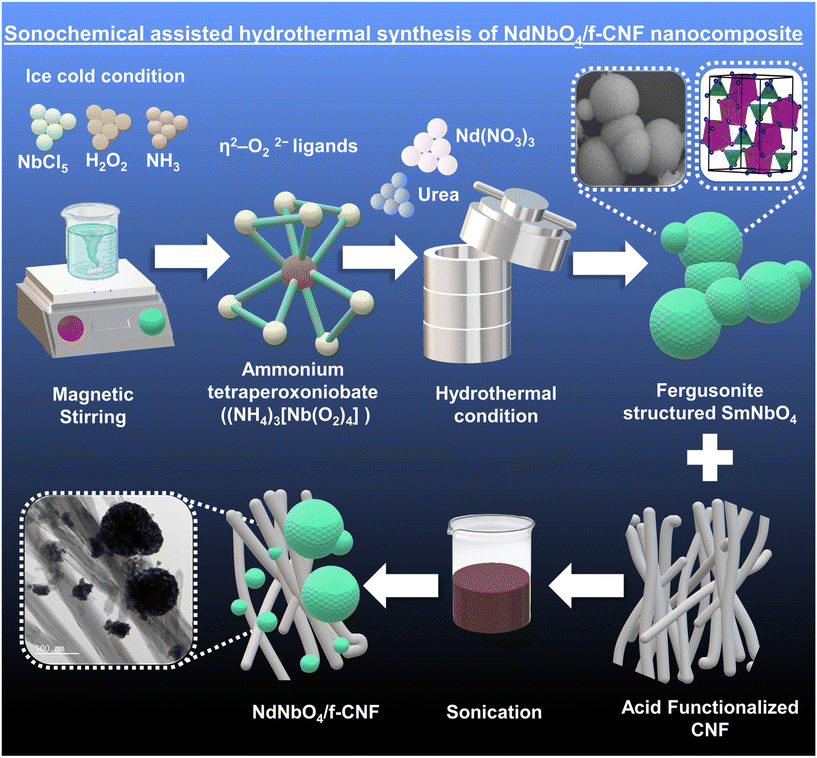
Neodymium niobate nanospheres on functionalized carbon nanofibers: a nanoengineering approach for highly sensitive vanillin detection†
I. Jenisha Daisy
Priscillal
and
Sea-Fue
Wang
 *
*
Department of Materials and Mineral Resources Engineering, National Taipei University of Technology, No. 1, Sec. 3, Chung-Hsiao East Rd., Taipei 106, Taiwan. E-mail: sfwang@ntut.edu.tw
First published on 28th May 2024
Abstract
Vanillin (VAN), the primary aroma compound in vanilla, contributes significantly to sensory delight; however, its unrestrained presence poses notable health risks. In response to the demanding concern regarding food safety, researchers have directed their efforts towards the detection of VAN, seeking sustainable strategies for contamination prevention. A groundbreaking solution has emerged in the form of a novel sensing platform, whose core lies on a finely tuned electrode, crafted through the incorporation of nano-sized NdNbO4 spheres onto carbon nanofibers (CNFs). This incorporation serves to augment the capabilities of a glassy carbon electrode (GCE), transforming it into a highly sensitive detector primed for vanillin detection. The NdNbO4/f-CNF nanocomposite embodies a paradigm of synergistic collaboration, wherein the nonlinear cumulative effects of synergism and quantum confinement impart exceptional performance characteristics. Notably, the sensor achieves a low detection limit of 6.3 nmol L−1, indicative of its remarkable sensitivity of 2.3 μA μ(mol L−1)−1 cm−2 and precision of 1.519 and 4.72%. Moreover, the sensor boasts a wide linear range spanning from 0.001 to 63.101 μmol L−1. These attributes, coupled with its discerning selectivity and robust stability, underscore its efficacy as a versatile tool for vanillin detection. Indeed, its successful deployment in monitoring food samples underscores its applicability across diverse culinary contexts, further cementing its status as a pivotal asset in safeguarding food quality and consumer well-being.
I. Jenisha Daisy Priscillal is currently doing her Ph.D. in Materials and Mineral Resources Engineering at the National Taipei University of Technology, Taiwan. Her research is mainly focused on bimetal oxides and nano-heterostructured carbon composites for electrochemical sensors and supercapacitors. Email: jenishadaisyi@gmail.com |
Sea-Fue Wang received his Ph.D. from the Pennsylvania State University and is currently serving as a distinguished professor in the Department of Materials and Mineral Resources Engineering at the National Taipei University of Technology. His works mainly focus on solid oxide fuel cells, electrochemical sensors, bioceramics, and lithium-ion batteries. Email: sfwang@ntut.edu.tw |
1. Introduction
In the contemporary culinary landscape, the allure of food hinges greatly on the nuanced play of flavors, often augmented by the judicious use of flavor enhancers. Among these, vanilla reigns supreme, lending its distinctive essence to a plethora of everyday indulgences such as cookies and chocolates. Vanillin (VAN, 4-hydroxy-3-methoxy benzaldehyde) is a key aroma compound in vanilla associated with positive emotional experiences and possessing anti-epileptic and anti-anxiety effects.1 Despite over 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons of annual production, less than 1% comes from natural vanilla orchids, with the majority synthesized chemically.2 Controlled vanillin intake offers antioxidant, antidepressant, anticancer, and anti-inflammatory benefits, particularly aiding anemic patients.3–5 However, excessive consumption can lead to liver, spleen, and kidney damage, as well as health issues like nausea, headaches, vomiting, and allergic reactions.3,6 To address these concerns, the China National Food Safety Standard and Joint FAO/WHO Expert Committee on Food Additive (JECFA) has set strict guidelines for vanillin consumption,7,8 emphasizing the need for rigorous monitoring of vanillin levels in food products.
000 tons of annual production, less than 1% comes from natural vanilla orchids, with the majority synthesized chemically.2 Controlled vanillin intake offers antioxidant, antidepressant, anticancer, and anti-inflammatory benefits, particularly aiding anemic patients.3–5 However, excessive consumption can lead to liver, spleen, and kidney damage, as well as health issues like nausea, headaches, vomiting, and allergic reactions.3,6 To address these concerns, the China National Food Safety Standard and Joint FAO/WHO Expert Committee on Food Additive (JECFA) has set strict guidelines for vanillin consumption,7,8 emphasizing the need for rigorous monitoring of vanillin levels in food products.
In recent days, numerous procedures have been described for the detection of vanillin, including gas chromatography,9 gas chromatography-mass spectrometry (GC-MS),10 high-performance liquid chromatography,11,12 liquid chromatography-mass spectrometry (LC-MS),13 chemiluminescence,14 capillary electrophoresis,15 and colorimetry.16 These existing methodologies necessitate a state-of-the-art lab environment, sophisticated instrumentation, tedious sample preparation, and a skilled operator for successful experimental execution.16,17 These methods fall short in practical applications due to issues like non-repeatability and the inability to conduct on-site testing. Conversely, modern electrochemical sensors are designed for field deployment, addressing space and power constraints with cost-effective instrumentation.18 The radical advancements in nanotechnology and nanoengineering have led to the discovery of eminent sensing platforms with a remarkable surface versus volume ratio.19 Among the prevailing sensing techniques, the voltammetry approach is more beneficial as it is inherently fast, strongly sensitive, and highly selective and offers minimal detection and quantification limits.20 The implementation of the aforementioned factors strongly depends on the electron transit, aspect ratio, potential window, and background current of the working electrode. A glassy carbon electrode (GCE) is chosen as the working electrode as it is a gas-impermeable conductive material resistive to erosion by chemicals. This electrode is capable of amplifying signals over a wide potential window ranging from +1.2 V to −0.8 V.21 The current challenge relies on finding electroactive materials whose sensing characteristics might be readily altered to create sensors with cross-selectivity and good stability which is obligatory for the construction of novel voltammetric sensors.
Recent advancements have introduced various GCE modifiers, including lanthanum nickelate anchored carbon fibers,22 CuO/NiO nanocomposites,23 MoS2 nanosheets on g-C3N4 nanotubes,24 and LaCoO3 with carbon nanofibers,25 for sensitive vanillin detection. Drawing inspiration from this literature, carbon nanofibers (CNFs) have been selected for exploration as an electrode modifier. The cone helix structure of CNFs undergoes structural transformation under vigorous conditions to form a stacked cone structure with a high aspect ratio. In order to activate the edge planes of CNFs, acid functionalization is carried out which introduces oxygen functionalities at the periphery of the carbon backbone which increases the surface-active group-to-volume ratio, thereby acting as an immobilization matrix.26
On the other hand, in the modern era, rare earth metal oxides (REMOs) are considered as prime candidates for surface modification due to their noteworthy catalytic behavior, rapid oxygen ion mobility, effective charge transfer, and high surface basicity.27 Rare earth niobates, a unique subset of REMOs, present challenges in their exploration as electrode materials due to their complex structure and synthesis methods. They comprise perovskite-like slabs with the formula ABO4, where A represents an f-block cation (e.g., Nd3+) and B typically refers to a tiny transition metal cation, such as niobium in its 5+ oxidation state. The complexity arises primarily from the potential coordination of the niobium cation, leading to reversible ferroelastic phase transitions and lattice deformations, resulting in significant macroscopic stresses within the lattice.28 The known niobates, with the exception of scheelites and fergusonites, are dominated by pentavalent Nb atoms in octahedral coordination. These niobates are recognized as fundamental mysteries of crystal chemistry because Nb5+ only exhibits tetrahedral coordination with oxygen and the interatomic gap between Nb–O and Nb–O in Nb2O5 is higher.29 This study aims to develop a simple hydrothermal technique that uses superheated aqueous solution processing under high pressure and temperature conditions to dissolve and recrystallize (recover) materials that are generally insoluble under ordinary circumstances.30 The goal is to produce the pure phase of NdNbO4 with valuable physiognomies.
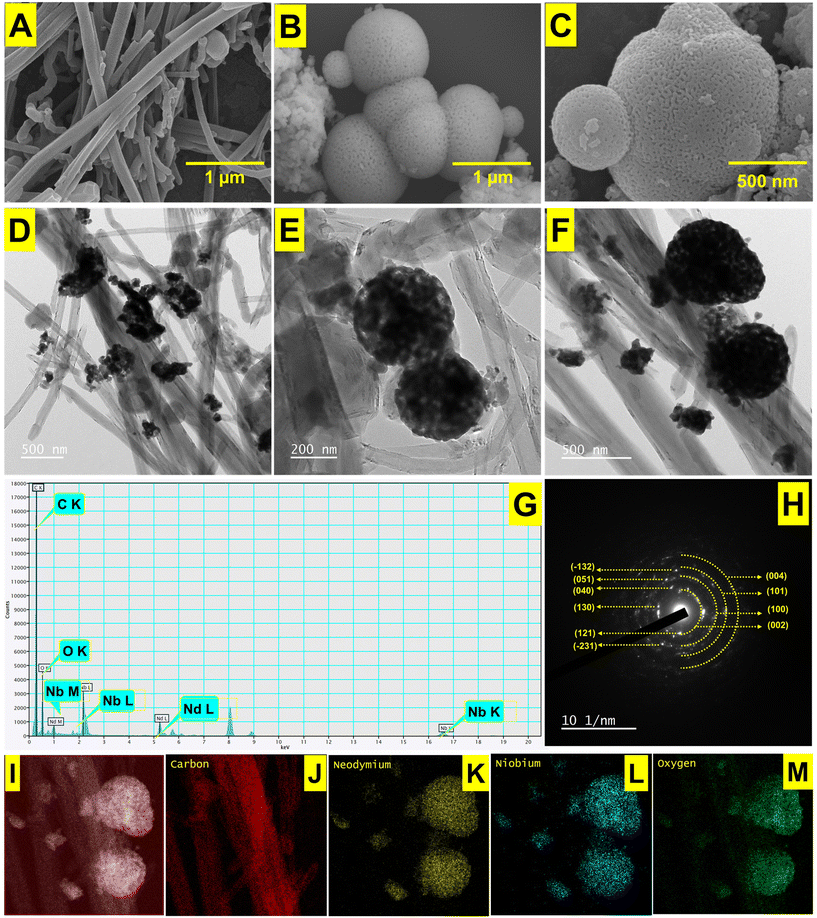
To enable NdNbO4 nanocrystalites to function as an efficient electrocatalyst, a CNF is used as an immobilization matrix. The nanocomposite prepared operates as a renowned transducer due to the cumulative effects of the individual constituents acquired through the formation of a hybrid heterojunction that enhances the conduits for electrochemical signal transmission. The NdNbO4/f-CNF nanocomposite is described here as an active electrode modification for the very sensitive detection of VAN. Through crystallographic, spectroscopic, and microscopic techniques, the produced nanocomposite was carefully studied. Additionally, the electrochemical parameters have been adjusted, and by expanding the experiments to food sample analysis, the practicability was examined.
2. Experimental section
2.1. Chemicals and reagents
Neodymium nitrate (Nd(NO3)3 ≥ 99% purity), niobium pentachloride (NbCl5 ≥ 99% purity), hydrogen peroxide (H2O2), and urea (CH4N2O) were purchased from Sigma Aldrich and carbon nanofibers (CNFs) were purchased from Rur-Grapheneox Company. All other necessary reagents and the abovementioned chemicals have been used without further refinement. The preparation of phosphate-buffered saline (PBS, 0.1 M) involved the utilization of sodium phosphate dibasic (Na2HPO4) and sodium dihydrogen phosphate (NaH2PO4). To adjust the pH of the buffer solution, 1 N HCl and 0.1 M NaOH solutions were employed. 0.5 mM ferricyanide [Fe(CN)6]3−/4− solution was prepared by adding potassium ferrocyanide (K4[Fe(CN)6]·3H2O) and potassium ferricyanide (K3[Fe(CN)6]) in 0.1 M KCl. All the experiments used ultrapure fresh water obtained from a Millipore water purification system (Milli-Q, specific resistivity > 18 MΩ cm, S.A., Molsheim, France).2.2. Instrumentation
Phase configurational analysis was carried out by X-ray diffraction (XRD) using the D2 Phaser XRD instrument. The chemical composition of the materials was quantitatively examined using X-ray photoelectron spectroscopy ESCA/Auger Laboratory (National Taiwan University, Taiwan). Structural morphology and elemental composition were investigated with the aid of energy-dispersive X-ray spectroscopy (EDX) incorporated with field-emission scanning electron microscopy (FESEM, JEOL JSM-7100F). Detailed crystallite information of the as-prepared nanocomposite was analyzed using high-resolution transmission electron microscopy (HRTEM) (H-7600, Hitachi, Japan) operating at 200 kV, alongside EDX (HORIBA EMAX XACT) spectroscopy.2.3. Synthesis of NdNbO4
In a typical synthetic procedure, a 50 mL homogeneous solution containing 0.05 M niobium pentachloride (NbCl5) was prepared and constantly maintained in an ice bath at 10 °C. While under the cold conditions, 15 mL of hydrogen peroxide was added dropwise to the prepared solution with continuous agitation. Subsequently, excess ammonia solution was slowly added to the reaction mixture until saturation, resulting in the formation of an ammonium tetra peroxo niobate complex ((NH4)3[Nb(O2)4]). Following the removal of the ice bath, 50 mL of 0.05 M Nd(NO3)3·6H2O solution was introduced and stirring was continued for 20 minutes. Eventually, 0.1 M urea was added to the reaction mixture, which was then transferred to a Teflon-lined autoclave and maintained under hydrothermal conditions for 24 hours at 180 °C. The suspension obtained post-hydrothermal processing was subjected to centrifugation to obtain the precipitate, which was then washed multiple times to eliminate impurities and achieve the desired NdNbO4 nanocryslalite.The formation of a peroxocomplex intermediate leads to the release of a considerable amount of Nb5+ ions in the reaction medium. Simultaneously, urea undergoes conversion to ammonia and isocyanic acid, which further decomposes into CO2 and NH3. Upon the addition of Nd(NO3)3 to the mixture, the reaction tends to promote the formation of neodymium niobium hydroxide carbonate. Under the influence of high temperature and pressure, this compound eliminates CO2 and H2O from carbonates and hydroxide moieties to precipitate out NdNbO4.
2.4. Functionalization of the CNF
Edge site activation of the pristine CNF was accomplished via acid functionalization. This process employs an oxidant consisting of concentrated nitric acid and concentrated sulfuric acid in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 volume ratio. The CNF was dispersed within the acidic mixture, resulting in the formation of a dense suspension, which was continuously stirred magnetically for 1 hour at 60 °C. Following evaporation, a thick black slurry was obtained. This slurry was then dispersed in DI water and subjected to centrifugation to eliminate the acidic mixture. This cycle was repeated multiple times, with the pH being monitored after each iteration using a pH meter. Once the slurry reaches a neutral pH, the residues were washed with ethanol and left to dry overnight. The resulting functionalized CNF exhibits enhanced solubility and electrical conductivity due to the introduction of oxygen functionalities.
3 volume ratio. The CNF was dispersed within the acidic mixture, resulting in the formation of a dense suspension, which was continuously stirred magnetically for 1 hour at 60 °C. Following evaporation, a thick black slurry was obtained. This slurry was then dispersed in DI water and subjected to centrifugation to eliminate the acidic mixture. This cycle was repeated multiple times, with the pH being monitored after each iteration using a pH meter. Once the slurry reaches a neutral pH, the residues were washed with ethanol and left to dry overnight. The resulting functionalized CNF exhibits enhanced solubility and electrical conductivity due to the introduction of oxygen functionalities.
2.5. Preparation of the NdNbO4/f-CNF composite
To retain the structural and morphological integrity, hydrothermally synthesized NdNbO4 nanoparticles were integrated into the network of functionalized CNF via sonochemical techniques. The weight ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) of NdNbO4 to f-CNF was accurately measured and dispersed in deionized water, leading to the formation of a colloidal solution. Upon centrifugation, the nanocomposite residues were effectively separated. Subsequently, the residues were dried, labeled based on the specific ratio, and then used for subsequent comprehensive characterization. This systematic preparation is demonstrated in Fig. 1.
1) of NdNbO4 to f-CNF was accurately measured and dispersed in deionized water, leading to the formation of a colloidal solution. Upon centrifugation, the nanocomposite residues were effectively separated. Subsequently, the residues were dried, labeled based on the specific ratio, and then used for subsequent comprehensive characterization. This systematic preparation is demonstrated in Fig. 1.
2.6. Fabrication of the electrode
Utilizing suitable electrode modifiers, drop casting emerges as a rapid and versatile technique for the construction of modified electrodes. Prior to the surface modification process, 3 mg of the as-prepared electrode modifier, NdNbO4/f-CNF, was dispersed in 1 mL of deionized water through 15 minutes of sonication. Subsequently, a GCE was polished using alumina powder (0.05 μm) to eliminate any adsorbed impurities. From the suspension containing the desired modifier, 6 mg μL−1 was carefully pipetted and cast onto the surface of the GCE. The droplet, upon contact with the electrode surface, experienced a surface tension gradient, resulting in an outward convective flow known as Marangoni flow influenced by interfacial forces. As the inward Marangoni flow velocity surpassed the outward capillary flow, the solute concentrated at the center, facilitating the adsorption of the desired particles and enabling surface modification. Following this, the coated electrodes were dried at 60 °C for 3 minutes and then prepared for electrochemical experimentation.2.7. Electrochemical characterization
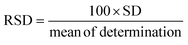 | (1) |
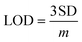 | (2) |
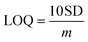 | (3) |
Here, SD is the standard deviation obtained from three measurements of the blank signal and m is the analytical sensitivity represented by the slope of the calibration plot.
2.8. Real sample preparation
For the real sample analysis, a chocolate bar and a milkshake were specifically chosen. A commercially available packet of each was obtained from local supermarkets in Taipei, Taiwan, and processed as real samples for the study.I. About 0.50 g of the chocolate bar was introduced into 20 mL of deionized water and heated to 60 °C in a water bath employing the double-boiling method to facilitate melting. Subsequently, the sample underwent centrifugation at 4000g force for 5 minutes, leading to the separation of the supernatant, which was then diluted in 0.1 M PBS for subsequent analysis.
II. Similarly, 1 mL of the milkshake was added to 5 mL of deionized water and further diluted in 10 mL of 0.1 M PBS, subsequently being directly utilized for the electrocatalytic evaluation of the prepared composite.
3. Results and discussion
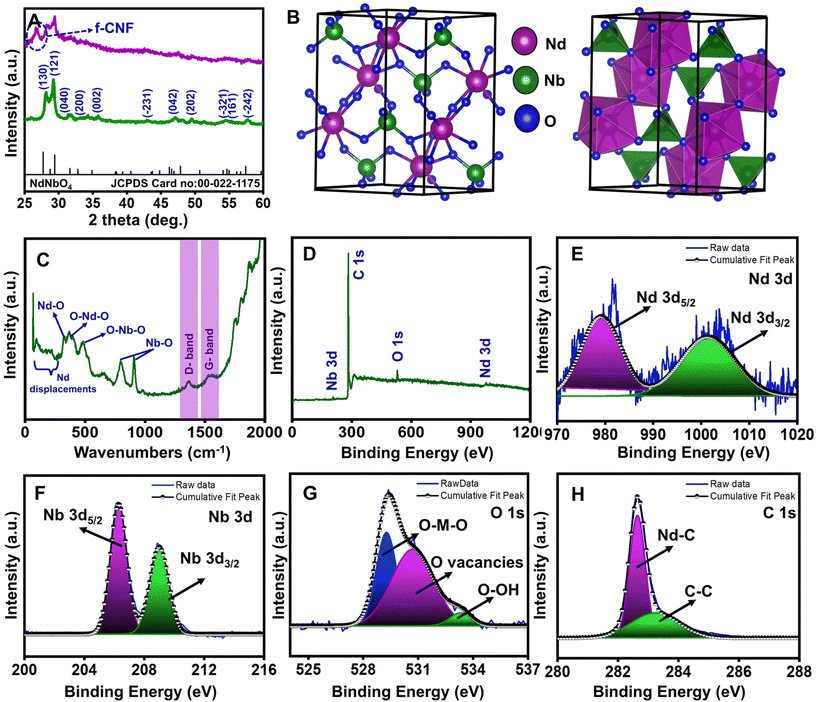
3.1. Characterization of the NdNbO4/f-CNF nanocomposite
The average crystallite size of NdNbO4 was calculated using the Debye–Scherrer formula expressed as:
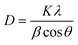 | (4) |
3.2. Electrocatalytic activity of the NdNbO4/f-CNF nanocomposite
EIS operates by introducing a signal of predetermined amplitude as a stimulus superimposed on the DC signal of the electrochemical system. This signal was then applied across a wide range of frequencies, and the resulting response was measured. The impedance analysis of a cell, featuring four terminals and three electrodes in potentiostatic mode, involved the utilization of a redox probe. A sinusoidal voltage, overlaying a DC potential aligned with the standard electrochemical potential (E°) of the redox reaction, was employed for this measurement.| [Fe(CN)6]3− + e− ↔ [Fe(CN)6]4− | (5) |
In accordance with Randel's equivalent circuit (refer to Fig. S1†), the impedance (ZF) of the faradaic process can be elucidated as a composite of the following two components:
| zF = RCt + Zw | (6) |
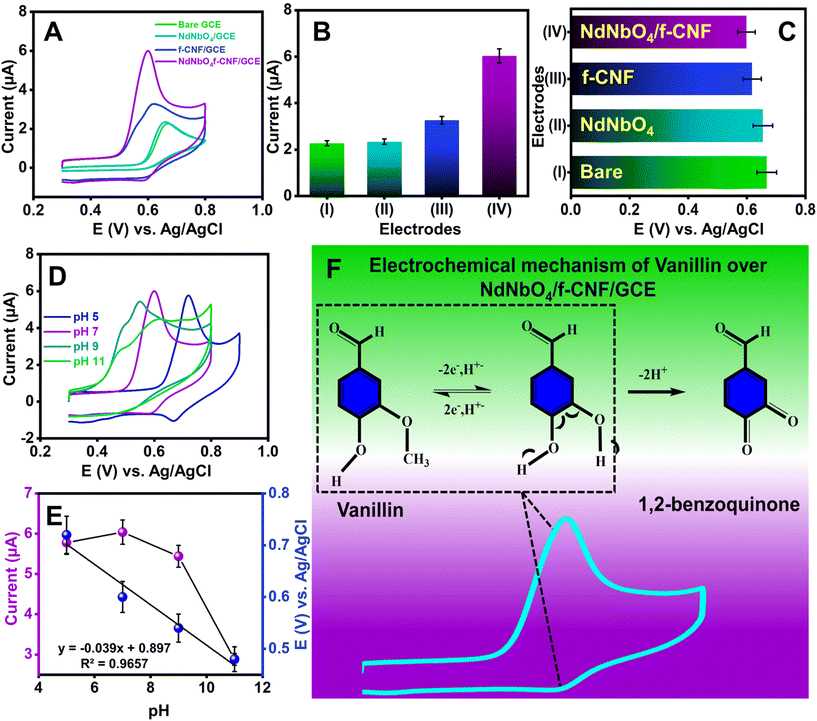
I. The charge-transfer resistance, denoted as Rct, correlates with the rate of the heterogeneous redox reaction, assuming the non-adsorption of oxidizing and reducing species on the electrode surface. The Nyquist plots presented in Fig. S1† depict that the unmodified GCE exhibited a higher Rct value (2128.61 Ω) compared to all other modified electrodes which indicates the necessity for electrode modification. Upon modification of the GCE with NdNbO4 and f-CNF, the Rct value significantly decreases to 655.84 and 149.67 Ω, respectively. This reduction is primarily attributed to the activation of the glassy carbon layer in the electrolyte through enhanced electron mobility provided by the electron-rich modifiers. Furthermore, the GCE modified with the NdNbO4/f-CNF nanocomposite exhibits a further reduction in the Rct value to 41.31 Ω. This reduction is ascribed to the formation of a hybrid heterojunction, facilitating rapid charge transportation between counterparts, along with improved electron transport at the electrified interface.
II. The Warburg impedance (ZW) is defined as the resistance encountered in transporting redox species across the electrode surface in the context of semi-infinite linear diffusion. The Nyquist plot's linear segment reflects the Warburg impedance. In the case of the NdNbO4/f-CNF nanocomposite, its Warburg line is positioned closer to the y-axis, indicating the presence of abundant conduits that enhance the transport of redox species. In stark contrast, the bare GCE exhibits poor transportation, evident from the substantial distance of its Warburg line from the y-axis.
These two parameters collectively emphasize the catalytic improvements achieved through the modification of the GCE with NdNbO4/f-CNF, revealing it as an electrocatalyst with enhanced capabilities for promoting fast and efficient electron mobilization in electrochemical processes.
Furthermore, the electrochemical characteristics of the GCE with various modifications were studied through CV in the same redox probe (5 mM Fe(CN)64−/Fe(CN)63− in 1 M KCl). Fig. S2† presents the CV profiles, featuring distinct redox peaks for the unmodified GCE, NdNbO4/GCE, f-CNF/GCE, and NdNbO4/f-CNF/GCE. The CV profile of the bare GCE revealed a suppressed redox peak current accompanied by a broad peak-to-peak separation, indicative of limited electron mobility at the electrode–electrolyte interface. The introduction of modifications, either with pure NdNbO4 or f-CNF, resulted in a substantial augmentation of peak current and a reduction in peak-to-peak separation. These enhancements are attributed to the highly responsive electrode processes occurring in the interlayer region. Notably, NdNbO4/f-CNF/GCE exhibited an extraordinary redox peak current with a minor peak-to-peak separation, highlighting the synergistic electrochemical advantages derived from the individual constituents within the nanocomposite. Fig. S3(A)† depicts the electrode's response to increasing scan rates, showing a consistent rise in peak current and distinct divergence in the potential. The relationship between the redox current and the square root of the scan rate was then plotted (Fig. S3(B)†), revealing a proportional correlation. The kinetics of the reversible reaction were characterized by regression equations for the anodic and cathodic scans, denoted as Ipa (μA) = 538.99 υ1/2 (mV s−1) − 0.6018 and Ipc (μA) = −474.84 υ1/2 (mV s−1) − 7.3577, respectively, with high regression coefficients of 0.9998 and 0.9996. To quantify the electrochemically active surface area of the fabricated sensor, eqn (3) was employed and the obtained EASA value for NdNbO4/f-CNF/GCE was found to be 1.45 cm2. The augmented electroactive surface proves advantageous for the electrocatalytic activity of the engineered electrode, facilitating improved electrolyte penetration.
3.3. Optimization of the sensing platform
| A + mH+ + ne− ↔ B | (7) |
The Nernst equation for the aforementioned process can be written as given below in eqn (8):
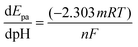 | (8) |
Fig. 4(E) supports the proportionality between the peak potential and the pH of the electrolyte, as indicated by the regression equation Ipa (μA) = −0.039 (X) + 0.897 (R2 = 0. 965). The slope of the linear model is used to estimate the m/n ratio. For this vanillin sensor, the m/n ratio was calculated to be approximately 1, indicating equivalent protons and electrons transfer.
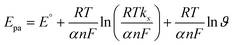 | (9) |
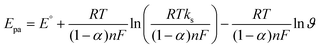 | (10) |
In the context of Laviron theory, the apparent charge transfer rate constant (ks) and the charge transfer coefficient (α) of a surface-confined redox couple can be evaluated using the variation of anodic and cathodic peak potentials as a function of the logarithm of the scan rate (eqn (9) and (10)). The obtained value of α is 0.6115, within the theoretical range of 0.3–0.7, allowing the determination of n as 2, consistent with previous findings. Further investigation into electrode activity involves plotting redox peak currents versus the square root of the scan rate, as shown in Fig. S5.† This analysis reveals that the electrode is primarily governed by surface-controlled kinetics at lower scan rates. However, at higher scan rates, charge carriers diffuse into the active layers of the electrode, leading to amplified signals. Consequently, it is elucidated that reactions occurring at NdNbO4/f-CNF/GCE depend on both diffusion and adsorption of vanillin towards the electrode. Additionally, a slight positive shift in the anodic peak and a slightly negative shift in the cathodic peak are observed.
3.4. Electrochemical detection of vanillin at NdNbO4/f-CNF/GCE
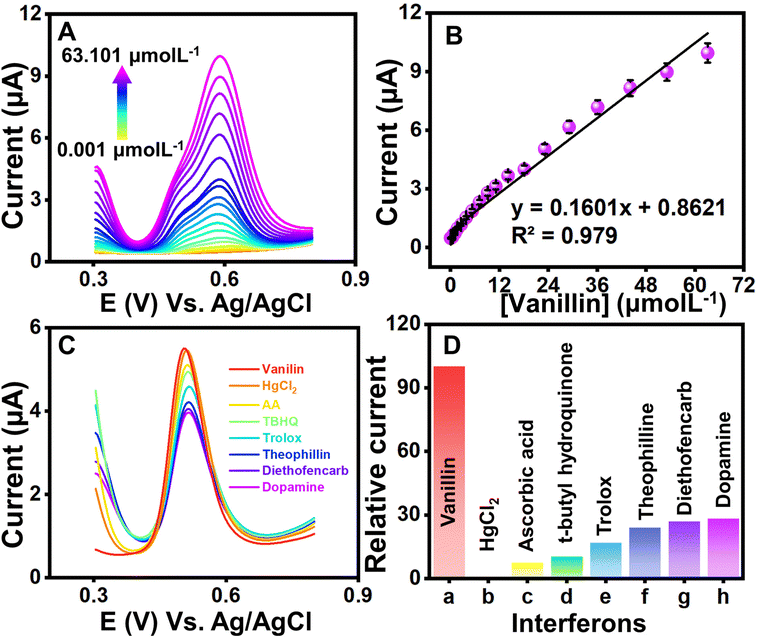
DPV, a potentiodynamic method, involves applying potential pulses along with a linear ramp potential, dependent on the analyte concentration. In the analysis, qualitative insights into vanillin are gleaned from the half-wave potential (E1/2). The DPV plot illustrates measured currents against applied potentials. Fig. 6(A) presents the DPV profile of NdNbO4/f-CNF/GCE, revealing variations with increasing vanillin concentrations. Due to vanillin's organic nature, its adsorption on the electrode surface produces enhanced current responses, minimizing the capacitive current.Optimizing DPV parameters led to a gradual peak current increase with increasing vanillin concentration, attributed to the sensor's antifouling properties. Linear regression demonstrated a proportional relationship between the measured current and the vanillin concentration, resulting in a regression equation: Ipa = 0.1601 [μmol L−1] + 0.8621 (R2 = 0.979) (Fig. 6(B)). The limit of detection (LOD) and the limit of quantification (LOQ) were determined using eqn (2) and (3).
Here, SD is the standard deviation of three blank signals and m is the regression model's slope. The fabricated sensor exhibited LOD and LOQ values of 6.3 and 21 nmol L−1, respectively. The sensor's linearity spanned from 0.001 to 63.101 μmol L−1 and sensitivity within linear ranges was found to be 2.3 μA μ(μmol L−1)−1 cm−2. All the optimized parameters of the proposed sensor have been compared with the existing reports in the literature, as mentioned in Table 1, and found that the superiority of the fabricated sensor is mainly attributed to the synchronously activated hybrid heterojunction and quantum confinement of the nanocomposite modifying traditional electrodes.
| Fabricated electrode | Method of detection | Linearity range (μM) | LOD (μM) | Real samples | Ref. |
|---|---|---|---|---|---|
| a ZnCr-layered double hydroxide and g-CN. b Graphene (GR) decorated with gold nanoparticles (AuNPs). c Iron phthalocyanine (FePc)-based metal–organic framework. d Poly methyl orange-modified graphene. e Fullerenes (FNTs) and functionalized multi-walled carbon nanotubes (f-MWCNTs). f Poly (titan yellow) and octoxynol-9-modified carbon nanotube paste electrode. g Molybdenum disulfide (MoS2) nanoparticle-decorated carbon nanofibers (MoS2-CNFs). h Poly(glutamic acid)/multi-walled carbon nanotube–graphite composite. i Lanthanum nickelate spheres embedded acid functionalized carbon nanofiber composite. j Molybdenum disulfide/polyaniline/functionalized multi-walled carbon nanotubes. k Polyaniline blended molybdenum sulfide-decorated graphene oxide. | |||||
| ZnCr-LDH@g-CN/GCEa | DPV | 0.001–143.2 | 0.009 | Ice cream and chocolate | 45 |
| AuNPs/GR/GCEb | DPV | 5–120 | 1.7 | Cookies | 46 |
| FePc MOF/GCEc | DPV | 0.22–29.14 | 0.05 | Tablet and human serum | 6 |
| PMOMGPEd | DPV | 10.0–35.0 | 0.073 | Vanilla essence | 47 |
| f-MWCNTs-FNTs/CPEe | CV | 0.05–9.0 | 0.034 | Vanilla sugar | 8 |
| PTY/OL/CNPEf | DPV | 2.0–40.0 | 0.049 | Biscuits | 48 |
| MoS2-CNF/GCEg | Amperometry | 0.3 to 135 | 0.15 | Cookie | 1 |
| Poly(GA)/(MWCNTs-GT)CPSh | DPV | 0.50–13.0 | 0.019 | Milkshake; cream cake; biscuit; vanilla extract. | 49 |
| CuO@SiO2-modified electrode | DPV | 0.05–1.2; 6.2–111.2 | 0.053 | Biscuits and chocolates | 50 |
| La2NiO4@CNF/GCEi | DPV | 0.005–235; 235–3035 | 0.0061 | Chocolate; ice cream | 22 |
| MoS2/PANI/f-CNTsj | DPV | 3.6–125 | 0.021 | Vanillin biscuit | 51 |
| MoS2/PANI@GOk | DPV | 6–86.6 | 0.043 | Commercial food and beverage samples | 52 |
| NdNbO4/f-CNF/GCE | DPV | 0.001 to 63.101 | 0.0063 | Milk chocolate and milkshake | This work |
3.5. Interference and precision studies
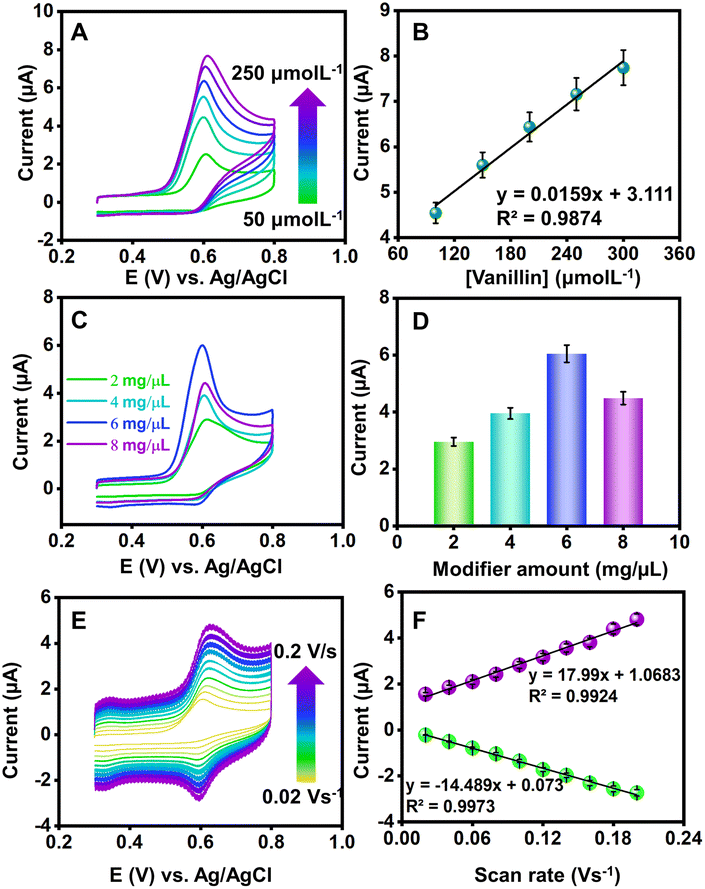
To investigate the potential impact of systematic errors originating from overlapping agents, DPV experiments were extended (Fig. 6(C)). Fig. 6(D) illustrates the relative error percentages in the current attributed to interferents like mercury ions, ascorbic acid, tertiary butyl hydroquinone, Trolox, theophylline, diethofencarb, and dopamine during vanillin sensing. Dopamine, a biomolecule, exerted maximum influence on current responses, while a food additive, vitamin and pesticides demonstrated a milder interference. The observed augmentation in the CV response was ascribed to the oxidative environment facilitating the mid-oxidation of functional groups attached to interfering moieties. However, it is crucial to note that the interference remains negligible and does not compromise the proposed sensor's performance. The precision of the fabricated sensor was assessed through CV experiments involving three distinct GCEs. Each GCE underwent identical modification employing the drop-casting method with a uniform amount of the NdNbO4/f-CNF nanocomposite. The CV profiles of the NdNbO4/f-CNF/GCE and the corresponding plot, which illustrates the relationship between the different GCEs and their relative current responses, are depicted in Fig. S6(A and B)† This comparative analysis highlights the variations in the current generated by each GCE, providing insight into the influence of the electrode composition and surface characteristics on the overall electrochemical activity of the NdNbO4/f-CNF/GCE composite. To quantify precision, the relative standard deviation (RSD) was calculated using eqn (1). For the proposed sensor, the calculated RSD was 1.519%, falling below the accepted error threshold, indicating the reproducibility of the sensor. The CV profile in Fig. S9(A)† was obtained by performing repeated measurements four times using the NdNbO4/f-CNF/GCE composite electrode. The corresponding plot in Fig. S7(B)† illustrates the relationship between these repetitive measurements and the observed current values. This analysis showcases the consistency of the electrode's electrochemical response. An RSD of 4.72% indicates a high degree of precision across the repeated measurements, underscoring the reliability of the NdNbO4/f-CNF/GCE composite in maintaining consistent electrochemical performance. Extensive cycling was conducted to thoroughly assess the durability and electrochemical stability of the composite electrode under continuous operation. The resulting CV profiles in Fig. S8† demonstrate the electrode's ability to maintain consistent current responses throughout the 100 cycles, reflecting its robustness and long-term stability. This indicates that the NdNbO4/f-CNF/GCE composite can reliably function in sensing applications without significant degradation in the performance over multiple uses.3.6. Real sample analysis
Extending on the DPV investigations, an in-depth exploration was conducted to assess the viability of the established electrochemical sensor crafted from NdNbO4/f-CNF/GCE for varying concentrations of vanillin in both milk chocolate and milk shake samples. The meticulously prepared sample underwent dilution with PBS, serving as the supporting electrolyte, wherein a sequential addition of vanillin was systematically performed. The analytical performance of the developed sensor within a real sample matrix was investigated for increasing concentrations of vanillin ranging from 50 to 250 μM, as illustrated in Fig. S9 and S10.† Utilizing the LRM, the correlation between the peak current and the concentration was elucidated through an equivalent plot, as shown in the inset of Fig. S9 and S10.†The LOD, calculated employing the standard deviation method for the electrochemical sensing of vanillin using NdNbO4/f-CNF/GCE, was determined to be 7.59 and 9.38 nmol L−1 in milk chocolate and milk shake samples. Consequently, the fabricated DPV sensor adeptly transforms the oxidation of vanillin on the surface of the NdNbO4/f-CNF-modified GCE into discernible signals in the form of peak current. This mechanism effectively quantifies the target analyte within the complex food sample matrix, confirming the sensor's efficacy in real-world applications.
4. Conclusion
This research underscores the application of a hydrothermal approach to synthesize nano-spheres of NdNbO4, which are then incorporated with f-CNF via sonication, culminating in a nanocomposite serving as an electrode modifier, maintaining its structural integrity. A comprehensive array of analytical methods, including XRD, Raman, FTIR, SEM, and TEM, was employed to investigate the improved characteristics resulting from the synergistic interactions among the counterparts of the nanocomposite. Voltammetry and impedance experiments unveiled that the NdNbO4/f-CNF-modified GCE manifested commendable electrocatalytic activity, enhancing electron transfer and furnishing enhanced reaction sites accessible to vanillin. The modified electrode exhibited notable electrochemical stability, resistance to fouling, low electrode/electrolyte impedance, and a substantial surface area. Evaluation encompassing LOD, selectivity, sensitivity, stability, reproducibility, and repeatability corroborated the sensing ability. Crucially, the practical utility of the developed sensor was validated through the successful detection of vanillin in food matrices, showcasing remarkable recovery rates. The fabrication of such sophisticated architectures, characterized by minimal energy consumption and negligible by-products, positions them as promising contenders for a diverse array of future applications.Conflicts of interest
The authors declare no conflicts of interest in this research work.Acknowledgements
This work was supported by the Ministry of Science and Technology (Special Research Project-MOST-108-2221-E-027-063).References
- M. Qianwen, D. Yaping, L. Li, W. Anqing, D. Dingding and Z. Yijun, J. Electroanal. Chem., 2019, 833, 297–303 CrossRef.
- N. J. Walton, M. J. Mayer and A. Narbad, Phytochemistry, 2003, 63, 505–515 CrossRef CAS PubMed.
- K. Murtada and V. Moreno, J. Electroanal. Chem., 2020, 861, 113988 CrossRef CAS.
- D. Zheng, C. Hu, T. Gan, X. Dang and S. Hu, Sens. Actuators, B, 2010, 148, 247–252 CrossRef CAS.
- Y. Sun, X. Jiang, H. Jin and R. Gui, Anal. Chim. Acta, 2019, 1083, 101–109 CrossRef CAS PubMed.
- J. Peng, L. Wei, Y. Liu, W. Zhuge, Q. Huang, W. Huang, G. Xiang and C. Zhang, RSC Adv., 2020, 10, 36828–36835 RSC.
- C. I. Fort, S. C. A. Cobzac and G. L. Turdean, Food Chem., 2022, 385, 132711 CrossRef CAS.
- L. Taouri, M. Bourouina, S. Bourouina-Bacha and D. Hauchard, J. Food Compos. Anal., 2021, 100, 103811 CrossRef CAS.
- M. Perini, S. Pianezze, L. Strojnik and F. Camin, J. Chromatogr., A, 2019, 1595, 168–173 CrossRef CAS PubMed.
- Z. Wang, G. Zeng, X. Wei, B. Ding, C. Huang and B. Xu, Food Anal. Methods, 2016, 9, 3360–3366 CrossRef.
- S. S. Hingse, S. B. Digole and U. S. Annapure, J. Anal. Sci. Technol., 2014, 5, 1–9 Search PubMed.
- M. Takahashi, S. Sakamaki and A. Fujita, Biosci., Biotechnol., Biochem., 2013, 77, 595–600 CrossRef CAS PubMed.
- Y. Shen, C. Han, B. Liu, Z. Lin, X. Zhou, C. Wang and Z. Zhu, J. Dairy Sci., 2014, 97, 679–686 CrossRef CAS PubMed.
- H. Duan, X. Li, L. Li, X. Wang, J. Feng, M. Sun and C. Luo, Anal. Methods, 2014, 6, 8706–8712 RSC.
- M. Ohashi, H. Omae, M. Hashida, Y. Sowa and S. Imai, J. Chromatogr., A, 2007, 1138, 262–267 CrossRef CAS PubMed.
- O. Folin and W. Denis, Ind. Eng. Chem., 1912, 4, 670–672 CrossRef.
- I. J. D. Priscillal and S.-F. Wang, Nanoscale, 2023, 15, 8693–8705 RSC.
- E. Bakker and M. Telting-Diaz, Anal. Chem., 2002, 74, 2781–2800 CrossRef CAS PubMed.
- B. J. Privett, J. H. Shin and M. H. Schoenfisch, Anal. Chem., 2010, 82, 4723–4741 CrossRef CAS PubMed.
- I. Baranowska, P. Markowski, A. Gerle and J. Baranowski, Bioelectrochemistry, 2008, 73, 5–10 CrossRef CAS.
- G. Pierini, S. Robledo, J. López, A. Tesio, H. Fernández, A. Granero and M. Zon, J. Mach. Form. Technol., 2018, 10, 15–49 Search PubMed.
- E. J. Nixon, R. Sakthivel, Z. A. Alothman, P. S. Ganesh and R.-J. Chung, Food Chem., 2023, 409, 135324 CrossRef CAS.
- S.-Z. Shah, I. H. Taqvi, S. Ameen, A. Mallah, J. A. Buledi, N. H. Khand and A. R. Solangi, Pure and Applied Chemistry, 2023 Search PubMed.
- R. Nehru, C.-W. Chen and C.-D. Dong, Carbon, 2023, 208, 410–420 CrossRef CAS.
- S. Vinoth and S.-F. Wang, New J. Chem., 2023, 47, 9229–9238 RSC.
- J. Huang, Y. Liu and T. You, Anal. Methods, 2010, 2, 202–211 RSC.
- J. Singh, A. Roychoudhury, M. Srivastava, P. R. Solanki, D. W. Lee, S. H. Lee and B. Malhotra, J. Mater. Chem. B, 2013, 1, 4493–4503 RSC.
- P. Sarin, R. W. Hughes, D. R. Lowry, Z. D. Apostolov and W. M. Kriven, J. Am. Ceram. Soc., 2014, 97, 3307–3319 CrossRef CAS.
- L. Brixner, J. Whitney, F. Zumsteg and G. Jones, Mater. Res. Bull., 1977, 12, 17–24 CrossRef CAS.
- K. Byrappa and T. Adschiri, Prog. Cryst. Growth Charact. Mater., 2007, 53, 117–166 CrossRef CAS.
- H. Zhao, S. Feng, W. Xu, Y. Shi, Y. Mao and X. Zhu, J. Mater. Chem., 2000, 10, 965–968 RSC.
- G. Blasse and A. Corsmit, J. Solid State Chem., 1973, 6, 513–518 CrossRef CAS.
- Y. Repelin, E. Husson, F. Bennani and C. Proust, J. Phys. Chem. Solids, 1999, 60, 819–825 CrossRef CAS.
- Q. Qiao, S. Singh, S.-L. Lo, Y. Li, J. Jin and L. Wang, J. Taiwan Inst. Chem. Eng., 2018, 84, 110–122 CrossRef CAS.
- T. Liu and H. Lei, Appl. Surf. Sci., 2017, 413, 16–26 CrossRef CAS.
- R. Chen, W. Wang, D. Jiang, X. Chu, X. Ma and Q. Zhan, J. Phys. Chem. Solids, 2018, 117, 28–35 CrossRef CAS.
- P. Yu, W. Guo, T. Gao, L. Su and J. Xu, Optik, 2018, 162, 102–107 CrossRef CAS.
- A. Lubenchenko, A. Batrakov, I. Shurkaeva, A. Pavolotsky, S. Krause, D. Ivanov and O. Lubenchenko, J. Surf. Invest.: X-Ray, Synchrotron Neutron Tech., 2018, 12, 692–700 CrossRef CAS.
- A. Dacca, G. Gemme, R. Parodi and L. Mattera, Part. Accel., 1998, 60, 103–120 CAS.
- C. Morant, J. Andrey, P. Prieto, D. Mendiola, J. Sanz and E. Elizalde, Phys. Status Solidi A, 2006, 203, 1069–1075 CrossRef CAS.
- B. M. Abu-Zied, Appl. Surf. Sci., 2019, 471, 246–255 CrossRef CAS.
- Y. Zhou, N. Wang, X. Qu, F. Huang, Y. Duan, X. Zhang, X. Dong and Z. Zhang, Nanoscale, 2019, 11, 19994–20005 RSC.
- I. J. Daisy Priscillal and S.-F. Wang, ACS Appl. Nano Mater., 2022, 5, 10331–10340 CrossRef CAS.
- I. J. D. Priscillal and S.-F. Wang, Microchem. J., 2023, 187, 108396 CrossRef CAS.
- S. Gopi and S.-F. Wang, Microchim. Acta, 2023, 190, 423 CrossRef CAS PubMed.
- X. Si, M. Han, W. Li, C. Bai, X. Xu and J. Xu, Curr. Anal. Chem., 2022, 18, 818–825 CrossRef CAS.
- A. B. Monnappa, J. G. G. Manjunatha, A. S. Bhatt and H. Nagarajappa, J. Sci.: Adv. Mater. Devices, 2021, 6, 415–424 CAS.
- G. Tigari, J. Manjunatha, E. S. D'Souza and N. Sreeharsha, ChemistrySelect, 2021, 6, 2700–2708 CrossRef CAS.
- N. Hareesha, J. Manjunatha, B. Amrutha, N. Sreeharsha, S. B. Asdaq and M. K. Anwer, Colloids Surf., A, 2021, 626, 127042 CrossRef CAS.
- A. Venkadesh, J. Mathiyarasu and S. Radhakrishnan, Mater. Today Chem., 2021, 22, 100554 CrossRef CAS.
- E. Murugan and A. Dhamodharan, Diamond Relat. Mater., 2022, 128, 109268 CrossRef CAS.
- E. Murugan, A. Dhamodharan and S. Saranya, Indian J. Chem. Technol., 2022, 28, 518–527 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4nr00521j |
| This journal is © The Royal Society of Chemistry 2024 |