Open Access Article
Open Access ArticleThiochromenocarbazole imide-based photosensitizers decorated with carbonic anhydrase inhibitors for the targeted treatment of hypoxic tumours†
Amina
Merabti
ab,
Darío Puchán
Sánchez
c,
Alessio
Nocentini
d,
Lamiaa M. A.
Ali
 b,
Christophe
Nguyen
b,
Christophe
Nguyen
 b,
Denis
Durand
b,
Kathleen
Hamon
b,
Tatiana
Ghanem
c,
Philippe
Arnoux
b,
Denis
Durand
b,
Kathleen
Hamon
b,
Tatiana
Ghanem
c,
Philippe
Arnoux
 e,
Pierre
Josse
c,
Céline
Frochot
e,
Pierre
Josse
c,
Céline
Frochot
 e,
Raivis
Zalubovskis
e,
Raivis
Zalubovskis
 f,
Sébastien
Richeter
f,
Sébastien
Richeter
 a,
Magali
Gary-Bobo
a,
Magali
Gary-Bobo
 b,
Claudiu T.
Supuran
b,
Claudiu T.
Supuran
 d,
Clément
Cabanetos
d,
Clément
Cabanetos
 c,
Jean-Yves
Winum
c,
Jean-Yves
Winum
 *b and
Sébastien
Clément
*b and
Sébastien
Clément
 *a
*a
aICGM, Univ Montpellier, CNRS, ENSCM, Montpellier, France. E-mail: sebastien.clement1@umontpellier.fr
bIBMM, Univ Montpellier, CNRS, ENSCM, Montpellier, France. E-mail: jean-yves.winum@umontpellier.fr
cUniv Angers, CNRS, MOLTECH-ANJOU, SFR MATRIX, F-49000 Angers, France
dNEUROFARBA Department, Pharmaceutical and Nutraceutical Section, University of Florence, Via U. Schiff 6, 50019 Sesto Fiorentino, Firenze, Italy
eUniversité de Lorraine, CNRS, LRGP, F-54000 Nancy, France
fLatvian Institute of Organic Synthesis, Aizkraukles 21, 1006, Riga, Latvia
First published on 21st February 2024
Abstract
PDT has gained growing interest as a prospective approach for cancer therapy, thanks to its minimal invasiveness and low systemic toxicity, making it a promising therapeutic option. Nevertheless, type II PDT is significantly influenced by the oxygen levels in the tumoral microenvironment. Consequently, the weak oxygen pressure encountered in hypoxic regions of solid tumours poses a significant challenge in cancer treatment. In this case, PDT efficiency is reduced and the existing hypoxia is intensified by oxygen consumption and vascular closure, activating the angiogenic factors and thus, potentially leading to cancer recurrence and progression. We describe here a series of thiochromenocarbazole imide (TCI) photosensitizers featuring carbonic anhydrase inhibitors (CAi) of the sulfonamide, coumarin and sulfocoumarine type, designed to alleviate the consequences of PDT-induced hypoxia by merging the advantages of hCA IX knockdowns with PDT. TCIs with coumarin and sulfocoumarin moieties showed selective inhibition against tumour-associated hCA IX and hCA XII, while TCI incorporating benzenesulfonamide moieties also showed activity against the off-target hCA II. In biological assays, the TCI photosensitizer incorporating coumarin-based CAi demonstrated minimal dark toxicity and showcased strong imaging and photodynamic therapy (PDT) effects in both in vitro and in vivo settings.
Introduction
Photodynamic therapy (PDT) has emerged as a promising therapeutic approach for solid cancer treatment, due to its minimal invasiveness, high safety and low systemic toxicity.1,2 A typical PDT process involves the in situ formation of cytotoxic reactive oxygen species (ROS) such as singlet oxygen (1O2), peroxide (O2−), superoxide (O2˙−) and the hydroxyl radical (HO˙) through the photoexcitation of a photosensitizer (PS), accumulated in the tumour, under appropriate light irradiation.3,4 The PDT process can be categorized into type I and type II depending on the photo-triggered reactions that occur between PS and the substances in its vicinity.3,4 Specifically, type I reaction involves either hydrogen atom abstraction or electron transfer, ultimately leading to the formation of radicals and hydrogen peroxide (H2O2) while type II leads to the generation of singlet oxygen (1O2) by energy transfer from the electronically excited triplet-state PS to the ground-state molecular oxygen (3O2).3,4 Type II PDT is the dominant mechanism since most of PSs are type II.3,4 Unfortunately, this dependency on the surrounding oxygen contradicts the inherent properties of tumour hypoxia.Hypoxia is a salient and important feature found in the microenvironment of solid tumours due to the fast cancer cell proliferation and irregular angiogenesis.5 The oxygen pressure typically falls below 10 mmHg in the hypoxic regions of the tumour in contrast to the 40–60 mmHg range found in most healthy tissues.6 Consequently, since type II PDT is highly dependent on oxygen concentrations, the hypoxic tumour microenvironment not only hampers the PDT efficiency but also exacerbates the existing hypoxia by consuming oxygen and vascular closure.7 Hypoxic cells respond to PDT-induced damages by activating signaling cascades mostly regulated by transcription factors HIFs (hypoxia-inducible factors 1 and 2, HIF-1/2) and by releasing pro-angiogenic growth factors which sustain survival, stimulate metastasis, invasiveness, and recurrence of tumour cells.8,9 Several strategies, including the direct delivery of exogenous oxygen to the tumour or the generation of oxygen in situ, have been developed to partially alleviate tumour hypoxia.10–13
However, these methods provide only a temporary oxygen supply and often result in modest degradation of HIF-1, which can significantly compromise PDT effectiveness. Consequently, the inhibition of the HIF-1 signalling pathway has emerged as a promising approach to mitigate tumour hypoxia.
Human carbonic anhydrase IX (hCA IX) and another related isoform, i.e. hCA XII, are transmembrane proteins regulated by hypoxia-inducible factor (HIF) transcription factors, leading to their overexpression under hypoxic conditions.14 These transmembrane isoforms hCA IX and hCA XII are members of the hCA family of zinc enzymes (CA, EC 4.2.1.1) which play a crucial role in regulating the pH balance within tumours, thereby contributing to the survival, proliferation, invasion, and metastasis of cancer cells.15 The overexpression and extracellular location of the active site of hCA IX/hCA XII in hypoxic cancer cells offer potential as a target for delivering PS to cancer cells in PDT, with the aim of addressing the limitations associated with this treatment approach. In 2017, Jung et al. described the synthesis of a BODIPY-based PS featuring hCA IX inhibitor (CAi) acetazolamide (AAZ).16 This PS, designed to specifically target hCA IX, exhibited significantly improved effectiveness in causing phototoxicity in vitro against the aggressive human breast cancer MDA-MB-231 cell line, both in standard culture and in a spheroid model. Moreover, its ability to suppress tumour growth in vivo was also demonstrated when tested on xenograft mice inoculated with MDA-MB-231 cells. Since this pioneering work, a series of CAi-PS systems including conjugated polymer,17 metal organic framework,18,19 porphyrins,20–22 and metal complexes were developed.23–26 We recently reviewed all these systems highlighting that a synergistic treatment that enhances the PDT effect against hypoxic cancer cells and reduces resistance can be achieved by combining a PS with a CAi.27 Nevertheless, although these studies have yielded promising results thus far, it is worth noting that comprehensive inhibition studies against recombinant proteins such as hCA IX, and hCA XII, as well as the cytosolic off-targets hCA I and hCA II, have only rarely been carried out.27
Herein, we design a series of CAi-PS hybrid systems incorporating as CAi either a classical benzenesulfonamide chemotype, known to strongly inhibit hCA isoforms with high potency, or coumarin and sulfocoumarin-based moieties more selective for hCA IX and XII and compare them with respect to their carbonic anhydrase inhibition activities and phototoxic effect.28–30 We opted to develop a metal-free PS based on the thiochromenocarbazole imide (TCI) core, derived from the N-annulation process of benzothioxanthene imide (BTI).31 The selection of the TCI core was driven by the existence of a nitrogen-containing cycle, allowing for extra and orthogonal functionalization through N-alkylation. Furthermore, its intriguing photophysical attributes, such as a high fluorescence quantum yield and noteworthy spin–orbit coupling (SOC), can be finely adjusted depending on the nature of the substituent. Compared to the parent BTI, TCI was found to be able to generate singlet oxygen (ΦΔ = 0.14 in CH2Cl2) due to increased intersystem crossing (ISC) transition.31 To increase the singlet oxygen generation quantum yield, we decided to introduce a bromine substituent, a well-known strategy to achieve efficient singlet oxygen sensitizers due to SOC.32 The synthesis, optical properties as well as detailed biological and PDT studies in a human breast adenocarcinoma (MDA-MB-231) cell line of this innovative CAi-TCI hybrid system were then investigated.
Results and discussion
Design and synthesis
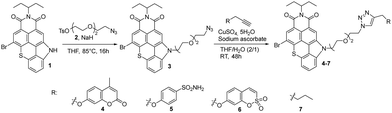
The synthetic route to a series of TCI PS featuring CAis is illustrated in Scheme 1. This series of PSs was achieved by formation of a 1,2,3-triazole bridge between a carbonic anhydrase inhibitor (coumarin, benzenesulfonamide and sulfocoumarin) and TCI-based PS through a Cu(I)-catalyzed 1,3-dipolar cycloaddition. A TCI PS without hCA IX inhibitor 7 was also prepared from hex-1-yne serving as a control system (Scheme 1). Azido-functionalized TCI PS 3 was prepared in 68% yield by N-alkylation of TCI 1 with 2-[2-(2-azidoethoxy)ethoxy]ethyl 4-methylbenzenesulfonate 2 in THF at 85 °C in the presence of sodium hydride. The structure of TCI 3 was confirmed by IR spectroscopy by the strong asymmetric stretching frequency at 2111 cm−1 related to the azido group. High-resolution ESI-TOF mass spectrometry (positive mode) also revealed the presence of the pseudo-molecular ion [M + H]+ at m/z = 622.1105 Da in agreement with the calculated one (calcd m/z = 622.1118 Da).Alkyne-substituted carbonic anhydrase inhibitors were then grafted to azido-functionalized TCI 3via a copper catalysed azide alkyne cycloaddition reaction in a mixture of THF–H2O, affording the TCI-CAi-based PSs 4–6 with yields ranging from 60% to 84%. A control system without a CA inhibitor was also elaborated through the reaction of TCI 3 with hex-1-yne (Scheme 1). The disappearance of the strong N3 stretching bands in the ATR-FTIR spectra of TCIs 4–7 confirmed that the cycloaddition occurred. The formation of the triazole ring was also corroborated by 1H NMR spectroscopy and by the presence of an additional singlet between 7.20 and 7.90 ppm. Additional signals corresponding to CAi moieties were also observed: two signals at 2.40 ppm (CH3) and 6.23 ppm (hydrogen close to the carbonyl) for the coumarin-based TCI 4, two doublets at 6.95 ppm and 7.79 ppm (hydrogens of the phenyl group) for the benzenesulfonamide-based TCI 5, signals between 6.75 and 7 ppm (aromatic hydrogens) for the sulfocoumarin-based TCI 6 and finally, signals corresponding to the CH2 and CH3 groups between 0.90 and 2.26 ppm for TCI 7. Finally, the presence of monocations [M + H]+ was also observed in the high-resolution ESI-TOF mass spectra (positive mode) of all CAi-TCI PSs 4–6 as well as the control PS 7.
Optical properties
All molecules were studied in diluted DMF solutions and their absorption, maximum emission wavelength, fluorescence quantum yield and lifetime were systematically studied. The absorption and emission spectra of CAi-TCI 4 are depicted in Fig. 1 as an example. All the CAi-TCI hybrid systems exhibit two broad absorption bands centered at around 410 nm and 480 nm corresponding to the absorption of the TCI central core. These CAi-TCI hybrid systems exhibit a broad emission band at 532 nm with moderate Stokes shift (∼50 nm) and fluorescence quantum yields between 0.13 and 0.20 using rose bengal as a reference (ΦF = 0.11 in EtOH).33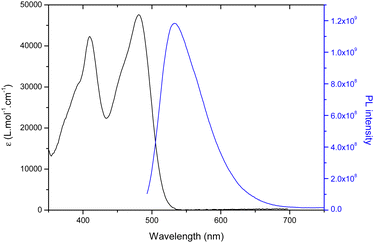 | ||
| Fig. 1 UV-visible absorption (black) and emission spectra (blue) of CAi-TCI 4 in DMF (C = 1.5 × 10−5 mol L−1, λexc = 435 nm). | ||
The ability of PSs 4–7 to generate 1O2 was then evaluated by monitoring its photoluminescence (PL) at 1270 nm in aerated DMF using rose bengal as a reference (ΦΔ = 0.40).33 As shown in Table 1, the nature of CAi had very little effect on the production of 1O2 with a 1O2 yield between 0.31–0.40 for all TCIs 4, 6, 7 whereas in the case of 5, a little lower 1O2 quantum yield is noticed. These yields are in the same range as that of rose bengal.34 Indeed, the presence of a halogen substituent, namely bromine, on the TCI core enables efficient singlet oxygen generation due to increased singlet-to-triplet inter-system crossing (ISC) owing to the so-called heavy atom effect.31
| Compounds | λ abs (nm) | ε (L mol−1 cm−1) | λ em (nm) | Φ F | τ obs (ns) | Φ Δ |
|---|---|---|---|---|---|---|
| a Rose bengal was used as a reference both for determining fluorescence quantum yields and 1O2 generation efficiency. b 1O2 generation efficiency. | ||||||
| 4 | 410 | 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
532 | 0.20 | 7.6 | 0.36 |
| 481 | 47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
|||||
| 5 | 412 | 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
532 | 0.18 | 7.0 | 0.25 |
| 483 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
|||||
| 6 | 413 | 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
532 | 0.13 | 9.8 | 0.31 |
| 483 | 43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
|||||
| 7 | 410 | 51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
532 | 0.18 | 6.7 | 0.39 |
| 480 | 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
|||||
Carbonic anhydrase inhibition assays
The CAi-TCI hybrid systems 4–6 as well as TCI PS without hCA IX inhibitor 7 were subsequently assessed for their inhibitory activity against carbonic anhydrases, using a stopped-flow assay method. Four clinically relevant human isoforms were investigated including the two cytosolic off-targets, hCA I and hCA II, along with two tumour-associated membrane-bound isoforms, hCA IX and hCA XII. The clinically used acetazolamide (AAZ) served as a reference drug (Table 2). As anticipated, the negative control 7, which lacks a pharmacophoric CA inhibitory moiety, exhibited no inhibitory activity (Ki > 100 μM) against the four tested isoforms. When compared to the standard drug AAZ, which effectively inhibits all four CA isoforms considered in this study, analogues 4, 5 and 6 exhibited lower effectiveness as hCA IX and XII inhibitors.| Compounds | K i (nM) | |||
|---|---|---|---|---|
| Cytosolic | Membrane-bound | |||
| hCA I | hCA II | hCA IX | hCA XII | |
| a Mean from three different measurements by a stopped flow technique (errors were in the range of 5–10% of the reported values). | ||||
| 4 | >100 μM | >100 μM | 86.2 | 45.3 |
| 5 | 89.5 | 28.7 | 42.9 | 21.3 |
| 6 | >100 μM | >100 μM | 76.9 | 48.2 |
| 7 | >100 μM | >100 μM | >100 μM | >100 μM |
| AAZ | 250 | 12 | 25 | 5.7 |
However, derivatives 4 and 6 displayed strong selectivity, as neither of them inhibited the cytosolic off-targets hCA I and hCA II. Specifically, coumarin derivative 4 and sulfocoumarin derivative 6 demonstrated identical activity profiles against the membrane-bound isoforms, with an inhibitory activity against hCA IX at 86.2 and 76.9 nM, respectively, and a slightly improved inhibitory activity against hCA XII, with Ki values of 45.3 and 48.2 nM, respectively. Although compound 5 displayed one of the best inhibition values against hCA IX and hCA XII, with inhibition constants of 42.9 and 21.3 nM, respectively, it exhibited the same inhibitory profile against hCA I and hCA II. The lack of selectivity of benzenesulfonamide towards the different CA isoforms is well-known28–30 and is due to the lack of additional interactions with hydrophobic and/or hydrophilic residues in the region of the active site, which influences the inhibitor binding but also, the selectivity.35,36 Given the absence of selectivity exhibited by compound 5, we considered only the selective inhibitors 4 and 6, along with the negative control 7, in the biological studies.
Biological studies in vitro
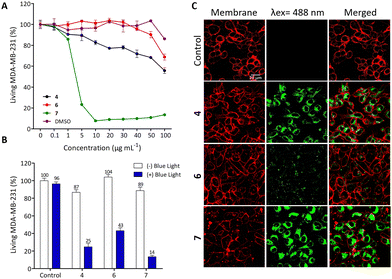
The biological potential of 4, 6 and 7 was studied both in vitro and in vivo. First, human breast cancer cell line (MDA-MB-231) was used to establish the dark cytotoxicity of these three compounds. To achieve this goal and in order to reduce the risk of precipitation due to solubilization of compounds in aqueous media, the compounds were first dissolved in DMSO followed by 10-fold dilutions in culture media. Fig. 2(A) describes the cell viability after 72 h of incubation with increasing concentrations of 4, 6 and 7 from 0.1 to 100 μg mL−1, corresponding to 0.12 to 142.21 μM (Fig. S24 in the ESI†). As observed, 4 and 6 exhibit relatively low toxicity in the studied condition while 7 is strongly toxic from 5 μg mL−1 (7.11 μM). In order to clearly demonstrate a PDT effect without any dark toxicity background, we performed the PDT experiments by incubating cells with only 0.5 μg mL−1 compound (Fig. 2(B)).For this, MDA-MB-231 cells were incubated 24 h with 0.5 μg mL−1 compound and excited during 30 s with a blue LED source (470 ± 22 nm). Two days later, living cells were quantified and Fig. 2(B) showed that compounds 4, 6 and 7 are very efficient in killing cancer cells by PDT with 75%, 57% and 86% of cell death induced by light excitation, respectively. Then, we decided to analyse the imaging potential of such compounds by incubating cells with the ligands at 10 μg mL−1 for 24 h. Fig. 2(C) highlights the strong fluorescence of compounds 4 and 7 and the localisation inside the cells, which suggests their potential for imaging of living cells.
These data demonstrate that 4 exhibits a low toxic level without specific excitation, a strong PDT and imaging potential. Compound 6 is not toxic in the studied conditions without excitation, but it is less efficient in PDT and particularly in imaging. Finally, while compound 7 may be deemed the most effective choice for imaging and PDT, its notable high toxicity above 5 μg mL−1 stems from its lack of specificity, as it can be internalized by all cells owing to its lipophilic properties.
Biological studies in vivo
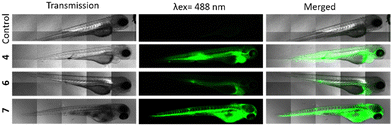
These highly encouraging results on cells led us to test their effectiveness in vivo, on zebrafish embryos. This is a particularly well-suited substitute animal model for small mammals to study biocompatibility, PDT, and imaging.37,38 We first injected the three compounds into the intravenous system of embryos. More precisely, 10 nL of 4, 6 and 7 at a concentration of 1 mg mL−1 in 5% glucose were injected in the tail vein of embryos at 72 hours post-fertilization (hpf). If we consider a total blood volume of around 80 nL for a 72 hpf embryo,39 the injection of 10 nL at 1 mg mL−1 allows to reach a final concentration of around 111 μg mL−1 (Fig. 3). As observed in vitro, at first sight, all compounds were luminescence and very well dispersed in the embryos, suggesting a good bioavailability, but analogue 6 was the least fluorescent. 4 was also well-dispersed and particularly luminescence when observed under the same condition of 6, that is 3 hours after injection and using a laser power of 30%.Finally, the negative control 7 exhibiting a tremendous brightness is visualized at only 1% laser power and immediately after injection because embryos died in the minutes following the injection. Taking all these results into account, we concluded that compound 4 was the most effective in terms of imaging and PDT, while maintaining a high level of safety. So, we decided to study its PDT activity in vivo. For this, MDA-MB-231 cells stably expressing red fluorescece protein were injected in the yolk of 30 hpf embryos.
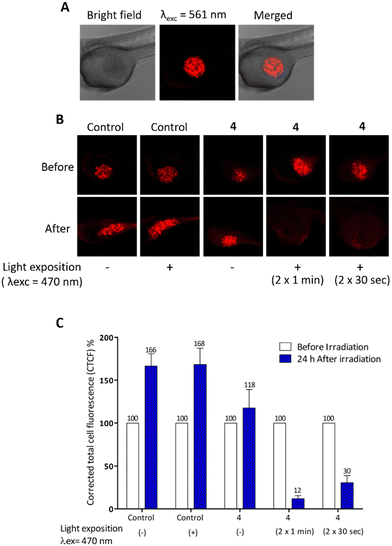
These MDA-MB-231 cells were previously treated (or not) with 10 μg mL−1 of compound 4 for 24 h (Fig. 4). Twenty-four hours after cell injection, embryos were imaged then, exposed to blue light for 2 min. One day later, embryos were imaged again to monitor the xenograft growth. By quantifying the fluorescence intensity of the xenograft before and after light exposure, results (Fig. 4) show the high efficiency of compound 4 in eradicating the tumours.
Conclusions
In summary, we successfully designed and synthesized thiochromenocarbazole imide derivatives incorporating carbonic anhydrase inhibitor moiety. Among the three CAi-TCI hybrid systems that were the subject of biological assays, compound 4, featuring the selective hCA IX/hCA XII coumarin inhibitor, displayed low dark toxicity and exhibited robust imaging and PDT effects both in vitro and in vivo. Interestingly, the negative-control compound 7, lacking pharmacophoric scaffold able to inhibit CA, surprisingly demonstrated significant imaging and PDT effects, albeit with high toxicity. The high lipophilicity of this non-targeted photosensitizer may account for its effectiveness, as it can readily penetrate various cell types, thereby explaining its significant toxicity. This underscores the importance of focusing on therapeutic targets, such as relevant carbonic anhydrase isoforms, to address this issue. Altogether, this study provides a valuable resource for the study of innovative photosensitizers linked to selective carbonic anhydrase inhibitors, with potential applications in imaging and phototherapy.Data availability
All of the additional information and experimental data are provided in the ESI.†Author contributions
Merabti A: methodology, investigation; Puchan Sanchez D: methodology, investigation; Nocentini A: methodology, investigation; Ali L. M. A.: methodology, investigation; Nguyen C: methodology, investigation; Durand D: methodology, investigation; Hamon K: methodology, investigation; Ghanem T: methodology, investigation; Arnoux P: methodology, investigation; Josse P: methodology, investigation; Frochot C: formal analysis, review & editing; Zalubovskis R: methodology, investigation; Richeter S: methodology, investigation; Gary-Bobo: formal analysis, writing – review & editing; Supuran CT: formal analysis, review & editing; Cabanetos C: formal analysis, review & editing; Winum J-Y: formal analysis, conceptualization, supervision, writing – review & editing; Clément S: formal analysis, conceptualization, supervision, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful to the University of Montpellier, the CNRS and the French Ministry of Research for financial support. The work is also partly supported by Ligue contre le Cancer funding to SC and JYW. A. M. thanks the Algerian Ministry of Education and Research for her PhD grant. We acknowledge the ZeNeuro platform (Inserm U1198), especially Nicolas Cubedo, and the imaging facility “Montpellier Ressources Imagerie” (MRI), member of the national infrastructure France-BioImaging supported by the French National Research Agency (ANR\u000210-INBS-04, “Investments for the future”). The ANR is also acknowledged for the financial support of BTXI-Apogee (ANR-20-CE05-0029) used for the development and synthesis of TCI precursors.Notes and references
- W. Jiang, M. Liang, Q. Lei, G. Li and S. Wu, Cancers, 2023, 15(3), 585 CrossRef CAS PubMed.
- H. O. Alsaab, M. S. Alghamdi, A. S. Alotaibi, R. Alzhrani, F. Alwuthaynani, Y. S. Althobaiti, A. H. Almalki, S. Sau and A. K. Iyer, Cancers, 2020, 12(10), 2793 CrossRef CAS PubMed.
- J. P. Celli, B. Q. Spring, I. Rizvi, C. L. Evans, K. S. Samkoe, S. Verma, B. W. Pogue and T. Hasan, Chem. Rev., 2010, 110(5), 2795 CrossRef CAS PubMed.
- S. Kwiatkowski, B. Knap, D. Przystupski, J. Saczko, E. Kedzierska, K. Knap-Czop, J. Kotlinska, O. Michel, K. Kotowski and J. Kulbacka, Biomed. Pharmacother., 2018, 106, 1098 CrossRef PubMed.
- A. Challapalli, L. Carroll and E. O. Aboagye, Clin. Transl. Imaging, 2017, 5(3), 225 CrossRef PubMed.
- J. M. Brown and W. R. Wilson, Nat. Rev. Cancer, 2004, 6, 437 CrossRef PubMed.
- K. R. Atkuri, L. A. Herzenberg, A.-K. Niemi and T. Cowan, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 4547 CrossRef CAS PubMed.
- V. Petrova, M. Annicchiarico-Petruzzelli, G. Melino and I. Amelio, Oncogenesis, 2018, 7(1), 10 CrossRef PubMed.
- G. Chen, K. Wu, H. Li, D. Xia and T. He, Front. Oncol., 2022, 12, 961637 CrossRef CAS PubMed.
- L. Larue, B. Myrzakhmetov, A. Ben-Mihoub, A. Moussaron, N. Thomas, P. Arnoux, F. Baros, R. Vanderesse, S. Acherar and C. Frochot, Pharmaceuticals, 2019, 12(4), 163 CrossRef CAS PubMed.
- Y. Wan, L.-H. Fu, C. Li, J. Lin and P. Huang, Adv. Mater., 2021, 33(48), e2103978 CrossRef PubMed.
- C. Zhang, X. Hu, L. Jin, L. Lin, H. Lin, Z. Yang and W. Huang, Adv. Healthcare Mater., 2023, 12(24), e2300530 CrossRef PubMed.
- N. Kwon, T. Guo, Z. Liu and J. Yoon, Angew. Chem., Int. Ed., 2018, 57(36), 11522 CrossRef PubMed.
- C. T. Supuran, Bioorg. Med. Chem. Lett., 2023, 93, 129411 CrossRef CAS PubMed.
- S. Kalinin, A. Malkova, T. Sharonova, V. Sharoyko, A. Bunev, C. T. Supuran and M. Krasavin, Int. J. Mol. Sci., 2021, 22(24), 13405 CrossRef CAS PubMed.
- H. S. Jung, J. Han, H. Shi, S. Koo, H. Singh, H.-J. Kim, J. L. Sessler, J. Y. Lee, J.-H. Kim and J. S. Kim, J. Am. Chem. Soc., 2017, 139(22), 7595 CrossRef CAS PubMed.
- Y. Jiang, J. Li, Z. Zeng, C. Xie, Y. Lyu and K. Pu, Angew. Chem., Int. Ed., 2019, 58(24), 8161 CrossRef CAS PubMed.
- W. Zhu, Y. Liu, Z. Yang, L. Zhang, L. Xiao, P. Liu, J. Wang, C. Yi, Z. Xu and J. Ren, J. Mater. Chem. B, 2018, 6, 265 RSC.
- W. Zhu, L. Zhang, Z. Yang, P. Liu, J. Wang, J. Cao, A. Shen, Z. Xu and J. Wang, Chem. Eng. J., 2019, 358, 969 CrossRef CAS.
- G.-L. Fan, P. Yuan, F.-A. Deng, L.-S. Liu, Y.-L. Miao, C. Wang, X.-Z. Qiu, X.-Y. Yu, H. Cheng and S.-Y. Li, ACS Appl. Bio. Mater., 2020, 3(9), 6124 CrossRef CAS PubMed.
- F. Wang, T. X. Meerovich, F. Hong, Z.-L. Chen and Y.-J. Yan, Dyes Pigm., 2022, 203, 110328 CrossRef CAS.
- A. Merabti, M. Roger, C. Nguyen, A. Nocentini, P. Gerbier, S. Richeter, M. Gary-Bobo, C. T. Supuran, S. Clément and J.-Y. Winum, Eur. J. Org. Chem., 2022, e202101538 CrossRef CAS.
- X. Su, W.-J. Wang, Q. Cao, H. Zhang, B. Liu, Y. Ling, X. Zhou and Z.-W. Mao, Angew. Chem., Int. Ed., 2022, 61, e202115800 CrossRef CAS PubMed.
- P. Kumar, P. Singh, S. Saren, J. Sayala, S. Sivakumar and A. K. Patra, Dalton Trans., 2022, 51, 18416 RSC.
- L. Hao, J. Wang, Z.-Y. Pan, Z.-W. Mao and C.-P. Tan, Chem. Commun., 2022, 58, 8069 RSC.
- H. S. Jung, S. Koo, M. Won, S. An, H. Park, J. L. Sessler, J. Han and J. S. Kim, Chem. Sci., 2023, 14, 1808 RSC.
- A. Merabti, S. Richeter, C. T. Supuran, S. Clément and J.-Y. Winum, Expert Opin. Ther. Targets, 2023, 27(9), 817, DOI:10.1080/14728222.2023.2255380.
- A. Angeli, F. Carta, A. Nocentini, J. Y. Winum, R. Zalubovskis, A. Akdemir, V. Onnis, W. M. Eldehna, C. Capasso, G. De Simone, S. M. Monti, S. Carradori, W. A. Donald, S. Dedhar and C. T. Supuran, Metabolites, 2020, 10(10), 412 CrossRef CAS PubMed.
- C. B. Mishra, M. Tiwari and C. T. Supuran, Med. Res. Rev., 2020, 40(6), 2485 CrossRef CAS PubMed.
- C. T. Supuran, Expert Opin. Ther. Pat., 2023, 33(11), 701, DOI:10.1080/13543776.2023.2245971.
- L. Abad Galán, J. M. Andrés Castán, C. Dalinot, P. Simón Marqués, P. Blanchard, O. Maury, C. Cabanetos, T. Le Bahers and C. Monnereau, Phys. Chem. Chem. Phys., 2020, 22, 12373 RSC.
- J. María Andrés Castán, C. Amruth, P. Josse, L. Abad Galan, P. Simón Marqués, M. Allain, O. Maury, T. Le Bahers, P. Blanchard, C. Monnereau, G. C. Welch and C. Cabanetos, Mater. Chem. Front., 2022, 6, 1912 RSC.
- P. G. Seybold, M. Gouterman and J. Callis, Photochem. Photobiol., 1969, 9(4), 229 CrossRef CAS PubMed.
- R. W. Redmond and J. N. Gamlin, Photochem. Photobiol., 1999, 70(4), 391 CrossRef CAS PubMed.
- V. Alterio, A. Di Fiore, K. D’Ambrosio, C. T. Supuran and G. De Simone, Chem. Rev., 2012, 112(8), 4421 CrossRef CAS PubMed.
- M. A. Pinard, B. Mahon and R. McKenna, BioMed Res. Int., 2015, 2015, 15 Search PubMed.
- Z. Chen, S. Pascal, M. Daurat, L. Lichon, C. Nguyen, A. Godefroy, D. Durand, L. M. A. Ali, N. Bettache, M. Gary-Bobo, P. Arnoux, J.-F. Longevial, A. D’Aléo, G. Marchand, D. Jacquemin and O. Siri, ACS Appl. Mater. Interfaces, 2021, 13(26), 30337 CrossRef CAS PubMed.
- N. Hamon, A. Roux, M. Beyler, J.-C. Mulatier, C. Andraud, C. Nguyen, M. Maynadier, N. Bettache, A. Duperray, A. Grichine, S. Brasselet, M. Gary-Bobo, O. Maury and R. Tripier, J. Am. Chem. Soc., 2020, 142(22), 10184 CrossRef CAS PubMed.
- E. Cörek, G. Rodgers, S. Siegrist, T. Einfalt, P. Detampel, C. M. Schlepütz, S. Sieber, P. Fluder, G. Schulz, H. Unterweger, C. Alexiou, B. Müller, M. Puchkov and J. Huwyler, Small, 2020, 16(31), 2000746 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section, optical properties, hCA inhibition assays, biological assays. See DOI: https://doi.org/10.1039/d3ma00926b |
| This journal is © The Royal Society of Chemistry 2024 |