Upcycling of thermosetting polymers into high-value materials
Binbo
Wang
bc,
Yi
Wang
a,
Shuai
Du
a,
Jin
Zhu
b and
Songqi
Ma
 *ab
*ab
aKey Laboratory of Synthetic and Biological Colloids, Ministry of Education, School of Chemical and Material Engineering, Jiangnan University, Wuxi, 214122, P. R. China. E-mail: songqi.ma@jiangnan.edu.cn
bLaboratory of Polymers and Composites, Ningbo Institute of Materials Technology and Engineering, Chinese Academy of Sciences, Ningbo, 315201, P. R. China
cUniversity of Chinese Academy of Sciences, Beijing, 100049, P. R. China
First published on 26th October 2022
Abstract
Thermosetting polymers, a large class of polymers featuring excellent properties, have been widely used and play an irreplaceable role in our life. Nevertheless, they are arduous to be recycled or reused on account of their permanently cross-linked networks, and the main recycling approaches used currently include energy recovery through incineration, utilization as fillers after mechanical grinding, and pyrolysis, which only reclaim a small fraction or partial value of thermosetting polymers and their downstream materials. In this minireview, we provide an overview of the efforts undertaken towards upcycling thermosetting polymers in recent years. The research progress on physical upcycling, carbonization, solvolysis and vitrimerization of thermoset waste to high-value materials, including oil–water separation materials, 3D printable materials, functional carbon materials (supercapacitors, photothermal conversion materials, and catalytic materials), additives, emulsifiers, biolubricants, and vitrimers, are summarized and discussed. Perspectives on the future development of the art of upcycling thermosets are also provided.
Introduction
Polymers have been widely used in various fields in our daily life since their invention. Thermosetting polymers (also known as thermosets) account for a significant fraction of polymer materials (around 18% of polymers) with a global annual output of around 65 million tons.1,2 This output is gradually increasing as a result of increasing consumption in several applications, including automobiles, navigation, and wind power. The most paramount feature of thermosets is the permanently cross-linked network, which endows them with excellent chemical resistance, thermal and mechanical properties, and dimensional stability; nevertheless, it also makes them formidable to be recycled, degraded, reprocessed, and dissolved. With the requirement for plastic recycling gradually becoming stricter in most countries, these difficult-to-recycle materials face a grim future. At present, incineration and landfill are the primary disposal methods for most thermoset waste, which cause severe pollution and tremendous resource waste.3To address these issues, various recycling methods have been proposed to achieve sustainable management of waste.4,5 They can be mainly classified into energy recycling, physical recycling and chemical recycling, and chemical recycling can be subdivided into pyrolysis and solvolysis. These recycling approaches, as well as their advantages and disadvantages, have been briefly discussed here. Energy recycling, the simplest recycling method, reclaims partial energy of the thermoset waste through incineration; however, the recycle value is very low and is often impacted by reinforcing fibers and fillers.6 This method is suitable for thermoset waste that gains low recycle value through other methods. Physical recycling does not involve chemical reactions during the recycling process.7 Generally, the scrap thermosets are mechanically pulverized and ground to obtain powders of size less than 50 μm.8 The recycled powders can be used for the preparation of new composites. Although this method is simple and has low energy consumption,9,10 the economic value of the recovered product is reduced, and it has no price advantage over traditional fillers, such as calcium carbonate. Therefore, this method is regarded as downcycling. Pyrolysis refers to the thermal degradation of thermoset waste in the range of 300–800 °C.11–13 The objective is to achieve gaseous or liquid small-molecule products to be used as fuels. While tar (a mixture of aromatic hydrocarbons with greater molecular weight greater than benzene) and carbon are also produced, the obtained fuels are often of low value. Thus, it is essentially a downcycle process although commercial processing levels have been achieved. Solvolysis refers to the degradation of thermosetting polymers in solvent systems. By using a wide range of solvents, temperatures, pressures, and catalysts, solvolysis aims to break specific covalent bonds of cross-linked networks to obtain oligomers (main products), monomers, or other chemicals.14–18 Due to the difference between the obtained oligomers and the raw materials of thermosets, they can generally replace only part of the raw materials, and excessive addition leads to a decline in material properties.19–21 Though solvolysis involves complex separation and purification steps, it is still considered the most prospective way to achieve closed-loop recycling of materials.
In general, the current mainstream management strategies used for thermoset waste are limited to cascaded or closed-loop recycling, and very less attention is paid to the high-value application of degradation products. With a deeper understanding of the recycling methods and recycled products, the above-mentioned recycling methods can realize thermoset waste upcycling. It should be noted that some recycling methods have issues like high energy consumption or inefficiency, which might not meet the standard of upcycling; however, they may also be considered upcycling due to their creativity and inspiration. The objectives of this minireview are to summarize the research progress on available upcycling methods, including physical upcycling, carbonization, solvolysis, and vitrimerization, for thermoset waste, discuss their advantages and disadvantages, and highlight the challenges facing the field and the prospects.
Physical upcycling
Physical upcycling, which is different from conventional physical recycling of low-value products, utilizes advanced recycling methods or unravels the unique properties of recycled products, realizing high-value reuse of thermoset waste.Epoxy resins22–24 outperform most other resin types in many aspects, including mechanical strength, chemical resistance, adhesion, low shrinkage, electrical insulation, dimensional stability, and processability. With the output of epoxy resin reaching 3.73 million tons in 2021,25 it is extremely significant to develop simple and efficient means of upcycling epoxy resin waste. In 2019, Xu, Wang, and coworkers26 developed a facile approach to transform epoxy resin waste into advanced materials. Controllable interpenetrated micro/nanopores were produced by the microwave-assisted swelling of epoxy resin waste in 1-methyl-2-pyrrolidinone (NMP) or the component solvent of NMP and dimethylacetamide (DMA). The obtained porous materials exhibited distinguished oil–water separation performance toward micro/nano oil–water emulsions with a combination of advantages including high strength, high flux, and high separation efficiency. The pore size of the porous materials can be easily tuned by changing the polarity of the swelling solvents to suit the requirements for the separation of various oil–water mixtures. This approach can be expanded to other types of thermoset waste. Although the commercial value of this method remains to be discussed, this study provides new insights into solving the increasingly serious issue of plastic waste and a new way to prepare high-efficiency oil–water emulsion separation materials.
In their follow-up work,27 it was found that even simple mechanical pulverization of thermoset waste can result in excellent oil–water separation materials with greatly reduced preparation costs. Epoxy resin (EP) waste could be converted into epoxy resin particles (CEPs) by simple mechanical crushing, and CEPs were used in the filter column for the direct separation of simple oil–water mixtures with separation flux up to 57325 L m−2 h−1 and surfactant-stabilized emulsions with droplets smaller than 10 μm (Fig. 1a and b). This upcycling strategy is also suitable for other thermoset waste. In combination with different adhesives, CEPs were used to develop superhydrophobic coatings for surface water storage, directional water transport (Fig. 1c), and water collection, and amine-cured CEP coatings were used to detect acid concentration (Fig. 1d), differentiate different types of acids and store information owing to their reversible color response to acid stimuli. The efficient utilization of pulverized particles obtained from thermosetting polymers may be the direction for future development. In 2022, Chi et al.28 prepared 3D printing inks from pulverized powders of commercial epoxy resin/graphene nanosheet composites. The physical properties of materials printed with the recycled inks remained unchanged over four consecutive recycling cycles. Moreover, both electrical (11.1 × 10−3 S cm−1) and thermal (1.2 W m K−1) conductivities remained at high levels. Based on the same recycled epoxy/graphene nanosheet powders, better results were obtained using the novel solid-state shear milling (S3M) equipment designed by Kang et al.29 The epoxy resin waste was not only ground into a powder with a size of 17 μm but also partially de-cross-linked. The surface of the powder was rich in hydroxyl and amino groups, which greatly improved the interfacial strength with the matrix and the properties of the materials when used as a filler. A composite material fabricated from the partially de-cross-linked waste epoxy resin powder (WEP), graphite nanosheet (GNP), fresh epoxy (EP) and hardener (Fig. 1e) exhibited great thermal conductivity (10.1 W m K−1) and excellent electromagnetic interference shielding performance (106.3 dB).30 These results demonstrate the great potential of S3M equipment in the mechanical upcycling of thermoset waste.
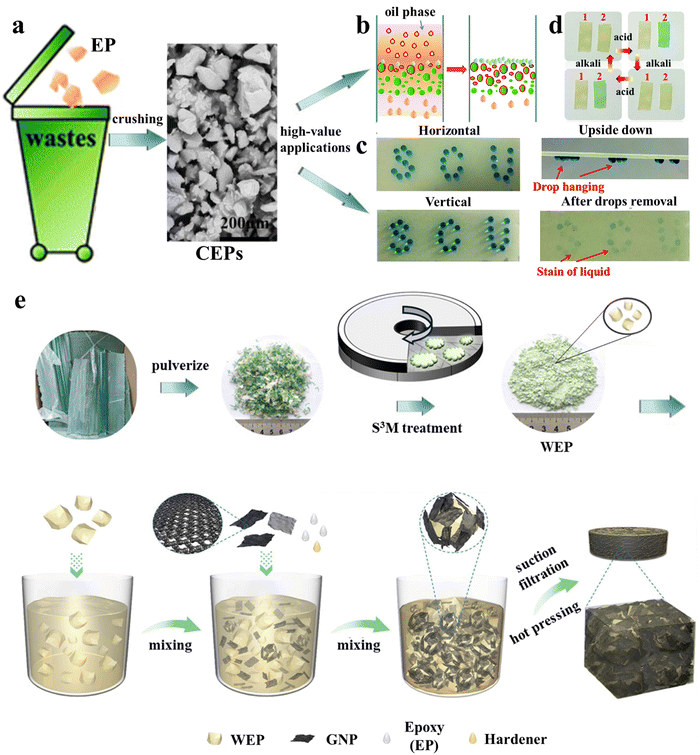 | ||
| Fig. 1 (a) Schematic of the conversion of epoxy resin (EP) waste into crushed epoxy resin particles (CEPs) through simple mechanical crushing and its various high-value applications: (b) oil–water separation, (c) water directional transport27 and (d) acidic liquid/gas detection. (e) The preparation process of waste epoxy resin powders (WEP) using S3M equipment and the GNP/WEP/EP composites29,30 (reproduced from ref. 29 with permission from the American Chemical Society and ref. 30 with permission from Elsevier). | ||
Therefore, the exploration of physical recycling methods for thermosets and the unique properties of the recycled products can ensure simplicity, high efficiency, and low pollution, thereby achieving the purpose of upcycling.
Carbonization upcycling
The preparation of functional carbon materials using various polymers (such as polyimide, polyetherimide, polyacrylonitrile, phenolic resin, and polyfurfuryl alcohol) and polymer mixtures as precursors has been widely reported. Therefore, the preparation of high-value carbon materials from waste resources might be a promising and sustainable solution. From the perspective of the carbon material preparation process, the size and morphology of carbon materials have a huge impact on their physicochemical properties, which determine their application in different fields.31–33 Carbon materials can be divided into zero-dimensional (nanospheres with sizes less than or close to 200 nm), one-dimensional (nanofibers with diameter less than 100 nm), two-dimensional (nanofilm or nanocoating), and three-dimensional forms (spheres more than 200 nm in size), foams, aerogels and monoliths.31 However, because of the non-remodeling properties of thermosetting polymers, it is difficult to obtain precursors in the zero-, one-, and two-dimensional forms. Thus, the carbon materials from thermoset waste are mainly three-dimensional.Moreover, micron-scale sphere precursors can be obtained by simple pulverization. Phenolic resin is the earliest resin to be synthesized at the industrial scale in the world and possesses excellent mechanical properties, electrical insulation properties, and corrosion resistance.26,27 Kuan et al.34 used scrap phenolic resin to prepare activated carbon microspheres with a highly porous structure by activating it with KOH and H3PO4 followed by microwave heating (Fig. 2a). KOH activation imparted activated carbon with a specific surface area of up to 924 m2 g−1, and modification with H3PO4 provided abundant phosphate functional groups, corresponding to high ammonium adsorption. This material has a unique value in the field of sewage treatment.
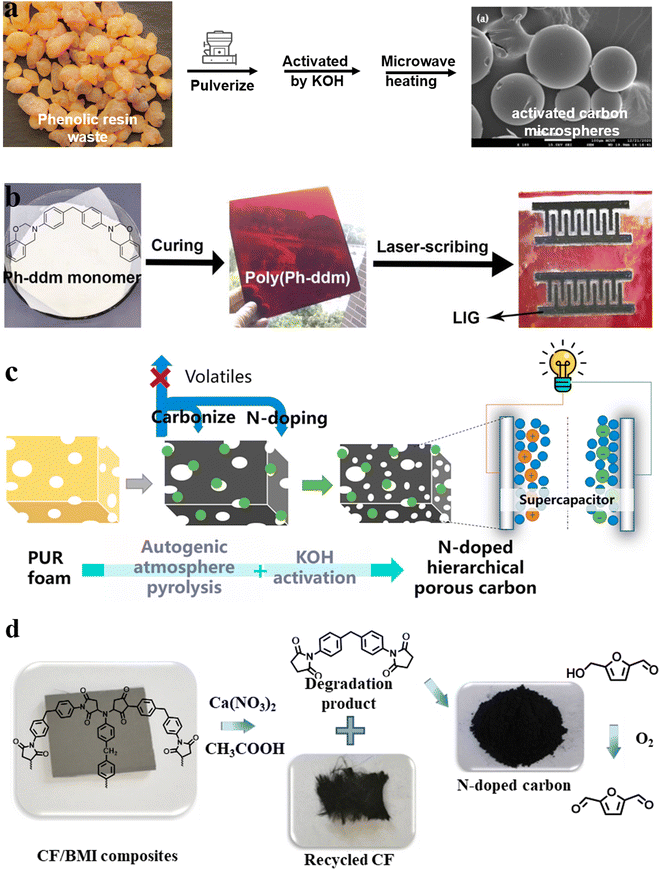 | ||
| Fig. 2 Schematic illustration of the preparation of (a) phenolic resin-based activated carbon microspheres,34 (b) benzoxazine (poly(Ph-ddm))-based laser-induced graphene (LIG)35 (reproduced from ref. 35 with permission from Elsevier), (c) N-doped hierarchical porous carbon (NHPC)36 (reproduced from ref. 36 with permission from Elsevier) and (d) N-doped carbon catalysts for the oxidization of 5-hydroxymethylfurfural (HMF) obtained from chemical degradation of carbon fiber-reinforced bismaleimide resin (CF/BMI) composites to 5-diformylfuran (DFF)37 (reproduced from ref. 37 with permission from Elsevier). | ||
A new type of thermosetting resin derived from phenolic resin, namely benzoxazine, retains its inherent excellent characteristics while avoiding the disadvantages of the phenolic resin. It also has the advantages of fire resistance and high carbon residue, which makes it suitable for the preparation of high-value carbon materials. In 2020, Liu's group35 directly generated graphene (laser-induced graphene (LIG)) patterns by engraving benzoxazine sheets with laser (Fig. 2b). Using a CO2 laser, LIGs with a sheet resistance of 35 Ω sq−1 and specific surface area of 883 m2 g−1 were prepared. Different from other reported LIG polymer matrices (polyimide,38 poly(ether ketone),39 polysulfone40 and Teflon41), benzoxazine showcased excellent comprehensive performance (chemical corrosion resistance, good processing performance, and high thermal stability), which make it a suitable LIG precursor. The obtained LIGs displayed excellent resistance to acid/alkali solutions and were used as supercapacitors and water-splitting electrodes, showing promising results in both cases. Afterward, the application of this kind of carbon materials was expanded to photothermal conversion,42–44 sewage treatment,45 and electromagnetic interference shielding.46 Despite these exciting results, no end-of-life commercial benzoxazines were used in these works, and more studies are needed to verify their generalizability.
Carbon foam also has a wide range of applications, such as high-temperature thermal insulation, catalyst supports, energy storage electrodes, etc.47 Polyurethane foam is used universally, with its production quantity reaching USD 42.9 billion in 2021.48 In addition, the representative carbamate bond in the structure of polyurethane makes it suitable for the preparation of N-atom-doped functional carbon materials (Fig. 2c).36 Commercial polyurethane foams have been used as sacrificial templates for the preparation of porous carbon materials since as early as 200449 as their foam morphology disappears after carbonization. After that, a lot of researchers used polyurethane foams as templates and added other carbon sources, such as poly(amide acid),49 resol,50 phenolic monomer-formaldehyde,51 Prussian blue nanotubes,52 and MnO2 nanotubes, to prepare porous carbon materials with different properties. Nevertheless, polyurethane itself was not recycled until 2015, when Wang et al.53 used KCO3 to activate polyurethane foam and used it as the carbon and nitrogen source to prepare a porous carbon material (yield: 28 wt%). After mixing the activated foam with elemental sulfur, a composite with large reversible capacity (1118 mA h g−1) and excellent cycling performances (460 mA h g−1 after 100 cycles at 5C (1C = 1670 mA g−1)) was obtained. Over time, the conversion degree of polyurethane to carbon materials was gradually improved by changing the type of activator, as well as the pre-carbonization and carbonization conditions, and the obtained carbon materials have been applied in a variety of fields, such as CO2 capture,54–56 electrocatalysis,57 supercapacitors,36,58 and others. For example, in the recent report by Jiang et al.,36 N-doped hierarchical porous carbon (NHPC) was prepared from polyurethane foams by the autogenic atmosphere pyrolysis (AAP)-KOH activation method, and an ultra-high carbon yield of 55.0%, which is more than 17 times the yield from traditional polyurethane carbonization, was achieved. The obtained NHPCs enhanced the performance of supercapacitors. The electrochemical measurement revealed that the NHPCs exhibited a high specific capacitance of 342 F g−1 (133 F cm−3) at 0.5 A g−1, low resistance, and outstanding cycling stability; moreover, the energy density and power density of the supercapacitor were improved to 11.3 W h kg−1 and 250 W kg−1, respectively. This work provides a possible solution to scrap polyurethane foams and energy shortage.
Low-value degradation products can also be used to prepare carbon materials. In 2020, Wang et al.37 prepared N-doped carbon catalysts from the chemical degradation products of carbon-fiber-reinforced bismaleimide resin (CF/BMI) composites. In the subcritical Ca(NO3)2/acetic system, the C–N bonds in the bismaleimide matrix were broken, resulting in carbon fibers and oligomers (Fig. 2d). Since the latter is rich in N element, the prepared carbon material exhibited a unique catalytic effect, and could be used to oxidize 5-hydroxymethylfurfural (HMF) to produce 5-diformylfuran (DFF) (yield: 89%).
Solvolysis upcycling
Solvolysis upcycling pays more attention to the special features of the degradation products themselves and applies them in high-value fields using less energy or simple modification methods.59Unsaturated polyester (UP)60,61 is formed by the polycondensation of a dibasic acid (or acid anhydride) and dihydric alcohol. Its main chain structure contains unique unsaturated bonds, which can undergo cross-linking reactions to form thermosetting resins. Because of its favorable physical properties, simple processing, and low cost, UP has been heavily used in many fields, such as agriculture, industries, transportation, and furniture, thus becoming one of the fast-growing thermosetting resins in recent years. As early as 2015, Nakagawa's team62 realized that in addition to separating raw materials from the degradation products of UP to regenerate new thermosets, high-value oligomers can be obtained by selectively destroying the cross-linked networks (Fig. 3a). In their work, the styrene-fumaric acid copolymer (SFC), which is similar to the styrene-maleic acid copolymer (a high value-added additive), was recovered with a high yield (99.6%) using subcritical water and soluble alkali to hydrolyze the thermosetting polyester matrix of the fiber-reinforced composites. The high-value copolymer of styrene and maleic anhydride could also be upcycled (yield: 44.16%) using an acidic catalysis system (p-toluenesulfonic acid/acetic acid/water) and used as an emulsifier.63 Inspired by this strategy, Xu, Wang and coworkers64 used a K2CO3/ethylene glycol catalyst system to selectively cleave the ester bond in UP and obtained a linear oligomer containing a carboxyl group and benzene ring structure with a yield of 64%. Then, amphiphilic aerogels were successfully prepared by cross-linking the oligomer with polyvinyl alcohol followed by freeze-drying. The prepared aerogels possessed a multi-level honeycomb pore structure and could simultaneously adsorb water and various oils. Further, they developed a simpler method for the direct conversion of UP into functional materials (Fig. 3b).65 First, UP was swollen using dichloromethane, and then, the pretreated UP was partially hydrolyzed in the ethylene glycol/water/K2CO3 reaction system at 140 °C for 12 h. The above two-step treatment imparted the material with a porous structure (porosity of 59.1%) and abundant carboxylate groups (15.9%), and this porous gel material was used for wastewater treatment. The gel material exhibited an outstanding methylene blue adsorption capacity (754.65 mg g−1) and excellent reusability with unchanged adsorption performance over 20 consecutive adsorption/desorption cycles. In addition, when loaded with the Fe2+ catalyst, it could also be used for the catalytic degradation of dyes to purify sewage. Afterward, a variety of processes, including microwave-assisted (KOH/water),66 low boiling point solvent (KOH/ethanol),67 synergistic catalytic (diethylenetriamine/NaOH/water),68 have been studied to achieve efficient and green preparation.
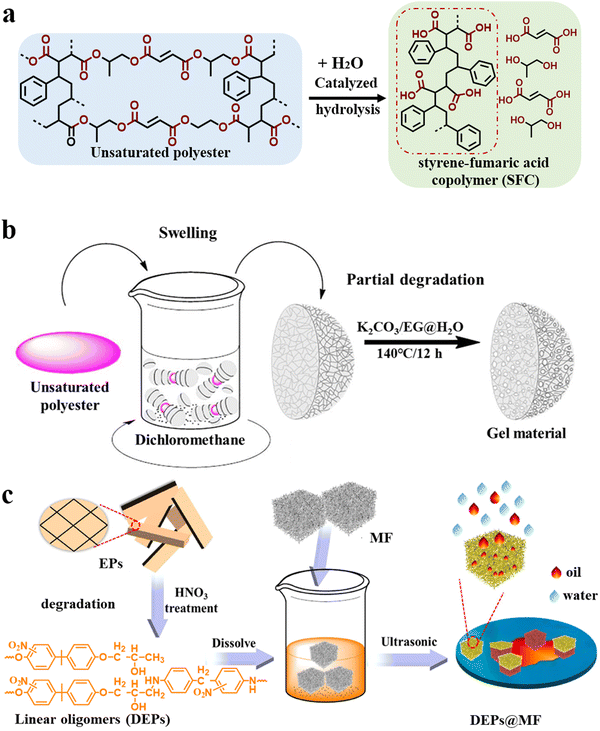 | ||
| Fig. 3 (a) Schematic of the upcycling of high-value oligomers (SFC) from unsaturated polyester.62 (b) Illustration of the preparation of oil–water separation gel materials from unsaturated polyester waste65 (reproduced from ref. 65 with permission from Elsevier). (c) Schematic of the fabrication of DEPs@MF from epoxy resin waste69 (reproduced from ref. 69 with permission from Elsevier). | ||
The chemical structure of epoxy resins is different from that of unsaturated polyester, and they can be mainly divided into anhydride-cured systems and amine-cured systems. Along the same lines, Kuang et al.70 used a zinc acetylacetonate/2-ethylhexanol solution to degrade an anhydride-cured epoxy resin and obtained a high-value dicarboxylate oligomer (yield: 40.4%), which could be used as biolubricant. Similarly, Xu, Wang and coworkers71 developed a straightforward approach for producing a porous epoxy resin in NMP via microwave-induced swelling and selective cleavage of the C–N bond under mild conditions to obtain a degraded epoxy resin (DEP) with high molecular weight. Besides, the DEP possessed excellent lipophilic and hydrophobic properties due to the retention of the hydrophobic skeleton structures, such as benzene rings, and strong binding with various substrates due to the hydroxyl and ether bonds. Therefore, DEP was utilized as a hydrophobic modifier and coated on porous melamine foam (MF) to obtain an efficient oil-absorbing material (DEP@MF) (Fig. 3c).69 DEP@MF could absorb various kinds of oils and organic solvents and exhibited a fast absorption rate (2 s absorption equilibrium) and large absorption capacity (116 g g−1). The absorbed oil could be recovered by simple mechanical extrusion, and the oil absorption capacity of DEP@MF remained relatively stable during 10 successive absorption cycles; therefore, DEP can be employed to achieve continuous oil–water separation. They also invented another solvolysis upcycling method involving acidic-ion-exchange resin-induced oxidative degradation of the amine-cured epoxy resin and water-induced phase separation of the degradation products to achieve microspheres. The microspheres exhibited great potential for dye removal in the field of wastewater treatment with a crystal violet absorption capacity of 270 mg g−1 and oil–water separation efficiency of 99.9% at a water flux of 57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 325 L m−2 h−1.72 DEPs have also been used in different fields according to different degradation methods. Recently, Xu, Wang and coworkers73 used only hydrogen peroxide to realize the degradation of epoxy resin. In the degradation process, the OH radicals attacked the C–N bond and destroyed the three-dimensional network in epoxy resin, finally obtaining oligomers with carboxyl, amino and hydroxyl groups. The end-functionalized DEP could be directly used as a supramolecular adhesive, which exhibited good and reversible adhesion to different substrates and good corrosion resistance to water, acid and organic solvents. In addition, the maximum lap shear strength (underwater) was 5.8 MPa, which is a high value among the reported underwater adhesives.
325 L m−2 h−1.72 DEPs have also been used in different fields according to different degradation methods. Recently, Xu, Wang and coworkers73 used only hydrogen peroxide to realize the degradation of epoxy resin. In the degradation process, the OH radicals attacked the C–N bond and destroyed the three-dimensional network in epoxy resin, finally obtaining oligomers with carboxyl, amino and hydroxyl groups. The end-functionalized DEP could be directly used as a supramolecular adhesive, which exhibited good and reversible adhesion to different substrates and good corrosion resistance to water, acid and organic solvents. In addition, the maximum lap shear strength (underwater) was 5.8 MPa, which is a high value among the reported underwater adhesives.
Solvolysis upcycling might be a very effective method that solves the problems of harsh reaction conditions, difficult product separation, and low product reuse value in solvent degradation.
Vitrimerization upcycling
The permanently cross-linked network of thermosets is the main reason for the recycling issue. Vitrimerization is a recently emerging technology that transforms conventional thermosets into vitrimers (Fig. 4a),74 a new class of innovative materials developed in the past ten years.75,76 Unlike thermosetting resins, vitrimers contain dynamic covalent bonds in place of the permanent cross-linking points, which impart excellent recyclability to the thermosets.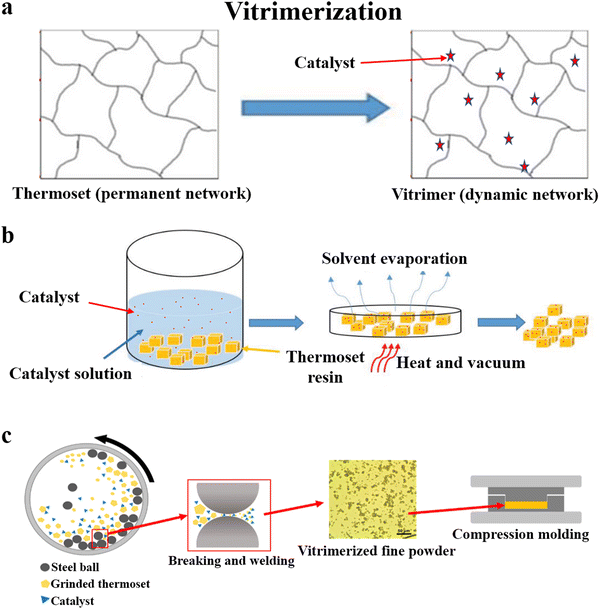 | ||
| Fig. 4 (a) Schematic of vitrimerization,74 and the methods used for achieving vitrimerization: (b) the swelling-drying method74 and (c) hot pressing after pulverization and mixing78 (reproduced from ref. 78 with permission from the American Chemical Society). | ||
In 2019, Manas-Zloczower and coworkers74 coined the concept of vitrimerization. After adding the catalyst (tin(II) 2-ethylhexanoate (Sn(Oct)2)) to PU and epoxy by the swelling-drying method, these materials could be processed by compression molding and extrusion (Fig. 4b). Based on this swelling-drying method, Ellison, Dichtel and coworkers77 achieved the vitrimerization of commercial PU foams by adding dibutyltin dilaurate to catalyze carbamate exchange. It was thus possible to quantitatively reprocess cross-linked PU foam continuously into a film using an industrially relevant twin screw extruder, and the air in the PU foam was removed during this process. However, the swelling-drying method uses a large amount of organic solvents, resulting in serious pollution and health hazards.
In their follow-up work in 2020, Manas-Zloczower and coworkers78,79 developed a simpler and more efficient method to achieve vitrimerization. They pulverized an anhydride-cured epoxy resin and zinc acetate using a planetary ball mill and then hot-pressed it to achieve vitrimerization (Fig. 4c). An increase in catalyst content made the network more rigid, leading to an increase in the modulus (from 1.5 GPa to 2.2 GPa) and a reduction in the elongation at break (from 11.1% to 1.4%), which might be attributed to the physical cross-linking points arising from the coordination of the metal catalyst. The thermal and mechanical properties of the material were maintained after recycling several times. Based on the transesterification reaction, this method was also used for the vitrimerization of unsaturated polyesters.80
Vitrimerization not only endows thermosets with reprocessability but also makes high-performance recycling possible. Unlike the functionalization methods described above, vitrimerization of thermosets easily accommodates fillers, which can readily improve performance. For instance, using cellulose nanocrystals in the vitrimerization of anhydride-cured epoxy resins enabled the large-scale production of nanocomposites with better performance (the glass transition temperature, tensile strength and elastic modulus were increased to 179 °C, 96 MPa and 4.5 GPa, respectively).81,82 The hydroxyl groups on the surface of the cellulose nanocrystals underwent transesterification with the ester bonds in the epoxy resin, so they participated in the reformation of the network during the hot-pressing process, further increasing the performance of the material.
Conclusions and perspective
Upcycling has been proven to be an effective strategy to conquer the current difficulties in recycling thermoset waste and increase the recycling value. This review summarizes the current advances in four categories of upcycling methods, namely physical upcycling, carbonization upcycling, solvolysis upcycling, and vitrimerization upcycling. High-value-added materials, including oil–water separation materials, 3D printing inks, supercapacitor electrodes, photothermal conversion materials, catalytic carbon materials, vitrimers, high-value additives, emulsifiers, and biolubricants, have been obtained via these approaches. Each upcycling method involves different processes, is suitable for certain thermosets, and has advantages and disadvantages (Table 1). Through the optimization of recycling methods and improving the value of recycled products, breakthroughs in the original downcycle have been achieved. Although significant advances have been achieved in these upcycling technologies, there are still quite a few challenges in realizing their industrialization. In this regard, we propose some future research directions, as follows.| Method | Process description | Thermoset type | Applications | Advantages | Disadvantages |
|---|---|---|---|---|---|
| Physical upcycling | Microwave-assisted swelling, mechanical crushing, solid-state shear milling | Epoxy resin | Oil–water separation material, acid detection, surface water storage, 3D printing inks, electrical and thermal conductivity material | Simple operation, low energy consumption, easy to scale up | Few application areas |
| Carbonization upcycling | Microwave heating, CO2 laser etching, autogenic atmosphere pyrolysis-KOH activation | Benzoxazine, phenolic resin, polyurethane, bismaleimide resin | Sewage treatment, photothermal conversion, electromagnetic interference shielding, CO2 capture, electrocatalysts, supercapacitor | Simple operation, easy to scale up | High-energy consumption, low conversion rate |
| Solvolysis upcycling | Supercritical fluid degradation (subcritical water), catalytic degradation after microwave assisted swelling (ethylene glycol, ethanol, water, 2-ethylhexanol, H2O2) | Epoxy resin, unsaturated polyester | Additive, emulsifier, biolubricants, oil–water separation material, adhesives | Relative mild reaction conditions, products with well-defined chemical structures | Contamination of organic solvents |
| Vitrimerization upcycling | Swelling-drying method, hot pressing after ball milling | Epoxy resin, polyurethane, unsaturated polyester | Vitrimer | Simple operation, excellent reprocessability | Reduced performance |
(1) Compared with the types of commercial thermosetting resins available, fewer types of resins are being studied currently, and the universality of various available methods needs to be further verified.
(2) The recycling of high-value products obtained after upcycling also requires attention. For instance, oil–water separation materials upcycled from thermosets might lose their oil–water separation ability after multiple uses; thus, dealing with such failed waste is still a problem.
(3) The combination of different upcycling methods may bring unexpected benefits. As an example, carbonization upcycling of thermosets can be achieved through solvolysis or physical pulverization of thermosets and carbonization.
(4) Carbonization upcycling of thermoset waste is a high-energy-consuming process, and the yield in carbonization is low. Designing suitable processes or highly efficient catalysts to reduce the carbonization temperature and improve carbonization efficiency for the conversion of thermoset waste should be a future direction.
(5) During the solvolysis of high-value oligomers obtained from thermoset waste, the upcycling yield is low; moreover, the upcycling processes often include catalytic degradation and purification, leading to high upcycling costs. These limit the industrialization of upcycling technologies. Thus, highly efficient catalyst systems or upcycling processes should be developed to achieve a high yield of high-value products and reduce the upcycling cost.
(6) Although various high-value materials and chemicals can be upcycled from thermoset waste, most researchers mainly focus on the upcycling process and the functions or features of the upcycled products and their overall properties; life cycle analysis, as well as market advantage, have been overlooked thus far, resulting in a lack of clarity in the industrialization process.
(7) In addition to upcycling thermoset waste into the above-mentioned high-value-added materials, including functional materials, vitrimers, high-value additives, emulsifiers, and biolubricants, efforts should be made to obtain high-performance materials after recycling, as high performance is the main characteristic of thermosets.
(8) The thermosets used in most upcycling studies were produced by the authors, while real thermoset waste has rarely been utilized. In reality, thermoset waste is much more complex than pure thermosets; it could be partially damaged during use and contain additives or fillers, which would increase the difficulty in achieving upcycling and impact the quality of the upcycled products. Thus, more efforts should be undertaken with real thermoset waste.
(9) In view of efficiency, green chemistry and sustainability, it is still indispensable to develop more upcycling methods. Despite the beneficial explorations on upcycling thermosetting polymers, there is still a long way to truly solve the environmental and resource problems triggered by waste resins.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are extremely grateful for the financial support from National Natural Science Foundation of China (No. 52073296), Research startup fund from Jiangnan University, and Zhejiang Provincial Ten Thousand Plan for Young Top Talents.References
- P. Shieh, W. Zhang, K. E. L. Husted, S. L. Kristufek, B. Xiong, D. J. Lundberg, J. Lem, D. Veysset, Y. Sun, K. A. Nelson, D. L. Plata and J. A. Johnson, Nature, 2020, 583, 542–547 CrossRef CAS PubMed.
- S. Ma and D. C. Webster, Prog. Polym. Sci., 2018, 76, 65–110 CrossRef CAS.
- D. J. Fortman, J. P. Brutman, G. X. De Hoe, R. L. Snyder, W. R. Dichtel and M. A. Hillmyer, ACS Sustainable Chem. Eng., 2018, 6, 11145–11159 CrossRef CAS.
- B. Wang, S. Ma, S. Yan and J. Zhu, Green Chem., 2019, 21, 5781–5796 RSC.
- Y. Liu, Z. Yu, B. Wang, P. Li, J. Zhu and S. Ma, Green Chem., 2022, 24, 5691–5708 RSC.
- S. Utekar, V. K. Suriya, N. More and A. Rao, Composites, Part B, 2021, 207, 108596 CrossRef CAS.
- V. Martinez Sanz, A. Morales Serrano and M. Schlummer, Waste Manage. Res., 2022, 0734242X221094917 Search PubMed.
- S. Gharde and B. Kandasubramanian, Environ. Technol. Innovation, 2019, 14, 100311 CrossRef.
- D. García, I. Vegas and I. Cacho, Constr. Build. Mater., 2014, 64, 293–300 CrossRef.
- K. Hamad, M. Kaseem and F. Deri, Polym. Degrad. Stab., 2013, 98, 2801–2812 CrossRef CAS.
- A. Kijo-Kleczkowska and A. Gnatowski, Energies, 2022, 15, 2114 CrossRef CAS.
- N. Sakthipriya, Sci. Total Environ., 2022, 809, 151160 CrossRef PubMed.
- P. Quicker, M. Seitz and J. Vogel, Waste Manage. Res., 2022, 40, 1494–1504 CrossRef CAS.
- J. P. Brutman, G. X. De Hoe, D. K. Schneiderman, T. N. Le and M. A. Hillmyer, Ind. Eng. Chem. Res., 2016, 55, 11097–11106 CrossRef CAS.
- D. K. Schneiderman, M. E. Vanderlaan, A. M. Mannion, T. R. Panthani, D. C. Batiste, J. Z. Wang, F. S. Bates, C. W. Macosko and M. A. Hillmyer, ACS Macro Lett., 2016, 5, 515–518 CrossRef CAS.
- M. Hong and E. Y. X. Chen, Nat. Chem., 2016, 8, 42–49 CrossRef CAS.
- X. Kuang, Y. Zhou, Q. Shi, T. Wang and H. J. Qi, ACS Sustainable Chem. Eng., 2018, 6, 9189–9197 CrossRef CAS.
- T. Liu, X. Guo, W. Liu, C. Hao, L. Wang, W. C. Hiscox, C. Liu, C. Jin, J. Xin and J. Zhang, Green Chem., 2017, 19, 4364–4372 RSC.
- Q. Mu, L. An, Z. Hu and X. Kuang, Polym. Degrad. Stab., 2022, 199, 109895 CrossRef CAS.
- T. Hanaoka, Y. Arao, Y. Kayaki, S. Kuwata and M. Kubouchi, ACS Sustainable Chem. Eng., 2021, 9, 12520–12529 CrossRef CAS.
- X. Zhao, X.-L. Wang, F. Tian, W.-L. An, S. Xu and Y.-Z. Wang, Green Chem., 2019, 21, 2487–2493 RSC.
- J. Chen, N. Chu, M. Zhao, F.-L. Jin and S.-J. Park, J. Appl. Polym. Sci., 2020, 137, 49592 CrossRef CAS.
- Z. Yu, S. Ma, Z. Tang, Y. Liu, X. Xu, Q. Li, K. Zhang, B. Wang, S. Wang and J. Zhu, Green Chem., 2021, 23, 6566–6575 RSC.
- P. Li, S. Ma, B. Wang, X. Xu, H. Feng, Z. Yu, T. Yu, Y. Liu and J. Zhu, Compos. Sci. Technol., 2022, 219, 109243 CrossRef CAS.
- X. Zhang, Analysis of supply and demand status of China's epoxy resin industry in 2021, an indispensable basic material in all industries, https://www.huaon.com/channel/trend/811384.html.
- F. Tian, Y. Yang, X.-L. Wang, W.-L. An, X. Zhao, S. Xu and Y.-Z. Wang, Mater. Horiz., 2019, 6, 1733–1739 RSC.
- X. Liu, F. Tian, X. Zhao, R. Du, S. Xu and Y.-Z. Wang, Mater. Horiz., 2021, 8, 234–243 RSC.
- H. Chi, Z. Lin, Y. Chen, R. Zheng, H. Qiu, X. Hu and H. Bai, ACS Appl. Mater. Interfaces, 2022, 14, 13758–13767 CrossRef CAS.
- P. Kang, S. Yang, S. Bai and Q. Wang, ACS Sustainable Chem. Eng., 2021, 9, 11778–11789 CrossRef CAS.
- P. Kang, Z. Jin, S. Yang and Q. Wang, Composites, Part A, 2022, 152, 106710 CrossRef CAS.
- I. Tiwari, P. Sharma and L. Nebhani, Mater. Today Chem., 2022, 23, 100734 CrossRef CAS.
- D. Torres, S. Pérez-Rodríguez, L. Cesari, C. Castel, E. Favre, V. Fierro and A. Celzard, Carbon, 2021, 183, 12–33 CrossRef CAS.
- C. Shaer, L. Oppenheimer, A. Lin and H. Ishida, Polymers, 2021, 13, 3775 CrossRef CAS.
- W.-H. Kuan, Y.-S. Hu, C.-Y. Chiu, K.-Y. Hung and S.-S. Chou, Catalysts, 2021, 11, 783 CrossRef CAS.
- L. Cao, S. Zhu, B. Pan, X. Dai, W. Zhao, Y. Liu, W. Xie, Y. Kuang and X. Liu, Carbon, 2020, 163, 85–94 CrossRef CAS.
- X. Zhou, L. Zhu, Y. Yang, L. Xu, X. Qian, J. Zhou, W. Dong and M. Jiang, Chemosphere, 2022, 300, 134552 CrossRef CAS PubMed.
- Y. Wang, J. Li, X. Zhou, T. Wang, Q. Zhao, H. Zhang, Y. Wang and X. Hou, Compos. Sci. Technol., 2020, 199, 108342 CrossRef CAS.
- Y.-S. Hsiao, C.-W. Chang-Jian, T.-Y. Huang, Y.-L. Chen, C.-W. Huang, J.-H. Huang, N.-J. Wu, S.-C. Hsu and C.-P. Chen, Chem. Eng. J., 2023, 451, 138656 CrossRef CAS.
- A. Lamberti, M. Serrapede, G. Ferraro, M. Fontana, F. Perrucci, S. Bianco, A. Chiolerio and S. Bocchini, 2D Mater., 2017, 4, 035012 CrossRef.
- S. P. Singh, Y. Li, J. Zhang, J. M. Tour and C. J. Arnusch, ACS Nano, 2018, 12, 289–297 CrossRef CAS.
- R. Ye, X. Han, D. V. Kosynkin, Y. Li, C. Zhang, B. Jiang, A. A. Martí and J. M. Tour, ACS Nano, 2018, 12, 1083–1088 CrossRef CAS PubMed.
- W. Zhao, Y. Jiang, W. Yu, Z. Yu and X. Liu, Small, 2022, 2202906 CrossRef CAS.
- Y. Peng, W. Zhao, F. Ni, W. Yu and X. Liu, ACS Nano, 2021, 15, 19490–19502 CrossRef CAS PubMed.
- W. Zhao, W. Yu, Y. Jiang, Z. Yu, G. Wang and X. Liu, Nano Energy, 2022, 100, 107477 CrossRef CAS.
- Y. Jiang, S. Wan, W. Zhao, W. Yu, S. Wang, Z. Yu, Q. Yang, W. Zhou and X. Liu, Carbon Lett., 2022, 32, 1047–1064 CrossRef.
- W. Yu, Y. Peng, L. Cao, W. Zhao and X. Liu, Carbon, 2021, 183, 600–611 CrossRef CAS.
- M. Inagaki, J. Qiu and Q. Guo, Carbon, 2015, 87, 128–152 CrossRef CAS.
- BlueWeave Consulting and Research Pvt Ltd, Polyurethane Foam Market to Surpass USD 70 Billion by 2028, https://www.blueweaveconsulting.com/press-release/polyurethane-foam-market-to-surpass-usd-70-billion-by-2028.
- M. Inagaki, T. Morishita, A. Kuno, T. Kito, M. Hirano, T. Suwa and K. Kusakawa, Carbon, 2004, 42, 497–502 CrossRef CAS.
- C. Xue, B. Tu and D. Zhao, Adv. Funct. Mater., 2008, 18, 3914–3921 CrossRef CAS.
- G. Nam, S. Choi, H. Byun, Y.-M. Rhym and S. E. Shim, Macromol. Res., 2013, 21, 958–964 CrossRef CAS.
- B. Kong, J. Tang, Z. Wu, J. Wei, H. Wu, Y. Wang, G. Zheng and D. Zhao, Angew. Chem., Int. Ed., 2014, 53, 2888–2892 CrossRef CAS PubMed.
- S. Xiao, S. Liu, J. Zhang and Y. Wang, J. Power Sources, 2015, 293, 119–126 CrossRef CAS.
- C. Ge, J. Song, Z. Qin, J. Wang and W. Fan, ACS Appl. Mater. Interfaces, 2016, 8, 18849–18859 CrossRef CAS.
- J. Duan, H. Fan and W. Shen, ChemistrySelect, 2016, 1, 3204–3207 CrossRef CAS.
- C. Ge, D. Lian, S. Cui, J. Gao and J. Lu, Processes, 2019, 7, 592 CrossRef CAS.
- G. Daniel, T. Kosmala, M. C. Dalconi, L. Nodari, D. Badocco, P. Pastore, A. Lorenzetti, G. Granozzi and C. Durante, Electrochim. Acta, 2020, 362, 137200 CrossRef CAS.
- C. Schneidermann, P. Otto, D. Leistenschneider, S. Gratz, C. Essbach and L. Borchardt, Beilstein J. Nanotechnol., 2019, 10, 1618–1627 CrossRef.
- W. An, X.-L. Wang, X. Liu, G. Wu, S. Xu and Y.-Z. Wang, Green Chem., 2022, 24, 701–712 RSC.
- Y. Yao, B. Wang, F. Zhao, Z. Hu and Y. Huang, ACS Appl. Polym. Mater., 2022, 4, 999–1009 CrossRef CAS.
- L. Feng, R. Li, H. Yang, S. Chen and W. Yang, Polymers, 2022, 14, 1127 CrossRef CAS PubMed.
- T. Nakagawa and M. Goto, Polym. Degrad. Stab., 2015, 115, 16–23 CrossRef CAS.
- N. Zhang, X. Hou, X. Cui, L. Chai, H. Li, H. Zhang, Y. Wang and T. Deng, J. Cleaner Prod., 2021, 296, 126492 CrossRef CAS.
- X.-L. Wang, W.-L. An, F. Tian, Y. Yang, X. Zhao, P.-P. Xu, S. Xu and Y.-Z. Wang, Waste Manage., 2021, 126, 89–96 CrossRef CAS PubMed.
- X.-L. Wang, W.-L. An, Y. Yang, Z.-Y. Hu, S. Xu, W. Liao and Y.-Z. Wang, Chem. Eng. J., 2019, 361, 21–30 CrossRef CAS.
- X.-L. Wang, W.-L. An, F. Tian, Y. Yang, H.-X. Xu, D. Wang, X. Zhao, P.-P. Xu, S. Xu and Y.-Z. Wang, J. Hazard. Mater., 2020, 384, 121465 CrossRef CAS PubMed.
- X.-L. Wang, W.-L. An, F. Tian, Y. Yang, X. Zhao, P.-P. Xu, S. Xu and Y.-Z. Wang, ACS Sustainable Chem. Eng., 2020, 8, 16010–16019 CrossRef CAS.
- W. An, X.-L. Wang, Y. Yang, H. Xu, S. Xu and Y.-Z. Wang, Green Chem., 2019, 21, 3006–3012 RSC.
- X. Liu, F. Tian, X. Zhao, R. Du, S. Xu and Y.-Z. Wang, Appl. Surf. Sci., 2020, 529, 147151 CrossRef CAS.
- X. Kuang, E. Guo, K. Chen and H. J. Qi, ACS Sustainable Chem. Eng., 2019, 7, 6880–6888 CrossRef CAS.
- F. Tian, X.-l Wang, Y. Yang, W. An, X. Zhao, S. Xu and Y.-Z. Wang, ACS Sustainable Chem. Eng., 2020, 8, 2226–2235 CrossRef CAS.
- X. Zhou, W. An, X. Xia, Y. Long, X. Liu, S. Xu and Y.-Z. Wang, ACS Sustainable Chem. Eng., 2022, 10, 5582–5589 CrossRef CAS.
- X. Liu, X. Zhao, W. An, R. Du, G. Wu, S. Xu, F. Zhang and Y.-Z. Wang, Mater. Horiz., 2022 10.1039/D2MH00781A.
- L. Yue, V. S. Bonab, D. Yuan, A. Patel, V. Karimkhani and I. Manas-Zloczower, Glob. Chall., 2019, 3, 1800076 CrossRef PubMed.
- N. J. Van Zee and R. Nicolaÿ, Prog. Polym. Sci., 2020, 104, 101233 CrossRef CAS.
- D. Montarnal, M. Capelot, F. Tournilhac and L. Leibler, Science, 2011, 334, 965–968 CrossRef CAS PubMed.
- D. T. Sheppard, K. Jin, L. S. Hamachi, W. Dean, D. J. Fortman, C. J. Ellison and W. R. Dichtel, ACS Cent. Sci., 2020, 6, 921–927 CrossRef CAS PubMed.
- L. Yue, H. Guo, A. Kennedy, A. Patel, X. Gong, T. Ju, T. Gray and I. Manas-Zloczower, ACS Macro Lett., 2020, 9, 836–842 CrossRef CAS PubMed.
- L. Yue, M. Amirkhosravi, X. Gong, T. G. Gray and I. Manas-Zloczower, ACS Sustainable Chem. Eng., 2020, 8, 12706–12712 CrossRef CAS.
- A. Bandegi, M. Amirkhosravi, H. Meng, M. K. R. Aghjeh and I. Manas-Zloczower, Glob. Chall., 2022, 2200036 CrossRef PubMed.
- L. Yue, K. Ke, M. Amirkhosravi, T. G. Gray and I. Manas-Zloczower, ACS Appl. Bio Mater., 2021, 4, 4176–4183 CrossRef CAS PubMed.
- L. Yue, M. Amirkhosravi, K. Ke, T. G. Gray and I. Manas-Zloczower, ACS Appl. Mater. Interfaces, 2021, 13, 3419–3425 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2023 |
