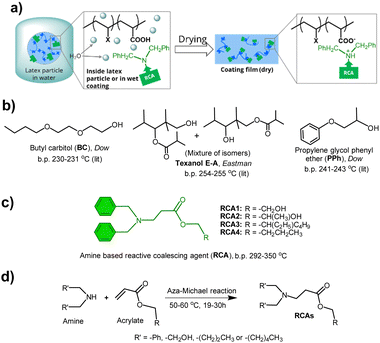
Open Access Article
Open Access ArticleAmine functional reactive coalescing agents (RCA) for emission-free waterborne paints and coatings†
Ranganathan Krishnan,
Jagjit Kaur,
Balamurugan Ramalingam * and
Satyasankar Jana
* and
Satyasankar Jana *
*
Institute of Sustainability for Chemicals, Energy and Environment (ISCE2), Agency for Science, Technology and Research (A*STAR), 1 Pesek Road, Singapore 627833, Republic of Singapore. E-mail: Satyasankar_jana@isce2.a-star.edu.sg; balamurugan_ramalingam@isce2.a-star.edu.sg
First published on 10th September 2024
Abstract
The unique characteristics of simple acid–base interactions have been exploited in designing new amine-functional reactive coalescing agents (RCAs). In addition to properties similar to commercial state-of-the-art coalescing agents (CAs), these RCAs possess reactivity that makes them suitable for producing true VOC-free/emission-free waterborne coatings.
Paints and coatings play a crucial role in sustainable development by protecting material surfaces, thereby extending their lifetime and improving performance along with other functional and aesthetic properties. This is particularly relevant in the automotive, transport vehicles, construction, electronics, aviation and marine industries, as well as low-carbon energy infrastructures such as windmills and solar cells. Among all major types of coatings, waterborne coatings are regarded as one of the most sustainable coatings due to the use of water as a dispersion medium. The global market size1a of waterborne coatings was USD 68.65 billion in 2023 and projected to reach USD 99.16 billion by 2030, growing at a CAGR of 5.21% due to rapid urbanization and industrialization. However, traditional waterborne coatings and formulations often contain up to 10% high boiling organic solvents as film-forming aids, also known as coalescing agents (CAs), that contribute to volatile and intermediate volatility organic compounds (VOCs and IVOCs). The total annual paint VOC and IVOC emissions from the US alone was recently estimated to be 48–155 Gg.1b Examples of some well-known CAs are provided in Fig. S1 in the ESI.† CAs play a crucial role in the film formation process by reducing the glass transition temperature (Tg) and minimum film formation temperature (MFFT) of the binder polymer of latex formulations, thereby enhancing chain diffusion during film formation. A successful film formation process is essential to avoid porosity and brittleness and to achieve desirable coating properties, such as gloss and scrub resistance, which impact the performance and longevity of the underlying substrates. However, these CAs are eventually released into the environment once the film formation is complete, contributing to VOCs. VOCs contribute to environmental pollution and are known to affect public health,2 especially when released from indoor coated surfaces. Although some current commercial CAs, such as Texanol E-A (see ESI† Fig. S1), exhibit very high boiling points (>250 °C) and low volatility, and are not classified as VOCs according to the European Union Decopaint Directive 2004/42/EC, they too eventually get released into the environment over a period of time. Therefore, both academia and industry are constantly seeking newer technologies3 and materials to overcome the emission of VOCs and other small molecule organic compounds (SOCs) from paints, coatings, and formulated products. Some of the main approaches to address this issue are: (i) use of reactive latices4 that react during the drying process of coatings; (ii) morphology control5 of latex particles, either using soft shell-hard core latex particles or the application of a blend of soft and hard latex particles; (iii) use of multiphase systems, where the hard phase is an inorganic filler6 and (iv) utilizing reactive coalescing agents (RCAs).7 Recently, another interesting concept has emerged involving the use of CO2-responsive polymer-based latex technology.8 However, among all these techniques, RCA technology has an added advantage because it may not require the synthesis of special polymer latices and can be used as a drop-in method.
RCAs act like traditional CAs by reducing the Tg and MFFT of the latex/polymer. However, unlike traditional CAs that simply evaporate after the drying process, RCAs chemically integrate into the polymer matrix of the coating during the drying process (Fig. 1b). This makes RCAs a valuable component in the formulation of eco-friendly and sustainable waterborne coatings. RCAs containing reactive functional groups such as dicyclopentenyloxyacrylate,9 2,2,4-trimethyl-3-oxopentanoate,10 glycidyl esters/ethers,11 and fatty acids12 (see ESI† Fig. S2) have been reported previously by several research groups. Recently, we have reported hydroxy ethyl sulfone (HES) RCAs,13 where reactivity is triggered by pH to produce reactive vinyl sulfone species that can easily react with the hydroxyl groups of latex polymers. The reactivity of HES-RCA with hydroxyl or amine-containing monomers/latex polymer was confirmed by 1H NMR spectroscopy. Our group has also earlier reported14 the recovery of Tg loss of acrylic copolymer, by both molecular dynamics (MD) simulation and experimental methods, when the RCA like molecules react with the polymer and permanently bond as a side chain.
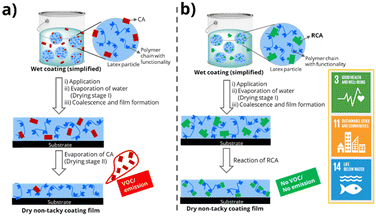 | ||
| Fig. 1 Comparative emission characteristics of waterborne coatings formulated using (a) traditional CAs and (b) RCAs and possible contribution to UN sustainable development goals. | ||
In this study, we present the design and synthesis of a novel class of high-boiling, low-volatility amine-functional15 reactive coalescing agents (RCAs). These RCAs react with carboxylic acid groups present in waterborne latices to produce emission-free waterborne coatings. The RCAs were synthesized using an atom-efficient aza-Michael addition reaction, characterized by various analytical techniques, and tested for their ability to reduce the minimum film formation temperature (MFFT) of several commercial latices. The reactivity between these RCAs and carboxylic acid-containing latex polymers through acid–base ionic interactions was confirmed by nuclear magnetic resonance (NMR) spectroscopy and head-space gas chromatography (HS-GC). Finally, a representative RCA was used to formulate a pigmented waterborne coating and was preliminarily evaluated for its hydrophilicity and UV resistance. This study highlights the potential of amine RCAs to create truly emission-free waterborne coatings, aligning with the United Nations sustainable development goals16 (Fig. 1).
In order to design new RCAs, we first examined the structures of commercial CAs which are presented in Fig. S1 (see ESI†). CAs are high boiling liquids with a wide range of polarity and compatibility to different latex polymers, and they often contain ether, ester and hydroxyl groups. These functional groups enable them to disperse in the aqueous phase of coating materials. Considering all these characteristics, our aim was to design similar high boiling molecules with tertiary amine reactive functionality that have a wide range of polymer compatibility and coalescing ability. We, therefore, first synthesized four model amine compounds, namely amine 1–4 (ESI,† Fig. S3), by reacting diethanolamine with 2-ethylhexyl acrylate and n-butyl acrylate, and 2-hydroxyethyl acrylate with dibutylamine and dihexylamine, respectively, via a catalyst-free, atom-efficient aza-Michael addition reaction. However, these model amine compounds possessed boiling points below 250 °C, designating them as VOCs according to the European Union directive 2004/42/EC. Although these molecules could potentially be reactive, we did not study them further as they might be released to the environment during storage, formulation, and application before drying of the coatings due to their low boiling points (<250 °C).
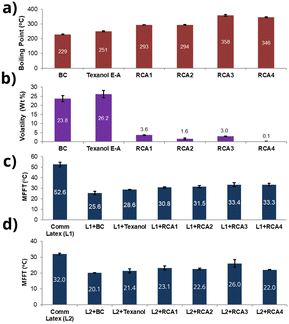
Interestingly, we identified that using dibenzylamine as a nucleophile instead of diethanolamine for the aza-Michael addition reaction produced amine compounds (RCA 1–4) with boiling points higher than 250 °C (Fig. 2c, d and 3a, and ESI,† Fig. S3), most likely due to the intermolecular π–π interaction of phenyl groups. The presence of aromatic groups in CA structures is not uncommon, as several commercial CAs, including Dowanol PPh and Eastman EHB contain phenyl groups (see, ESI† Fig. S1). As shown in Fig. 3, all RCA 1–4 had significantly higher boiling points and lower volatility (measured according to EPA method 24, % weight loss at 110 °C, 1 h) compared to industry-best CAs such as butyl carbitol (BC) and Texanol E-A.
These amine-functional RCAs were then investigated for their coalescing characteristics, specifically their ability to reduce the minimum film formation temperature (MFFT) using a wide range of commercial latices. A couple of waterborne acrylic latices, Joncryl 538A (L1) and N-2398 (L2), obtained from two different commercial sources were utilized for this investigation. The MFFTs of L1 and L2 were found to be 52.6 °C and 32.0 °C, respectively. When 2 wt% of RCA 1–4 was mixed with L1 and L2, the MFFT was reduced to 30.8–33.4 °C for L1 and 21.4–26.0 °C for L2 (Fig. 3c and d). The results were comparable to those obtained using commercial CAs like BC and Texanol E-A (Fig. 3c and d).
We presume that the amine group present in RCA 1–4 possibly interacts with the acid (–COOH) functional groups in the latex. To understand the interaction of the amine of RCA1 and acid group in latex, initially we used methacrylic acid (MAA) as a mimic for the acid group of latex polymer. The amine–acid interaction between RCA1 and methacrylic acid (MAA) monomer was investigated using 1H and 13C nuclear magnetic resonance (NMR) spectroscopy. As seen in Fig. S4 (see ESI†), the broad 1H peak of the –COOH group of MAA in the 1H NMR spectra disappeared when mixed with an equimolar amount of RCA1. Similarly, the carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) peak of MAA at 173.0 ppm in the 13C NMR spectra shifted upfield to 172.5 ppm when mixed with equimolar RCA1 (Fig. S4 in ESI†).
O) peak of MAA at 173.0 ppm in the 13C NMR spectra shifted upfield to 172.5 ppm when mixed with equimolar RCA1 (Fig. S4 in ESI†).
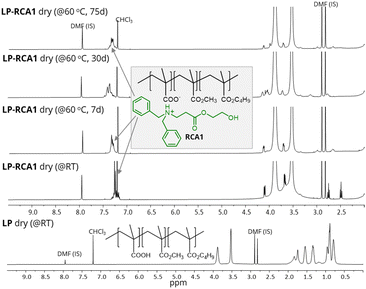
Next, to investigate the reactivity of RCA1 with –COOH containing latex, an in-house latex with known acid composition was synthesized. Most commercial acrylic waterborne latices contain carboxylic acid groups; however, they were not used in the current study to prevent interference from other unknown additives present in them. The in-house waterborne latex polymer (LP) with the composition of poly(nBMA52.8-co-MMA42.2-co-MAA5) was synthesized via emulsion polymerization using ammonium persulfate as a free radical initiator (see ESI†). All methacrylate monomers, namely nbutyl methacrylate (nBMA), methyl methacrylate (MMA), and methacrylic acid (MAA), were utilized to avoid composition drift14 during polymerization. The polymerization continued until complete conversion of these vinyl monomers was achieved, which was confirmed with NMR spectroscopy. Finally, a latex with 39% solid content, latex particle size of 63 nm, Mw 281 kDa and an MFFT of 56 °C was obtained. The addition of 2 wt% of RCA1 to LP produced an LP-RCA1 (wet) blend (see ESI,† Fig. S5) with a reduced MFFT of 33 °C. This LP-RCA1 (wet) blend was then dried at room temperature to yield LP-RCA1 dry (@RT) sample. The presence of 1H NMR peaks at 7.13–7.27, 4.06, 3.6, 2.7 and 2.5 ppm confirmed the presence of RCA1 in the LP-RCA1 dry (@RT) sample. The composition of RCA1 in the LP-RCA1 dry (@RT) sample did not change over many months, as confirmed by 1H NMR spectroscopy.
To check further whether any emission was occurring from the LP-RCA1 dry (@RT) sample, an accelerated approach was adopted. The LP-RCA1 dry (@RT) sample powder was placed in an open glass bottle and heated in a convection oven at 60 °C for 75 days. A quantitative estimation from the 1H NMR spectra of the LP-RCA1 dry (@60 °C, 75 d) sample using an internal standard confirmed that there was hardly any loss of RCA1 during this heating process (Fig. 4). Additionally, it was observed that the 1H NMR signals of RCA1 of LP-RCA1 dry (@60 °C) samples broadened over the period of heating (Fig. 4), indicating that the RCA1 molecule was getting strongly attached to the –COOH group of LP. The above observation aligns with our proposed working mechanism (Fig. 2a) for the reactivity of RCAs with latex. Heating the LP-RCA1 dry (@RT) sample powder at a further higher temperature of 110 °C for 4 days showed similar results (Fig. S6 in ESI†). In contrast, when a control sample of LP–PPh dry (@RT) sample (PPh is a traditional CA) was heated similarly, the PPh completely evaporated off from the sample (Fig. S6 in ESI†), leaving no trace of PPh in the heated sample. This observation was expected, because PPh is not a reactive coalescing agent like RCA1.
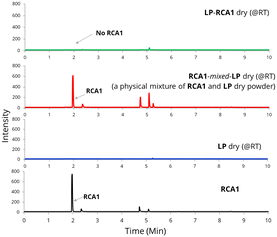
Additionally, the LP-RCA1 dry (@RT) sample was analysed using head-space gas chromatography (HS-GC). Both neat RCA1 and a physical mixture of RCA1 with the LP dry (@RT) sample (i.e. RCA1-mixed-LP dry (@RT)) exhibited a peak at 2 minutes (Fig. 5). Interestingly, the LP-RCA1 dry (@RT) sample did not show the RCA1 peak at 2 minutes, presumably due to the strong interaction/reaction between RCA1 and LP. Mixing RCA1 with LP in the wet form of latex facilitated strong molecular interaction/reaction of the amine group of RCA1 with the –COOH group of LP. Similar results, indicating stronger RCA1-latex polymer interaction, were obtained when RCA1 was blended with commercial latices L2 and L3 (Fig. S7 in ESI†). However, commercial controls like PPh and BC, which are non-reactive coalescing agents, were emitted from the blend samples when heated during HS-GC analysis, as seen in Fig. S7 in the ESI.†
Finally, a pigmented coating was formulated (procedure in ESI†) using a commercial latex L3 (YS800AP, MFFT 27.2 °C), rutile TiO2, and dispersing agent BYK-154, with or without RCAs. The coating formulated with RCA1 (2 wt% in wet) produced defect-free, continuous, and non-tacky films (Fig. S8 in ESI†) that did not show any blistering, defects, or tackiness even after being immersed in water for 2 days. The dry white coating film did not significantly change its coloration even after 100 hours of UV light exposure (ESI,† Fig. S9) despite the amine nature of RCA1 and, additionally, RCA1 proved to retard the thermo-oxidative coloration of polymer when heated at 110 °C (see ESI,† Fig. S10) similar to hindered amine stabilizers.17
In summary, a novel class of amine-functional RCAs15 was conceptualized and synthesized. These amine-functional RCAs are readily synthesizable, possessing notable high boiling points and exhibiting low volatility. RCAs were subjected to comprehensive characterization and assessed for their capacity to lower the MFFT of commercial latices. The reactivity of these amine RCAs with carboxylic acid-containing latices was studied using NMR spectroscopy and head-space gas chromatography (HS-GC). Subsequently, a representative RCA was applied in formulating a pigmented waterborne coating, which underwent preliminary evaluation for its hydrophilicity and UV resistance properties. This investigation highlights the potential of such amine RCAs in the development of genuinely emission-free waterborne paints, coatings and formulations (except for the application in a highly acidic or basic environment), offering promise for their role in advancing the UN's sustainable development goals.
This work was funded by the Agency for Science, Technology and Research (A*STAR), Singapore under the Environmentally Friendly Specialty Products Programme (Science and Engineering Research Council grant no. 152 80 00044). The authors are thankful to Prof. Alexander M. Van Herk, program manager of this research programme for his suggestions and feedback during the research work and Mr Zhao Wenguang for his support on SEC analysis. The authors are also grateful to BASF Singapore, NIPSEA Singapore, BYK Singapore and Tronox Singapore for providing free samples for this work.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) https://www.pcimag.com/articles/112266-the-rise-of-water-based-coatings, 2024; (b) R. Tanzer-Gruener, P. Ethi Rajan, L. D. Dugan, M. E. Bier, A. L. Robinson and A. A. Presto, Environ. Sci. Technol., 2022, 56, 11236–11245 CrossRef CAS PubMed.
- (a) X. Zhou, X. Zhou, C. Wang and H. Zhou, Chemosphere, 2023, 313, 137489 CrossRef CAS PubMed; (b) Z. Mo, S. Lu and M. Shao, Environ. Pollut., 2021, 269, 115740 CrossRef CAS PubMed; (c) O. C. Ulker, O. Ulker and S. Hiziroglu, Coatings, 2021, 11, 806 CrossRef CAS.
- (a) N. Jiménez, N. Ballard and J. M. Asua, Macromol. Mater. Eng., 2024, 2400026 CrossRef; (b) M. F. Cunningham, J. D. Campbell, Z. Fu, J. Bohling, J. G. Leroux, W. Mabeee and T. Robert, Green Chem., 2019, 21, 4919–4926 RSC.
- (a) J. W. Taylor and M. A. Winnik, J. Coat. Technol. Res., 2004, 1, 163–190 CrossRef CAS; (b) A. B. Foster, P. A. Lovell and M. A. Rabjohns, Polymer, 2009, 50, 1654–1670 CrossRef CAS.
- E. Limousin, N. Ballard and J. M. Asua, J. Appl. Polym. Sci., 2019, 47608 CrossRef.
- (a) E. W. S. Hagan, M. N. Charalambides, C. R. T. Young, T. J. S. Learner and S. Hackney, Polymer, 2011, 52, 1662 CrossRef CAS; (b) V. Alvarez and M. Paulis, Prog. Org. Coat., 2017, 112, 210 CrossRef CAS; (c) D. K. Makepeace, P. Locatelli, C. Lindsay, J. M. Adams and J. L. Keddie, J. Colloid Interface Sci., 2018, 523, 45 CrossRef CAS PubMed; (d) S. Ugur, M. S. Sunay and Ö. Pekcan, Polym. Compos., 2014, 35, 2376 CrossRef CAS.
- (a) https://www.pcimag.com/articles/83117-formulating-solutions-meeting-voc-regulations-with-coalescents; (b) https://www.epa.gov/greenchemistry/presidential-green-chemistry-challenge-2005-designing-greener-chemicals-award.
- (a) J. Ho, B. Mudraboyina, C. Spence-Elder, R. Resendes, M. F. Cunningham and P. G. Jessop, Green Chem., 2018, 20, 1899–1905 RSC; (b) M. F. Cunningham and P. G. Jessop, Macromolecules, 2019, 52, 6801–6816 CrossRef CAS.
- W. D. Weir, US Pat., 6509494 B1, 2003 Search PubMed.
- J. T. Maddox, M. D. Clark and R. L. Eagan, US Pat., 2012/0157614 A1, 2012 Search PubMed.
- (a) J. Hulkko, S. Koskimies, K. Rissanen, M. Marttina, R. Kylakoski, M. Loija and L. Paajanen, WO Pat., 2005/090501 A1, 2005 Search PubMed; (b) M. Lahtinen, E. Glad, S. Koskimies, F. Sundholm and K. Rissanen, J. App. Polym. Sci., 2003, 87, 610 CrossRef CAS.
- (a) D. G. Speece Jr., E. K. Eisenhart, M. D. Bowe, W. D. Weir and M. A. H. Wolfersberger, EP Pat., 1106593 B1, 2004 Search PubMed; (b) P. Bloom, WO Pat., 2007/094922 A2, 2007 Search PubMed; (c) O. C. Ziemann, A. P. Marks and M. S. Gebhard, EP Pat., 1447432 A1, 2004 Search PubMed; (d) J. V. Barbosa, E. Veludo, J. Moniz, F. D. Magalhães and M. M. S. M. Bastos, Eur. J. Lipid Sci. Technol., 2012, 114, 1175–1182 CrossRef CAS.
- J. Kaur, R. Krishnan, B. Ramalingam and S. Jana, RSC Adv., 2020, 10, 17171–17179 RSC.
- M. Klähn, R. Krishnan, J. M. Phang, F. C. H. Lim, A. M. van Herk and S. Jana, Polymer, 2019, 179, 121635 CrossRef.
- S. Jana, WO Pat., 2021/150170 A1, 2021 Search PubMed.
- https://sdgs.un.org/goals.
- (a) P. Gijsman, Polym. Degrad. Stab., 2017, 145, 2–10 CrossRef CAS; (b) T. P. Sassi and R. B. Gupta, US Pat., 6492521 B2, 2002 Search PubMed; (c) P. Gijsman and M. Gitton-Chevalier, Polym. Degrad. Stab., 2003, 81, 483–489 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4cc03147d |
| This journal is © The Royal Society of Chemistry 2024 |