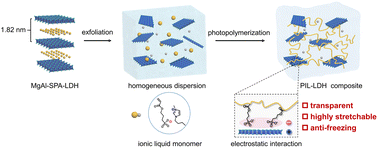
Stretchable ionic conductors show great promise for various applications including electronic skins, soft robotics, energy storage devices, actuators, and bio-integrated electronics. Solid-state ionic conductors do not suffer from the dehydration or liquid leakage problems that are typically encountered in hydrogels or ionogels, providing a promising platform for fabricating soft electronics with enhanced stability. However, the design and fabrication of solid-state ionic conductors with high ionic conductivity and excellent mechanical robustness still remain a great challenge. Here, we report a novel synthesis strategy toward the fabrication of solid-state stretchable ionic conductors by seamlessly incorporating layered double hydroxide (LDH) nanosheets into a poly(ionic liquid) (PIL) matrix. In this manner, the LDH nanosheets can form effective electrostatic interactions with the PIL chains, leading to the formation of solid-state ionic conductors with enhanced mechanical strength and toughness without compromising the ionic conductivity. The resultant PIL-LDH composite with an optimal LDH content exhibits high stretchability (>600%), ultra-low temperature tolerance (−81 °C), excellent transparency, and superior ionic conductivity (>3 mS cm−1). Wearable strain and pressure sensors were further demonstrated using the newly developed solid-state ionic conductors to monitor a variety of mechanical deformations with high sensitivity and durability. This study offers a new strategy toward the fabrication of high-performance solid-state ionic conductors for soft and wearable electronics that can stably function under extreme conditions.