y. Lu, Y. P. Huang, J. Fu, S. Y. Mo, C. Wei, Y. E. Miao and T. X. Liu, J. Mater. Chem. A, 2015, 3, 23299–23306 RSC.
- Y. Yang, A. Centrone, L. Chen, F. Simeon, T. A. Hatton and G. C. Rutledge, Carbon, 2011, 49, 3395–3403 CrossRef CAS.
- M. X. Guo, J. X. Guo, D. Z. Jia, H. Y. Zhao, Z. P. Sun, X. L. Song and Y. H. Li, J. Mater. Chem. A, 2015, 3, 21178–21184 RSC.
- T. Y. Liu, Z. P. Zhou, Y. C. Guo, D. Guo and G. L. Liu, Nat. Commun., 2019, 10, 675 CrossRef CAS PubMed.
- L. Cao, X. H. Zhou, Z. H. Li, K. M. Su and B. W. Cheng, J. Power Sources, 2019, 413, 376–383 CrossRef CAS.
- T. Y. Liu, J. Serrano, J. Elliott, X. Z. Yang, W. Cathcart, Z. X. Wang, Z. He and G. L. Liu, Sci. Adv., 2020, 6, eaaz0906 CrossRef CAS PubMed.
- H. Liu, C. Y. Cao, F. F. Wei, Y. Jiang, Y. B. Sun, P. P. Huang and W. G. Song, J. Phys. Chem. C, 2013, 117, 21426–21432 CrossRef CAS.
- L. Fan, L. Yang, X. Y. Ni, J. Han, R. Guo and C. F. Zhang, Carbon, 2016, 107, 629–637 CrossRef CAS.
- D. G. Lee, J. H. Kim and B. H. Kim, Chem. Eng. J., 2015, 263, 62–70 CrossRef CAS.
- Y. M. Sun, R. B. Sills, X. L. Hu, Z. W. Seh, X. Xiao, H. H. Xui, W. Luo, H. Y. Jin, Y. Xin, T. Q. Li, Z. L. Zhang, J. Zhou, W. Cai, Y. H. Huang and Y. Cui, Nano Lett., 2015, 15, 3899–3906 CrossRef CAS PubMed.
- X. H. Zhou, K. M. Su, W. M. Kang, B. W. Cheng, Z. H. Li and Z. Jiang, Chem. Eng. J., 2020, 397, 10 Search PubMed.
- L. Wang, G. H. Zhang, X. J. Zhang, H. M. Shi, W. Zeng, H. Zhang, Q. Liu, C. C. Li, Q. H. Liu and H. G. Duan, J. Mater. Chem. A, 2017, 5, 14801–14810 RSC.
- S. K. Park, J. S. Park and Y. C. Kang, J. Mater. Chem. A, 2018, 6, 1028–1036 RSC.
- L. F. Chen, Y. Lu, L. Yu and X. W. Lou, Energy Environ. Sci., 2017, 10, 1777–1783 RSC.
- Y. Yang, B. w. Deng, X. Liu, Y. Li, B. Yin and M. b. Yang, Electrochim. Acta, 2019, 324, 134891 CrossRef CAS.
- H. P. Yang, Y. Wu, Q. Lin, L. D. Fan, X. Y. Chai, Q. L. Zhang, J. H. Liu, C. X. He and Z. Q. Lin, Angew. Chem., Int. Ed., 2018, 57, 15476–15480 CrossRef CAS PubMed.
- Y. C. Yao, H. L. Wu, L. Huang, X. Y. Li, L. Yu, S. Z. Zeng, X. R. Zeng, J. W. Yang and J. Z. Zou, Electrochim. Acta, 2017, 246, 606–614 CrossRef CAS.
- Y. Ouyang, X. B. Zhu, F. Li, F. L. Lai, Y. Wu, Y. E. Miao and T. X. Liu, Appl. Surf. Sci., 2019, 475, 211–218 CrossRef CAS.
- Y. W. Liu, Q. Liu, L. Wang, X. F. Yang, W. Y. Yang, J. J. Zheng and H. L. Hou, ACS Appl. Mater. Interfaces, 2020, 4777–4786 CrossRef CAS PubMed.
- J. G. Ju, L. T. Zhang, H. S. Shi, Z. J. Li, W. M. Kang and B. W. Cheng, Appl. Surf. Sci., 2019, 484, 392–402 CrossRef CAS.
- J. G. Ju, Y. Lv, L. Y. Wang, W. C. Liu, Z. J. Li, W. M. Kang and B. W. Cheng, J. Electrochem. Soc., 2019, 166, A1223–A1230 CrossRef CAS.
- J. G. Ju, H. J. Zhao, W. M. Kang, N. N. Tian, N. P. Deng and B. W. Cheng, Electrochim. Acta, 2017, 258, 116–123 CrossRef CAS.
- N. P. Deng, J. G. Ju, J. Yan, X. H. Zhou, Q. Q. Qin, K. Zhang, Y. Y. Liang, Q. X. Li, W. M. Kang and B. W. Cheng, ACS Appl. Mater. Interfaces, 2018, 10, 12626–12638 CrossRef CAS PubMed.
- J. H. Yan, K. Q. Dong, Y. Y. Zhang, X. Wang, A. A. Aboalhassan, J. Y. Yu and B. Ding, Nat. Commun., 2019, 10, 1–9 CrossRef CAS PubMed.
- T. S. He, Q. Y. Su, Z. Yildiz, K. D. Cai and Y. J. Wang, Electrochim. Acta, 2016, 222, 1120–1127 CrossRef CAS.
- X. F. Yang, Y. Yu, N. Yan, H. Z. Zhang, X. F. Li and H. M. Zhang, J. Mater. Chem. A, 2016, 4, 5965–5972 RSC.
- Y. X. Zhao, Q. X. Lai, Y. Wang, J. J. Zhu and Y. Y. Liang, ACS Appl. Mater. Interfaces, 2017, 9, 16178–16186 CrossRef CAS PubMed.
- Z. P. Zhou, T. Y. Liu, A. U. Khan and G. L. Liu, Mol. Syst. Des. Eng., 2020, 5, 153–165 RSC.
- J. M. Serrano, T. Y. Liu, A. U. Khan, B. Botset, B. J. Stovall, Z. Xu, D. Guo, K. Cao, X. Hao, S. F. Cheng and G. L. Liu, Chem. Mater., 2019, 31, 8898–8907 CrossRef CAS.
- Y. Li, B. Zou, C. W. Hu and M. H. Cao, Carbon, 2016, 99, 79–89 CrossRef CAS.
- A. Choudhury, J. H. Kim, S. Sinha Mahapatra, K. S. Yang and D. J. Yang, ACS Sustainable Chem. Eng., 2017, 5, 2109–2118 CrossRef CAS.
- C. Ma, Y. J. Li, J. L. Shi, Y. Song and L. Liu, Chem. Eng. J., 2014, 249, 216–225 CrossRef CAS.
- C. Ma, L. Q. Wu, M. Dirican, H. Cheng, J. J. Li, Y. Song, J. L. Shi and X. W. Zhang, Appl. Surf. Sci., 2021, 537, 11 Search PubMed.
- Y. Li, C. X. Lu, S. C. Zhang, F. Y. Su, W. Z. Shen, P. C. Zhou and C. L. Ma, J. Mater. Chem. A, 2015, 3, 14817–14825 RSC.
- Q. Li, J. Wang, C. Liu, S. M. Fakhrhoseini, D. Liu, L. Zhang, W. Lei and M. Naebe, Adv. Sci., 2019, 6, 1900762 CrossRef CAS PubMed.
- D. Deng, X. Liao and B. Shi, ChemSusChem, 2008, 1, 298–301 CrossRef CAS PubMed.
- Y. Liu, Z. J. Shi, Y. F. Gao, W. D. An, Z. Z. Cao and J. R. Liu, ACS Appl. Mater. Interfaces, 2016, 8, 28283–28290 CrossRef CAS PubMed.
- Y. Huang, L. Peng, Y. Liu, G. Zhao, J. Y. Chen and G. Yu, ACS Appl. Mater. Interfaces, 2016, 8, 15205–15215 CrossRef CAS PubMed.
- G. L. Cao, Y. M. Yan, T. Liu, D. Rooney, Y. F. Guo and K. N. Sun, Carbon, 2015, 94, 680–686 CrossRef CAS.
- Q. Wang, F. Y. Liu, Z. Y. Jin, X. R. Qiao, H. C. Huang, X. Chu, D. Xiong, H. T. Zhang, Y. Liu and W. Q. Yang, Adv. Funct. Mater., 2020, 30, 2002580 CrossRef CAS.
- H. N. Zhang, A. N. Li, J. Wang, Y. Zhang, Z. Zhao, H. f. Zhao, M. Cheng, C. w. Wang, J. y. Wang and S. C. Zhang, Carbon, 2018, 126, 500–506 CrossRef CAS.
- X. H. Zhang, H. X. Li, B. Qin, Q. Wang, X. H. Xing, D. H. Yang, L. e. Jin and Q. Cao, J. Mater. Chem. A, 2019, 7, 3298–3306 RSC.
- K. Lu, Z. Hu, J. Ma, H. Ma, L. Dai and J. Zhang, Nat. Commun., 2017, 8, 527 CrossRef PubMed.
- R. J. Zou, Q. Liu, G. J. He, M. F. Yuen, K. B. Xu, J. Q. Hu, I. P. Parkin, C. S. Lee and W. J. Zhang, Adv. Energy Mater., 2017, 7, 1601363 CrossRef.
- Q. Li, Z. A. Zhang, Z. P. Guo, Y. Q. Lai, K. Zhang and J. Li, Carbon, 2014, 78, 1–9 CrossRef CAS.
- F. Wu, K. Yang, Q. Li, T. R. Shah, M. Ahmad, Q. Y. Zhang and B. L. Zhang, Carbon, 2021, 173, 918–931 CrossRef CAS.
- W. J. Ma, M. Li, X. Zhou, J. H. Li, Y. M. Dong and M. F. Zhu, ACS Appl. Mater. Interfaces, 2019, 11, 9283–9290 CrossRef CAS PubMed.
- G. Wu, P. F. Tan, X. J. Wu, L. Peng, H. Y. Cheng, C. F. Wang, W. Chen, Z. Y. Yu and S. Chen, Adv. Funct. Mater., 2017, 27, 1702493 CrossRef.
- Z. Xu, Y. Zhang, P. G. Li and C. Gao, ACS Nano, 2012, 6, 7103–7113 CrossRef CAS PubMed.
- X. H. Zheng, K. Zhang, L. Yao, Y. P. Qiu and S. R. Wang, J. Mater. Chem. A, 2018, 6, 896–907 RSC.
- Toray Innovation by Chemistry, https://www.toray.com/global/news/details/20191118000542.html, accessed 18 November, 2019 Search PubMed.
- T. Mihara, K. Tanaka, K. Takeuchi, T. Horiguchi, US Pat., US10011487B2, 2018.
- J. J. Hao, C. C. Lu, P. C. Zhou and D. H. Li, Thermochim. Acta, 2013, 569, 42–47 CrossRef CAS.
- L. F. Lei, F. J. Pan, A. Lindbrathen, X. P. Zhang, M. Hillestad, Y. Nie, L. Bai, X. Z. He and M. D. Guiver, Nat. Commun., 2021, 12, 268 CrossRef CAS PubMed.
- W. C. Jiang, L. Y. Li, J. Q. Pan, R. A. Senthil, X. Jin, J. Q. Cai, J. Wang and X. G. Liu, J. Power Sources, 2019, 438, 226936 CrossRef CAS.
- X. H. Duan, C. Srinivasakannan, X. Wang, F. Wang and X. Y. Liu, J. Taiwan Inst. Chem. Eng., 2017, 70, 374–381 CrossRef CAS.
- S. H. Yang, Z. Z. Han, F. Y. Zheng, J. Sun, Z. S. Qiao, X. P. Yang, L. Li, C. C. Li, X. F. Song and B. Q. Cao, Carbon, 2018, 134, 15–21 CrossRef CAS.
- L. Chen, T. Ji, L. W. Mu and J. H. Zhu, Carbon, 2017, 111, 839–848 CrossRef CAS.
- W. L. Zhang, R. Guo, J. Sun, L. Q. Dang, Z. H. Liu, Z. B. Lei and Q. J. Sun, J. Colloid Interface Sci., 2019, 553, 705–712 CrossRef CAS PubMed.
- M. L. Li, H. Y. Xiao, T. Zhang, Q. R. Li and Y. F. Zhao, ACS Sustainable Chem. Eng., 2019, 7, 4716–4723 CrossRef CAS.
- C. Nieto-Delgado, D. Partida-Gutierrez and J. R. Rangel-Mendez, J. Cleaner Prod., 2019, 213, 650–658 CrossRef CAS.
- Y. R. Liang, D. C. Wu and R. W. Fu, Sci. Rep., 2013, 3, 1119 CrossRef PubMed.
- X. Q. Zhang, Y. Zhong, X. H. Xia, Y. Xia, D. H. Wang, C. A. Zhou, W. J. Tang, X. L. Wang, J. Wu and J. P. Tu, ACS Appl. Mater. Interfaces, 2018, 10, 13598–13605 CrossRef CAS PubMed.
- T. T. Yang, Y. L. Xi, Y. K. Zhu, J. Z. Li, X. X. Pan, V. Y. Izotov, Q. Guo and W. Han, J. Mater. Sci.: Mater. Electron., 2017, 28, 17592–17600 CrossRef CAS.
- N. Salim, X. Jin, S. Mateti, H. Lin, V. Glattauer, B. Fox and J. Ramshaw, Mater. Today Adv., 2019, 1, 100005 CrossRef.
- W. Wang, Y. Sun, B. Liu, S. G. Wang and M. H. Cao, Carbon, 2015, 91, 56–65 CrossRef CAS.
- W. Z. Shen, T. P. Hu, P. Y. Wang, H. Z. Sun and W. B. Fan, ChemPlusChem, 2014, 79, 284–289 CrossRef CAS PubMed.
- K. Sagisaka, A. Kondo, T. Iiyama, M. Kimura, H. Fujimoto, H. Touhara and Y. Hattori, Chem. Phys. Lett., 2018, 710, 118–122 CrossRef CAS.
- Q. Li, J. Wang, C. Liu, S. M. Fakhrhoseini, D. Liu, L. Zhang, W. Lei and M. Naebe, Adv. Sci., 2019, 6, 1900762 CrossRef CAS PubMed.
- Y. Q. Zhang, Y. Cong, J. Zhang, X. K. Li, Y. J. Li, Z. J. Dong, G. M. Yuan, J. Zhang and Z. W. Cui, Surf. Coat. Technol., 2018, 349, 384–391 CrossRef CAS.
- J. Wang, C. J. Cui and W. Z. Qian, Carbon, 2019, 154, 1–6 CrossRef.
- G. B. Lou, Y. T. Wu, X. Q. Zhu, Y. Z. Lu, S. Yu, C. h. Yang, H. Chen, C. Guan, L. Li and Z. Shen, ACS Appl. Mater. Interfaces, 2018, 10, 42503–42512 CrossRef CAS PubMed.
- Y. Han, Y. Z. Lu, S. H. Shen, Y. Zhong, S. Liu, X. H. Xia, Y. X. Tong and X. H. Lu, Adv. Funct. Mater., 2019, 29, 1806329 CrossRef.
- Y. Fu, J. Hu, Q. Wang, K. K. Li and L. M. Zhou, Carbon, 2019, 150, 76–84 CrossRef CAS.
- W. Q. Kong, G. Wang, M. Zhang, X. D. Duan, J. W. Hu and X. F. Duan, Desalination, 2019, 459, 1–9 CrossRef CAS.
- Z. Zhao, Z. K. Yuan, Z. S. Fang, J. H. Jian, J. Li, M. J. Yang, C. S. Mo, Y. Zhang, X. H. Hu, P. Li, S. Y. Wang, W. Hong, Z. K. Zheng, G. F. Ouyang, X. D. Chen and D. S. Yu, Adv. Sci., 2018, 5, 1800760 CrossRef PubMed.
- H. F. Wang, C. Tang, B. Wang, B. Q. Li, X. Y. Cui and Q. Zhang, Energy Storage Mater., 2018, 15, 124–130 CrossRef.
- Q. X. Li, J. S. Church, M. Naebe and B. L. Fox, Composites, Part A, 2016, 90, 174–185 CrossRef.
- Q. H. Zhang, J. W. Liu, R. Sager, L. M. Dai and J. Baur, Compos. Sci. Technol., 2009, 69, 594–601 CrossRef CAS.
- Y. Q. Zhang, X. H. Xia, X. Cao, B. W. Zhang, N. H. Tiep, H. Y. He, S. Chen, Y. Z. Huang and H. J. Fan, Adv. Energy Mater., 2017, 7, 1700220 CrossRef.
- J. Zeng, X. X. Ji, Y. H. Ma, Z. X. Zhang, S. G. Wang, Z. H. Ren, C. Y. Zhi and J. Yu, Adv. Mater., 2018, 30, 1705380 CrossRef PubMed.
- H. J. Chu, C. Y. Lee and N. H. Tai, RSC Adv., 2016, 6, 111465–111471 RSC.
- J. N. Zhang, X. L. Zhang, Y. C. Zhou, S. J. Guo, K. X. Wang, Z. Q. Liang and Q. Xu, ACS Sustainable Chem. Eng., 2014, 2, 1525–1533 CrossRef CAS.
- L. Wang, Y. Dong, Y. Zhang, Z. Y. Zhang, K. Chi, H. Yuan, A. S. Zhao, J. H. Ren, F. Xiao and S. Wang, NPG Asia Mater., 2016, 8, e337 CrossRef.
- Z. Xu and C. Gao, Nat. Commun., 2011, 2, 571 CrossRef PubMed.
- H. P. Cong, X. C. Ren, P. Wang and S. H. Yu, Sci. Rep., 2012, 2, 613 CrossRef PubMed.
- Z. L. Dong, C. C. Jiang, H. H. Cheng, Y. Zhao, G. Q. Shi, L. Jiang and L. T. Qu, Adv. Mater., 2012, 24, 1856–1861 CrossRef CAS PubMed.
- Z. Xu, Y. J. Liu, X. L. Zhao, L. Peng, H. Y. Sun, Y. Xu, X. B. Ren, C. H. Jin, P. Xu, M. Wang and C. Gao, Adv. Mater., 2016, 28, 6449 CrossRef CAS PubMed.
- G. Q. Xin, T. K. Yao, H. T. Sun, S. M. Scott, D. L. Shao, G. K. Wang and J. Lian, Science, 2015, 349, 1083–1087 CrossRef CAS PubMed.
- Z. Xu, H. Y. Sun, X. L. Zhao and C. Gao, Adv. Mater., 2013, 25, 188–193 CrossRef CAS PubMed.
- Z. Xu and C. Gao, Acc. Chem. Res., 2014, 47, 1267–1276 CrossRef CAS PubMed.
- S. H. Chen, W. J. Ma, Y. H. Cheng, Z. Weng, B. Sun, L. Wang, W. P. Chen, F. Li, M. F. Zhu and H. M. Cheng, Nano Energy, 2015, 15, 642–653 CrossRef CAS.
- S. H. Aboutalebi, R. Jalili, D. Esrafilzadeh, M. Salari, Z. Gholamvand, S. A. Yamini, K. Konstantinov, R. L. Shepherd, J. Chen, S. E. Moulton, P. C. Innis, A. I. Minett, J. M. Razal and G. G. Wallace, ACS Nano, 2014, 8, 2456–2466 Search PubMed.
- X. Jiang, Z. Ren, Y. Fu, Y. Liu, R. Zou, G. Ji, H. Ning, Y. Li, J. Wen, H. J. Qi, C. Xu, S. Fu, J. Qiu and N. Hu, ACS Appl. Mater. Interfaces, 2019, 11, 37051–37059 CrossRef CAS PubMed.
- W. H. Cai, T. Lai and J. S. Ye, J. Mater. Chem. A, 2015, 3, 5060–5066 RSC.
- Y. N. Meng, Y. Zhao, C. G. Hu, H. H. Cheng, Y. Hu, Z. P. Zhang, G. Q. Shi and L. T. Qu, Adv. Mater., 2013, 25, 2326–2331 CrossRef CAS PubMed.
- Y. W. Ma, P. Li, J. W. Sedloff, X. Zhang, H. B. Zhang and J. Liu, ACS Nano, 2015, 9, 1352–1359 CrossRef CAS PubMed.
- Z. Lu, J. Foroughi, C. Y. Wang, H. R. Long and G. G. Wallace, Adv. Energy Mater., 2018, 8, 1702047 CrossRef.
- H. Park, R. B. Ambade, S. H. Noh, W. Eom, K. H. Koh, S. B. Ambade, W. J. Lee, S. H. Kim and T. H. Han, ACS Appl. Mater. Interfaces, 2019, 11, 9011–9022 CrossRef CAS PubMed.
- S. L. Wang, N. S. Liu, J. Su, L. Y. Li, F. Long, Z. G. Zou, X. L. Jiang and Y. H. Gao, ACS Nano, 2017, 11, 2066–2074 CrossRef CAS PubMed.
- W. J. Ma, W. F. Li, M. Li, Q. H. Mao, Z. H. Pan, J. Hu, X. Li, M. F. Zhu and Y. G. Zhang, Adv. Funct. Mater., 2021, 31, 2100195 CrossRef CAS.
- T. Huang, P. He, R. R. Wang, S. W. Yang, J. Sun, X. M. Xie and G. Q. Ding, Adv. Funct. Mater., 2019, 29, 1903732 CrossRef CAS.
- G. Y. Chen, Y. L. Ai, I. T. Mugaanire, W. J. Ma, B. S. Hsiao, K. Hou and M. F. Zhu, J. Power Sources, 2020, 450, 227637 CrossRef CAS.
- F. C. Meng, W. B. Lu, Q. W. Li, J. H. Byun, Y. Oh and T. W. Chou, Adv. Mater., 2015, 27, 5113–5131 CrossRef CAS PubMed.
- B. Fang, D. Chang, Z. Xu and C. Gao, Adv. Mater., 2020, 32, 1902664 CrossRef CAS PubMed.
- J. Niu, R. Shao, M. Y. Liu, Y. X. Zan, M. L. Dou, J. J. Liu, Z. P. Zhang, Y. Q. Huang and F. Wang, Adv. Funct. Mater., 2019, 29, 1905095 CrossRef CAS.
- J. Deng, M. M. Li and Y. Wang, Green Chem., 2016, 18, 4824–4854 RSC.
- L. H. Zhang, Y. M. Shi, Y. Wang and N. R. Shiju, Adv. Sci., 2020, 7, 1902126 CrossRef CAS PubMed.
- S. Jiang, J. L. Li, J. Fang and X. G. Wang, Small, 2021, 17, 1903760 CrossRef CAS PubMed.
- D. V. Lam, K. Jo, C. H. Kim, J. H. Kim, H. J. Lee and S. M. Lee, ACS Nano, 2016, 10, 11351–11359 CrossRef CAS PubMed.
- W. Raza, F. Z. Ali, N. Raza, Y. W. Luo, K. H. Kim, J. H. Yang, S. Kumar, A. Mehmood and E. E. Kwon, Nano Energy, 2018, 52, 441–473 CrossRef CAS.
- S. Kang, K. Lim, H. Park, J. B. Park, S. C. Park, S. P. Cho, K. Kang and B. H. Hong, ACS Appl. Mater. Interfaces, 2018, 10, 1033–1038 CrossRef CAS PubMed.
- S. Moon, D. H. Kim, J. H. Kwak, S. M. Lee, H. D. Lim, K. Kang, H. J. Jin and Y. S. Yun, Appl. Surf. Sci., 2021, 537, 148037 CrossRef CAS.
- H. Qian, A. R. Kucernak, E. S. Greenhalgh, A. Bismarck and M. S. P. Shaffer, ACS Appl. Mater. Interfaces, 2013, 5, 6113–6122 CrossRef CAS PubMed.
- Y. Yang, Y. X. Liu, Y. Li, B. W. Deng, B. Yin and M. B. Yang, J. Mater. Chem. A, 2020, 8, 17257–17265 RSC.
- J. Cherusseri and K. K. Kar, J. Mater. Chem. A, 2015, 3, 21586–21598 RSC.
- Z. Li, Z. Xu, Y. J. Liu, R. Wang and C. Gao, Nat. Commun., 2016, 7, 13684 CrossRef CAS PubMed.
- Q. P. Cao, M. N. Zhu, J. A. Chen, Y. Y. Song, Y. Li and J. H. Zhou, ACS Appl. Mater. Interfaces, 2020, 12, 1210–1221 CrossRef CAS PubMed.
- Z. Cui, S. A. He, Q. Liu, G. Q. Guan, W. L. Zhang, C. T. Xu, J. Q. Zhu, P. Feng, J. Q. Hu, R. J. Zou and M. F. Zhu, Adv. Sci., 2020, 7, 1903045 CrossRef CAS PubMed.
- G. Y. Xu, B. Ding, J. Pan, J. P. Han, P. Nie, Y. Zhu, Q. Sheng and H. Dou, J. Mater. Chem. A, 2015, 3, 23268–23273 RSC.
- J. G. Kim, H. C. Kim, N. D. Kim and M. S. Khil, Composites, Part B, 2020, 186, 107825 CrossRef CAS.
- Y. J. Ma, X. G. Zhang, Z. Liang, C. L. Wang, Y. Sui, B. Zheng, Y. C. Ye, W. J. Ma, Q. Zhao and C. L. Qin, Electrochim. Acta, 2020, 337, 135800 CrossRef CAS.
- W. Na, J. Jun, J. W. Park, G. Leea and J. Jang, J. Mater. Chem. A, 2017, 5, 17379–17387 RSC.
- C. Ma, J. Sheng, C. L. Ma, R. R. Wang, J. Q. Liu, Z. Y. Xie and J. L. Shi, Chem. Eng. J., 2016, 304, 587–593 CrossRef CAS.
- F. J. Miao, C. L. Shao, X. H. Li, K. X. Wang and Y. C. Liu, J. Mater. Chem. A, 2016, 4, 4180–4187 RSC.
- T. P. Mofokeng, Z. N. Tetana and K. I. Ozoemena, Carbon, 2020, 169, 312–326 CrossRef CAS.
- L. F. Chen, X. D. Zhang, H. W. Liang, M. G. Kong, Q. F. Guan, P. Chen, Z. Y. Wu and S. H. Yu, ACS Nano, 2012, 6, 7092–7102 CrossRef CAS PubMed.
- D. He, L. Wu, Y. C. Yao, J. Zhang, Z. H. Huang and M. X. Wang, Appl. Surf. Sci., 2020, 507, 145108 CrossRef CAS.
- L. Chen, Z. Y. Wen, L. N. Chen, W. P. Wang, Q. Ai, G. M. Hou, Y. H. Li, J. Lou and L. J. Ci, Carbon, 2020, 158, 456–464 CrossRef CAS.
- M. N. Zhu, H. Liu, Q. P. Cao, H. Zheng, D. Xu, H. Y. Guo, S. M. Wang, Y. Li and J. H. Zhou, ACS Sustainable Chem. Eng., 2020, 8, 12831–12841 CrossRef CAS.
- D. Z. Zhu, K. Cheng, Y. W. Wang, D. M. Sun, L. H. Gan, T. Chen, J. X. Jiang and M. X. Liu, Electrochim. Acta, 2017, 224, 17–24 CrossRef CAS.
- R. Y. Shi, C. P. Han, X. F. Xu, X. Y. Qin, L. Xu, H. F. Li, J. Q. Li, C. P. Wong and B. H. Li, Chem.–Eur. J., 2018, 24, 10460–10467 CrossRef CAS PubMed.
- Y. N. Liu, J. N. Zhang, H. T. Wang, X. H. Kang and S. W. Bian, Mater. Chem. Front., 2019, 3, 25–31 RSC.
- B. Dahal, T. Mukhiya, G. P. Ojha, A. Muthurasu, S. H. Chae, T. Kim, D. Kang and H. Y. Kim, Electrochim. Acta, 2019, 301, 209–219 CrossRef CAS.
- K. B. Huang, M. Li, Z. H. Chen, Y. Y. Yao and X. W. Yang, Electrochim. Acta, 2015, 158, 306–313 CrossRef CAS.
- Y. S. Yun, C. Im, H. H. Park, I. Hwang, Y. Tak and H. J. Jin, J. Power Sources, 2013, 234, 285–291 CrossRef CAS.
- L. J. Zhang, G. L. Xia, Z. P. Guo, D. L. Sun, X. G. Li and X. B. Yu, J. Power Sources, 2016, 324, 294–301 CrossRef CAS.
- T. F. Qin, S. L. Peng, J. X. Hao, Y. X. Wen, Z. L. Wang, X. F. Wang, D. Y. He, J. C. Zhang, J. Hou and G. Z. Cao, Adv. Energy Mater., 2017, 7, 1700409 CrossRef.
- Q. Liu, J. W. Zhou, C. H. Song, X. L. Li, Z. P. Wang, J. Yang, J. L. Cheng, H. Li and B. Wang, Energy Storage Mater., 2020, 24, 495–503 CrossRef.
- H. Y. Wang, C. M. Xu, Y. Q. Chen and Y. Wang, Energy Storage Mater., 2017, 8, 127–133 CrossRef.
- S. D. Liu, D. Q. Gao, J. F. Li, K. San Hui, Y. Yin, K. N. Hui and S. C. Jun, J. Mater. Chem. A, 2019, 7, 26618–26630 RSC.
- A. Amiri, K. Bashandeh, M. Naraghi and A. A. Polycarpou, Chem. Eng. J., 2021, 409, 11 CrossRef.
- G. Z. Sun, X. Zhang, R. Z. Lin, J. Yang, H. Zhang and P. Chen, Angew. Chem., Int. Ed., 2015, 54, 4651–4656 CrossRef CAS PubMed.
- X. M. Qiu, X. Zhang and L. Z. Fan, J. Mater. Chem. A, 2018, 6, 16186–16195 RSC.
- C. X. Zhou, T. T. Gao, Y. J. Wang, Q. L. Liu, Z. H. Huang, X. X. Liu, M. Q. Qing and D. Xiao, Small, 2019, 15, 1803469 CrossRef PubMed.
- N. P. Deng, Y. Feng, G. Wang, X. X. Wang, L. Y. Wang, Q. X. Li, L. T. Zhang, W. M. Kang, B. W. Cheng and Y. Liu, Chem. Eng. J., 2020, 401, 125976 CrossRef CAS.
- C. Tran, R. Singhal, D. Lawrence and V. Kalra, J. Power Sources, 2015, 293, 373–379 CrossRef CAS.
- J. H. Liu, X. Y. Xu, J. L. Yu, J. L. Hong, C. Liu, X. Ouyang, S. Lei, X. Meng, J. N. Tang and D. Z. Chen, Electrochim. Acta, 2019, 314, 9–19 CrossRef CAS.
- Z. A. Zhang, J. Zhang, X. X. Zhao and F. H. Yang, Carbon, 2015, 95, 552–559 CrossRef CAS.
- X. T. Ding, Y. Zhao, C. G. Hu, Y. Hu, Z. L. Dong, N. Chen, Z. P. Zhang and L. T. Qu, J. Mater. Chem. A, 2014, 2, 12355–12360 RSC.
- G. X. Qu, J. L. Cheng, X. D. Li, D. M. Yuan, P. N. Chen, X. L. Chen, B. Wang and H. S. Peng, Adv. Mater., 2016, 28, 3646–3652 CrossRef CAS PubMed.
- Y. Chen, J. G. Bai, D. Y. Yang, P. Sun and X. Li, Electrochim. Acta, 2020, 330, 135205 CrossRef CAS.
- H. W. Wang, H. Yi, C. R. Zhu, X. F. Wang and H. J. Fan, Nano Energy, 2015, 13, 658–669 CrossRef CAS.
- A. Stein, Z. Y. Wang and M. A. Fierke, Adv. Mater., 2009, 21, 265–293 CrossRef CAS.
- G. W. Hu, K. Z. Zhong, R. H. Yu, Z. H. Liu, Y. Y. Zhang, J. S. Wu, L. Zhou and L. Q. Mai, J. Mater. Chem. A, 2020, 8, 13285–13291 RSC.
- Y. C. Liu, N. Zhang, X. B. Liu, C. C. Chen, L. Z. Fan and L. F. Jiao, Energy Storage Mater., 2017, 9, 170–178 CrossRef.
- M. Liu, N. P. Deng, J. G. Ju, L. L. Fan, L. Y. Wang, Z. J. Li, H. J. Zhao, G. Yang, W. M. Kang, J. Yan and B. W. Cheng, Adv. Funct. Mater., 2019, 29, 1905467 CrossRef CAS.
- Z. L. Xu, J. K. Kim and K. Kang, Nano Today, 2018, 19, 84–107 CrossRef CAS.
- Z. L. Xu, J. Park, C. Yoon, H. Kim and K. Kang, Small Methods, 2019, 3, 1800227 CrossRef CAS.
- J. Y. Rao, N. S. Liu, Z. Zhang, J. Su, L. Y. Li, L. Xiong and Y. H. Gao, Nano Energy, 2018, 51, 425–433 CrossRef CAS.
- R. Z. Chen, Y. Hu, Z. Shen, P. Pan, X. He, K. S. Wu, X. W. Zhang and Z. L. Cheng, J. Mater. Chem. A, 2017, 5, 12914–12921 RSC.
- H. I. Cho, Y. C. Jeong, J. H. Kim, Y. S. Cho, T. Kim, S. J. Yang and C. R. Park, ACS Nano, 2018, 12, 11106–11119 CrossRef CAS PubMed.
- T. Le, H. Tian, J. Cheng, Z. H. Huang, F. Kang and Y. Yang, Carbon, 2018, 138, 325–336 CrossRef CAS.
- J. Chen, J. Z. Wang, A. I. Minett, Y. Liu, C. Lynam, H. K. Liu and G. G. Wallace, Energy Environ. Sci., 2009, 2, 393–396 RSC.
- W. H. Li, M. S. Li, M. Wang, L. C. Zeng and Y. Yu, Nano Energy, 2015, 13, 693–701 CrossRef CAS.
- Y. Zhong, D. L. Chao, S. J. Deng, J. Y. Zhan, R. Y. Fang, Y. Xia, Y. D. Wang, X. L. Wang, X. H. Xia and J. P. Tu, Adv. Funct. Mater., 2018, 28, 1706391 CrossRef.
- S. J. Wang, R. T. Wang, Y. Bian, D. D. Jin, Y. B. Zhang and L. Zhang, Nano Energy, 2019, 55, 173–181 CrossRef CAS.
- M. S. Balogun, W. T. Qiu, F. Y. Lyu, Y. Luo, H. Meng, J. T. Li, W. J. Mai, L. Q. Mai and Y. X. Tong, Nano Energy, 2016, 26, 446–455 CrossRef CAS.
- C. Deng, S. Zhang, H. F. Wang and G. M. Zhang, Nano Energy, 2018, 49, 419–433 CrossRef CAS.
- F. Liu, J. S. Meng, F. J. Xia, Z. A. Liu, H. Y. Peng, C. L. Sun, L. H. Xu, G. Van Tendeloo, L. Q. Mai and J. S. Wu, J. Mater. Chem. A, 2020, 8, 18079–18086 RSC.
- D. Y. Liu, L. Yang, Z. Y. Chen, G. Q. Zou, H. S. Hou, J. G. Hu and X. B. Ji, Sci. Bull., 2020, 65, 1003–1012 CrossRef CAS.
- D. P. Li, L. N. Dai, X. H. Ren, F. J. Ji, Q. Sun, Y. M. Zhang and L. J. Ci, Energy Environ. Sci., 2021, 14, 424–436 RSC.
- L. C. Yue, H. T. Zhao, Z. G. Wu, J. Liang, S. Y. Lu, G. Chen, S. Y. Gao, B. H. Zhong, X. D. Guo and X. P. Sun, J. Mater. Chem. A, 2020, 8, 11493–11510 RSC.
- J. Cui, S. S. Yao, M. Ihsan-Ul-Haq, J. X. Wu and J. K. Kim, Adv. Energy Mater., 2019, 9, 1802777 CrossRef.
- Z. Y. Wang, Q. X. Li, S. Qin, D. Liu, P. Zhang, D. Hegh, J. Z. Zhang, M. Naebe, W. W. Lei and J. M. Razal, J. Power Sources, 2021, 489, 229464 CrossRef CAS.
- D. H. Li, C. X. Lv, L. Liu, Y. Z. Xia, X. L. She, S. J. Guo and D. J. Yang, ACS Cent. Sci., 2015, 1, 261–269 CrossRef CAS PubMed.
- X. X. Ji, Z. J. Lin, J. Zeng, Y. H. Lin, Y. B. Mu, S. G. Wang, Z. G. Ren and J. Yu, Carbon, 2020, 158, 394–405 CrossRef CAS.
- Y. C. Liu, N. Zhang, L. F. Jiao and J. Chen, Adv. Mater., 2015, 27, 6702 CrossRef CAS PubMed.
- H. C. Gu, L. P. Yang, Y. Zhang, C. Y. Wang, X. Zhang, Z. J. Xie, J. P. Wei and Z. Zhou, Energy Storage Mater., 2019, 21, 203–209 CrossRef.
- L. W. Ji, M. Gu, Y. Y. Shao, X. L. Li, M. H. Engelhard, B. W. Arey, W. Wang, Z. M. Nie, J. Xiao, C. M. Wang, J. G. Zhang and J. Liu, Adv. Mater., 2014, 26, 2901–2908 CrossRef CAS PubMed.
- X. He, Y. Hu, R. Z. Chen, Z. Shen, K. S. Wu, Z. L. Cheng and P. Pan, Chem. Eng. J., 2019, 360, 1020–1029 CrossRef CAS.
- D. T. Ma, Y. L. Li, H. W. Mi, S. Luo, P. X. Zhang, Z. Q. Lin, J. Q. Li and H. Zhang, Angew. Chem., Int. Ed., 2018, 57, 8901–8905 CrossRef CAS PubMed.
- G. L. Xia, L. J. Zhang, F. Fang, D. L. Sun, Z. P. Guo, H. K. Liu and X. B. Yu, Adv. Funct. Mater., 2016, 26, 6188–6196 CrossRef CAS.
- Q. C. Zhang, Z. Y. Zhou, Z. H. Pan, J. Sun, B. He, Q. L. Li, T. Zhang, J. X. Zhao, L. Tang, Z. X. Zhang, L. Wei and Y. G. Yao, Adv. Sci., 2018, 5, 1801462 CrossRef PubMed.
- G. L. Xia, L. J. Zhang, X. W. Chen, Y. Q. Huang, D. L. Sun, F. Fang, Z. P. Guo and X. B. Yu, Energy Storage Mater., 2018, 14, 314–323 CrossRef.
- J. Yan, M. Liu, N. P. Deng, L. Y. Wang, A. Sylvestre, W. M. Kang and Y. X. Zhao, Nanoscale Adv., 2021, 3, 1136–1147 RSC.
- Y. Y. Liang, W. M. Kang, C. L. Zhong, N. P. Deng and B. W. Cheng, Chem. Eng. J., 2021, 403, 9 CrossRef.
- J. Xia, L. Liu, S. Jamil, J. J. Xie, H. X. Yan, Y. T. Yuan, Y. Zhang, S. Nie, J. Pan, X. Y. Wang and G. Z. Cao, Energy Storage Mater., 2019, 17, 1–11 CrossRef.
- C. L. Zhang, Z. H. Jiang, B. R. Lu, J. T. Liu, F. H. Cao, H. Li, Z. L. Yu and S. H. Yu, Nano Energy, 2019, 61, 104–110 CrossRef CAS.
- W. N. Ren, H. F. Zhang, C. Guan and C. W. Cheng, Adv. Funct. Mater., 2017, 27, 1702116 CrossRef.
- Q. Chen, S. Sun, T. Zhai, M. Yang, X. Y. Zhao and H. Xia, Adv. Energy Mater., 2018, 8, 1800054 CrossRef.
- Z. L. Ren, J. Wen, W. Liu, X. P. Jiang, Y. H. Dong, X. L. Guo, Q. N. Zhao, G. P. Ji, R. H. Wang, N. Hu, B. H. Qu and C. H. Xu, Nano-Micro Lett., 2019, 11, 66 CrossRef PubMed.
- C. L. Lai, Z. Z. Zhang, Y. F. Xu, J. Y. Liao, Z. H. Xu, Z. Y. Yi, J. Y. Xu, J. C. Bao and X. S. Zhou, J. Mater. Chem. A, 2021, 9, 1487–1494 RSC.
- J. Zhu, T. Wang, F. R. Fan, L. Mei and B. A. Lu, ACS Nano, 2016, 10, 8243–8251 CrossRef CAS PubMed.
- X. Z. Sun, W. H. Li, X. W. Zhong and Y. Yu, Energy Storage Mater., 2017, 9, 112–118 CrossRef.
- G. He, B. Mandlmeier, J. Schuster, L. F. Nazar and T. Bein, Chem. Mater., 2014, 26, 3879–3886 CrossRef CAS.
- T. Jin, Y. C. Liu, Y. Li, K. Z. Cao, X. J. Wang and L. F. Jiao, Adv. Energy Mater., 2017, 7, 1700087 CrossRef.
- J. S. Lee, J. Jun, J. Jang and A. Manthiram, Small, 2017, 13, 1602984 CrossRef.
- F. F. Wang, N. Zhang, X. D. Zhao, L. X. Wang, J. Zhang, T. S. Wang, F. F. Liu, Y. C. Liu and L. Z. Fan, Adv. Sci., 2019, 6, 1900649 CrossRef PubMed.
- L. C. Zeng, Y. Yao, J. N. Shi, Y. Jiang, W. H. Li, L. Gu and Y. Yu, Energy Storage Mater., 2016, 5, 50–57 CrossRef.
- N. P. Deng, Y. Liu, Q. X. Li, J. Yan, W. W. Lei, G. Wang, L. Y. Wang, Y. Y. Liang, W. M. Kang and B. W. Cheng, Energy Storage Mater., 2019, 23, 314–349 CrossRef.
- H. J. Zhao, N. P. Deng, J. Yan, W. M. Kang, J. G. Ju, Y. L. Ruan, X. Q. Wang, X. P. Zhuang, Q. X. Li and B. W. Cheng, Chem. Eng. J., 2018, 347, 343–365 CrossRef CAS.
- H. X. Wang, B. Zhang, X. Q. Zeng, L. J. Yan, J. C. Zheng, M. Ling, Y. Hou, Y. Y. Lu and C. D. Liang, J. Power Sources, 2020, 473, 8 Search PubMed.
- L. C. Zeng, W. C. Zeng, Y. Jiang, X. Wei, W. H. Li, C. L. Yang, Y. W. Zhu and Y. Yu, Adv. Energy Mater., 2015, 5, 1401377 CrossRef.
- L. L. Lin, F. Pei, J. Peng, A. Fu, J. Q. Cui, X. L. Fang and N. F. Zheng, Nano Energy, 2018, 54, 50–58 CrossRef CAS.
- Y. C. Liu, N. Zhang, F. F. Wang, X. B. Liu, L. F. Jiao and L. Z. Fan, Adv. Funct. Mater., 2018, 28, 1801917 CrossRef.
- D. T. Ma, Y. L. Li, J. B. Yang, H. W. Mi, S. Luo, L. B. Deng, C. Y. Yan, P. X. Zhang, Z. Q. Lin, X. Z. Ren, J. Q. Li and H. Zhang, Nano Energy, 2018, 43, 317–325 CrossRef CAS.
- Y. Yao, R. Xu, M. L. Chen, X. L. Cheng, S. F. Zeng, D. J. Li, X. F. Zhou, X. J. Wu and Y. Yu, ACS Nano, 2019, 13, 4695–4704 CrossRef CAS PubMed.
- Y. Hao, L. Y. Wang, Y. Y. Liang, B. Q. He, Y. F. Zhang, B. W. Cheng, W. M. Kang and N. P. Deng, Nanoscale, 2019, 11, 21324–21339 RSC.
- R. Xu, Y. Yao, H. Y. Wang, Y. F. Yuan, J. W. Wang, H. Yang, Y. Jiang, P. C. Shi, X. J. Wu, Z. Q. Peng, Z. S. Wu, J. Lu and Y. Yu, Adv. Mater., 2020, 32, 11 Search PubMed.
- H. Pan, Z. B. Cheng, J. Q. Chen, R. H. Wang and X. J. Li, Energy Storage Mater., 2020, 27, 435–442 CrossRef.
- J. S. Lee, W. Kim, J. Jang and A. Manthiram, Adv. Energy Mater., 2017, 7, 1601943 CrossRef.
- X. Li, X. C. Hu, L. Zhou, R. Wen, X. Xu, S. L. Chou, L. B. Chen, A. M. Cao and S. X. Dou, J. Mater. Chem. A, 2019, 7, 11976–11984 RSC.
- X. F. Wang, Y. H. Tang, P. H. Shi, J. C. Fan, Q. J. Xu and Y. L. Min, Chem. Eng. J., 2018, 334, 1642–1649 CrossRef CAS.
- W. C. Ren, W. Ma, M. M. Umair, S. F. Zhang and B. T. Tang, ChemSusChem, 2018, 11, 2695–2702 CrossRef CAS.
- S. Feng, J. H. Song, S. F. Fu, C. Z. Zhu, Q. R. Shi, M. K. Song, D. Du and Y. H. Lin, J. Mater. Chem. A, 2017, 5, 23737–23743 RSC.
- Q. L. Gao, Z. X. Yuan, L. X. Dong, G. F. Wang and X. B. Yu, Electrochim. Acta, 2018, 270, 417–425 CrossRef CAS.
- F. L. Tong, J. X. Guo, Y. L. Pan, H. B. A. Liu, Y. Lv, X. Y. Wu, D. Z. Jia, X. J. Zhao and S. C. Hou, J. Colloid Interface Sci., 2021, 586, 371–380 CrossRef CAS.
- J. S. Lee, M. S. Jo, R. Saroha, D. S. Jung, Y. H. Seon, J. S. Lee, Y. C. Kang, D. W. Kang and J. S. Cho, Small, 2020, 16, 2002213 CrossRef CAS PubMed.
- Q. Y. Xia, H. Yang, M. Wang, M. Yang, Q. B. Guo, L. M. Wan, H. Xia and Y. Yu, Adv. Energy Mater., 2017, 7, 1701336 CrossRef.
- L. Y. Chai, J. X. Wang, H. Y. Wang, L. Y. Zhang, W. T. Yu and L. Q. Mai, Nano Energy, 2015, 17, 224–232 CrossRef CAS.
- X. Hu, G. B. Zhong, J. W. Li, Y. J. Liu, J. Yuan, J. X. Chen, H. B. Zhan and Z. H. Wen, Energy Environ. Sci., 2020, 13, 2431–2440 RSC.
- H. J. Tian, T. Y. Wang, F. Zhang, S. Q. Zhao, S. Wan, F. R. He and G. X. Wang, J. Mater. Chem. A, 2018, 6, 12816–12841 RSC.
- A. P. Wang, S. Kadam, H. Li, S. Q. Shi and Y. Qi, npj Comput. Mater., 2018, 4, 26 CrossRef.
- Y. J. Wei, Y. Q. Tao, Z. K. Kong, L. Liu, J. T. Wang, W. M. Qiao, L. C. Ling and D. H. Long, Energy Storage Mater., 2016, 5, 171–179 CrossRef.
- D. S. Su, G. D. Wen, S. C. Wu, F. Peng and R. Schlögl, Angew. Chem., Int. Ed., 2017, 56, 936–964 CrossRef CAS PubMed.
- J. T. Wang, C. Y. Ke, X. F. Jia, C. Ma, X. J. Liu, W. M. Qiao and L. C. Ling, Appl. Catal., B, 2021, 283, 119650 CrossRef CAS.
- S. Yasuda, L. Yu, J. Kim and K. Murakoshi, Chem. Commun., 2013, 49, 9627–9629 RSC.
- D. Shin, B. Jeong, B. S. Mun, H. Jeon, H. J. Shin, J. Baik and J. Lee, J. Phys. Chem. C, 2013, 117, 11619–11624 CrossRef CAS.
- Q. X. Lai, Y. X. Zhao, Y. Y. Liang, J. P. He and J. H. Chen, Adv. Funct. Mater., 2016, 26, 8334–8344 CrossRef CAS.
- M. G. Wu, Y. Q. Wang, Z. X. Wei, L. Wang, M. Zhuo, J. T. Zhang, X. P. Han and J. M. Ma, J. Mater. Chem. A, 2018, 6, 10918–10925 RSC.
- Y. F. Zhao, J. Q. Zhang, K. F. Li, Z. M. Ao, C. Y. Wang, H. Liu, K. N. Sun and G. X. Wang, J. Mater. Chem. A, 2016, 4, 12818–12824 RSC.
- J. Z. Huang, J. C. Han, T. L. Gao, X. H. Zhang, J. J. Li, Z. J. Li, P. Xu and B. Song, Carbon, 2017, 124, 34–41 CrossRef CAS.
- K. Dong, J. Liang, Y. Y. Wang, Z. Q. Xu, Q. Liu, Y. L. Luo, T. S. Li, L. Li, X. F. Shi, A. M. Asiri, Q. Li, D. W. Ma and X. P. Sun, Angew. Chem., Int. Ed., 2021, 60, 10583–10587 CrossRef CAS PubMed.
- Y. F. Chen, Q. Liu and J. C. Wang, J. Mater. Chem. A, 2016, 4, 5553–5560 RSC.
- Q. Shi, Y. Ma, L. Qin, B. Tang, W. Y. Yang and Q. Liu, ChemElectroChem, 2019, 6, 2924–2930 CrossRef CAS.
- C. Hang, J. Zhang, J. W. Zhu, W. Q. Li, Z. K. Kou and Y. H. Huang, Adv. Energy Mater., 2018, 8, 1703539 CrossRef.
- S. Liu, C. Zhao, Z. Y. Wang, H. J. Ding, H. P. Deng, G. Yang, J. F. Li and H. L. Zheng, Chem. Eng. J., 2020, 386, 124015 CrossRef CAS.
- M. R. Haider, W. L. Jiang, J. L. Han, H. M. A. Sharif, Y. C. Ding, H. Y. Cheng and A. J. Wang, Appl. Catal., B, 2019, 256, 117774 CrossRef CAS.
- Y. X. Kong, T. T. Qiu and J. Qiu, Appl. Surf. Sci., 2013, 265, 352–357 CrossRef CAS.
- Z. Han, Y. Y. Yu, Y. B. Zhang, B. Dong, A. G. Kong and Y. K. Shan, J. Mater. Chem. A, 2015, 3, 23716–23724 RSC.
- Y. E. Miao, J. J. Yan, Y. Ouyang, H. Y. Lu, F. L. Lai, Y. Wu and T. X. Liu, Appl. Surf. Sci., 2018, 443, 266–273 CrossRef CAS.
- X. L. Yang, W. J. Zou, Y. H. Su, Y. H. Zhu, H. L. Jiang, J. H. Shen and C. z. Li, J. Power Sources, 2014, 266, 36–42 CrossRef CAS.
- L. M. Dai, Y. H. Xue, L. T. Qu, H. J. Choi and J. B. Baek, Chem. Rev., 2015, 115, 4823–4892 CrossRef CAS PubMed.
- S. Akula and A. K. Sahu, ACS Appl. Mater. Interfaces, 2020, 12, 11438–11449 CrossRef CAS PubMed.
- B. Dahal, S. H. Chae, A. Muthurasu, T. Mukhiya, J. Gautam, K. Chhetri, S. Subedi, G. P. Ojha, A. P. Tiwari, J. H. Lee and H. Y. Kim, J. Power Sources, 2020, 453, 227883 CrossRef CAS.
- A. Mulyadi, Z. Zhang, M. Dutzer, W. Liu and Y. L. Deng, Nano Energy, 2017, 32, 336–346 CrossRef CAS.
- J. Zeng, Y. B. Mu, X. X. Ji, Z. J. Lin, Y. H. Lin, Y. H. Ma, Z. X. Zhang, S. G. Wang, Z. H. Ren and J. Yu, J. Mater. Sci., 2019, 54, 14495–14503 CrossRef CAS.
- L. Yang, X. Y. Xiao, Z. Yang, Y. Cai, B. Xie, N. Zhao, X. Li, Y. Wang, M. F. Liu and X. Z. Wang, Int. J. Hydrogen Energy, 2018, 43, 15135–15143 CrossRef CAS.
- W. Zhang, Z. Y. Wu, H. L. Jiang and S. H. Yu, J. Am. Chem. Soc., 2014, 136, 14385–14388 CrossRef CAS PubMed.
- K. N. Wood, R. O'Hayre and S. Pylypenko, Energy Environ. Sci., 2014, 7, 1212–1249 RSC.
- Q. Chen, X. F. Tan, Y. G. Liu, S. B. Liu, M. F. Li, Y. L. Gu, P. Zhang, S. J. Ye, Z. Z. Yang and Y. Y. Yang, J. Mater. Chem. A, 2020, 8, 5773–5811 RSC.
- Y. P. Lei, Q. Shi, C. Han, B. Wang, N. Wu, H. Wang and Y. D. Wang, Nano Res., 2016, 9, 2498–2509 CrossRef CAS.
- L. Liu, Y. P. Zhu, M. Su and Z. Y. Yuan, Chemcatchem, 2015, 7, 2765–2787 CrossRef CAS.
- C. H. Wang, J. Kim, M. Kim, H. Lim, M. Zhang, J. You, J. H. Yun, Y. Bando, J. S. Li and Y. Yamauchi, J. Mater. Chem. A, 2019, 7, 13743–13750 RSC.
- Y. She, J. Liu, H. K. Wang, L. Li, J. S. Zhou and M. K. H. Leung, Nano Res., 2020, 13, 2175–2182 CrossRef.
- J. C. Lee, J. Y. Kim, W. H. Joo, D. Hong, S. H. Oh, B. Kim, G. D. Lee, M. Kim, J. Oh and Y. C. Joo, J. Mater. Chem. A, 2020, 8, 11632–11641 RSC.
- Z. M. Tang, Y. X. Zhao, Q. X. Lai, J. Zhong and Y. Y. Liang, Nano-Micro Lett., 2019, 11, 33 CrossRef CAS PubMed.
- X. Ma, X. Zhao, J. S. Huang, L. T. Sun, Q. Li and X. R. Yang, ACS Appl. Mater. Interfaces, 2017, 9, 21747–21755 CrossRef CAS PubMed.
- Z. Wang, S. J. Peng, Y. X. Hu, L. L. Li, T. Yan, G. R. Yang, D. X. Ji, M. Srinivasan, Z. J. Pan and S. Ramakrishna, J. Mater. Chem. A, 2017, 5, 4949–4961 RSC.
- Y. S. Chen, W. H. Zhang, Z. Y. Zhu, L. L. Zhang, J. Y. Yang, H. H. Chen, B. Zheng, S. Li, W. N. Zhang, J. S. Wu and F. W. Huo, J. Mater. Chem. A, 2020, 8, 7184–7191 RSC.
- W. Peng, X. X. Yang, L. C. Mao, J. H. Jin, S. L. Yang, J. J. Zhang and G. Li, Chem. Eng. J., 2021, 407, 11 Search PubMed.
- G. Fu, Y. Chen, Z. Cui, Y. Li, W. Zhou, S. Xin, Y. Tang and J. B. Goodenough, Nano Lett., 2016, 16, 6516–6522 CrossRef CAS PubMed.
- H. Zhu, L. Gu, D. N. Yu, Y. j. Sun, M. Wan, M. Zhang, L. Wang, L. N. Wang, W. W. Wu, J. M. Yao, M. L. Du and S. J. Guo, Energy Environ. Sci., 2017, 10, 321–330 RSC.
- D. D. Yin, C. Han, X. J. Bo, J. Liu and L. P. Guo, J. Colloid Interface Sci., 2019, 533, 578–587 CrossRef CAS PubMed.
- Y. N. Zhang, X. B. Hou, X. Li, D. W. Li, F. L. Huang and Q. F. Wei, J. Colloid Interface Sci., 2020, 578, 805–813 CrossRef CAS PubMed.
- Y. L. Niu, X. Teng, S. Q. Gong and Z. F. Chen, J. Mater. Chem. A, 2020, 8, 13725–13734 RSC.
- P. Wei, X. P. Sun, Q. R. Liang, X. G. Li, Z. M. He, X. S. Hu, J. X. Zhang, M. H. Wang, Q. Li, H. Yang, J. T. Han and Y. H. Huang, ACS Appl. Mater. Interfaces, 2020, 12, 31503–31513 CrossRef CAS PubMed.
- W. R. Cheng, X. F. Lu, D. Y. Luan and X. W. Lou, Angew. Chem., Int. Ed., 2020, 59, 18234–18239 CrossRef CAS PubMed.
- A. Wang, Y. Hu, H. Wang, Y. Cheng, T. Thomas, R. Ma and J. Wang, Mater. Today Phys., 2021, 17, 10 Search PubMed.
- D. X. Ji, J. G. Sun, L. D. Tian, A. Chinnappan, T. R. Zhang, W. Jayathilaka, R. Gosh, C. Baskar, Q. Y. Zhang and S. Ramakrishna, Adv. Funct. Mater., 2020, 30, 10 Search PubMed.
- R. Q. Zhong, Y. X. Wu, Z. B. Liang, W. H. Guo, C. X. Zhi, C. Qu, S. Gao, B. J. Zhu, H. Zhang and R. Q. Zou, Carbon, 2019, 142, 115–122 CrossRef CAS.
- T. T. Liu, M. Li, X. J. Bo and M. Zhou, J. Colloid Interface Sci., 2019, 533, 709–722 CrossRef CAS PubMed.
- Z. Li, B. Chen, X. Wang, J. Nie and G. Ma, J. Colloid Interface Sci., 2019, 546, 231–239 CrossRef CAS PubMed.
- H. Wang, C. Sun, Y. J. Cao, J. T. Zhu, Y. Chen, J. Guo, J. Zhao, Y. H. Sun and G. F. Zou, Carbon, 2017, 114, 628–634 CrossRef CAS.
- Z. Y. Wu, X. X. Xu, B. C. Hu, H. W. Liang, Y. Lin, L. F. Chen and S. H. Yu, Angew. Chem., Int. Ed., 2015, 54, 8179–8183 CrossRef CAS PubMed.
- Y. Y. Ma, A. Sumboja, W. J. Zang, S. Y. Yin, S. X. Wang, S. J. Pennycook, Z. K. Kou, Z. L. Liu, X. Li and J. Wang, ACS Appl. Mater. Interfaces, 2018, 11, 1988–1995 CrossRef PubMed.
- G. Y. Ren, X. Y. Lu, Y. Li, Y. Zhu, L. M. Dai and L. Jiang, ACS Appl. Mater. Interfaces, 2016, 8, 4118–4125 CrossRef CAS PubMed.
- W. Peng, Y. Wang, X. X. Yang, L. C. Mao, J. H. Jin, S. L. Yang, K. Fu and G. Li, Appl. Catal., B, 2020, 268, 10 CrossRef.
- W. Zhang, X. Yao, S. Zhou, X. Li, L. Li, Z. Yu and L. Gu, Small, 2018, 14, e1800423 CrossRef PubMed.
- B. C. Hu, Z. Y. Wu, S. Q. Chu, H. W. Zhu, H. W. Liang, J. Zhang and S. H. Yu, Energy Environ. Sci., 2018, 11, 2208–2215 RSC.
- L. L. Wu, Q. S. Wang, J. Li, Y. Long, Y. Liu, S. Y. Song and H. J. Zhang, Small, 2018, 14, e1704035 CrossRef.
- Y. X. Zhao, Q. X. Lai, J. J. Zhu, J. Zhong, Z. M. Tang, Y. Luo and Y. Y. Liang, Small, 2018, 14, 9 Search PubMed.
- M. Q. Wang, C. Ye, H. Liu, M. W. Xu and S. J. Bao, Angew. Chem., Int. Ed., 2018, 57, 1963–1967 CrossRef CAS PubMed.
- T. T. Zheng, K. Jiang, N. Ta, Y. F. Hu, J. Zeng, J. Y. Liu and H. T. Wang, Joule, 2019, 3, 265–278 CrossRef CAS.
- R. Lang, W. Xi, J. C. Liu, Y. T. Cui, T. B. Li, A. F. Lee, F. Chen, Y. Chen, L. Li, L. Li, J. Lin, S. Miao, X. Y. Liu, A. Q. Wang, X. D. Wang, J. Luo, B. T. Qiao, J. Li and T. Zhang, Nat. Commun., 2019, 10, 234 CrossRef PubMed.
- C. Cheng, S. Li, Y. Xia, L. Ma, C. Nie, C. Roth, A. Thomas and R. Haag, Adv. Mater., 2018, 30(40), 1802669 CrossRef.
- Y. H. He, H. Guo, S. Hwang, X. X. Yang, Z. Z. He, J. Braaten, S. Karakalos, W. T. Shan, M. Y. Wang, H. Zhou, Z. X. Feng, K. L. More, G. F. Wang, D. Su, D. A. Cullen, L. Fei, S. Litster and G. Wu, Adv. Mater., 2020, 32, 14 Search PubMed.
- L. Roldán, Y. Marco and E. García-Bordejé, ChemSusChem, 2017, 10, 1139–1144 CrossRef.
- H. Yang, Q. Lin, C. Zhang, X. Yu, Z. Cheng, G. Li, Q. Hu, X. Ren, Q. Zhang, J. Liu and C. He, Nat. Commun., 2020, 11, 593 CrossRef CAS PubMed.
- A. A. Taha, A. A. Hriez, Y. N. Wu, H. Wang and F. Li, J. Colloid Interface Sci., 2014, 417, 199–205 CrossRef CAS PubMed.
- D. P. Xue, H. C. Xia, W. F. Yan, J. A. Zhang and S. C. Mu, Nano-Micro Lett., 2020, 13, 5 CrossRef PubMed.
- W. J. Tian, H. Y. Zhang, X. G. Duan, H. Q. Sun, G. S. Shao and S. B. Wang, Adv. Funct. Mater., 2020, 30, 1909265 CrossRef CAS.
- J. J. Zhang, Y. X. Li, L. Li, W. L. Li and C. F. Yang, ACS Sustainable Chem. Eng., 2018, 6, 12893–12905 CrossRef CAS.
- S. Kang and J. Hwang, Chem. Eng. J., 2021, 406, 127158 CrossRef CAS.
- H. Singh, S. Q. Zhuang, B. Ingis, B. B. Nunna and E. S. Lee, Carbon, 2019, 151, 160–174 CrossRef CAS.
- D. Banham, S. Ye, K. Pei, J. Ozaki, T. Kishimoto and Y. Imashiro, J. Power Sources, 2015, 285, 334–348 CrossRef CAS.
- J. Herranz, F. Jaouen, M. Lefevre, U. I. Kramm, E. Proietti, J. P. Dodelet, P. Bogdanoff, S. Fiechter, I. Abs-Wurmbach, P. Bertrand, T. M. Arruda and S. Mukerjee, J. Phys. Chem. C, 2011, 115, 16087–16097 CrossRef CAS PubMed.
- C. H. Choi, C. Baldizzone, J. P. Grote, A. K. Schuppert, F. Jaouen and K. J. J. Mayrhofer, Angew. Chem., Int. Ed., 2015, 54, 12753–12757 CrossRef CAS PubMed.
- L. J. Yang, N. Larouche, R. Chenitz, G. X. Zhang, M. Lefevre and J. P. Dodelet, Electrochim. Acta, 2015, 159, 184–197 CrossRef CAS.
- G. X. Zhang, R. Chenitz, M. Lefevre, S. Sun and J. P. Dodelet, Nano Energy, 2016, 29, 111–125 CrossRef CAS.
- L. Osmieri, R. Estudero-Cid, M. Armandi, A. Videla, J. L. G. Fierro, P. Ocon and S. Specchia, Appl. Catal., B, 2017, 205, 637–653 CrossRef CAS.
- C. H. Choi, H. K. Lim, M. W. Chung, G. Chon, N. R. Sahraie, A. Altin, M. T. Sougrati, L. Stievano, H. S. Oh, E. S. Park, F. Luo, P. Strasser, G. Drazic, K. J. J. Mayrhofer, H. Kim and F. Jaouen, Energy Environ. Sci., 2018, 11, 3176–3182 RSC.
- J. K. Li, M. T. Sougrati, A. Zitolo, J. M. Ablett, I. C. Oguz, T. Mineva, I. Matanovic, P. Atanassov, Y. Huang, I. Zenyuk, A. Di Cicco, K. Kumar, L. Dubau, F. Maillard, G. Drazic and F. Jaouen, Nat. Catal., 2021, 4, 18 Search PubMed.
- C. X. Zhao, J. N. Liu, J. Wang, D. Ren, B. Q. Li and Q. Zhang, Chem. Soc. Rev., 2021, 50, 7745–7778 RSC.
- X. Y. Zhang, B. Gao, A. E. Creamer, C. C. Cao and Y. C. Li, J. Hazard. Mater., 2017, 338, 102–123 CrossRef CAS PubMed.
- B. N. Zheng, X. D. Lin, X. C. Zhang, D. C. Wu and K. Matyjaszewski, Adv. Funct. Mater., 2020, 30, 1907006 CrossRef CAS.
- H. L. Hou, G. Shao, W. Y. Yang and W. Y. Wong, Prog. Mater. Sci., 2020, 113 Search PubMed.
- J. G. Zhou, Z. L. Sun, M. Q. Chen, J. T. Wang, W. M. Qiao, D. H. Long and L. C. Ling, Adv. Funct. Mater., 2016, 26, 5368–5375 CrossRef CAS.
- W. Wang, A. Saeed, J. Y. He, Z. J. Wang, D. Y. Zhan, Z. X. Li, C. M. Wang, Y. F. Sun, F. Tao and W. H. Xu, J. Colloid Interface Sci., 2020, 578, 304–314 CrossRef CAS PubMed.
- K. N. Dinh, Z. X. Pei, Z. W. Yuan, V. C. Hoang, L. Wei, Q. W. Huang, X. Z. Liao, C. T. Liu, Y. Chen and Q. Y. Yan, J. Mater. Chem. A, 2020, 8, 7297–7308 RSC.
- J. M. Serrano, A. U. Khan, T. Y. Liu, Z. Xu, A. R. Esker and G. L. Liu, Adv. Mater. Interfaces, 2020, 7, 2000507 CrossRef CAS.
- L. Xiong, X. F. Wang, L. Li, L. Jin, Y. G. Zhang, S. L. Song and R. P. Liu, Energy Fuels, 2019, 33, 12558–12567 CrossRef CAS.
- W. Z. Shen, S. C. Zhang, Y. He, J. F. Li and W. B. Fan, J. Mater. Chem., 2011, 21, 14036–14040 RSC.
- W. H. Zheng, J. T. Hu, Z. S. Han, E. Diesel, Z. X. Wang, Z. Zheng, C. Y. Ba, J. Langer and J. Economy, Chem. Eng. J., 2016, 287, 54–61 CrossRef CAS.
- W. H. Zheng, J. T. Hu, Z. S. Han, Z. X. Wang, Z. Zheng, J. Langer and J. Economy, Chem. Commun., 2015, 51, 9853–9856 RSC.
- F. K. Mahar, L. L. He, K. Wei, M. Mehdi, M. L. Zhu, J. Gu, K. Q. Zhang, Z. Khatri and I. Kim, Chemosphere, 2019, 225, 360–367 CrossRef CAS PubMed.
- J. Gutierrez-Martinez, C. Nieto-Delgado, M. Avalos-Borja, E. Basiuk and J. R. Rangel-Mendez, Sep. Purif. Technol., 2021, 257, 11 CrossRef.
- M. A. Riaz, Z. W. Yuan, A. Mahmood, F. Liu, X. Sui, J. S. Chen, Q. W. Huang, X. Z. Liao, L. Wei and Y. Chen, Sens. Actuators, B, 2020, 319, 10 CrossRef.
- X. L. Sun, Z. Liu and Z. L. Cheng, J. Hazard. Mater., 2021, 403, 13 Search PubMed.
- N. F. Attia, M. Jung, J. Park, H. Jang, K. Lee and H. Oh, Chem. Eng. J., 2020, 379, 122367 CrossRef CAS.
- H. C. Bi, Z. Y. Yin, X. H. Cao, X. Xie, C. L. Tan, X. Huang, B. Chen, F. T. Chen, Q. L. Yang, X. Y. Bu, X. H. Lu, L. T. Sun and H. Zhang, Adv. Mater., 2013, 25, 5916–5921 CrossRef CAS PubMed.
- Z. M. Song, X. F. Liu, X. Sun, Y. Li, X. Y. Nie, W. K. Tang, R. H. Yu and J. L. Shui, Carbon, 2019, 151, 36–45 CrossRef CAS.
- I. Abdalla, A. Elhassan, J. Y. Yu, Z. L. Li and B. Ding, Carbon, 2020, 157, 703–713 CrossRef CAS.
- X. D. Zuo, P. Xu, C. Y. Zhang, M. Z. Li, X. Y. Jiang and X. G. Yue, Ceram. Int., 2019, 45, 4474–4481 CrossRef CAS.
- M. Perovic, Q. Qin and M. Oschatz, Adv. Funct. Mater., 2020, 30, 201908371 CrossRef.
- S. S. Gao, J. Liu, J. Luo, X. Mamat, S. Sambasivam, Y. T. Li, X. Hu, T. Wågberg and G. Z. Hu, Microchim. Acta, 2018, 185, 282 CrossRef PubMed.
- T. T. Liang, L. Zou, X. G. Guo, X. Q. Ma, C. K. Zhang, Z. Zou, Y. H. Zhang, F. X. Hu, Z. S. Lu, K. L. Tang and C. M. Li, Adv. Funct. Mater., 2019, 29, 1903026 CrossRef CAS.
- J. S. Im, S. C. Kang, S. H. Lee and Y. S. Lee, Carbon, 2010, 48, 2573–2581 CrossRef CAS.
- J. Bai, L. P. Wu, X. J. Wang and H. M. Zhang, Electrochim. Acta, 2015, 185, 142–147 CrossRef CAS.
- J. H. Cha, S. J. Choi, S. Yu and I. D. Kim, J. Mater. Chem. A, 2017, 5, 8725–8732 RSC.
- H. J. Guan, Y. F. Zhao, J. Zhang, Y. Liu, S. G. Yuan and B. Zhang, Sens. Actuators, B, 2018, 261, 354–363 CrossRef CAS.
- K. Mondal, M. A. Ali, C. Singh, G. Sumana, B. D. Malhotra and A. Sharma, Sens. Actuators, B, 2017, 246, 202–214 CrossRef CAS.
- Y. P. Huang, Y. E. Miao, S. S. Ji, W. W. Tjiu and T. X. Liu, ACS Appl. Mater. Interfaces, 2014, 6, 12449–12456 CrossRef CAS PubMed.
- R. J. Lü, K. Y. Shi, W. Zhou, L. Wang, C. G. Tian, K. Pan, L. Sun and H. G. Fu, J. Mater. Chem., 2012, 22, 24814–24820 RSC.
- D. D. Yin, J. Liu, X. J. Bo and L. P. Guo, Anal. Chim. Acta, 2020, 1093, 35–42 CrossRef CAS PubMed.
- X. G. Feng, S. R. Lin, M. Li, X. J. Bo and L. P. Guo, Anal. Chim. Acta, 2017, 984, 96–106 CrossRef CAS PubMed.
- X. W. Yu, H. H. Cheng, M. Zhang, Y. Zhao, L. T. Qu and G. Q. Shi, Nat. Rev. Mater., 2017, 2, 17046 CrossRef CAS.
- S. J. Choi, D. M. Lee, H. Yu, J. S. Jang, M. H. Kim, J. Y. Kang, H. S. Jeong and I. D. Kim, Sens. Actuators, B, 2019, 290, 293–301 CrossRef CAS.
- S. J. Choi, H. Yu, J. S. Jang, M. H. Kim, S. J. Kim, H. S. Jeong and I. D. Kim, Small, 2018, 14, 1703934 CrossRef PubMed.
- L. W. Chen, X. T. Ding, J. F. Zeng, L. Jiao, C. B. Wu, Y. Z. Wang, Q. Han and L. T. Qu, Sci. Bull., 2019, 64, 718–722 CrossRef CAS.
- J. S. Jang, H. Yu, S. J. Choi, W. T. Koo, J. Lee, D. H. Kim, J. Y. Kang, Y. J. Jeong, H. Jeong and I. D. Kim, ACS Appl. Mater. Interfaces, 2019, 11, 10208–10217 CrossRef CAS PubMed.
- T. Khudiyev, J. T. Lee, J. R. Cox, E. Argentieri, G. Loke, R. Yuan, G. H. Noel, R. Tatara, Y. Yu, F. Logan, J. Joannopoulos, Y. Shao-Horn and Y. Fink, Adv. Mater., 2020, 32, 2004971 CrossRef CAS PubMed.
- B. Bian, D. Shi, X. B. Cai, M. J. Hu, Q. Q. Guo, C. H. Zhang, Q. Wang, A. X. Sun and J. Yang, Nano Energy, 2018, 44, 174–180 CrossRef CAS.
- S. Ma, Y. X. Wang, Z. Y. Liu, M. N. Huang, H. Yang and Z. Xu, Chem. Eng. Sci., 2019, 205, 181–189 CrossRef CAS.
- L. Li, X. F. Wang, J. J. Zhong, X. Qian, S. L. Song, Y. G. Zhang and D. H. Li, Ind. Eng. Chem. Res, 2018, 57, 11608–11616 CrossRef CAS.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2022 |