Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Nanoarchitectonics on Z-scheme and Mott–Schottky heterostructure for photocatalytic water oxidation via dual-cascade charge-transfer pathways†
Yao
Li
 a,
Siyuan
Liu
a,
Runlu
Liu
a,
Jian
Pan
a,
Siyuan
Liu
a,
Runlu
Liu
a,
Jian
Pan
 b,
Xin
Li
c,
Jianyu
Zhang
a,
Xiaoxiao
Zhang
a,
Yixin
Zhao
b,
Xin
Li
c,
Jianyu
Zhang
a,
Xiaoxiao
Zhang
a,
Yixin
Zhao
 c,
Dawei
Wang
c,
Dawei
Wang
 b,
Hengdao
Quan
d and
Shenmin
Zhu
b,
Hengdao
Quan
d and
Shenmin
Zhu
 *a
*a
aState Key Laboratory of Metal Matrix Composites, School of Materials Science and Engineering, Shanghai Jiao Tong University, Shanghai 200240, P. R. China. E-mail: smzhu@sjtu.edu.cn
bParticles and Catalysis Research Group, School of Chemical Engineering, University of New South Wales, Sydney 2052, Australia
cSchool of Environmental Science and Engineering, Shanghai Jiao Tong University, Shanghai 200240, China
dSchool of Chemical Engineering and Environment, Beijing Institute of Technology, Beijing 100081, China
First published on 10th May 2023
Abstract
The bottleneck for water splitting to generate hydrogen fuel is the sluggish oxidation of water. Even though the monoclinic-BiVO4 (m-BiVO4)-based heterostructure has been widely applied for water oxidation, carrier recombination on dual surfaces of the m-BiVO4 component have not been fully resolved by a single heterojunction. Inspired by natural photosynthesis, we established an m-BiVO4/carbon nitride (C3N4) Z-scheme heterostructure based on the m-BiVO4/reduced graphene oxide (rGO) Mott–Schottky heterostructure, constructing the face-contact C3N4/m-BiVO4/rGO (CNBG) ternary composite to remove excessive surface recombination during water oxidation. The rGO can accumulate photogenerated electrons from m-BiVO4 through a high conductivity region over the heterointerface, with the electrons then prone to diffuse along a highly conductive carbon network. In an internal electric field at the heterointerface of m-BiVO4/C3N4, the low-energy electrons and holes are rapidly consumed under irradiation. Therefore, spatial separation of electron–hole pairs occurs, and strong redox potentials are maintained by the Z-scheme electron transfer. These advantages endow the CNBG ternary composite with over 193% growth in O2 yield, and a remarkable rise in ·OH and ·O2− radicals, compared to the m-BiVO4/rGO binary composite. This work shows a novel perspective for rationally integrating Z-scheme and Mott–Schottky heterostructures in the water oxidation reaction.
Introduction
Since pressing energy and environmental issues are afflicting modern society, humans depend more and more on new sources of renewable energy.1 To this end, converting inexhaustible solar energy into chemical bonds through artificial photosynthesis, e.g., water reduction into hydrogen fuel, is desirable for energy storage.2 Along with the process of photocatalytic water reduction, photocatalytic water oxidation (PWO) determines the overall reaction rate of water splitting. As a thermodynamically uphill process, the PWO reaction is a four-proton/four-electron step with sluggish kinetics, involving the dissociation of O–H chemical bonds as well as the formation of O–O chemical bonds.3,4 Targeting high-efficiency PWO, monoclinic bismuth vanadate (m-BiVO4) has stood out as one of the finest PWO catalysts since Kudo's report in 1999.5 To date, m-BiVO4 has been extensively studied due to its lone-pair effect, local electric field, appropriate band structure, earth abundance, and low toxicity.6–8 Despite vigorous development of single m-BiVO4, its PWO performance still falls short of expectations, owing to hampered carrier transfer and excessive carrier recombination, particularly on the surface and/or interface.9 Among the booming examples of modified tactics, heterointerface charge modulation has proven to be a useful method to elevate charge separation within photocatalysts.10 Nevertheless, there is still a lot of room to reconstruct m-BiVO4-based composites via heterojunction engineering,11 which should fine-tune the surface and/or interface properties with regard to the PWO reaction.The Mott–Schottky heterostructure constructed by coupling semiconductors and reduced graphene oxide (rGO) is supposed to exert the distinct advantages of each component.12,13 Meanwhile, the heterointerface between adjacent components works for charge transfer and separation. With regard to m-BiVO4, rGO possessing surface oxygen-containing groups (OCGs) offers the prerequisites for anchoring m-BiVO4. Upon intimate coupling, the rGO component can darken the composite for light absorption, speed up electron transfer for high conductivity, and supply abundant active sites with large specific surface areas.14 Significantly, a high conductivity region at the heterointerface of m-BiVO4/rGO ensures spontaneous electron transfer over the Mott–Schottky heterojunction.15,16 Therefore, when the m-BiVO4 component enters into the excited state, its photogenerated electrons are conducive to flow toward rGO and can diffuse along a carbon network. Even though heterointerface charge modulation is implemented in the current m-BiVO4/rGO Mott–Schottky heterostructure, the m-BiVO4 component with a two-dimensional (2D) layered morphology in fact possesses another bare surface, which is not in contact with the rGO component. In this context, some photogenerated charge carriers still undergo surface recombination on the m-BiVO4 component, while others migrate to the heterointerface between m-BiVO4 and rGO.17,18 Therefore, without hindering the existing interfacial interaction and electron transfer in the m-BiVO4/rGO Mott–Schottky heterostructure, how to further overcome this surface recombination on the m-BiVO4 component remains challenging.
Green plants are known to convert CO2 and H2O into O2 and carbohydrates through natural photosynthesis, whereby photogenerated electrons are transported under a Z-scheme mode.19 Mimicking natural photosynthesis to build an artificial Z-scheme heterostructure is an optimal route to suppress surface recombination on a single semiconductor.20,21 The carbon nitride (C3N4) candidate, as a typical 2D photocatalytic material, belongs to a redox-complementary component for the direct Z-scheme heterostructure.22–25 Once the m-BiVO4/rGO (BG) Mott–Schottky heterostructure has been acquired, further coupling of C3N4 with the m-BiVO4 component may additionally construct the m-BiVO4/C3N4 Z-scheme heterostructure. Between m-BiVO4 and C3N4, the 2D/2D face-to-face contact heterointerface featuring an effective charge transfer is a promising candidate to tackle surface recombination on the m-BiVO4 component.26 However, so far, no studies have established a Z-scheme heterostructure based on a Mott–Schottky heterostructure. Given that one surface of m-BiVO4 is in contact with rGO to form a heterointerface, the other bare surface of m-BiVO4 provides an opportunity to bear the m-BiVO4/C3N4 Z-scheme heterostructure. This additional 2D/2D intimate coupling can be predicted according to the large contact area, limited crystal boundary, and strong electronic coupling effect between the dimension-matched 2D layered materials.27,28 Profiting from the synergistic role of the Mott–Schottky and Z-scheme heterostructure, especially the spatial separation of carriers and the sustained high redox potentials of components in the latter, the C3N4/m-BiVO4/rGO (CNBG) ternary composite with face-to-face contact is speculated to achieve excellent PWO performance.
Herein, using the m-BiVO4/rGO Mott–Schottky heterostructure as a prototype, we also established the m-BiVO4/C3N4 Z-scheme heterostructure. The obtained CNBG ternary composite with face-to-face contact is postulated to solve the excessive surface recombination during the PWO reaction. To study the synergistic role of this dual heterostructure, particularly the virtues of Z-scheme electron transfer, extensive investigation of physical structures, chemical status, optoelectrical features, and catalytic performance was performed, and the related PWO mechanism is further illustrated. As expected, the CNBG ternary composite shows over 193% growth in O2 yield compared with the binary composite and this PWO performance boost is validated by the increase in reactive oxygen species.
Results and discussion
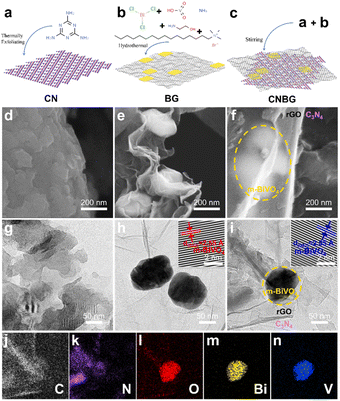
CNBG was fabricated using a multi-step process. In detail, the pristine C3N4 was synthesized via thermal polymerization from pre-heated melamine and chloride precursors, and the BG binary composite was obtained via hydrothermal treatment. The CNBG ternary composite was synthesized by simply mixing C3N4 and BG in deionized water followed by freeze-drying. To investigate the formation of CNBG, the zeta potentials of pristine C3N4, BG, and CNBG were measured in aqueous solution (Fig. S1†). The decrease in values from −8.7 mV for BG to −30.9 mV for CNBG is due to the incorporated C3N4 (−41.5 mV), certifying that the CNBG ternary composite was smoothly obtained based on the BG binary composite.The physical structures were unveiled by microstructure models (Fig. 1a–c), scanning electron microscopy (SEM), and transmission electron microscopy (TEM) images (Fig. 1d–i). The pristine C3N4 shows irregular, lamellar-like, and stacking layers with a rough surface (Fig. 1d and g), and these structural characteristics accord with C3N4 reported previously.29,30 In terms of the BG binary composite, the sheet-shaped BiVO4 is uniformly distributed on rGO, the size of the BiVO4 nanosheets exceeds 100 nm, and the surface of rGO seems wrinkled and smooth (Fig. 1e and h). After the incorporation of C3N4 in the BG binary composite, the BG is covered by C3N4 to form the CNBG ternary composite, which is like a “sandwich”—the top layer is the smooth rGO with translucence under electron beam imaging, the bottom layer is the C3N4 with a rough surface, and the m-BiVO4 nanosheets with size over 100 nm are co-wrapped between the top and bottom layers, in perspective view (Fig. 1f and i). The face-to-face contact of the three components in CNBG, in particular, is desirable for heterointerface electron transfer, whereas their similar Brunauer–Emmett–Teller (BET) specific surface areas imply that the incorporated C3N4 makes no difference to the overall surface area of CNBG (Fig. S2†).
Elemental mapping images show the homogeneous distribution of C, N, O, Bi, and V elements across the CNBG (Fig. 1j–n). The existing areas of C and N elements are consistent with C3N4, the shapes of the Bi, O, and V elements are identical to m-BiVO4 nanosheets, and the partial regions of C and O elements can be attributed to rGO. These images corroborate a ternary hybrid architecture, namely the m-BiVO4 co-wrapped by C3N4 and rGO with intimate contact. The characterization of BG and CNBG from high-resolution TEM (HRTEM) shows an interplanar spacing of 2.55 Å for the (002) spacing of m-BiVO4 (insets in Fig. 1h and i), while the lattice fringes of C3N4 cannot be detected due to the poor crystallinity. Therefore, the incorporation of C3N4 shows no impact on the morphology and crystal structure of m-BiVO4.
To further reveal the crystal structure information, X-ray diffraction (XRD) peaks for BG and CNBG, except for a peak at 27.8°, are both indexed to the m-BiVO4 (Fig. 2a), and their Raman bands (100 to 1000 cm−1), except for a peak at 734 cm−1, are also assigned to m-BiVO4 (Fig. 2b). These results agree with the HRTEM images and validate the m-BiVO4 being highly crystalline and phase pure, excluding any impact from the incorporated C3N4 on the crystal structure of m-BiVO4. Distinct from the BG binary composite, the CNBG ternary composite shows a small peak for the C3N4 (002) plane at 27.8° (in Fig. 2a), which belongs to the interlayer stacking of the conjugated aromatic segments.31 However, this peak in CNBG is much weaker than that in pristine C3N4, indicating decreased crystallinity within the C3N4 framework after its incorporation. A similar phenomenon is apparent in the Raman spectra (Fig. 2b), where the CNBG ternary composite presents a spike at 734 cm−1 and a broad peak (1000 to 2250 cm−1) relative to the BG, which are consistent with in-plane bending and disordered graphitic carbon–nitrogen vibrations, respectively .32 These additional peaks also emerge for pristine C3N4, suggesting the structure of C3N4 is preserved in CNBG after its incorporation. All these findings unambiguously reflect that the CNBG ternary composite was subtly constructed, and the sandwich-like structure with face-to-face contact is potentially useful for photocatalytic reactions.
 | ||
| Fig. 2 Characterization of the chemical status for the C3N4, BG, and CNBG. (a) XRD patterns. (b) Raman spectra. High-resolution XPS spectra of (c) Bi 4f, (d) V 2p, (e) O 1s, and (f) N 1s. | ||
To glean precise information on the heterointerface between m-BiVO4 and C3N4 in CNBG, the surface chemical status was analyzed by X-ray photoelectron spectroscopy (XPS). The change in binding energy reflects the interfacial chemical interaction and the different electron densities in the semiconductor–semiconductor heterojunction. The electron density increases when the binding energy shifts negatively, and decreases when the binding energy shifts positively.33 As shown in Fig. 2c, the Bi 4f spectrum of CNBG shows two peaks at 164.2 and 158.9 eV assigned to the binding energies of Bi–O chemical bonds; however, these peaks are negatively shifted compared with BG. Parallel negative shifts can be found in V 2p and O 1s spectra as well; the binding energies of the V–O and V–C chemical bonds appear at 523.4 and 516.5 eV, 524.5 and 517.4 eV in CNBG, respectively, while these peaks are located at higher values in BG (Fig. 2d). The three peaks at 533.1, 531.2, and 529.8 eV in CNBG (Fig. 2e) correspond to small molecules, bridging hydroxyls, and lattice oxygen,34 respectively, and together they are reduced relative to those in BG. In contrast, the N 1s spectra show an opposite trend (Fig. 2f). The binding energies of the C–N–H, N–(C)3, and C–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C chemical bonds in CNBG are located at 404.1, 401.2, and 399.2 eV, respectively, which are higher values than those for pristine C3N4. These results ascertain that the Bi–O–N and V–O–N chemical bonds link the m-BiVO4/C3N4 heterostructure in the CNBG ternary composite. The electrons migrate from C3N4 to m-BiVO4 as a result of the Fermi level equilibrium upon hybridization.26,35
C chemical bonds in CNBG are located at 404.1, 401.2, and 399.2 eV, respectively, which are higher values than those for pristine C3N4. These results ascertain that the Bi–O–N and V–O–N chemical bonds link the m-BiVO4/C3N4 heterostructure in the CNBG ternary composite. The electrons migrate from C3N4 to m-BiVO4 as a result of the Fermi level equilibrium upon hybridization.26,35
The C 1s spectra uncover whether the interfacial chemical interaction and the electron transfer are affected by the incorporation of C3N4 (Fig. S3†). The C 1s spectrum of graphene oxide (GO) was fitted into three peaks at 288.3 eV (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O chemical bonds), 287.4 eV (C–O chemical bonds), and 285.3 eV (C
O chemical bonds), 287.4 eV (C–O chemical bonds), and 285.3 eV (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C chemical bonds).36 After in situ growth of m-BiVO4 on rGO, the C 1s spectrum of BG was divided into two peaks, at 285.8 eV (C–O chemical bonds) and 284.7 eV (C
C chemical bonds).36 After in situ growth of m-BiVO4 on rGO, the C 1s spectrum of BG was divided into two peaks, at 285.8 eV (C–O chemical bonds) and 284.7 eV (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C chemical bonds). Compared with BG, the C 1s spectrum of CNBG shows an additional peak at 288.6 eV (N
C chemical bonds). Compared with BG, the C 1s spectrum of CNBG shows an additional peak at 288.6 eV (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–N chemical bonds).37 Conforming to the Bi 4f, V 2p, O 1s, and N 1s spectra results, the C 1s spectral analysis reconfirms that the C3N4 was incorporated into CNBG. In addition, there is an overall shift in the peaks over both BG and CNBG relative to GO, indicating the emergence of chemical bonds involving C atoms, i.e., the V–C chemical bonds in the m-BiVO4/rGO Mott–Schottky heterostructure for both BG and CNBG (Fig. 2d). As a result, the higher Fermi level of rGO compared with that of m-BiVO4 results in an electron rearrangement,38,39 leading to a high conductivity region as well as downward band bending within m-BiVO4. When the m-BiVO4 absorbs visible light to generate electron–hole pairs, the rGO can accept electrons from m-BiVO4 through this high conductivity region in both BG and CNBG. A similar binding energy shift was found between black phosphorus (BP) and m-BiVO4/BP in the P 2p spectra in a previous study,40 indicating a strong interfacial chemical interaction between BP and m-BiVO4, as well as the electron transfer from m-BiVO4 to BP during photocatalytic reactions. Thus, the incorporated C3N4 makes no difference to both the V–C chemical bonds in CNBG and the electron transfer over the Mott–Schottky heterojunction.
C–N chemical bonds).37 Conforming to the Bi 4f, V 2p, O 1s, and N 1s spectra results, the C 1s spectral analysis reconfirms that the C3N4 was incorporated into CNBG. In addition, there is an overall shift in the peaks over both BG and CNBG relative to GO, indicating the emergence of chemical bonds involving C atoms, i.e., the V–C chemical bonds in the m-BiVO4/rGO Mott–Schottky heterostructure for both BG and CNBG (Fig. 2d). As a result, the higher Fermi level of rGO compared with that of m-BiVO4 results in an electron rearrangement,38,39 leading to a high conductivity region as well as downward band bending within m-BiVO4. When the m-BiVO4 absorbs visible light to generate electron–hole pairs, the rGO can accept electrons from m-BiVO4 through this high conductivity region in both BG and CNBG. A similar binding energy shift was found between black phosphorus (BP) and m-BiVO4/BP in the P 2p spectra in a previous study,40 indicating a strong interfacial chemical interaction between BP and m-BiVO4, as well as the electron transfer from m-BiVO4 to BP during photocatalytic reactions. Thus, the incorporated C3N4 makes no difference to both the V–C chemical bonds in CNBG and the electron transfer over the Mott–Schottky heterojunction.
When electrons transfer toward rGO, its degree of reduction determines the electron diffusion ability. In the C 1s spectra (Fig. S3†), the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–O chemical bonds of GO represent the OCGs, contributing to the in situ growth of m-BiVO4. For both BG and CNBG, the C–O peaks shifted more than the C
O and C–O chemical bonds of GO represent the OCGs, contributing to the in situ growth of m-BiVO4. For both BG and CNBG, the C–O peaks shifted more than the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C peaks, causing shorter distances between the C–O and C
C peaks, causing shorter distances between the C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C peaks than for GO. This phenomenon indicates that some OCGs of GO turned into V–C chemical bonds in both BG and CNBG. Moreover, the absence of C
C peaks than for GO. This phenomenon indicates that some OCGs of GO turned into V–C chemical bonds in both BG and CNBG. Moreover, the absence of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O peaks and diminished C–O peaks relative to GO indicate the removal of other OCGs, which agrees with the results of the Raman spectra. As the Raman bands range from 1000 to 2000 cm−1 (Fig. S4†), both D band (sp3 carbon defects) and G band (sp2 carbon atoms) are derived from the carbon network within the rGO. The lower intensity ratio (ID/IG) in BG (0.79) than in GO (1.37) reveals that the rGO is partially reduced in BG. Although a broad peak induced by disordered graphitic carbon–nitrogen vibrations overlaps with both the D band and G band in CNBG, a similar degree of reduction of rGO in CNBG can be speculated based on the lower ID/IG in BG, as well as a facile composite process in CNBG. Therefore, Raman spectra and C 1s spectra elaborate an effective degree of reduction of rGO in both BG and CNBG, achieving a high conductivity and electron diffusion capacity for the carbon network after accepting electrons.
O peaks and diminished C–O peaks relative to GO indicate the removal of other OCGs, which agrees with the results of the Raman spectra. As the Raman bands range from 1000 to 2000 cm−1 (Fig. S4†), both D band (sp3 carbon defects) and G band (sp2 carbon atoms) are derived from the carbon network within the rGO. The lower intensity ratio (ID/IG) in BG (0.79) than in GO (1.37) reveals that the rGO is partially reduced in BG. Although a broad peak induced by disordered graphitic carbon–nitrogen vibrations overlaps with both the D band and G band in CNBG, a similar degree of reduction of rGO in CNBG can be speculated based on the lower ID/IG in BG, as well as a facile composite process in CNBG. Therefore, Raman spectra and C 1s spectra elaborate an effective degree of reduction of rGO in both BG and CNBG, achieving a high conductivity and electron diffusion capacity for the carbon network after accepting electrons.
An assessment of the energy band structure of the components is crucial to evaluate the origin of the charge carrier behavior. The photoresponse range of the photocatalysts was disclosed by UV-visible (UV-vis) absorption spectra (Fig. 3a), and pristine C3N4 and BG display discrete optical absorption edges attributed to their intrinsic band gap transitions. As for CNBG, there is a ladder-like optical absorption edge resulting from the overlapped band gap transitions of the C3N4 and BG components, which is consistent with the color change of the three photocatalysts (Fig. S5†). Furthermore, the CNBG exhibits similar absorption features to pristine C3N4 and BG, implying that the C3N4 linked with m-BiVO4 at the heterointerface is not incorporated into the m-BiVO4 lattice. This inference agrees with the XRD and Raman results. The optical band gap of C3N4 and BG can be quantitatively analyzed from Tauc plots (i.e., the curve of (αhv)rvs. hv, where r = 2) for direct band gap semiconductors of m-BiVO4 (ref. 41) and C3N4.42 As shown in Fig. 3b, the optical band gap of BG is 2.47 eV and is smaller than that of C3N4 (2.69 eV), which is in harmony with their discrete optical absorption edges. Despite the larger optical band gap of C3N4 than of BG, both C3N4 and BG are able to realize visible light absorption. Hence, for the CNBG ternary composite, the m-BiVO4 component and C3N4 component concurrently trigger abundant photogenerated carriers upon visible light irradiation.
Besides the optical band gap, proper matching of the conduction band (CB) and valence band (VB) positions of the components with the redox potentials of photocatalytic reactions cannot be neglected. Measured by XPS VB spectra (Fig. S6a†), the VB positions of BG and C3N4 are +1.59 eV and +1.29 eV, respectively. Therefore, the VB position of the m-BiVO4 component in both BG and CNBG meets the thermodynamic demands for the PWO. Based on (αhv)rvs. hv curves and XPS VB spectra, the CB positions of BG and C3N4 were calculated as −0.88 eV and −1.40 eV, respectively. Hence, the approximate band positions of BG and C3N4 were drawn vs. a normal hydrogen electrode (NHE, pH = 7) in Fig. S6b.† Once the C3N4 was incorporated into the BG binary composite, a staggered energy band structure formed with the Fermi level of C3N4 much higher than that of m-BiVO4.23,43 The electrons of C3N4 would be transferred toward m-BiVO4via the Bi–O–N and V–O–N chemical bonds until the Fermi level equilibrium was reached, as corroborated by the Bi 4f, V 2p, O 1s, and N 1s spectral results. This spontaneous electron redistribution creates an electron depletion layer with upward band bending in C3N4, and an electron accumulation layer with downward band bending in m-BiVO4. Hence, an internal electric field (IEF) with a direction from C3N4 to m-BiVO4 appears at the heterointerface. Under visible light irradiation, both C3N4 and m-BiVO4 components became excited states with electron transition from the VB to the CB. The IEF can promote the transfer of electrons in the CB of m-BiVO4 across the heterointerface to consume holes in the VB of C3N4, i.e., a Z-scheme electron-transfer pathway.26,35 In consequence, spatial separation of electron–hole pairs occurs in the heterointerface between m-BiVO4 and C3N4 in CNBG, preserving the strong reducing electrons in the CB of C3N4 and the strong oxidizing holes in the VB of m-BiVO4.
To illustrate the positive effect of the Z-scheme process on PWO activity, the activity was evaluated under visible light irradiation. Controlled tests revealed no O2 yield without photocatalysts or visible light. The CNBG exhibited an O2 yield of 1000.8 μmol g−1 (Fig. 3c), over 193% of that of BG, in the first 5 h, while a gap in the PWO performance between BG and a mixture of BG and C3N4 was small (within experimental error). Therefore, a strong interfacial chemical reaction, together with the Z-scheme process, explains the enhanced performance in CNBG. The pristine C3N4 showed scarce O2 yield owing to its weak PWO driving force induced by an unsuitable VB position. Also, the apparent quantum efficiency (AQE) was qualitatively analyzed with the help of different band-pass filters (Fig. 3d). The enhanced PWO performance of CNBG explains the maximum of 26.35% at 420 nm and is 1.42-fold higher than that of BG. As the light wavelength continues to increase, the gradually reduced AQE of both BG and CNBG validates that the PWO reaction is dependent on visible light absorption. However, the sharply decreased AQE of CNBG began at 475 nm, compared with BG. Since the m-BiVO4 component can be excited by visible light even up to 520 nm, whereas visible light beyond 475 nm cannot fully excite the C3N4 component (Fig. 3a), the photogenerated holes in the C3N4 component were deficient in annihilating photogenerated electrons in the m-BiVO4 component. This deduction supports the proposed Z-scheme pathway in CNBG. A comparison of the AQE of CNBG with the applied photocatalysts is provided in Table S1,† certifying that the Z-scheme heterostructure along with the Mott–Schottky heterostructure enables a superior PWO performance for CNBG relative to previous photocatalysts.
Except for the pristine C3N4 with scarce O2 yield, both BG and CNBG were collected to measure their durability. As shown in Fig. 3e, they both exhibited continuous O2 yield in each cycle. Considering that loss of photocatalyst in each cycle caused a decrease in performance, the CNBG still maintained over 374% of the performance of BG in the fifth cycle. XRD patterns and the TEM images confirm that the crystal phase and morphology of CNBG were sustained after the cycling tests (Fig. S7†), proving its reusability and photostability. To survey the impact of the incorporated amount of C3N4 on the PWO performance, CNBGs with different C3N4/BG molar ratios were prepared as reference samples (Fig. 3f). Evidently, the PWO performance continually decreased when the molar ratio exceeded 1.00. This downward trend is attributed to the excessive incorporation of C3N4 on the surface of the m-BiVO4 component causing shielding of the incident light,44 and thus inhibiting visible light absorption of the m-BiVO4 component and the Z-scheme electron transfer inside the CNBG.
Molecular oxygen activation measurements were performed to uncover the influence of the Z-scheme process on reactive oxygen species. Assisted by 5,5-dimethyl-L-pyrroline-N-oxide (DMPO), ·O2− radicals and ·OH radicals were both detected. Electron spin resonance (ESR) signals from the DMPO−·O2− adduct with light were found for pristine C3N4, BG, and CNBG (Fig. 4a), with ·O2− radicals from O2 reduction by electrons; while peaks from the DMPO−·OH adduct with light were observed for BG and CNBG (Fig. 4b), with ·OH radicals originating from OH−/H2O oxidation by holes. By contrast, almost no DMPO−·OH adduct formed with light for pristine C3N4, which agrees with its scarce O2 yield under irradiation and was due to the VB position being too negative thermodynamically to oxidize OH−/H2O. Thereby ·OH radicals were mainly gathered in the m-BiVO4 component, while ·O2− radicals were accumulated in the rGO component, or the C3N4 component in CNBG, supporting that the electron transfer follows the Mott–Schottky and Z-scheme pathway. Notably, the stronger signals from two adducts for CNBG than for BG affirm that more photogenerated electrons and holes were formed in CNBG, which can be ascribed to the increased spatial separation of the electron–hole pairs and the improved redox capability. Hence, based on the Mott–Schottky heterostructure of the BG binary composite, an additional Z-scheme heterostructure endows the CNBG ternary composite with formidable molecular oxygen activation ability, as well as superior PWO performance.
 | ||
| Fig. 4 Characterization of reactive oxygen species for the C3N4, BG, and CNBG. ESR signals of (a) ·O2− radicals and (b) ·OH radicals with and without visible light irradiation (λ > 420 nm). | ||
Considering the prominent photocatalytic behavior of CNBG, we carried out controlled experiments to probe the reaction mechanism. To begin with photoluminescence (PL) spectra were obtained to survey the electron-transfer pathway (Fig. 5a). The pristine C3N4 and BG displayed two peaks centered at 469 and 511 nm, respectively, assigned to the intrinsic band gap emission. On the other hand, the CNBG showed a broad peak from 445 to 485 nm, which is not matched with the peaks of either pristine C3N4 or BG. The emission from the broad peak was assigned to the multiple heterointerface recombination of electrons in the CB of the m-BiVO4 component and of holes in the VB of the C3N4 component via Z-scheme electron transfer. In addition, the CNBG showed stronger fluorescence intensity than the BG. Although the high conductivity region that favors electron transfer exists over the Mott–Schottky heterostructure, the incorporated C3N4 can accelerate radiative recombination via the Z-scheme process, leading to fluorescence enhancement for CNBG. Moreover, time-resolved PL (TRPL) spectra were applied to further investigate the electron-transfer pathway (Fig. 5b). Based on the parameters from biexponential fitting (Table S2†), the fluorescence lifetime (τav) value decreased from 4.86 ns for BG to 3.38 ns for CNBG. Despite a high conductivity region over the Mott–Schottky heterostructure, the decreased τav reconfirms an additional Z-scheme process that quenches low-energy electrons and holes in CNBG, in accord with the fluorescence enhancement described above. Therefore, the strong reducing electrons in the C3N4 component and strong oxidizing holes in the m-BiVO4 component are maintained with spatial separation, ultimately strengthening the photocatalytic behavior of the CNBG. As for the pristine C3N4, the strongest fluorescence intensity and the shortest τav imply its intrinsic high recombination rate of charge carriers without construction of a Z-scheme heterostructure.
Photoelectrochemical measurements were also employed to gain insight into the reaction mechanism. The electrochemical impedance spectroscopy (EIS) Nyquist plots were recorded under irradiation, and the arc radius was related to the interfacial transfer resistance (Fig. 5c). A smaller arc radius for CNBG than for BG verified the significance of an additional Z-scheme process in CNBG; namely, the spatial separation of strong reducing electrons and strong oxidizing holes in respective components raised the interfacial transfer efficiency. Measurements of transient photocurrent responses were conducted to evaluate the effect of the appended Z-scheme process on the photoelectron conversion efficiency (Fig. 5d). The photocurrent intensity of CNBG was 2.85 times that of BG, proving that more carriers are generated in double semiconductors and spatially separated via the Z-scheme process in CNBG. The almost unchanging photocurrent intensity with repeated on/off cycles illustrates the ideal photostability of CNBG. In addition to the results of the PL and TRPL spectra, the biggest arc radius and the lowest photocurrent intensity reconfirm the serious charge carrier recombination in pristine C3N4.
Taking these results together, the PWO mechanism over CNBG is summarized as follows (Fig. 6): (1) on constructing the CNBG ternary composite, the sandwich-like hybrid architecture with chemical bonding fulfills the requirement for intimate face-to-face contact of the three components; (2) until the Fermi level equilibrium is reached, a high conductivity region exists at the m-BiVO4/rGO heterointerface and an IEF appears at the m-BiVO4/C3N4 heterointerface; (3) both C3N4 and m-BiVO4 components achieve excited states, with electron transition from the VB to the CB upon visible light irradiation; (4) the rGO accepts some photogenerated electrons from m-BiVO4 through a high conductivity region, and these electrons soon rapidly diffuse along the carbon network within rGO; (5) the IEF, meanwhile, promotes other photogenerated electrons in the CB of m-BiVO4, consuming the low-energy photogenerated holes in the VB of C3N4via the Z-scheme process; (6) the feasible spatial separation of electron–hole pairs sustains plenty of strong oxidizing holes in the VB of m-BiVO4, accomplishing the subsequent PWO reaction. As a consequence, an innovative strategy is proposed to develop multicomponent photocatalysts with dual-cascade charge-transfer pathways, aimed at high-efficiency artificial photosynthesis.
 | ||
| Fig. 6 Schematic illustration of the PWO mechanism over CNBG. Here, EC, EV, and EF represent CB, VB, and Fermi levels, respectively. | ||
Conclusions
Taking the unresolved surface recombination on the m-BiVO4 component in the BG binary composite into account, we mimicked natural photosynthesis to establish a new m-BiVO4/C3N4 Z-scheme heterostructure based on the m-BiVO4/rGO Mott–Schottky heterostructure. As a result, the face-contact CNBG ternary composite was elaborately constructed for PWO. In the heterointerface between m-BiVO4 and rGO, the rGO accepted photogenerated electrons from m-BiVO4 through a high conductivity region, achieving further electron diffusion along a carbon network. Noteworthy are the heterointerface between m-BiVO4 and C3N4, the IEF-promoted electrons in the CB of m-BiVO4 consuming holes in the VB of C3N4 upon irradiation, the spatial separation of the strong reducing electrons in the CB of C3N4, and the strong oxidizing holes in the VB of m-BiVO4. With these merits, the CNBG showed an O2 yield of 1000.8 μmol g−1, which is over 193% that of BG, together with an AQE of 26.35% at 420 nm, which is 1.42-fold higher than that of BG. In addition, the CNBG possessed excellent reusability and photostability. We have not only incorporated C3N4 into the BG binary composite to resolve carrier recombination on the bare surface of the m-BiVO4 component, but also unravelled the synergistic role of Mott–Schottky and Z-scheme heterostructures in a ternary composite, thus emulating the interfacial chemistry in artificial photosynthesis.Experimental section
Materials synthesis
All chemicals were of analytical grade and were used without further purification. A GO sol–gel solution was prepared according to a previous study,45 and the pristine C3N4 was obtained by thermally exfoliating a mixture of pre-heated melamine and chloride according to a previous report.46In a typical synthesis of m-BiVO4 grown in situ on rGO (BG), a GO sol–gel solution (containing 100 mg of GO) was dispersed in 85 mL of deionized water. Then, 631 mg BiCl3 and 400 mg cetyltrimethylammonium bromide were dissolved into the suspension by stirring for 4 h. Afterwards 234 mg NH4VO3 was dissolved into the suspension and stirred for 1 h. Soon after, excess 5 M aqueous ethanolamine was added into this suspension until pH 10, then the pH was mediated to 6.2 by adding 2 M hydrochloric acid. The solution was poured into an autoclave and heated at 160 °C for 12 h. After cooling, the product was centrifuged, washed, and collected by freeze-drying.
In a typical synthesis of CNBG, 70 mg of pristine C3N4 was dispersed in 80 mL of deionized water, and 80 mg of pristine BG was added into the mixed solution under stirring for 24 h. The product was centrifuged, washed, and collected by freeze-drying. Other reference samples were prepared under the same conditions but with different C3N4/BG molar ratios ranging from 0.75 to 1.50.
Material characterization
Zeta potentials were measured in deionized water using a Zetasizer Nano ZS90. SEM images were recorded on a JEOL JSM-6360LV electron microscope. TEM and HRTEM were performed on a JEOL JEM-ARM200F electron microscope. The crystal phase was confirmed by X-ray diffraction using a Rigaku D/max 2550VL/PC system. Raman spectra were performed using a LabRAM HR system at 633 nm. XPS spectra were collected on an ESCALAB 250Xi instrument; the binding energies were referred to the C 1s peak from adventitious carbon. UV-vis absorption spectra were recorded with a PerkinElmer Lambda 750S UV-Vis-NIR spectrophotometer. The BET specific surface area was measured by using a nitrogen adsorption apparatus. The ESR spectra were acquired on a Bruker Elexsys E580 spectrometer with DMPO as the spin-trapping reagent. PL spectra were obtained on a Shimadzu RF-5301PC spectrophotometer at 365 nm. The fluorescence lifetime was quantified by the TRPL decay spectra on an Edinburgh FLS1000 spectrophotometer at 365 nm.Photoelectrochemical measurements
EIS spectra and transient photocurrent responses were obtained with 0.5 M Na2SO4 electrolytes in a standard three-electrode cell on a CHI 600E electrochemical workstation (Chenhua, China). Indium tin oxide (ITO) deposited with synthesized samples, platinum wires, and Ag/AgCl electrodes were utilized as the working electrodes, counter electrodes, and reference electrodes, respectively. To prepare the working electrode, 10 mg of sample and 500 μL of 5 wt% Nafion solution were dispersed in 5 mL of water–ethanol solution (Vwater/Vethanol = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) to form a homogeneous slurry, which was then spin-coated on 1.5 cm × 3.0 cm ITO and exposed to air. All working electrodes were prepared with the same conditions to guarantee the same loading. The EIS measurements were obtained under a constant potential of 0.6 V with irradiation, and the amplitude of the applied sine wave potential in each case was 5 mV. The transient photocurrent responses as the light switched on or off were measured at a constant potential of 0.6 V. The measurements were completed at room temperature. The potential in each case was 5 mV. The transient photocurrent responses as the light switched on or off were measured at a constant potential of 0.6 V. The measurements were completed at room temperature.
4) to form a homogeneous slurry, which was then spin-coated on 1.5 cm × 3.0 cm ITO and exposed to air. All working electrodes were prepared with the same conditions to guarantee the same loading. The EIS measurements were obtained under a constant potential of 0.6 V with irradiation, and the amplitude of the applied sine wave potential in each case was 5 mV. The transient photocurrent responses as the light switched on or off were measured at a constant potential of 0.6 V. The measurements were completed at room temperature. The potential in each case was 5 mV. The transient photocurrent responses as the light switched on or off were measured at a constant potential of 0.6 V. The measurements were completed at room temperature.
Activity evaluations
The PWO activity of samples was tested with a PLS-SXE300 Labsolar-6A system (Perfectlight, China). A 30 mg portion of the sample was poured into 50 mL of 0.15 M Fe(NO3)3 solution in a Pyrex reaction vessel equipped with a quartz lid cooled by recirculating water. Soon after, the reaction setup was degassed to remove air, and the reaction solution was magnetically stirred and then irradiated under visible light. The O2 yield was recorded using a gas chromatograph (Panna G2090A), which was equipped with a thermal conductivity detector (column temperature at 60 °C, inlet temperature at 150 °C, test temperature at 125 °C). All peaks were examined against corresponding standards, and each test was conducted three times in parallel to obtain an average value.AQE measurements
The AQE was qualitatively analyzed under the same conditions as the activity evaluations, except for the insertion of band-pass filters (420, 450, 475, 500, 520 nm). The light intensity was measured at seven different points to obtain an average value using a PL-MW2000 optical power meter. The AQE was calculated using the following formula:31where α = 4 for the O2 yield, M is the amount of O2 molecule, NA is Avogadro's constant, ℏ is Planck's constant, c is the speed of light, S is the irradiation area, P is the irradiation intensity, t is the irradiation time, and λ is the wavelength of the light.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by the National Natural Science Foundation of China [51672173, U1733130], Shanghai Science and Technology Committee [21ZR1435700, 18520744700, 18JC1410500], Translational Medicine National Key Science and Technology Infrastructure (Shanghai) Open Project [TMSK2020128], the Open Project of Key Laboratory of Green Chemical Engineering Process of Ministry of Education (GCP202107).Notes and references
- Q. Wang, J. Warnan, S. Rodríguez-Jiménez, J. J. Leung, S. Kalathil, V. Andrei, K. Domen and E. Reisner, Molecularly engineered photocatalyst sheet for scalable solar formate production from carbon dioxide and water, Nat. Energy, 2020, 5(9), 703–710 CrossRef CAS.
- Q. Wang and K. Domen, Particulate photocatalysts for light-driven water splitting: mechanisms, challenges, and design strategies, Chem. Rev., 2020, 120(2), 919–985 CrossRef CAS PubMed.
- Y. Fang, Y. Hou, X. Fu and X. Wang, Semiconducting polymers for oxygen evolution reaction under light illumination, Chem. Rev., 2022, 122(3), 4204–4256 CrossRef CAS PubMed.
- Y. Chen, Y. Sun, M. Wang, J. Wang, H. Li, S. Xi, C. Wei, P. Xi, G. E. Sterbinsky, J. W. Freeland, A. C. Fisher, J. W. Ager, Z. Feng and Z. J. Xu, Lattice site-dependent metal leaching in perovskites toward a honeycomb-like water oxidation catalyst, Sci. Adv., 2021, 7(50), 1–13 Search PubMed.
- A. Kudo, K. Omori and H. Kato, A novel aqueous process for preparation of crystal form-controlled and highly crystalline BiVO4 powder from layered vanadates at room temperature and its photocatalytic and photophysical properties, J. Am. Chem. Soc., 1999, 121(49), 11459–11467 CrossRef CAS.
- Y. Qi, J. Zhang, Y. Kong, Y. Zhao, S. Chen, D. Li, W. Liu, Y. Chen, T. Xie, J. Cui, C. Li, K. Domen and F. Zhang, Unraveling of cocatalysts photodeposited selectively on facets of BiVO4 to boost solar water splitting, Nat. Commun., 2022, 13(1), 484 CrossRef CAS PubMed.
- Y. Park, K. J. McDonald and K.-S. Choi, Progress in bismuth vanadate photoanodes for use in solar water oxidation, Chem. Soc. Rev., 2013, 42(6), 2321–2337 RSC.
- S. Yue, L. Chen, M. Zhang, Z. Liu, T. Chen, M. Xie, Z. Cao and W. Han, Electrostatic field enhanced photocatalytic CO2 conversion on BiVO4 nanowires, Nano-Micro Lett., 2021, 14(1), 15 CrossRef PubMed.
- B. Zhang, X. Huang, Y. Zhang, G. Lu, L. Chou and Y. Bi, Unveiling the activity and stability origin of BiVO4 photoanodes with FeNi oxyhydroxides for oxygen evolution, Angew. Chem., Int. Ed., 2020, 59(43), 18990–18995 CrossRef CAS PubMed.
- X. Ning, B. Lu, Z. Zhang, P. Du, H. Ren, D. Shan, J. Chen, Y. Gao and X. Lu, An efficient strategy for boosting photogenerated charge separation by using porphyrins as interfacial charge mediators, Angew. Chem., Int. Ed., 2019, 58(47), 16800–16805 CrossRef CAS PubMed.
- Q. Pan, A. Li, Y. Zhang, Y. Yang and C. Cheng, Rational design of 3D hierarchical ternary SnO2/TiO2/BiVO4 arrays photoanode toward efficient photoelectrochemical performance, Adv. Sci., 2020, 7(3), 1902235 CrossRef CAS PubMed.
- S. Liu, J. Pan, X. Li, X. Meng, H. Yuan, Y. Li, Y. Zhao, D. Wang, J. Ma, S. Zhu and L. Kong, In situ modification of BiVO4 nanosheets on graphene for boosting photocatalytic water oxidation, Nanoscale, 2020, 12(27), 14853–14862 RSC.
- K. Zhang, H. Su, H. Wang, J. Zhang, S. Zhao, W. Lei, X. Wei, X. Li and J. Chen, Atomic-scale Mott–Schottky heterojunctions of boron nitride monolayer and graphene as metal-free photocatalysts for artificial photosynthesis, Adv. Sci., 2018, 5(7), 1800062 CrossRef PubMed.
- C. Bie, H. Yu, B. Cheng, W. Ho, J. Fan and J. Yu, Design, fabrication, and mechanism of nitrogen-doped graphene-based photocatalyst, Adv. Mater., 2021, 33(9), 2003521 CrossRef CAS PubMed.
- H. Tan, H. A. Tahini, X. Wen, R. J. Wong, X. Tan, A. Iwase, A. Kudo, R. Amal, S. C. Smith and Y. H. Ng, Interfacing BiVO4 with reduced graphene oxide for enhanced photoactivity: a tale of facet dependence of electron shuttling, Small, 2016, 12(38), 5295–5302 CrossRef CAS PubMed.
- S. Liu, X. Li, X. Meng, T. Chen, W. Kong, Y. Li, Y. Zhao, D. Wang, S. Zhu, W. A. Cheema and J. Pan, Enhanced visible/near-infrared light harvesting and superior charge separation via 0D/2D all-carbon hybrid architecture for photocatalytic oxygen evolution, Carbon, 2020, 167, 724–735 CrossRef CAS.
- Q. Meng, B. Zhang, L. Fan, H. Liu, M. Valvo, K. Edström, M. Cuartero, R. d. Marco, G. A. Crespo and L. Sun, Efficient BiVO4 photoanodes by postsynthetic treatment: remarkable improvements in photoelectrochemical performance from facile borate modification, Angew. Chem., Int. Ed., 2019, 58(52), 19027–19033 CrossRef CAS PubMed.
- S. Chen, D. Huang, P. Xu, X. Gong, W. Xue, L. Lei, R. Deng, J. Li and Z. Li, Facet-engineered surface and interface design of monoclinic scheelite bismuth vanadate for enhanced photocatalytic performance, ACS Catal., 2020, 10(2), 1024–1059 CrossRef CAS.
- Y. Tachibana, L. Vayssieres and J. R. Durrant, Artificial photosynthesis for solar water-splitting, Nat. Photonics, 2012, 6(8), 511–518 CrossRef CAS.
- W. Zhang, A. R. Mohamed and W.-J. Ong, Z-scheme photocatalytic systems for carbon dioxide reduction: where are we now?, Angew. Chem., Int. Ed., 2020, 59(51), 22894–22915 CrossRef CAS PubMed.
- J. Abdul Nasir, A. Munir, N. Ahmad, T. u. Haq, Z. Khan and Z. Rehman, Photocatalytic Z-scheme overall water splitting: recent advances in theory and experiments, Adv. Mater., 2021, 33(52), 2105195 CrossRef CAS PubMed.
- H. Xu, X. She, T. Fei, Y. Song, D. Liu, H. Li, X. Yang, J. Yang, H. Li, L. Song, P. M. Ajayan and J. Wu, Metal–oxide-mediated subtractive manufacturing of two-dimensional carbon nitride for high-efficiency and high-yield photocatalytic H2 evolution, ACS Nano, 2019, 13(10), 11294–11302 CrossRef CAS PubMed.
- P. Xia, S. Cao, B. Zhu, M. Liu, M. Shi, J. Yu and Y. Zhang, Designing a 0D/2D S-scheme heterojunction over polymeric carbon nitride for visible-light photocatalytic inactivation of bacteria, Angew. Chem., Int. Ed., 2020, 59(13), 5218–5225 CrossRef CAS PubMed.
- Q. Liu, H. Tian, Z. Dai, H. Sun, J. Liu, Z. Ao, S. Wang, C. Han and S. Liu, Nitrogen-doped carbon nanospheres-modified graphitic carbon nitride with outstanding photocatalytic activity, Nano-Micro Lett., 2020, 12(1), 24 CrossRef CAS PubMed.
- Y. Wu, P. Xiong, J. Wu, Z. Huang, J. Sun, Q. Liu, X. Cheng, J. Yang, J. Zhu and Y. Zhou, Band engineering and morphology control of oxygen-incorporated graphitic carbon nitride porous nanosheets for highly efficient photocatalytic hydrogen evolution, Nano-Micro Lett., 2021, 13(1), 48 CrossRef PubMed.
- Y. Jiang, H. Chen, J. Li, J. Liao, H. Zhang, X. Wang and D. Kuang, Z-scheme 2D/2D heterojunction of CsPbBr3/Bi2WO6 for improved photocatalytic CO2 reduction, Adv. Funct. Mater., 2020, 30(50), 2004293 CrossRef CAS.
- J. Ran, W. Guo, H. Wang, B. Zhu, J. Yu and S. Qiao, Metal-free 2D/2D phosphorene/g-C3N4 van der Waals heterojunction for highly enhanced visible-light photocatalytic H2 production, Adv. Mater., 2018, 30(25), 1800128 CrossRef PubMed.
- S. Cao, B. Shen, T. Tong, J. Fu and J. Yu, 2D/2D heterojunction of ultrathin MXene/Bi2WO6 nanosheets for improved photocatalytic CO2 reduction, Adv. Funct. Mater., 2018, 28(21), 1800136 CrossRef.
- L. Jiang, X. Yuan, G. Zeng, J. Liang, X. Chen, H. Yu, H. Wang, Z. Wu, J. Zhang and T. Xiong, In-situ synthesis of direct solid-state dual Z-scheme WO3/g-C3N4/Bi2O3 photocatalyst for the degradation of refractory pollutant, Appl. Catal., B, 2018, 227, 376–385 CrossRef CAS.
- D. Liu, D. Chen, N. Li, Q. Xu, H. Li, J. He and J. Lu, Surface engineering of g-C3N4 by stacked BiOBr sheets rich in oxygen vacancies for boosting photocatalytic performance, Angew. Chem., Int. Ed., 2020, 59(11), 4519–4524 CrossRef CAS PubMed.
- Y. Wang, X. Liu, J. Liu, B. Han, X. Hu, F. Yang, Z. Xu, Y. Li, S. Jia, Z. Li and Y. Zhao, Carbon quantum dot implanted graphite carbon nitride nanotubes: excellent charge separation and enhanced photocatalytic hydrogen evolution, Angew. Chem., Int. Ed., 2018, 57(20), 5765–5771 CrossRef CAS PubMed.
- Y. Wang, X. Liu, X. Han, R. Godin, J. Chen, W. Zhou, C. Jiang, J. F. Thompson, K. B. Mustafa, S. A. Shevlin, J. R. Durrant, Z. Guo and J. Tang, Unique hole-accepting carbon-dots promoting selective carbon dioxide reduction nearly 100% to methanol by pure water, Nat. Commun., 2020, 11(1), 2531 CrossRef CAS PubMed.
- F. Xu, K. Meng, B. Cheng, S. Wang, J. Xu and J. Yu, Unique S-scheme heterojunctions in self-assembled TiO2/CsPbBr3 hybrids for CO2 photoreduction, Nat. Commun., 2020, 11(1), 4613 CrossRef CAS PubMed.
- S. Wang, P. Chen, Y. Bai, J. H. Yun, G. Liu and L. Wang, New BiVO4 dual photoanodes with enriched oxygen vacancies for efficient solar-driven water splitting, Adv. Mater., 2018, 30(20), 1800486 CrossRef PubMed.
- J. Wang, L. Xu, T. Wang, R. Li, Y. Zhang, J. Zhang and T. Peng, Porphyrin conjugated polymer grafted onto BiVO4 nanosheets for efficient Z-scheme overall water splitting via cascade charge transfer and single-atom catalytic sites, Adv. Energy Mater., 2021, 11(7), 2003575 CrossRef CAS.
- C. Gao, S. Chen, Y. Wang, J. Wang, X. Zheng, J. Zhu, L. Song, W. Zhang and Y. Xiong, Heterogeneous single-atom catalyst for visible-light-driven high-turnover CO2 reduction: the role of electron transfer, Adv. Mater., 2018, 30(13), 1704624 CrossRef PubMed.
- X. Li, J. Hu, T. Yang, X. Yang, J. Qu and C. Li, Efficient photocatalytic H2-evolution coupled with valuable furfural-production on exquisite 2D/2D LaVO4/g-C3N4 heterostructure, Nano Energy, 2022, 92, 106714 CrossRef CAS.
- Y. Yu, Y. Zhao, S. Ryu, L. E. Brus, K. S. Kim and P. Kim, Tuning the graphene work function by electric field effect, Nano Lett., 2009, 9(10), 3430–3434 CrossRef CAS PubMed.
- J. Eichhorn, C. Kastl, J. K. Cooper, D. Ziegler, A. M. Schwartzberg, I. D. Sharp and F. M. Toma, Nanoscale imaging of charge carrier transport in water splitting photoanodes, Nat. Commun., 2018, 9(1), 1–8 CrossRef CAS PubMed.
- M. Zhu, Z. Sun, M. Fujitsuka and T. Majima, Z-scheme photocatalytic water splitting on a 2D heterostructure of black phosphorus/bismuth vanadate using visible light, Angew. Chem., Int. Ed., 2018, 57(8), 2160–2164 CrossRef CAS PubMed.
- Z. Tian, P. Zhang, P. Qin, D. Sun, S. Zhang, X. Guo, W. Zhao, D. Zhao and F. Huang, Novel black BiVO4/TiO2−x photoanode with enhanced photon absorption and charge separation for efficient and stable solar water splitting, Adv. Energy Mater., 2019, 9(27), 1901287 CrossRef.
- Y. Wang, P. Du, H. Pan, L. Fu, Y. Zhang, J. Chen, Y. Du, N. Tang and G. Liu, Increasing solar absorption of atomically thin 2D carbon nitride sheets for enhanced visible-light photocatalysis, Adv. Mater., 2019, 31(40), 1807540 CrossRef CAS PubMed.
- J. K. Cooper, S. Gul, F. M. Toma, L. Chen, P.-A. Glans, J. Guo, J. W. Ager, J. Yano and I. D. Sharp, Electronic structure of monoclinic BiVO4, Chem. Mater., 2014, 26(18), 5365–5373 CrossRef CAS.
- L. Zhang, C. Yang, Z. Xie and X. Wang, Cobalt manganese spinel as an effective cocatalyst for photocatalytic water oxidation, Appl. Catal., B, 2018, 224, 886–894 CrossRef CAS.
- S. Eigler, M. Enzelberger-Heim, S. Grimm, P. Hofmann, W. Kroener, A. Geworski, C. Dotzer, M. Röckert, J. Xiao, C. Papp, O. Lytken, H.-P. Steinrück, P. Müller and A. Hirsch, Wet chemical synthesis of graphene, Adv. Mater., 2013, 25(26), 3583–3587 CrossRef CAS PubMed.
- J. Zhang, C. Yu, J. Lang, Y. Zhou, B. Zhou, Y. Hu and M. Long, Modulation of Lewis acidic-basic sites for efficient photocatalytic H2O2 production over potassium intercalated tri-s-triazine materials, Appl. Catal., B, 2020, 277, 119225 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Zeta potentials of the C3N4, BG, and CNBG (Fig. S1). Specific surface areas of the C3N4, BG, and CNBG (Fig. S2). High-resolution C 1s XPS spectra of the GO, BG, and CNBG (Fig. S3). Raman spectra of the GO and BG (Fig. S4). Digital photographs of the C3N4, CNBG, and BG (Fig. S5). XPS valence band spectra of the C3N4 and BG, and energy band structure diagrams of the C3N4 and BG (Fig. S6). Characterizations for the CNBG before or after cycling tests (Fig. S7). Comparison of AQE of the CNBG with reported photocatalysts (Table S1). Time-resolved PL decay curves were fitted by a biexponential function to calculate the fluorescence lifetime for the C3N4, BG, and CNBG (Table S2). See DOI: https://doi.org/10.1039/d3na00182b |
| This journal is © The Royal Society of Chemistry 2023 |