Open Access Article
Open Access ArticleRecent development of end-of-life strategies for plastic in industry and academia: bridging their gap for future deployment†
Jackie
Zheng‡
ab,
Md
Arifuzzaman‡
a,
Xiaomin
Tang
 a,
Xi Chelsea
Chen
a,
Xi Chelsea
Chen
 *a and
Tomonori
Saito
*a and
Tomonori
Saito
 *a
*a
aChemical Sciences Division, Oak Ridge National Laboratory, Oak Ridge, TN 37831, USA. E-mail: chenx@ornl.gov; saitot@ornl.gov
bBredesen Center for Interdisciplinary Research and Education, University of Tennessee Knoxville, Knoxville, TN 37966, USA
First published on 6th April 2023
Abstract
Plastics have advanced society as a lightweight, inexpensive material of choice, and consequently over 400 million metric tons of plastics are produced each year. The difficulty with their reuse, due to varying chemical structures and properties, is leading to one of the major global challenges of the 21st century—plastic waste management. While mechanical recycling has been proven successful for certain types of plastic waste, most of these technologies can only recycle single types of plastics at a time. Since most recycling collection streams today have a mixture of different plastic types, additional sorting is required before the plastic waste can be processed by recyclers. To combat this problem, academics have devoted their efforts to developing technologies such as selective deconstruction catalysts or compatibilizer for commodity plastics and new types of upcycled plastics. In this review, the strengths and challenges of current commercial recycling processes are discussed, followed by examples of the advancement in academic research. Bridging a gap to integrate new recycling materials and processes into current industrial practices will improve commercial recycling and plastic waste management, as well as create new economies. Furthermore, establishing closed-loop circularity of plastics by the combined efforts of academia and industry will contribute toward establishing a net zero carbon society by significant reduction of carbon and energy footprints. This review serves as a guide to understand the gap and help to create a path for new discovery in academic research to be integrated into industrial practices.
1. Introduction
The volume of yearly plastic production has exceeded 400 million metric tons (Mt)1 and had an estimated value of $593 billion in 2021.2 This demand will continue to grow at a projected rate of 3.7% for the next decade. Plastics are a critical material for the advancement of society, but improper end-of-life management of plastic-based materials is also detrimental to society. Only ∼9% of global plastics are recycled today,3 with the rest being incinerated for energy, ending up in landfills, or even worse, littered into the environment, polluting our lands and oceans. While recycling technologies to process single component plastic waste streams already exist on a commercial scale, plastics currently account for over 20% of the waste in landfills due to the lack of technology to sort and separate mixed plastic streams because of size limitations as well as mixed materials that cannot be physically separated.Most plastics today are made from monomers that are synthesized from fossil fuels. Small molecules extracted from crude oil are chemically modified via multi-step reactions to form monomers, which can then be polymerized into polymers of various structures and chemistries. The processes used to make monomers and then polymers are associated with high energy consumption, an estimated 3.2 quadrillion Btus per year, and 104 MMTCO2 equivalent greenhouse gas (GHG) emissions in the U.S. alone.4 The most common commercially available polymers include polypropylene (PP), polyethylene (PE), polyethylene terephthalate (PET), polyurethanes (PU), and polystyrenes (PS), with global production volumes of 68, 166, 33, 27, and 25 Mt, respectively.5 Among them, PE, PP and PET represent the most recycled plastics, which occupy 95.2% of recycled materials (Fig. 1). Since the chemistry of each polymer is different, the physical and mechanical properties are also different, thereby requiring different processing conditions to recycle each type of plastic.
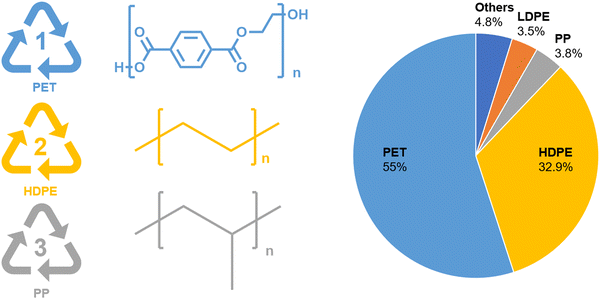 | ||
| Fig. 1 Polyethylene terephthalate (PET), high density polyethylene (HDPE), polypropylene (PP), and low density polypropylene (LDPE) are some of the most commercially recycled polymers today with PET and HDPE making up the majority of the plastic recycling market.6 Adapted in part from theroundup.org. | ||
Mechanical recycling is the most commercially used recycling method, but has its limitations due to its sorting challenges, decreased physical properties, and the need for additives to allow recycled materials to be used again. As such, chemical recycling is being developed as a complementary method to mechanical recycling. One of the most prevalent techniques currently used to chemically recycle polymers is pyrolysis, where plastics are heated to high temperatures to yield chemicals that would otherwise be extracted from fossil fuels. Academically, there is a large effort toward chemical recycling with advanced techniques such as pyrolysis, depolymerization, and biobased plastics, on which large quantities of publications are seen each year.
In this short review, the current efforts and advantages in industrial recycling processes will be discussed as well as their unmet needs. Then, recent academic efforts relating to novel recycling methods as well as the design of recyclable materials will be featured. This review will primarily focus on recycling efforts for PET and polyolefins such as PP and PE, since those commodity polymers are some of the most used and recycled consumer plastics that will allow for comparison between industry and academia. There is a gap between what industry needs and what academia offers. This review will help academics understand the current major challenges that industrial recycling processes have as well as highlight technologies and research that have been developed in academic laboratories that can have strong sustainability and economic impacts.
2. Industry efforts
2.1 Sorting of waste feedstock
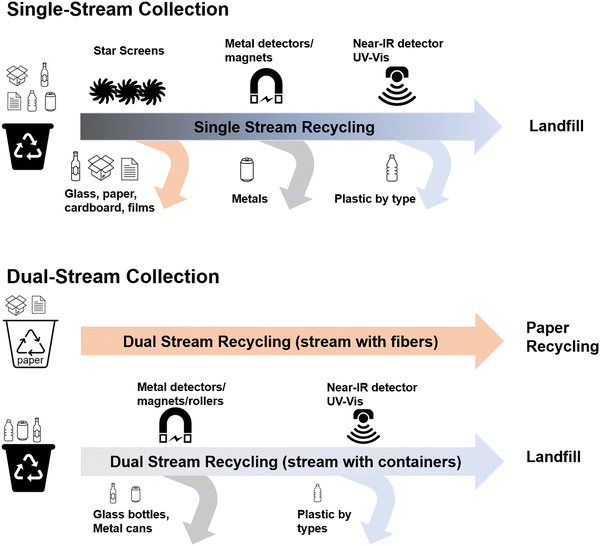
Curbside recycling in the U.S. generally comes in two forms: single-stream and dual-stream (Fig. 2). In single stream recycling, paper-based waste and old corrugated cardboard are combined with glass, metal, and plastic containers. To extract the plastic waste, materials are processed at materials recovery facilities (MRFs). Plastic only makes up a small portion of single-stream feedstocks and must be separated from the single stream feedstock before plastic recycling can occur. On the other hand, dual stream recycling is collected as two different feedstocks, where one has paper-based recyclables and another includes glasses, metals, and plastics. Dual-stream recycling yields a higher percentage of plastics but requires effort from consumers to separate their recyclables as well as the extra collection cost of having specialized types of collection trucks for each type of recyclable.Regardless of single or dual stream, plastics must be further separated out at MRFs before mechanical recycling can be done. Sorting is conducted manually or via a machine/AI driven separation process, where an AI can optically detect the type of plastic by shape of the object or using near infrared (NIR) detectors that are able to detect the types of chemical bonds in a polymer to determine the type of plastic. In a modernized system, blasts of air are used to move the plastics to positive and negative sorting lines, where the blast of air can push the plastic into another line or allow it to drop down and pass to a different line. There are also optical systems being developed by companies in which robotic arms are incorporated that can quickly remove contamination along the lines.
While sorting for mechanical processes continues to be improved, there are still various challenges that make certain plastics extremely hard to recycle. The shape and the size of plastic containers can be a hindrance for the sortation process. For example, the shape of clamshell takeout containers (i.e., containers that might be used for salad or other take out) make it hard for the machine to separate them via blasts of air due to the shape of the material and air resistance, causing the material to fly in unexpected trajectories. Additionally, even though the plastic type for clamshells is classified as e.g., PET or similar analogues, they sometimes have slightly different chemical structures giving an altered glass transition temperature (Tg) and melting point that shifts their optimal processing temperature. These types of containers frequently have adhesives and labels on their surface, which also causes issues during mechanical recycling.
The color of an object can also cause issues during sortation. Since NIR relies on the reflection of light off the material into detectors, plastic objects with additives like carbon black are hard for NIR detectors to pick up. Additionally, if the color of the object blends in with the conveyor belt it's on, optical systems can have trouble detecting the material too. Another color related challenge is that some MRFs use the color and shape of known objects to detect the type of material. If a certain type of consumer good comes in a specific color and shape, MRFs will use that information in their optical sorting system. However, if the manufacturer were to change the material from PP to PET, machines will continue to sort the consumer product as if it were PP, causing a contamination of their bale of sorted plastics. Contamination causes the quality and value of the bale to drop, which can even result in the entire bale being rejected by buyers. Following the separation of plastic waste by polymer type, they can be sold to downstream recyclers.
2.2 PET recycling
PET is a versatile plastic that has a wide range of applications from food containers to thermoforms for manufacturing and even textiles. However, due to the durability of PET, it poses a significant long-term persistence problem in the environment if not disposed of and recycled properly. Only 14% of consumer PET is recovered for recycling after being used, and just 2% is recycled appropriately and put back into circulation.7 PET is commercially recycled via both mechanical and chemical recycling.Prior to 2016, a large amount of plastic waste in the U.S. was sold to China. But when China's National Sword program was announced, U.S. companies had to start recycling more PET domestically. Companies such as DAK Americas, a subsidiary of Alpek, and Indorama Ventures are some of the larger mechanical recycling companies in the U.S. that process PET. According to the 2018 annual report, polyesters account for 74% of Alpek's (DAK's parent company) sales. Alpek's capacity for PET production is 2.46 Mt while recycled PET (rPET) only has a capacity of 70 kt.8 Indorama Venture Limited (IVL) also recycles PET via a mechanical process, however they also have a subsidiary to their business, where PET flakes are melted and spun into recycled fibers and yarn under the brand DEJA. 317 kt of PET was recycled by Indorama in 20219 into the form of resins, fibers, and flakes.
2.2.2.1 Pyrolysis. Pyrolysis is a method of thermally decomposing polymers into small molecules. By applying high temperature and pressure to a polymer in the presence of a catalyst and the absence of oxygen, the polymer structure can be broken down into lower molecular weight components. The pyrolysis of PET often entails the random scission of the polymer into chemicals of varying sizes. However, the high temperature will cause a rearrangement via a hydrogen bond transfer and finally a decarboxylation, resulting in the production of CO2, which can cause an increase of emissions as well as interference with the process. As such, PET is not a typical polymer target for pyrolysis.10
2.2.2.2 Depolymerization. Solvolysis, also known as chemical depolymerization, is a technique that involves the use of chemicals in order to break the polymer down into its constituent monomers. In comparison to pyrolysis, PET deconstruction can take place at temperatures lower than pyrolysis, since it involves the breaking of hetero-atom bonds in the polymer backbone. There are various approaches that can be taken in order to solvolyze PET (Fig. 3), and the end products of this deconstruction vary greatly depending on which approach is used.
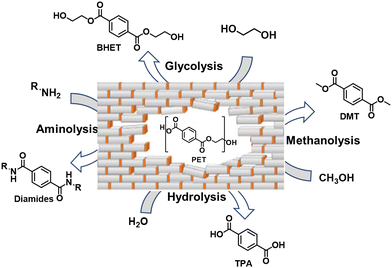 | ||
| Fig. 3 PET can be deconstructed using various techniques to obtain different monomers with various functional groups as end products. | ||
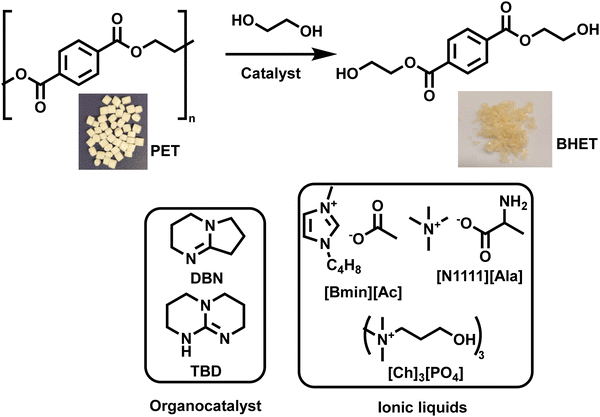
Glycolysis is the oldest technique used to depolymerize PET. Glycolysis of PET by catalytic transesterification occurs with an overabundance of a glycol at temperatures between 110 and 270 °C.11 The glycolysis of PET with ethylene glycol (EG) produces bis(2-hydroxyethyl) terephthalate (BHET) (Fig. 3). BHET is a favorable product for PET synthesis as it is an intermediate in the reaction of terephthalic acid (TA) with EG. There are several companies globally that currently have glycolysis plants to produce BHET or methanolysis plants to produce dimethyl terephthalate (DMT) (Table 1).
| Company name | Country | Established | Capacity (kt a−1) |
|---|---|---|---|
| Eastman Chemical Co | USA | 2022 | 100 |
| CuRE Technology | Netherlands | 2020 | 25 |
| IFP Energies nouvelles | France | 2020 | 22 |
| Ioniqa Technologies | Netherlands | 2019 | 10 |
PET can also be industrially depolymerized by the process of hydrolysis, which can be done in an acidic, alkaline, or even neutral aqueous environment, where water is used to cleave the ester bond in the backbone of the polymer. The process generally takes place at high temperatures and pressures and yields a final product of TA and EG. Some companies that depolymerize PET via hydrolysis are listed in Table 2.
| Company name | Country | Established | Capacity (kt a−1) |
|---|---|---|---|
| Carbios | France | 2011 | 50–100 |
| Gr3n | Switzerland | 2021 | 30 |
2.3 Polyolefins
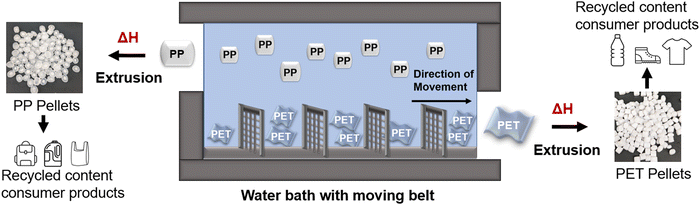
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 while the density of HDPE is 0.94–0.96 g cm−3. LDPE, on the other hand, is slightly harder to separate as the density falls between 0.91–0.93 g cm−3. PET has a density of 1.38 g cm−3 which allows for the separation of polyolefin and PET flakes by submerging the plastics into water and using a grated moving belt to remove the PET flakes that sink in the water (Fig. 4).
12 while the density of HDPE is 0.94–0.96 g cm−3. LDPE, on the other hand, is slightly harder to separate as the density falls between 0.91–0.93 g cm−3. PET has a density of 1.38 g cm−3 which allows for the separation of polyolefin and PET flakes by submerging the plastics into water and using a grated moving belt to remove the PET flakes that sink in the water (Fig. 4).
Following the sortation of the different types of plastics, the different types of plastics are washed and dried before heating and extruding as recycled pellets. Recycled pellets can then be used by downstream manufacturers to make consumer goods to be resold. While this is generally the case for plastic recycling, certain types of plastic waste have to be processed differently. Plastic bags, generally made from LDPE, can sometimes cause issues due to the nature of bags. As plastic bags get shredded by rotating machinery, it is possible for a bag to wrap around the rotating parts, resulting in the processing lines being turned off to deal with the caught bags.
One of the largest mechanical recyclers of HDPE and PP in the U.S. is KW Plastics which has a processing volume of over 450 kt of plastics per year.13 Additionally, some of the recycled plastics that KW Plastics produces are FDA approved food grade PP. Natura PCR is one of the largest processors of plastic films and shrink wraps in the world and has a goal to reach an annual processing capacity of 180 kt of plastic film within the next five years.14 WM Inc. reported a processing volume of 13.6 Mt for combined PP, PE, and PET waste.15
Companies involved in pyrolysis are proliferating at a rapid rate, particularly those involved in the production of fuel or syngas. Some innovations that have recently been developed for pyrolysis include more efficient heating of the waste plastic feedstock using supercritical steam by Mura Technology. Since steam can penetrate the bulk material faster than heat transfer occurs through the material, the processing time can be shortened, saving both time and money. The following selection of companies highlights a few of the most recent developments, with a focus on the recycling of plastics into building blocks, monomers, or naphtha and is listed in Table 3.
| Company | Country | Feedstock | Major products |
|---|---|---|---|
| Agilyx | USA | Mixed plastics | Synthetic crude oil |
| Mura Technology | UK | Flexible, multi-layered materials | Crude oil |
| BASF (ChemCycling) | Germany | Mixed plastic | Synthetic crude oil |
| LyondellBasell | Netherlands | PP, PE | Oxyfuels |
| Brightmark LLC | USA | Mixed plastics | Synthetic crude oil |
| Nexus Circular LLC | USA | HDPE, LDPE, PP, PS | Synthetic crude oil |
| Plastic Energy Ltd | UK | Mixed plastics | Synthetic crude oil |
| New Hope Energy | USA | Mixed plastics | Synthetic crude oil |
| OMV | Austria | Mixed plastics | Synthetic crude oil |
| Indaver NV | Belgium | Polyolefins | Waxes |
One challenge that the pyrolysis industry faces is the concern that the process seems very much like burning plastic for energy or fuel. Since a plastic waste feedstock is heated to high temperature and an oil is produced, there is a public misconception that plastic is being burned for fuel in the process. In reality, the products are useful molecules used to make other industrially relevant chemicals. As such, there has been a lot of pushback by environmentalists as well as policymakers, where some states do not recognize pyrolysis as a recycling process and is classified as a manufacturing process instead. Additionally, pyrolysis also tends to generate various byproducts such as waxes, crude oil, syngas, or even fuel, as well as hydrocarbons that get cleaved too short to be useful.
Pyrolysis is a technology that is still being developed; there is a lot of research in both academia and industry to develop catalysts that will enable more controlled length of polymer chain cleavage, and catalysts that will allow pyrolysis to be done at lower temperatures or even shorter reaction times. Overall, pyrolysis has potential and high research activities can be seen in many petrochemical companies such as ExxonMobil and Shell investing in the development of their own pyrolysis plants.
2.4 Mixed plastics
In addition to multilayer packaging, blended materials are also a challenge. Consumer waste such as carpets and textile are often blended or woven together. They are generally not collected by recyclers due to the inability to process the materials as a whole. There are some carpet collectors that will collect nylon carpets due to the availability of feasible processes to separate nylon fibers, which can be spun into recycled nylon fibers. However, the same does not apply to PET carpets, and any PET carpets that have been recycled need to be further processed to separate the PET face fibers from the PP backing. Textiles are very rarely recycled, and instead get reused or downcycled into other products. As such, there is virtually no established process for consumer wastes such as textiles and PET carpets in the current recycling industry.
3. Academic efforts
Chemical upcycling of polymers has the promise of altering the paradigm for discarded plastic from waste to a valued resource. Significant basic research is needed to advance toward a circularity of plastics, in which the chemical ingredients of plastics are reformed into polymers or recycled to give them another life. The basic research conducted in universities and national laboratories has the potential to quickly transform the process of recycling plastic waste into useful fuels, chemicals, and materials.3.1 Efforts to improve mechanical recycling
One of the main challenges for traditional mechanical recycling is reduced properties upon recycling, i.e. downcycling. For example, typical causes for degradation of mechanical strength upon mechanical recycling are related to the decrease in molecular weight (MW) due to repeated grinding and heating of the polymer. Additionally, some polymers, such as PE and PP, iPP in particular, have a very similar chemical composition and density, making it difficult to separate the two types of plastic into a single stream for mechanical sorting. The two polymers have very different physical properties including Tg, melting point and phase behaviors. Due to their property differences, it is hard to make a good blend of the two polymers as there will be a tendency to phase separate, causing a drastic decrease in mechanical strength when mixed. Therefore, items made from direct mechanical recycling of mixed plastics typically exhibit undesirable end-use qualities. In order to mitigate the challenges of blending or separating these types of polymers in a mixed feedstock, a number of studies on compatibilizers have been conducted.For instance, compatibilization of PE/iPP blends by PE-PP multiblock copolymers greatly improves interface adhesion as well as prevents droplet coalescence during the melt-extrusion process, thereby changing brittle materials into mechanically robust blends. As depicted in Fig. 5, the addition of 1 to 5% PE-b-PP-b-PE-b-PP tetrablock copolymer drastically reduces the size of the dispersed PP phase in an HDPE matrix. The strengthening of the interface, together with droplet size reduction, has transformed the HDPE/PP blend from brittle to tough and ductile. This study indicates that these block copolymer compatibilizers could enhance the recycling of HDPE and PP by reducing the need for sorting.19
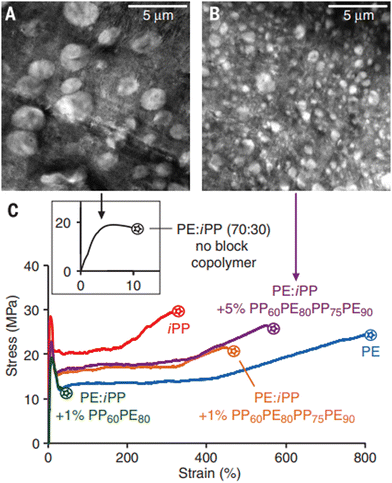 | ||
| Fig. 5 Electron microscopy images of a 70/30 blend of HDPE and PP (A) without any block copolymer and (B) with 5 wt% of tetrablock copolymer. (C) The blend without any block copolymer is brittle and there is only modest improvement with the inclusion of 1 wt% of a diblock copolymer. The use of the tetrablock copolymer results in a blend with mechanical properties comparable to the pure (HDPE and PP) components.19 Reproduced with permission from ref. 19. Copyright 2017 Science. | ||
Another example to improve recycling rates of immiscible polymer blends is using PET-b-PE multiblock copolymers (PETPE MBCPs) (Fig. 6), which have been shown to effectively improve the adhesion between the otherwise weak interfaces in melt reprocessed PET/PE blends. PET/PE (80/20 wt%) blends with less than 0.5 wt% of PETPE MBCP, which were melt mixed to simulate the recycling of mixed plastic waste, were found to be superior to those of pure PET. By understanding the mechanism in which the compatibilizer functions, highly immiscible PET/PE blends can be blended due to PETPE MBCPs acting as an interface adhesive, enabling the viability of recycling PET/PE mixed waste streams.20,21
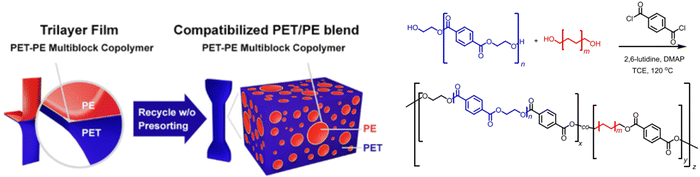 | ||
| Fig. 6 Compatibilization of PET-PE multiblock copolymer and synthetic scheme for the preparation of PET-PE MBCP. Reproduced with permission from ref. 20. Copyright 2020 ACS Appl. Mater. Interfaces. | ||
3.2 Catalyst design to deconstruct polyolefin and condensation polymers
Catalytic deconstruction provides selective functionalization or degradation of one kind of polymer possibly within a mixture or composite to recover particular chemical components. For instance, tandem catalysis demonstrates the high yield conversion of waste polyolefins to liquid alkylaromatics by Pt/g-Al2O3 (up to 80 wt%). In a verification experiment, a low MW PE (0.118 g, Mw = 3.5 kg mol−1, Đ = 1.9) was mixed with Pt/g-Al2O3 in a 10 ml mini-autoclave without solvent or additional H2. The liquid/wax products (80% by mass) were recovered after 24 h at 280 °C by dissolving them in hot CHCl3 (Fig. 7).22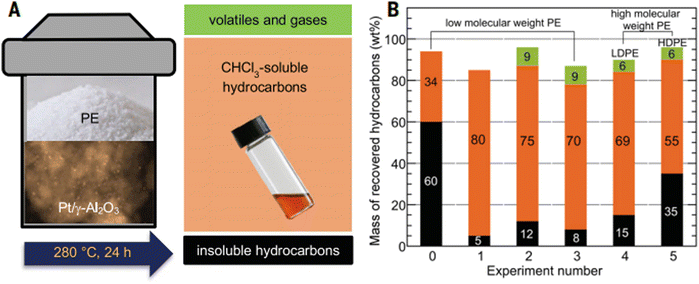 | ||
| Fig. 7 Solvent-free conversion of various types of PE. (A) Schematic of reactor and product fractions, with photographs of the powdered polymer and liquid products, as well as a transmission electron micrograph of the catalyst. (B) Hydrocarbon distributions after 24 hours at 280 °C. Reactions of a low molecular-weight PE (Mw = 3.52 kg mol−1, Đ = 1.90) in an unstirred mini-autoclave reactor: (0) catalyzed by g-Al2O3 (no gas recovery); (1) catalyzed by Pt/g-Al2O3 (no gas recovery); (2) catalyzed by Pt/g-Al2O3 (with gas recovery). Reactions catalyzed by Pt/g-Al2O3 in a stirred autoclave reactor with gas recovery: (3) low-molecular-weight PE; (4) LDPE bag (Mw = 94.5 kg mol−1, Đ = 7.37); (5) HDPE bottle cap (Mw = 53.5 kg mol−1, Đ = 3.61). Orange represents CHCl3 soluble hydrocarbons, black represents CHCl3 insoluble residues, and green represents recovered gases including H2 and light hydrocarbons (C1–C8). Reproduced with permission from ref. 22. Copyright 2020 Science. | ||
Many nitrogen-containing nucleophiles, including amines and anilines, can also be used for PET deconstruction catalysts in addition to metal catalysts. Use of a readily available guanidine catalyst 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD), enabled organocatalytic glycolysis of PET. Waste PET beverage bottles were deconstructed in 3.5 h at 190 °C with an excess of ethylene glycol (EG), yielding BHET as the predominant product (Fig. 8). Computational analyses showed that TBD and EG facilitated the depolymerization of PET by forming and activating hydrogen bonds with the polymer.11 A protic ionic salt, combining TBD and methane sulfonic acid (MSA) at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio was further developed for PET depolymerization, while exhibiting high catalyst stability at temperatures up to 400 °C without degradation. In the presence of EG, PET can be successfully depolymerized by TBD:MSA in less than 2h at 180 °C under an inert atmosphere. Reusing the TBD
1 molar ratio was further developed for PET depolymerization, while exhibiting high catalyst stability at temperatures up to 400 °C without degradation. In the presence of EG, PET can be successfully depolymerized by TBD:MSA in less than 2h at 180 °C under an inert atmosphere. Reusing the TBD![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MSA catalyst more than five times does not significantly degrade its ability to mediate the self-condensation of BHET and produce fresh PET with characteristics similar to those of virgin PET.11,23,24 Additionally, the same technology can be used to deconstruct polycarbonates to obtain monomers such as bisphenol A.
MSA catalyst more than five times does not significantly degrade its ability to mediate the self-condensation of BHET and produce fresh PET with characteristics similar to those of virgin PET.11,23,24 Additionally, the same technology can be used to deconstruct polycarbonates to obtain monomers such as bisphenol A.
Metal based catalysts have also been reported to show significant advancement in the deconstruction of various plastics. Using a combined tandem catalytic and biological process, mixed plastics are transformed into single products (Fig. 9). The first catalytic step employs metal-promoted autoxidation to depolymerize mixed or single polymers of HDPE, PS, and PET into oxygenated intermediates (dicarboxylic acid, benzoic acid, and terephthalic acid) that are advantageous substrates for further bioconversion. In the biological stage, a robust designed bacterial strain such as Pseduomonas putida converts the mixed oxygenates into β-ketoadipate monomer, which can be polymerized to polyhydroxyalkanoates, a biologically synthesized polyester that has industrial applications.25
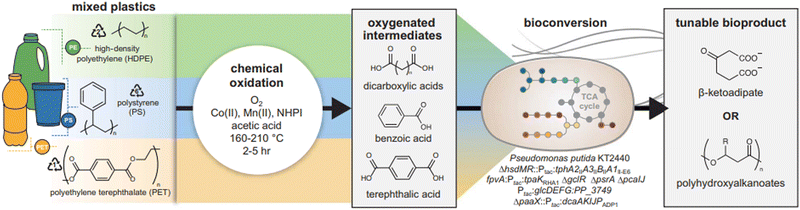 | ||
| Fig. 9 Upcycling of mixed plastic waste through tandem chemical oxidation and bioconversion. Metal-promoted autoxidation simultaneously deconstructs multiple polymers, generating a mixture of oxygenated intermediates that are advantageous substrates for bioconversion. An engineered P. putida strain funnels the heterogeneous mixture of oxygenates into a single target product. Reproduced with permission from ref. 25. Copyright 2022 Science. | ||
3.3 Materials designed for recycling
New designs are necessary for new generation of recyclable or upcycled polymers that have properties equal or better than those of today's materials. To establish close-loop circularity of plastics via mechanical recycling and chemical upcycling, new plastics must be purposefully designed to have a specific set of physical and mechanical properties as well as a mechanism to efficiently breakdown by atomic- and energy-efficient processes at the end of life. In this section, we will highlight some promising areas of materials development, beyond traditional polymer, that offer potential solutions in the future plastics economy.One method to develop materials that are better suited for circularity is to design monomers that can be polymerized into a polymer and can be depolymerized to form the same monomer. For example, a polymer system based on γ-butyrolactone (GBL) has been reported to be polymerizable at room temperature to yield high molecular weight poly(γ-butyrolactone). The structure of the synthesized polymer exists as either a linear or a cyclic structure and can be readily depolymerized via thermolysis at high temperatures or chemolysis in the presence of a catalyst (Fig. 10).26 Similarly, a bridged bicyclic thiolactone (BTL) monomer can be polymerized via ring opening polymerization to obtain a ductile tough plastic, where poly(BTL) can be deoligomerized and ring closed to reform the original BTL monomer.27 Since these polymers can be depolymerized to reform the exact same monomer as used in the synthesis, recovered monomers can be easily integrated back into the manufacturing process of the polymer.
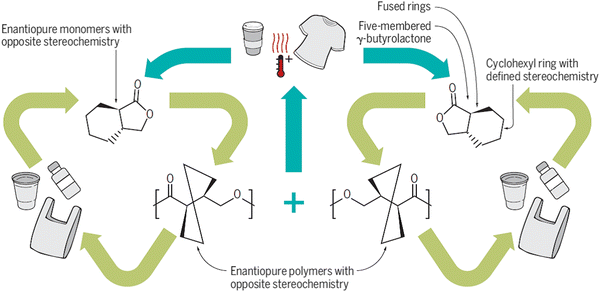 | ||
| Fig. 10 Polymers synthesized from γ-butyrolactone exist as two enantiomers and when blended, this allows for materials with improved thermal stability which can be recycled repeatedly without losing mechanical properties. Reproduced with permission from ref. 28. Copyright 2018 Science. | ||
A closed-loop recyclability of monomer to polymer, then to monomer, has also been reported for cyclic acetals.29 The use of reversible-deactivation cationic ring opening polymerization enables control of the polymerization of cyclic acetals (Fig. 11A) to produce polyacetals with controlled molecular weights of 38–182 kg mol−1. The synthesized poly(1,3-dioxolane) exhibits desirable chemical and thermal stability, suitable for consumer products (Fig. 11B). Moreover, they can be depolymerized at relatively low temperatures to reform cyclic acetal monomers with the presence of a Lewis acid catalyst. Monomers can be separated after the depolymerization by simple distillation even in the presence of various other polymers and be used to repolymerize polymers with properties identical to virgin polymers.
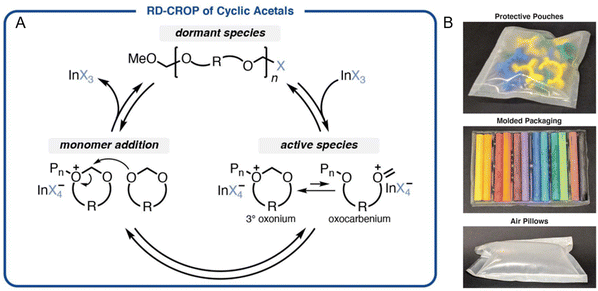 | ||
| Fig. 11 (A) Mechanism for reversible-deactivation cationic ring opening polymerization of cyclic acetal monomers to produce polyacetals. (B) Polyacetal can be used to make consumer products such as protective pouches, packaging, and air pillows. Reproduced in part with permission from ref. 29. Copyright 2021 Science. | ||
Polyethylene-like materials containing readily depolymerizable functional groups have also been synthesized from bio-based building blocks (Fig. 12A). The polymers made from this material are able to have high molecular weights (Mw = 300 kg mol−1, Mn 90 kg mol−1) with mechanical properties similar to HDPE, while the polymer can be broken into repolymerizable fragments by reacting at 120 °C in ethanol.30 The reported polymers can also be used as a filament for 3D printing, and the printed products include snap on smartphone cases and even a 3D printed cup which can hold boiling water while still maintaining its shape and dimensions (Fig. 12B).
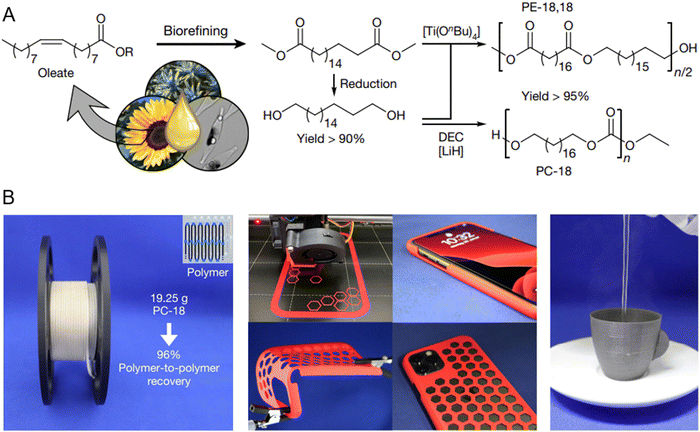 | ||
| Fig. 12 (A) Biobased materials can be used to make building blocks to make high molecular weight polyethylene-like materials. (B) Polymers can be made into filaments used to 3D print products such as smartphone cases and cups that can hold boiling water while maintaining shape and dimensions. Reproduced in part with permission from ref. 30. Copyright 2021 Nature. | ||
Thermoset materials possess crosslinked networks which provide excellent mechanical properties, but they are conventionally not amenable to mechanical recycling or solution processing due to the presence of permanent covalent networks. Covalent adaptive networks (CANs) allow acrosslinked network to be processable and recyclable, while exhibiting thermoset-like properties, due to the presence of dynamic dissociative or associative exchange groups. The dynamic bonds act as movable or reversible anchor points in the network, enabling the bulk material to be reshaped or replaced, sometimes without stimulus (self-healing).31–34 Epoxy networks with alcohol and ester functionalized components were first reported to exhibit dynamic exchange via transesterification processes, and CANs were named as vitrimers.35 The epoxy-based material exhibits robust mechanical properties at room temperature and when placed in solvents at high temperatures, only swelled and did not dissolve. The material was proven to be reprocessed by injection molding. To enhance the recyclability of epoxy-based vitrimers, polyurea/epoxy vitrimers with exchangeable disulfide bonds were recently designed. The tailored design allowed lower reprocessing temperatures compared to conventional disulfide-based epoxy-based vitrimers. The material can be reprocessed via a heat press in as little time as 1 minute and multi-cycle recycling maintains its tensile strength of ∼60 MPa even after being reprocessed 6 times, a stark contrast to the conventional vitrimer, losing 63% loss of tensile strength. The vitrimer can be used in conjunction with carbon fibers, while providing the reprocessability and repairability of the carbon fiber composites.36
CANs of diketoenamine chemistries have also been used in closed-loop recycling of polymer network systems (Fig. 13A). Diketoenamine bonds from triketones and amines, both aromatic and aliphatic, react spontaneously and can form network structures. Under strong acidic aqueous conditions, the diketoenamine bond hydrolyzes and reforms the triketone and an ammonium salt.37 Due to the depolymerization conditions, it is possible to extract polydiketoenamine based polymers from a mixed plastic waste feedstock by mixing with a strong acid (Fig. 13B). The acidic solution containing the deconstructed polydiketoenamine, now triketone monomers, can be precipitated and pure monomer is recovered. The monomers can then be repolymerized and used to make more polydiketoenamine based products, exhibiting the same mechanical properties as before and enabling circularity due to the reformation of the diketoenamine bonds. In addition to amines and ketones, this type of dynamic bond can also be seen between imines and hydrogens to exhibit self-healing behavior.38,39
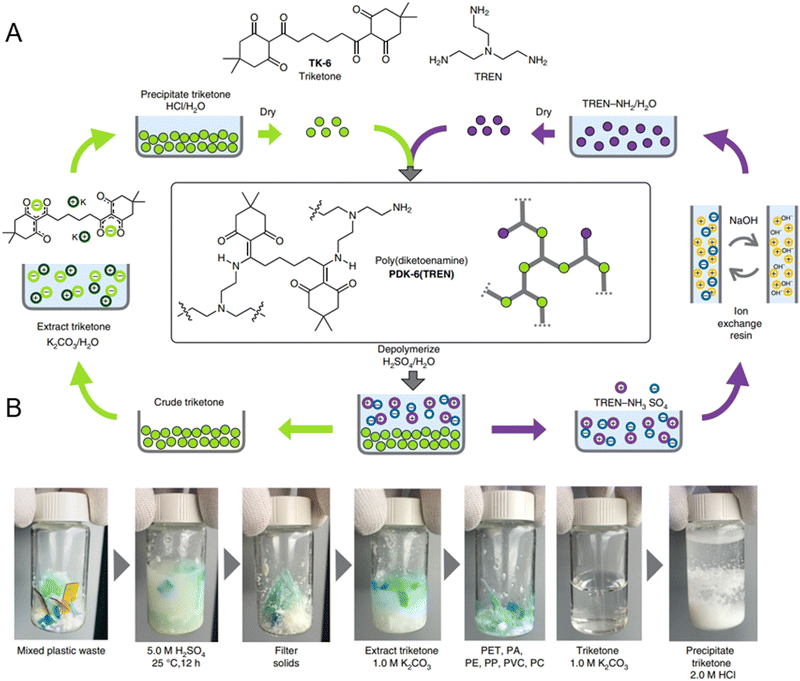 | ||
| Fig. 13 (A) Mechanism for polydiketoenamine synthesis and depolymerization. (B) Polydiketoenamine based polymers can be extracted from a mixed plastic waste source to obtain pure triketone. Reproduced in part with permission from ref. 37. Copyright 2019 Nature Chem. | ||
Recyclable and malleable polycyanurate based network polymers have also been reported. Triazine containing monomers, such 2,4,6-triethoxy-1,3,5-triazine (TETA) monomer synthesized from isocyanates, are polymerized to obtain polycyanurates (Fig. 14A), which exhibit strong mechanical properties with varying ductility based on the structure of monomer used.40 The polycyanurates can also be depolymerized to reform the triazine monomer by refluxing in ethanol, and the depolymerization can be accelerated with the addition of K2CO3. The recovered monomers can be used to repolymerize materials of similar properties as the original virgin polymer (Fig. 14B).
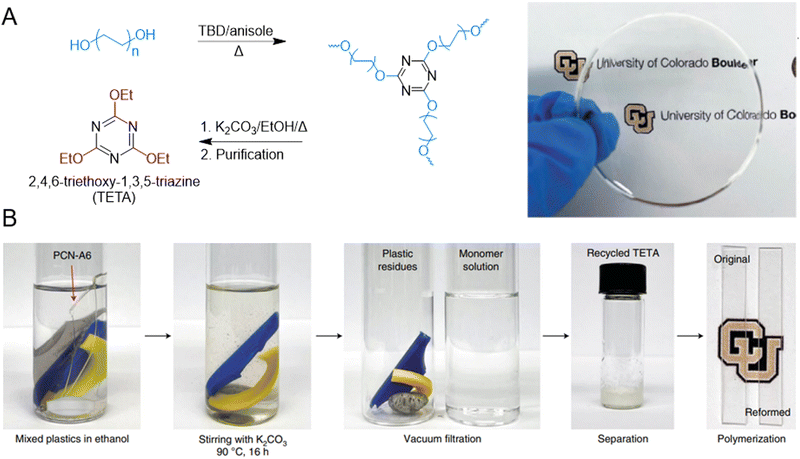 | ||
| Fig. 14 (A) Depolymerization and synthesis of polycyanurate network polymers. (B) Polycyanurates can be depolymerized in the presence of ethanol and K2CO3 added as a catalyst. Reproduced in part with permission from ref. 40. Copyright 2022 Nature Chem. | ||
Polyolefin-based commodity plastic has been upcycled by CANs, providing a processable and recyclable crosslinked network with enhanced properties. Commercial thermoplastic HDPE has been transformed to high-performance vitrimers by grafting dioxaborolane-functionalized maleimides onto HDPE, followed by cross-linking with a bis-dioxaborolane metathesis onto pendant dioxaborolane units in the polymer (Fig. 15A). The upcycled HDPE vitrimers exhibit improved melt strength, dimension stability and show resistance to cracking in the presence of solvents.41 Additionally, they exhibited enhanced polymer–polymer adhesion due to the presence of dynamic dioxaborolane (Fig. 15B).
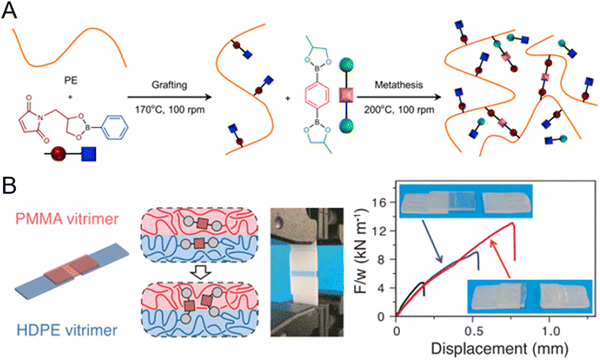 | ||
| Fig. 15 (A) Conversion of commercial HDPE to vitrimers via metathesis with dioxaborolane. (B) Adhesion between the two vitrimers is also enhanced due to the presence of dynamic dioxaborolane bonds. Reproduced in part with permission from ref. 41. Copyright 2017 Science. | ||
Similarly, dynamic covalent bonds of boronic ester can be used to upcycle triblock styrene–ethylene–butylene–styrene (SEBS) thermoplastic elastomers. The boronic ester groups on SEBS can reversibly bind with hydroxyls on the surface of different fillers and substrates, leading to exceptional adhesion, surpassing the adhesive strength of various commercial adhesives (Fig. 16A). The dynamic interaction also improved their tensile strength, toughness, and temperature service window while maintaining recyclability.42 Upcycling commodity plastics via CANs can also be applied to additive manufacturing with a closed loop. An engineering plastic, acrylonitrile butadiene styrene (ABS) and its waste are first upcycled to ABS-vitrimer. The ABS-vitrimer exhibits superior mechanical performance (e.g., 2× toughness, 1.8× tensile strength) and chemical resistance compared with untreated ABS. Importantly, ABS-vitrimer or their mixture with ABS can be readily reprinted multiple times by fused filament fabrication (FFF) (Fig. 16B). This easy adoption to FFF enables the manufacturing of intricate 3D structures unachievable through conventional molding or casting. Furthermore, the waste of ABS-vitrimer can be easily separated from mixed plastic waste by dissolution for multi-cycle reuse, and the dissolved ABS can be again upcycled to ABS-vitrimer. The multiple paths of chemical upcycling and separation, coupled with facile additive manufacturing offer an effective and realistic carbon-efficient closed-loop circular plastic manufacturing.43
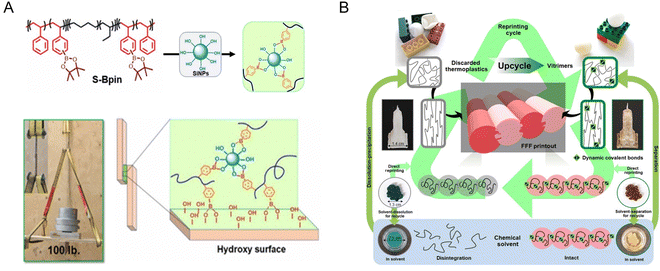 | ||
| Fig. 16 (A) Tough adhesive from upcycled commodity polymer SEBS. (B) Closed-loop additive manufacturing via upcycled ABS vitrimers. The photographs show structures printed from the untreated ABS (Neat-ABS) (left) and the ABS-vitrimer (right) of a building (top) and an oak leaf before (middle) and after (bottom) immersion in tetrahydrofuran (THF) for 48 hours. Reproduced with permission from ref. 42 and 43. Copyright 2021 Sci. Adv. and 2022 Sci. Adv. | ||
4. Summary and outlook
4.1 Commercial recycling is mainly mechanical recycling
The majority of commercial plastic recycling today is mechanical recycling. While the process has been well developed and can be fast and inexpensive for common plastics, such as plastic bottles, it is typically limited to feedstocks of a single type of plastic. Sorting is required in order to obtain a single type of desired plastic feedstock at high percentage, which can involve several detectors such as NIR and UV-Vis to differentiate between plastic type and color. There are some limitations to these detectors to distinguish polymers that might be chemically similar but have significantly different physical and mechanical properties. The difference in physical and mechanical properties can result in a macro-phase separated blend and a large shift or incompatibility in the process temperature and conditions, causing issues with the melting and extrusion of plastics in various industrial machinery.Another challenge for mechanical recycling is that recycled plastics generally exhibit lower mechanical strength due to the repeated heating and extrusion process as well as degradation during use. Therefore, in order to ensure achieving sufficient properties of recycled plastic resins, the recycled resins are typically blended with virgin polymers or with additives that can increase the strength of the material. Complex types of plastic waste are also a challenge for mechanical recyclers. Blended or mixed plastic waste that has several types of polymers are combined in a way that cannot be easily mechanically separated. Such plastic waste is essentially of no value to mechanical recyclers since the material is generally not processable. Waste sources such as multilayer packaging and textiles fall into this category.
4.2 Academia is mostly focusing on chemical recycling
While a lot of promising technology is developed in academia, potential commercialization paths are not frequently considered when designing new technology. Chemical recycling has significant potential to address feedstock concerns, such as mixed plastic waste that cannot be separated via mechanical sorting. However, the process generally costs significantly more compared to mechanical recycling and the monomers produced from chemical recycling have a higher associated cost than producing the conventional petroleum-based monomer. While some of the chemical recycling process indicates significantly lowering associated GHG emissions and energy consumption, few companies are willing to pay a higher price for the green premium offered.There is often a large uncertainty about what the scaling up process of academic research looks like and how feasible commercial streams of waste plastics are in the scaled-up operation. Unlike the simulated feedstock used in laboratory experiments, real waste has significantly higher levels and more types of contaminations. For example, while a PET deconstruction catalyst can deconstruct a PET water bottle with fewer sorting steps and can tolerate high levels of contamination, certain contaminants in real wastes may completely hinder the deconstruction reaction. Even if the catalyst was tested on a water bottle, even with the presence of labels and some amount of water to simulate water being left in the bottle, it does not account for the unexpected contaminations that a bottle may encounter during its lifetime (e.g. cigarette butts or chewing gum). Though these contaminations could be trivial, there could be unintended effects at a scaled up commercial scale.
Another strength of academic research is developing new classes of polymer materials. Polymers can be designed to have more sustainable functionality such as the incorporation of dynamic bonds, which can reform, extend the lifespan of materials, and reduce the amount of waste disposed. There are also polymers being developed that can be depolymerized under certain conditions to obtain the same monomer used to make the virgin polymer, enabling simpler recycling processes. To add even more appeal to this type of polymer, some polymers can even be made using previously deconstructed waste plastic to further improve lifecycle assessments. However, with the development of new materials, it is important to consider the potential impact of materials used on their adoptability and scalability toward industry adoption as well as the mitigation of unintended adverse environment impacts.
4.3 Bridging the gap
Recycling rate and effort are significantly different across the world—even within the U.S., it is done differently based on region. Since most of the U.S. recycling infrastructure was developed in the 1950s and 60s, some systems and technologies are outdated particularly for the collection and sorting of plastic waste. While there are new technologies that can be retrofitted into existing MRFs to improve sorting processes, waste containing mixed or blended plastics or new types of polymers still proves to be a challenge for mechanical recycling. At the same time, academia across the world has innovated and developed a variety of recycling and upcycling technologies that can work with these challenging plastic waste feedstocks. However, there is some disconnect between the technologies academic research offers and the needs of industries, and this gap needs to be closed.There are several ways that academic research can improve plastic recycling including designing materials that can be more easily recycled, developing new types of polymers that have better end-of-life processing, and developing very efficient methods to break plastics down to valuable chemicals or monomers. By designing materials such as packaging to have fewer types of polymers or that are readily delaminated, mechanical recycling could be more effective at creating valuable recycled materials from waste. Designing polymers that have dynamic bonds, which can be broken and reformed, can also improve the mechanical recycling as well as extend the longevity due to its healing capability, particularly if the materials can be reformed in simple ways such as applying heat.
Another approach is developing methods to deconstruct plastic waste back into monomers or valuable chemicals. Efficient chemical recycling processes for recovering monomers from plastic waste can reduce the amount of fossil fuel needed to make polymers that can be a favorable process if the monomer is used in the manufacturing of the polymer easily integrated in the polymer manufacturing supply chain.
There are many important aspects to consider when considering commercializing academic research. Not only is life cycle assessment important to consider and report in publications, but it should also be reported in a standardized way so that many types of research can be more comparable. Additionally, it is critical that the developed technology considers the potential environmental side effects of the technology developed, such as excessive use of resources in energy or water, or the use of extremely toxic chemicals. Transparency of data is also important when thinking about commercialization. While technologies might work in the lab environment, more often than not, the same conditions are nearly impossible to recreate in the real world. Feedstocks that are collected and sorted by MRFs and reclaimers will likely not contain as high a percentage of the desired type of plastic waste as hoped for, due to the associated cost or feasibility of sorting processes.
Chemical recycling processes are also still very expensive—the investment required to build a chemical recycling facility is significantly higher than mechanical recycling. For example, in 2021, Eastman invested $250 M to construct their Kingsport, Tennessee, USA methanolysis facility,44 which has an annual processing capacity of 100 kt of plastic waste. Eastman also invested up to $1 B to build a similar plant in Normandy, France, which would have a processing capacity of 160 kt y−1.45 Similarly, Encina announced in 2022 a $1.1 B investment plan for building a state-of-the-art manufacturing facility in Point Town, Pennsylvania, USA with a processing volume of 449 kt of plastic waste.46 Additionally, due to chemical recycling processes being more complex and newer than mechanical recycling, the estimated minimum selling price for products of processes such as glycolysis and methanolysis is $0.96–1.04 kg−1, almost double the cost of mechanically recycled plastic, $0.54 kg−1.47 Thus, academic efforts to improve efficiency or processing speed/volume of chemical recycling will reduce the cost, and make chemical recycling much more impactful in the economy.
Advancing recycling efforts is not a task that can be done by academic research or the recycling industry independently. By communicating and bridging the gap of what academic research offers and what the recycling industry needs, we can work together to mitigate the amount of plastic waste being discarded into landfills and the environment. Understanding how the technology works in the lab and how to integrate it into current industrial processes is critical, and the considerations of supply chains and cost minimization will navigate the deployment. Furthermore, establishment of new technology with closed-loop plastic circularity will be able to lower carbon and energy footprint toward industrial decarbonization. Bridging the gap in plastic recycling will accelerate innovation and help us to solve the global challenge of plastic waste management.
Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
This research was supported by the US Department of Energy, Office of Science, Materials Sciences and Engineering Division. The concept was initiated by the Laboratory Directed Research and Development Program of Oak Ridge National Laboratory, managed by UT Battelle, LLC, for the U.S. Department of Energy (DOE), under contract DE-AC05-00OR22725. The authors also acknowledge DOE Energy I-Corps program for giving us an opportunity to conduct interviews with experts in industry.References
- Visual Feature Beat Plastic Pollution, https://unep.org/interactive/beat-plastic-pollution/, (accessed 14 December 2022).
- Plastic market size worldwide 2030, https://www.statista.com/statistics/1060583/global-market-value-of-plastic/, (accessed 14 December 2022).
- https://www.oecd.org/environment/plastic-pollution-is-growing-relentlessly-as-waste-management-and-recycling-fall-short.htm, (accessed 14 December 2022).
- S. R. Nicholson, N. A. Rorrer, A. C. Carpenter and G. T. Beckham, Manufacturing energy and greenhouse gas emissions associated with plastics consumption, Joule, 2021, 5, 673–686 CrossRef CAS.
- Primary plastic production by polymer type, https://ourworldindata.org/grapher/plastic-production-polymer, (accessed 15 December 2022).
- A. Ruiz, 25 Plastic Waste Statistics That Will Shock You, https://theroundup.org/plastic-waste-statistics/.
- E. Boon, A global momentum to rethink the plastic system, Brussel, Belgium, 2018 Search PubMed.
- Alpek, Fourth Quarter 2018 (4Q18), 2019.
- Recycling Indorama Ventures, https://sustainability.indoramaventures.com/en/environmental/recycling/at-a-glance, (accessed 15 December 2022).
- T. Yoshioka, G. Grause, C. Eger, W. Kaminsky and A. Okuwaki, Pyrolysis of poly(ethylene terephthalate) in a fluidised bed plant, Polym. Degrad. Stab., 2004, 86, 499–504 CrossRef CAS.
- K. Fukushima, O. Coulembier, J. M. Lecuyer, H. A. Almegren, A. M. Alabdulrahman, F. D. Alsewailem, M. A. Mcneil, P. Dubois, R. M. Waymouth, H. W. Horn, J. E. Rice and J. L. Hedrick, Organocatalytic depolymerization of poly(ethylene terephthalate), J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 1273–1281 CrossRef CAS.
- Sorting Recyclable Plastics by Density, https://chem.libretexts.org/Ancillary_Materials/Exemplars_and_Case_Studies/Exemplars/Environmental_and_Green_chemistry/Sorting_Recyclable_Plastics_by_Density, (accessed 15 December 2022).
- Nationwide Plastics Recycler and Resin Supplier, https://www.kwplastics.com/about-us/, (accessed 15 December 2022).
- WM to acquire majority stake in plastic film recycling business Natura PCR from Avangard, https://www.wastedive.com/news/wm-avangard-plastic-natura-pcr-film-dow/631710/, (accessed 15 December 2022).
- C. Staub, Waste Management increased plastics collection in 2020, https://resource-recycling.com/plastics/2021/10/20/waste-management-increased-plastics-collection-in-2020/, (accessed 15 December 2022).
- Our Process PureCycle Polypropylene Plastic Recycling, https://www.purecycle.com/our-process, (accessed 15 December 2022).
- PureCycle Technologies Provides Third Quarter 2022 Update, https://ir.purecycle.com/news-events/press-releases/detail/62/purecycle-technologies-provides-third-quarter-2022-update, (accessed 15 December 2022).
- Potato Chips Packaging by Sonoco, https://sonocoasia.com/products/rigid-paper-containers/insights/potato-chips-packaging, (accessed 15 December 2022).
- J. M. Eagan, J. Xu, R. Di Girolamo, C. M. Thurber, C. W. Macosko, A. M. LaPointe, F. S. Bates and G. W. Coates, Combining polyethylene and polypropylene: Enhanced performance with PE/i PP multiblock polymers, Science, 2017, 355, 814–816 CrossRef CAS PubMed.
- K. Nomura, X. Peng, H. Kim, K. Jin, H. J. Kim, A. F. Bratton, C. R. Bond, A. E. Broman, K. M. Miller and C. J. Ellison, Multiblock Copolymers for Recycling Polyethylene–Poly(ethylene terephthalate) Mixed Waste, ACS Appl. Mater. Interfaces, 2020, 12, 9726–9735 CrossRef CAS PubMed.
- X. Tang, C. Liu, J. Keum, J. Chen, B. E. Dial, Y. Wang, W.-Y. Tsai, W. Bras, T. Saito, C. C. Bowland and X. C. Chen, Upcycling of semicrystalline polymers by compatibilization: mechanism and location of compatibilizers, RSC Adv., 2022, 12, 10886–10894 RSC.
- F. Zhang, M. Zeng, R. D. Yappert, J. Sun, Y.-H. Lee, A. M. LaPointe, B. Peters, M. M. Abu-Omar and S. L. Scott, Polyethylene upcycling to long-chain alkylaromatics by tandem hydrogenolysis/aromatization, Science, 2020, 370, 437–441 CrossRef CAS PubMed.
- C. Jehanno, M. M. Pérez-Madrigal, J. Demarteau, H. Sardon and A. P. Dove, Organocatalysis for depolymerisation, Polym. Chem., 2019, 10, 172–186 RSC.
- C. Jehanno, J. Demarteau, D. Mantione, M. C. Arno, F. Ruipérez, J. L. Hedrick, A. P. Dove and H. Sardon, Selective Chemical Upcycling of Mixed Plastics Guided by a Thermally Stable Organocatalyst, Angew. Chem., Int. Ed., 2021, 60, 6710–6717 CrossRef CAS PubMed.
- K. P. Sullivan, A. Z. Werner, K. J. Ramirez, L. D. Ellis, J. R. Bussard, B. A. Black, D. G. Brandner, F. Bratti, B. L. Buss, X. Dong, S. J. Haugen, M. A. Ingraham, M. O. Konev, W. E. Michener, J. Miscall, I. Pardo, S. P. Woodworth, A. M. Guss, Y. Román-Leshkov, S. S. Stahl and G. T. Beckham, Mixed plastics waste valorization through tandem chemical oxidation and biological funneling, Science, 2022, 378, 207–211 CrossRef CAS PubMed.
- J.-B. Zhu, E. M. Watson, J. Tang and E. Y.-X. Chen, A synthetic polymer system with repeatable chemical recyclability, Science, 2018, 360, 398–403 CrossRef CAS PubMed.
- C. Shi, M. L. McGraw, Z.-C. Li, L. Cavallo, L. Falivene and E. Y.-X. Chen, High-performance pan-tactic polythioesters with intrinsic crystallinity and chemical recyclability, Sci. Adv., 2020, 6, eabc0495 CrossRef CAS PubMed.
- H. Sardon and A. P. Dove, Plastics recycling with a difference, Science, 2018, 360, 380–381 CrossRef CAS PubMed.
- B. A. Abel, R. L. Snyder and G. W. Coates, Chemically recyclable thermoplastics from reversible-deactivation polymerization of cyclic acetals, Science, 2021, 373, 783–789 CrossRef CAS PubMed.
- M. Häußler, M. Eck, D. Rothauer and S. Mecking, Closed-loop recycling of polyethylene-like materials, Nature, 2021, 590, 423–427 CrossRef PubMed.
- S. J. Rowan, S. J. Cantrill, G. R. L. Cousins, J. K. M. Sanders and J. F. Stoddart, Dynamic covalent chemistry, Angew. Chem., Int. Ed., 2002, 41, 898–952 CrossRef PubMed.
- P. Chakma and D. Konkolewicz, Dynamic Covalent Bonds in Polymeric Materials, Angew. Chem., Int. Ed., 2019, 58, 9682–9695 CrossRef CAS PubMed.
- G. M. Scheutz, J. J. Lessard, M. B. Sims and B. S. Sumerlin, Adaptable Crosslinks in Polymeric Materials: Resolving the Intersection of Thermoplastics and Thermosets, J. Am. Chem. Soc., 2019, 141, 16181–16196 CrossRef CAS PubMed.
- N. Roy, B. Bruchmann and J.-M. Lehn, DYNAMERS: dynamic polymers as self-healing materials, Chem. Soc. Rev., 2015, 44, 3786–3807 RSC.
- D. Montarnal, M. Capelot, F. Tournilhac and L. Leibler, Silica-Like Malleable Materials from Permanent Organic Networks, Science, 2011, 334, 965–968 CrossRef CAS PubMed.
- Z. Zhou, S. Kim, C. C. Bowland, B. Li, N. Ghezawi, E. Lara-Curzio, A. Hassen, A. K. Naskar, M. A. Rahman and T. Saito, Unraveling a path for multi-cycle recycling of tailored fiber-reinforced vitrimer composites, Cell Rep. Phys. Sci., 2022, 3, 101036 CrossRef CAS.
- P. R. Christensen, A. M. Scheuermann, K. E. Loeffler and B. A. Helms, Closed-loop recycling of plastics enabled by dynamic covalent diketoenamine bonds, Nat. Chem., 2019, 11, 442–448 CrossRef CAS PubMed.
- F. Song, Z. Li, P. Jia, M. Zhang, C. Bo, G. Feng, L. Hu and Y. Zhou, Tunable “soft and stiff”, self-healing, recyclable, thermadapt shape memory biomass polymers based on multiple hydrogen bonds and dynamic imine bonds, J. Mater. Chem. A, 2019, 7, 13400–13410 RSC.
- Z. Zhang, D. Lei, C. Zhang, Z. Wang, Y. Jin, W. Zhang, X. Liu and J. Sun, Strong and Tough Supramolecular Covalent Adaptable Networks with Room-Temperature Closed-Loop Recyclability, Adv. Mater., 2022, 2208619 Search PubMed.
- Z. Lei, H. Chen, C. Luo, Y. Rong, Y. Hu, Y. Jin, R. Long, K. Yu and W. Zhang, Recyclable and malleable thermosets enabled by activating dormant dynamic linkages, Nat. Chem., 2022, 14, 1399–1404 CrossRef CAS PubMed.
- M. Röttger, T. Domenech, R. van der Weegen, A. Breuillac, R. Nicolaÿ and L. Leibler, High-performance vitrimers from commodity thermoplastics through dioxaborolane metathesis, Science, 2017, 356, 62–65 CrossRef PubMed.
- M. A. Rahman, C. Bowland, S. Ge, S. R. Acharya, S. Kim, V. R. Cooper, X. C. Chen, S. Irle, A. P. Sokolov, A. Savara and T. Saito, Design of tough adhesive from commodity thermoplastics through dynamic crosslinking, Sci. Adv., 2021, 7, eabk2451 CrossRef CAS PubMed.
- S. Kim, M. A. Rahman, M. Arifuzzaman, D. B. Gilmer, B. Li, J. K. Wilt, E. Lara-Curzio and T. Saito, Closed-loop additive manufacturing of upcycled commodity plastic through dynamic cross-linking, Sci. Adv., 2022, 8, eabn6006 CrossRef CAS PubMed.
- Plastic-to-Plastic Molecular Recycling Facility in Kingsport, https://www.eastman.com/en/media-center/news-stories/2021/governor-lee-announce-plastic-recycling-facility, (accessed 25 January 2023).
- Eastman Negotiates to Build Molecular Recycling Plant in France, https://www.eastman.com/en/media-center/news-stories/2022/eastman-exclusive-negotiation-site-normandy, (accessed 25 January 2023).
- Encina Announces Major Investment in New Advanced Recycling Manufacturing Facility, https://www.ptonline.com/news/encina-announces-major-investment-in-new-advanced-recycling-manufacturing-facility, (accessed 25 January 2023).
- T. Uekert, A. Singh, J. S. DesVeaux, T. Ghosh, A. Bhatt, G. Yadav, S. Afzal, J. Walzberg, K. M. Knauer, S. R. Nicholson, G. T. Beckham and A. C. Carpenter, Technical, Economic, and Environmental Comparison of Closed-Loop Recycling Technologies for Common Plastics, ACS Sustainable Chem. Eng., 2023, 11, 965–978 CrossRef CAS.
Footnotes |
| † This manuscript has been authored by UT-Battelle, LLC, under contract DE-AC05-00OR22725 with the US Department of Energy (DOE). The US government retains and the publisher, by accepting the article for publication, acknowledges that the US government retains a nonexclusive, paid-up, irrevocable, worldwide license to publish or reproduce the published form of this manuscript, or allow others to do so, for US government purposes. DOE will provide public access to these results of federally sponsored research in accordance with the DOE Public Access Plan (https://energy.gov/downloads/doe-publicaccess-plan). |
| ‡ Equal contribution. |
| This journal is © The Royal Society of Chemistry 2023 |