Open Access Article
Open Access ArticleSolvent-free hydroboration of alkenes and alkynes catalyzed by rhodium-ruthenium nanoparticles on carbon nanotubes†
Mateus P.
Nunes‡
a,
Dhanaji V.
Jawale‡
b,
Fábio G.
Delolo
 a,
Maria H.
Araujo
a,
Edmond
Gravel
a,
Maria H.
Araujo
a,
Edmond
Gravel
 *b,
Eric
Doris
*b,
Eric
Doris
 *b and
Eufrânio N.
da Silva Júnior
*b and
Eufrânio N.
da Silva Júnior
 *a
*a
aInstitute of Exact Sciences, Department of Chemistry, Federal University of Minas Gerais, Belo Horizonte, 31270-901, MG, Brazil. E-mail: eufranio@ufmg.br
bUniversité Paris-Saclay, CEA, INRAE, Département Médicaments et Technologies pour la Santé (DMTS), SCBM, 91191, Gif-sur-Yvette, France. E-mail: edmond.gravel@cea.fr; eric.doris@cea.fr
First published on 7th February 2023
Abstract
A heterogeneous catalyst consisting of bimetallic rhodium-ruthenium particles immobilized on carbon nanotubes was used in the hydroboration reaction and proved highly effective for a variety of alkenes and alkynes. The reactions were carried out with low catalytic loadings (0.04 mol%), under solvent-free conditions, and at room temperature. In addition, to demonstrate its recyclability, the catalyst was recovered by a simple centrifugation process and reused over 5 consecutive cycles without losing any activity.
Organoboron reagents are versatile intermediates in the synthesis of bioactive compounds, used for example in cross-coupling reactions.1 Alkylboranes and alkenylboranes can be produced by catalytic hydroboration of alkenes or alkynes by adding B–H to olefins in an atom-efficient process.2 In this context, homogeneous catalytic systems based on noble metals (e.g. Ir,3 Pt,4 Pd,5 Ru,6 and Rh7), rare-earth metals (e.g. La, Sm, Y, Yb, Sc),8 but also earth-abundant metals9 (e.g. Fe,10 Co,11 Cu,12 Ni,13 Mn14) have been reported as catalysts for the hydroboration of unsaturated hydrocarbons. However, these systems usually rely on the use of additives such as ligands and/or bases and cannot be readily recycled. In addition to environmental aspects, the non-recyclability of the catalysts is a major drawback for cost-effective industrial applications.
Unlike homogeneous catalyst complexes, heterogeneous catalysts are more sustainable because they can be readily recovered from reaction mixtures and reused. However, heterogeneous catalysts have only been scarcely explored in the hydroboration of alkenes and alkynes.15
Among the strategies to optimize the catalytic performance of heterogeneous systems, our groups have previously investigated the immobilization of metallic particles on carbonaceous materials.16 We demonstrated that the immobilization of metal nanoparticles on multi-walled carbon nanotubes (CNT) can provide a significantly enhanced catalytic activity in various organic transformations.
More recently, we also reported the assembly of bimetallic rhodium–ruthenium nanoparticles (RhRu NP) on CNT. The RhRuCNT nanohybrid was investigated in the efficient hydrothiolation17 and hydrophosphinylation18 of various alkenes and alkynes, under mild reaction conditions (Scheme 1). In the above nanohybrid, rhodium and ruthenium acted synergistically, thus providing better performances than either of the metals taken individually. With these features in mind, we report here the use of the RhRuCNT nanohybrid catalyst in the hydroboration of alkenes and alkynes at room temperature and under solvent-free conditions.
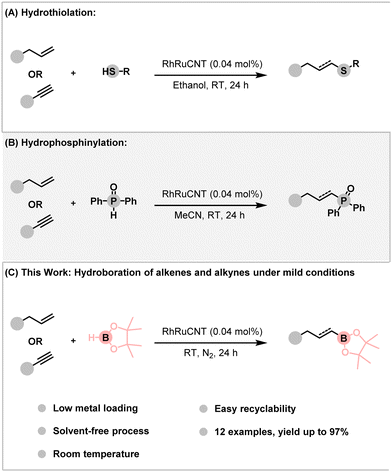 | ||
| Scheme 1 Overview of transformations catalyzed by RhRuCNT nanohybrid catalyst. (A) Hydrothiolation, (B) Hydrophosphinylation, and (C) this work: Hydroboration. | ||
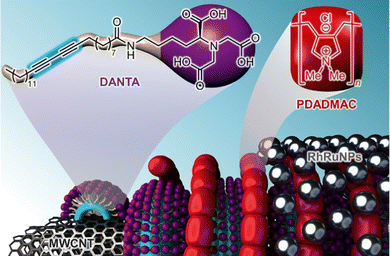
The preparation of the RhRuCNT catalyst, as well as the other tested catalysts (i.e. RuCNT, RhCNT, RhRu NP), was carried out using our previously reported procedure (see ESI† for details).19 In brief, RhRuCNT was assembled in water using a layer-by-layer strategy and the following steps: i) adsorption and photo-polymerization of a primary layer made of an anionic amphiphile (DANTA) at the surface of multiwalled-carbon nanotubes (MWCNT), ii) deposition of a secondary layer made of a cationic polymer (PDADMAC), and iii) deposition of the preformed bimetallic nanoparticles (RhRuNPs).20 In this system (Fig. 1), the polyammonium network served as a tridimensional anchoring and stabilizing environment for RhRu nanoparticles. The RhRuCNT catalyst was recovered as an aqueous suspension whose metal concentration was measured by inductively coupled plasma mass spectrometry (ICP-MS, [Ru] = 4.3 mM and [Rh] = 3.8 mM).
To evaluate the RhRuCNT nanohybrid material in the hydroboration reaction, we selected 1-hexene (1a) and pinacolborane (HBpin, 2) as model substrates (Table 1). The reaction was performed under an inert nitrogen atmosphere, without solvent, and at room temperature for 24 h. In the presence of 0.04 mol% of RhRuCNT, the hydroborylated product (3a) was obtained as a single regioisomer in 96% yield (Table 1, entry 1). On the other hand, the monometallic hybrids RuCNT and RhCNT afforded only 19% and 60% yield, respectively (entries 2 and 3). The combination of RhCNT and RuCNT slightly improved the catalytic performance, although not to the extent of RhRuCNT (entry 4). These results suggest a cooperative effect between Rh and Ru21 in which the more electron-deficient ruthenium could be responsible for the activation of the unsaturated substrates, while rhodium may act as the main catalytic species. This synergy accounts for the higher conversion observed for RhRuCNT.22 The reaction mechanism might follow a classical 3-step sequence involving: i) oxidative addition of the B–H bond to the metal center, followed by ii) insertion of the alkene into the rhodium–hydride bond, and iii) reductive elimination (Scheme S1, ESI†). Although two regioisomeric compounds can be formed, we observed only the C-terminal-borylation of the substrate.
| Entry | Catalyst | Yieldb (%) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a Reaction conditions: 1a (1.0 mmol), 2 (1.1 mmol), catalyst (0.04 mol%), room temperature (RT), inert atmosphere (N2), neat, 24 h. b Isolated yield. c 0.02 mol% Ru + 0.02 mol% Rh. NR: no reaction. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1 | RhRuCNT | 96 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2 | RuCNT | 19 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3 | RhCNT | 60 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4 | RuCNT + RhCNT | 73c | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5 | Colloidal RhRu NPs | NR | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6 | CNT | NR | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
It is also important to highlight the key role played by nanotubes in the process as, in addition to providing high specific surface area, CNT are electronically active and can stabilize transient oxidation states of the supported metals.21 In fact, the reaction performed in the presence of unsupported colloidal RhRu NPs (without CNT) showed no conversion (entry 5). The same comment applies to a reaction run with CNT devoid of any metal (entry 6).
In order to support the hypothesis of a heterogeneous catalytic process, a control reaction was run under the above-mentioned conditions (Table 1, Entry 1) except that, after 3 h, the mixture was filtered on a membrane (0.45 μm) to remove the supported catalyst. 1H-NMR of the filtrate indicated 27% conversion of the substrate. After stirring the filtered mixture for another 24 h, no additional conversion was detected, suggesting that the transformation takes place at the surface of the hybrid and is not due to soluble metallic species released from the hybrid. Moreover, neither Ru nor Rh was detected by ICP-MS analysis of the filtered reaction mixture.
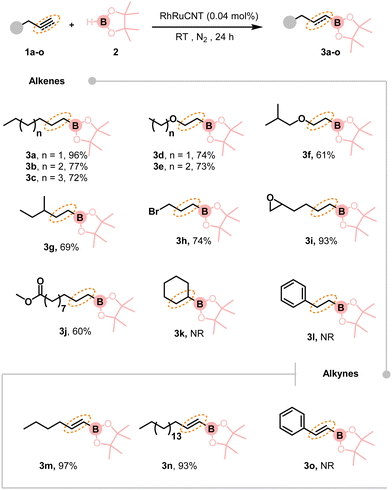
Next, we applied the RhRuCNT system to a range of substrates in order to evaluate the versatility and generality of this methodology (Scheme 2). First, we evaluated aliphatic olefins with an extended saturated hydrocarbon chain. The corresponding products 3b and 3c were obtained with 77 and 72% yield, starting from 1-heptene and 1-octene, respectively. Ether-containing alkylboranes 3d-3f were obtained in 61 to 74% yield from the corresponding vinyl-ethers, and the branched hydroborylated product 3g from 3-methyl-1-pentene in 69% yield. The developed methodology also proved compatible with various groups that can be used for further functionalization, such as a terminal bromide (3h), an epoxide (3i), and a methyl ester (3j). The products were obtained in good to excellent yields (74–93%). Nevertheless, cyclohexene (3k) and styrene (3l), exemplifying an internal olefin and an aromatic one, did not react under our reaction conditions.
Yet, the same protocol was applied to the hydroboration of alkynes in order to access vinylborane derivatives. Aliphatic vinylboranes 3m and 3n were produced in 97 and 93% yield, starting from 1-hexyne and 1-octadecyne, respectively. Unfortunately, the methodology was not effective to convert phenylacetylene into compound 3o. While the hydroboration reaction of alkynes is known for issues both in terms of regioselectivity and stereoselectivity,23 using our RhRuCNT catalyst, trans-configuration anti-Markovnikov products were exclusively obtained for all the alkyne substrates that were investigated.
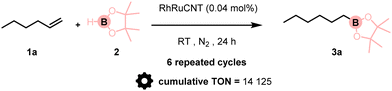
As stated above, in addition to environment-friendly conditions, the efficient recycling of the metal-based catalysts is of key importance for the development of sustainable chemical processes. We thus turned our attention to performing recycling and kinetics experiments to evaluate the stability of the RhRuCNT in the hydroboration reaction of 1-hexene 1a with HBpin 2. After completion of the reaction, ethanol (0.5 mL) was added to the mixture and the suspension was centrifuged. The supernatant (containing the product) was collected and the pellet was washed with ethanol (2 × 2 mL). The combined organic phases were worked-up to provide compound 3a. The recovered catalyst pellet was dried under reduced pressure before it was used in the next run by simply adding fresh reagents. This process was repeated five more times with no significant decrease in the catalytic activity (Table 2). Yield above 90% was still obtained in the sixth run. A cumulative TON (turnover number) of 14 125 was calculated from this set of experiments. ICP-MS analysis of the mother liquors showed no significant metal leaching. In addition, transmission electron microscopy (TEM) and X-ray photoelectron spectroscopy (XPS) indicated no alteration of the supported catalyst (Fig. S27 and S28, ESI†). Finally, the hydroboration reaction rate using RhRuCNT was measured through the calculation of a turnover frequency (TOF) – (moles of 1-hexene converted per mole of RhRuCNT per hour) – which reached a value of 225 h−1 after 3 h of reaction (27% yield).
| Cycle # | Yieldb (%) | TONc | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a Reaction conditions: 1a (1.0 mmol), 2 (1.1 mmol), catalyst (0.04 mol%), room temperature (RT), inert atmosphere (N2), neat, 24 h. b Isolated yield. c TON – turnover number (mol of 1-hexene converted per mol of RhRuCNT). | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1 (fresh) | 96 | 2400 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2 | 96 | 2400 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3 | 95 | 2375 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4 | 93 | 2325 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5 | 94 | 2350 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6 | 91 | 2275 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
In conclusion, a novel procedure using a heterogeneous catalyst for the hydroboration of alkenes and alkynes under mild conditions is presented. The catalytic system based on RhRuCNT operates with low catalytic loadings (0.04 mol%), solvent-free, and at room temperature. The RhRuCNT catalyst can transform a range of substrates with high selectivity and yield of the desired products. In addition, the catalyst showed high stability in recycling studies with no significant loss of catalytic performance. The RhRuCNT hybrid compares favourably with recently reported heterogeneous catalytic systems (see Table S2, ESI†) that, in many cases, require either heating15a,24,25 or the use of toxic solvents and/or additives.26–29 This new application expands the versatility of RhRuCNT-based nanohybrids in the field of heterogeneous catalysis.
D.V.J. thanks the “Make our Planet Great Again” program and the “Initiative de Recherche Stratégique MOMENTOM” for financial support. E.N.S.J acknowledges funding from CNPq (PQ 309774/2020-9 and Universal Project 405052/2021-9), CAPES, FAPEMIG (PPM-00635-18 and Rede de Pesquisa e Inovação para Bioengenharia de Nanossistemas-RED-00282-16), Return Fellowship of the Alexander von Humboldt Foundation (AvH), Royal Society of Chemistry for the research fund grant (R19-9781) and INCT-Catálise/CNPq/FAPESC. We would also like to thank Prof. Eduardo N. dos Santos and Prof. Elena V. Gusevskaya from UFMG for their support, as well as Dr Frédéric Fossard from CNRS-ONERA for HRSTEM experiments.
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) C. Sandford and V. K. Aggarwal, Chem. Commun., 2017, 53, 5481 RSC; (b) L. Xu, S. Zhang and P. Li, Chem. Soc. Rev., 2015, 44, 8848 RSC; (c) A. Suzuki, Angew. Chem., Int. Ed., 2011, 50, 6722 CrossRef CAS PubMed; (d) A. Suzuki and Y. Yamamoto, Chem. Lett., 2011, 40, 894 CrossRef CAS.
- (a) K. Saha and S. Ghosh, Dalton Trans., 2022, 51, 2631 RSC; (b) X. Yang, S. J. Kalita, S. Maheshuni and Y.-Y. Huang, Coord. Chem. Rev., 2019, 392, 35 CrossRef CAS.
- (a) H. Zhao, Q. Gao, Y. Zhang, P. Zhang and S. Xu, Org. Lett., 2020, 22, 2861 CrossRef CAS PubMed; (b) M. W. Drover, L. L. Schafer and J. A. Love, Angew. Chem., Int. Ed., 2016, 55, 3181 CrossRef CAS PubMed.
- (a) X.-H. Yu, K. Cao, Y. Huang, J. Li and G. Chang, Chem. Commun., 2014, 50, 4585 RSC; (b) T. Ishiyama, N. Matsuda, N. Miyaura and A. Suzuki, J. Am. Chem. Soc., 1993, 115, 11018 CrossRef CAS.
- (a) F. Sun, S. Tan, H.-J. Cao, J. Xu, V. I. Bregadze, D. Tu, C. Lu and H. Yan, Angew. Chem., Int. Ed., 2022, 61, e202207125 CAS; (b) M. Yang, Y. Yu, W. Ma, Y. Feng, G. Zhang, Y. Wu, F. Zhou, Y. Yang and D. Liu, RSC Adv., 2022, 12, 9815 RSC.
- (a) S. Kisan, V. Krishnakumar and C. Gunanathan, ACS Catal., 2017, 7, 5950 CrossRef CAS; (b) A. Kaithal, B. Chatterjee and C. Gunanathan, Org. Lett., 2015, 17, 4790 CrossRef CAS PubMed.
- (a) W. Zhao, K.-Z. Chen, A.-Z. Li and B.-J. Li, J. Am. Chem. Soc., 2022, 144, 13071 CrossRef CAS PubMed; (b) Y.-X. Tan, F. Zhang, P.-P. Xie, S.-Q. Zhang, Y.-F. Wang, Q.-H. Li, P. Tian, X. Hong and G.-Q. Lin, J. Am. Chem. Soc., 2019, 141, 12770 CrossRef CAS PubMed.
- K. Nie, Y. Han, C. Wang and X. Cheng, Appl. Organomet. Chem., 2022, 36(3), e6570 CrossRef CAS.
- J. V. Obligacion and P. J. Chirik, Nat. Rev. Chem., 2018, 2, 15 CrossRef CAS PubMed.
- (a) W. Su, R.-X. Qiao, Y.-Y. Jiang, X.-L. Zhen, X. Tian, J.-R. Han, S.-M. Fan, Q. Cheng and S. Liu, ACS Catal., 2020, 10, 11963 CrossRef CAS; (b) X. Chen, Z. Cheng and Z. Lu, Org. Lett., 2017, 19, 969 CrossRef CAS PubMed.
- (a) M. Hu, B. B. Tan and S. Ge, J. Am. Chem. Soc., 2022, 144, 15333 CrossRef CAS PubMed; (b) J. Pecak, S. Fleissner, L. F. Veiros, E. Pittenauer, B. Stöger and K. Kirchner, Organometallics, 2021, 40, 278 CrossRef CAS PubMed.
- (a) J. M. Medina, T. Kang, T. G. Erbay, H. Shao, G. M. Gallego, S. Yang, M. Tran-Dubé, P. F. Richardson, J. Derosa, R. T. Helsel, R. L. Patman, F. Wang, C. P. Ashcroft, J. F. Braganza, I. McAlpine, P. Liu and K. M. Engle, ACS Catal., 2019, 9, 11130 CrossRef CAS PubMed; (b) D. Gao, Y. Xiao, M. Liu, Z. Liu, M. K. Karunananda, J. S. Chen and K. M. Engle, ACS Catal., 2018, 8, 3650 CrossRef CAS PubMed.
- (a) G. Vijaykumar, M. Bhunia and S. K. Mandal, Dalton Trans., 2019, 48, 5779 RSC; (b) J.-F. Li, Z.-Z. Wei, Y.-Q. Wang and M. Ye, Green Chem., 2017, 19, 4498 RSC.
- (a) S. Weber, D. Zobernig, B. Stöger, L. F. Veiros and K. Kirchner, Angew. Chem., Int. Ed., 2021, 60, 24488 CrossRef CAS PubMed; (b) P. Ghosh and A. J. von Wangelin, Angew. Chem., Int. Ed., 2021, 60, 16035 CrossRef CAS PubMed.
- For examples of heterogeneous hydroboration reactions, see: (a) W.-H. Li, J. Yang, H. Jing, J. Zhang, Y. Wang, J. Li, J. Zhao, D. Wang and Y. Li, J. Am. Chem. Soc., 2021, 143, 15453 CrossRef CAS PubMed; (b) S. Thoka, M. Madasu, C. F. Hsia, S. Y. Liu and M. H. Huang, Chem. – Asian J., 2017, 12, 2318 CrossRef CAS PubMed; (c) K. Manna, P. Ji, F. X. Greene and W. Lin, J. Am. Chem. Soc., 2016, 138, 7488 CrossRef CAS PubMed; (d) B. Mohan and K. H. Park, Appl. Catal., A, 2016, 519, 78 CrossRef CAS; (e) S. B. Hong, M. Y. Liu, W. Zhang, Q. Zeng and W. Deng, Tetrahedron Lett., 2015, 56, 2297 CrossRef CAS; (f) J. Zhao, Z. Q. Niu, H. Fu and Y. D. Li, Chem. Commun., 2014, 50, 2058 RSC; (g) A. Grirrane, A. Corma and H. Garcia, Chem. – Eur. J., 2011, 17, 2467 CrossRef CAS PubMed; (h) R. Cano, D. J. Ramon and M. Yus, J. Org. Chem., 2010, 75, 3458 CrossRef CAS PubMed.
- (a) D. V. Jawale, V. Geertsen, F. Miserque, P. Berthault, E. Gravel and E. Doris, Green Chem., 2021, 23, 815 RSC; (b) P. Prakash, D. De Masi, V. Geertsen, F. Miserque, H. Li, I. N. N. Namboothiri, E. Gravel and E. Doris, ChemistrySelect, 2017, 2, 5891 CrossRef CAS; (c) P. Prakash, R. A. Kumar, F. Miserque, V. Geertsen, E. Gravel and E. Doris, Chem. Commun., 2018, 54, 3644 RSC; (d) G. A. M. Jardim, Í. A. O. Bozzi, W. X. C. Oliveira, C. Mesquita-Rodrigues, R. F. S. Menna-Barreto, R. A. Kumar, E. Gravel, E. Doris, A. L. Braga and E. N. da Silva Júnior, New J. Chem., 2019, 43, 13751 RSC.
- D. V. Jawale, J. A. Tchuiteng Kouatchou, F. Fossard, F. Miserque, V. Geertsen, E. Gravel and E. Doris, Green Chem., 2022, 24, 1231 RSC.
- D. V. Jawale, F. Fossard, F. Miserque, V. Geertsen, E. Doris and E. Gravel, Catal. Sci. Technol., 2022, 12, 4983 RSC.
- J. John, E. Gravel, A. Hagège, H. Li, T. Gacoin and E. Doris, Angew. Chem., Int. Ed., 2011, 50, 7533 CrossRef CAS PubMed.
- Y. Wang, J. Ren, K. Deng, L. Gui and Y. Tang, Chem. Mater., 2000, 12, 1622 CrossRef CAS.
- G. M. A. Rahman, D. M. Guldi, E. Zambon, L. Pasquato, N. Tagmatarchis and M. Prato, Small, 2005, 1, 527 CrossRef CAS PubMed.
- (a) K. Loza, M. Heggen and M. Epple, Adv. Funct. Mater., 2020, 30, 1909260 CrossRef CAS; (b) N. Toshima and T. Yonezawa, New J. Chem., 1998, 1179 RSC.
- S. Rej, A. Das and T. K. Panda, Adv. Synth. Catal., 2021, 363, 4818 CrossRef CAS.
- J. Zhang, Z. Wang, W. Chen, Y. Xiong, W.-C. Cheong, L. Zheng, W. Yan, L. Gu, C. Chen, Q. Peng, P. Hu, D. Wang and Y. Li, Chemistry, 2020, 6, 725 CrossRef CAS.
- L. J. Zhang, J. C. Yuan, L. J. Ma, Z. Y. Tang and X. M. Zhang, J. Catal., 2021, 401, 63 CrossRef CAS.
- S. Furukawa, M. Ieda and K.-I. Shimizu, ACS Catal., 2019, 9, 5096 CrossRef CAS.
- J.-S. Jia, T.-X. Wu, Y.-J. Fu, Z.-R. Hu, H.-T. Tang, Y.-M. Pan and F.-P. Huang, Adv. Synth. Catal., 2022, 364, 1873 CrossRef CAS.
- S. Saini, D. S. Gavali, R. Bhawar, R. Thapa, R. S. Dhayal and S. K. Bose, Catal. Sci. Technol., 2023, 13, 147 RSC.
- Z.-L. Wu, X. Lan, N. Gao, X. Kang, Z. Wang, T. Hu and B. Zhao, J. Catal., 2021, 404, 250 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: experimental procedures and NMR data. See DOI: https://doi.org/10.1039/d2cc06864h |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2023 |