Unlocking new possibilities: application of MXenes in 3D bioprinting for advanced therapy
Yusuf Olatunji
Waidi
 * and
Nipun
Jain
* and
Nipun
Jain

Department of Materials Engineering, Indian Institute of Science, Bangalore 560012, India. E-mail: yusufwaidi@iisc.ac.in
First published on 2nd October 2024
Abstract
3D bioprinting has become a leading contender among additive manufacturing techniques in biomedicine, offering the potential to create functional tissues and organs that could eliminate the need for transplants. However, for complex tissues like muscle, neural, bone, and heart, bioinks need significant improvements in properties like printability, mechanical strength, and functionalities crucial for mimicking natural tissues. Nanomaterial-based bioinks offer exciting possibilities. Among these, MXenes stand out due to their excellent biocompatibility, abundant surface groups for cell interaction, conductivity for electrical stimulation, and photothermal properties. This review delves into the potential of MXenes in 3D bioprinting. We explore the advantages of 3D printing for MXene-based biofabrication, followed by a deep dive into MXenes’ properties that make them ideal for tissue engineering and regeneratice medicine. We also provide a concise overview of various 3D bioprinting techniques and the essential criteria for bioinks employed in this process. We then discuss the diverse applications of these MXene-incorporated bioprinted constructs. Finally, we address the current challenges and future directions in this promising field. This comprehensive analysis will provide valuable insights for researchers exploring the exciting potential of nanomaterials beyond MXenes in 3D bioprinting for biomedicine advancements.
1. Introduction
The 3D bioprinting fabrication technique is revolutionizing the field of medical science and tissue engineering. This cutting-edge technology utilizes a layer-by-layer deposition to meticulously assemble biomaterials and living cells, ultimately creating functional 3D biological structures.1,2 Compared to traditional techniques, 3D bioprinting offers significant benefits. It leverages automation for consistent, high-precision fabrication while also enabling customization to meet the specific needs of each application.3 This has significantly propelled bioengineering advancements in numerous key areas. One of the most promising applications of 3D bioprinting lies in transplantation. By creating personalized tissues and organs, it holds the potential to alleviate the critical shortage of donor organs.4 Beyond transplantation, 3D bioprinting offers a powerful tool for drug development. Researchers can utilize this technique to create more complex and human-like tissue models for drug testing, leading to more reliable and translatable drug discovery processes.5 Additionally, 3D bioprinting allows for the development of sophisticated in vitro models that mimic human tissues more accurately, opening doors for disease research and therapeutic screening.6 The potential extends beyond medical applications. The technology is even being explored to create sustainable meat alternatives, offering a glimpse into a future with more environmentally friendly food sources.MXenes are a family of inorganic carbides, nitrides, and carbonitrides synthesized from MAX phase precursors.7,8 They have the general formula Mn+1XnTx, consisting of alternating transition metals (M) and carbon/nitrogen (X) layers. The outermost groups of M occupy multiple locations depending on Tx and M within the hexagonal crystal lattice.9,10 Recent advancements have enabled the inclusion of multiple transition metals in both ordered and disordered states, expanding the chemical and structural complexity of MXenes.11 They are revolutionary 2D nanomaterials that have captivated scientists with their unique properties and adaptable structure. Their high aspect ratio, large surface area, and tunable composition make them promising candidates across diverse fields like energy, sustainability, medicine, sensing, and catalysis.12 Notably, MXenes’ excellent electrical conductivity and hydrophilic nature position them perfectly for developing next-generation biocompatible materials in biomedicine and bioengineering. This potential extends beyond conductivity. MXenes’ surfaces readily attach molecules, enabling the creation of customized materials with tailored properties.13 The versatility, biocompatibility, and abundance of MXenes compared to materials like graphene make them frontrunners in developing sustainable and effective solutions for a wide range of applications such as infected bone,14 muscle,15 and nerve16 regeneration. However, biomedical engineering and biomedicine studies are still exploring their full potential, with only a few MXene forms currently in use.
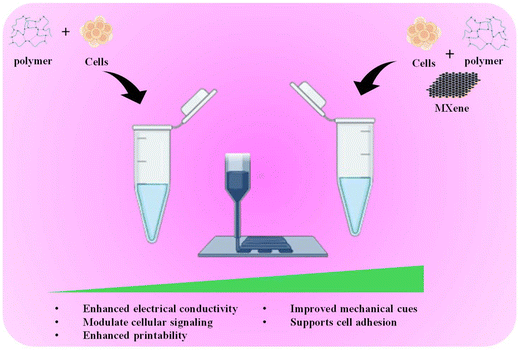
Although the ability to print living cells with 3D bioprinting holds immense promise for various biomedical applications, the current bioinks often struggle to balance printability and cell health, with compromises made on one front to improve the other. This is further complicated by their limited ability to create intricate vascular networks within bioprinted tissues, hindering the development of the vital blood flow necessary for nutrient delivery and waste removal.17 Additionally, the inherent limitations of single bioink formulations in mimicking the diverse properties of different tissues pose a significant challenge in replicating complex organs for regenerative medicine applications. Studies have shown that MXenes can enhance the printability and mechanical strength of bioinks, while simultaneously introducing electrical conductivity, a crucial property for mimicking tissues like muscles and nerves.18 Furthermore, MXenes exhibit good biocompatibility, supporting healthy cell growth within the bioprinted construct (Fig. 1). By integrating MXenes into bioink formulations, researchers are opening doors to developing next-generation bioinks to address these critical limitations. These advanced bioinks hold the potential to create bioprinted tissues and organs with superior functionality and intricate details, allowing for significant progress in the field of regenerative medicine.
MXenes show enormous promise in creating bioinks with diverse biomedical applications. However, despite this potential, there is a lack of a comprehensive article on why MXene-encapsulated inks are valuable for various 3D bioprinting purposes. To bridge this gap, this article provides a timely review. This article explores the advantages of 3D printing for creating MXene-based bioprinted structures, examining MXenes’ suitability for scaffolds, their applications, and the remaining challenges and promising future in this field. This comprehensive review will be a valuable asset for researchers exploring the use of MXenes in 3D bioprinting for future studies.
2. Bioprinting techniques and bioink requirements
2.1. Different bioprinting techniques
3D bioprinting techniques can be categorized into extrusion-based, jetting-based, and vat photopolymerization-based (Fig. 2), as outlined in the ISO/ASTM 52900:2015-12 standard.19 Each technique has its unique mechanism and working principle.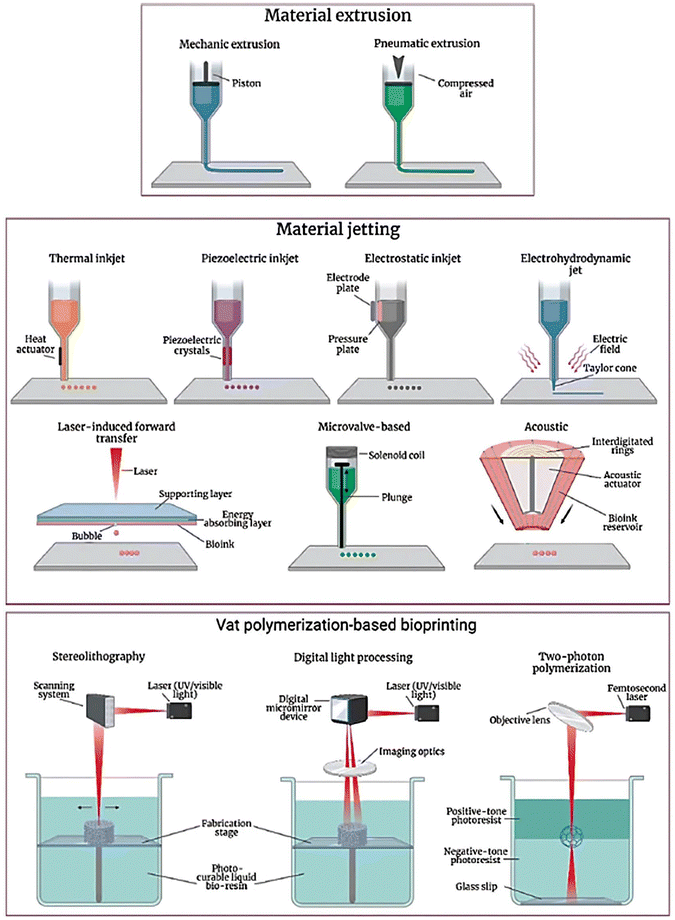 | ||
| Fig. 2 An image showing 3D bioprinting techniques. The fundamental principle of 3D bioprinting remains consistent across various technological approaches, even though they may differ in specific methods. Reproduced from ref. 20, under CC BY 4.0. | ||
Extrusion-based bioprinting (EBB) involves the precise deposition of cell-laden bioinks through a computer-controlled nozzle.21 This method offers several advantages, including its ability to create complex 3D structures with high accuracy and repeatability. EBB is widely used due to its versatility, cost-effectiveness, and potential for producing a variety of biological constructs, from cells and tissues to organ modules and tissue/organ-on-a-chip devices.22 The flexibility of EBB is due to its ability to use various bioink types and nozzle sizes, allowing for precise control over the deposited material. This versatility has made EBB valuable for fundamental research and applied applications.
Jetting-based bioprinting is another powerful bioprinting technique commonly used in the biomedical field. This non-contact technique deposits nanoliter-sized bioink droplets in a precise, layer-by-layer fashion.23 This allows for precise control over cell arrangement, growth factors, and drugs, making it ideal for wound healing, regenerative medicine, and tissue engineering applications. The contactless nature of jetting-based bioprinting enables precise manipulation of cell–cell and cell–matrix interactions, facilitating the creation of physiologically relevant structures.24 By varying the droplet quantity and placement, researchers can fine-tune the properties of printed constructs. Jetting-based bioprinting often employs low-viscosity (3–10 mPa s) inks, resulting in thin and simple 3D structures. This technique encompasses various methods, including inkjet-based, laser-based, acoustic-based, microvalve-based, and electrohydrodynamic jet bioprinting.23
Vat photopolymerization is a 3D printing technique that involves curing liquid bioinks layer-by-layer using light. Photoinitiators within the bioink interact with light, generating radicals that initiate polymerization and solidify the exposed material. This process offers high precision and speed, making it suitable for various tissue engineering applications.25 Researchers have explored different photoinitiators to optimize crosslinking and create intricate tissue constructs.26 Unlike traditional cell-seeding methods, direct bioprinting allows for the bioprinting of small amounts of cell-encapsulated bioinks without significant shear stress. This technique can achieve nanoscale resolution (∼100 nm), enabling the fabrication of biomimetic structures that closely resemble the extracellular matrix (ECM) found in native tissues.27 In a nutshell, each technique has its own unique advantages and limitations, making them suitable for different applications and research objectives.
2.2. Bioink requirements
Selecting the optimal bioink for 3D bioprinting is contingent upon the specific printing technique, intended application, desired properties, and inherent limitations of the bioink itself.28 Hydrogels, a common choice for bioinks, offer a complex interplay between physical and biological characteristics. While higher hydrogel concentrations enhance mechanical properties, they can hinder cell movement within the material. The rheological properties of hydrogels, particularly for extrusion-based printing, significantly influence printability.29 High concentrations can increase extrusion pressure and difficulty, while low concentrations may reduce accuracy. Beyond the rheological properties, bioinks must be compatible with tissue regeneration and provide necessary nutrients for cell survival. In jetting-based bioprinting, surface tension, viscosity, and inertia govern droplet formation and deposition. A delicate balance of these forces is essential for successful droplet ejection; excessive viscosity can prevent droplet detachment, while insufficient viscosity may result in satellite droplet formation and unpredictable placement.30 For vat-based bioprinting, the bioink typically consists of a water-based solution that undergoes methacrylation for crosslinking. The selected bioink must meet stringent criteria, including biocompatibility, appropriate degradation kinetics, and desirable mechanical and structural properties.31 Key parameters for bioink formulation encompass viscosity, functionality, and curing times. Viscosity, in particular, influences printing accuracy, with low-viscosity bio-resins being advantageous for vat-based processes but potentially leading to reduced cell uniformity and sedimentation.32 Overall, the selection of a bioink for 3D bioprinting requires careful consideration of various factors, including the printing technique, desired tissue properties, and the bioink's rheological and biological characteristics. By optimizing bioink formulations and understanding the interplay between bioink properties and printing processes, researchers can advance the field of 3D bioprinting and create functional tissue constructs for regenerative medicine applications.3. MXenes for 3D bioprinting
Electrically conductive hydrogels have emerged as promising devices for improving cell signaling due to their ability to conduct electricity.33 However, most currently used biomaterial inks lack this key property. Researchers are incorporating conductive materials like metal nanoparticles and polymers into bioinks to address this issue. However, popular choices like graphene oxide lose conductivity after oxidation.34 MXenes offer a more attractive alternative due to their unique, tunable chemistry. Adjusting the M and X elements within their structure allows MXenes to be customized for specific applications.35 Additionally, MXenes’ surface chemistry allows control over their hydrophilicity, a crucial factor for biocompatibility. A recent study successfully incorporated Ti3C2 MXene nanosheets into HA/Alg hydrogels. This has resulted in the development of novel bioinks with excellent printability, shape retention, and enhanced mechanical characteristics due to stronger interactions between MXenes and the hydrophilic polymers within the ink.36 These findings highlight the exciting potential of MXenes for creating next-generation bioinks with superior electrical conductivity and printability, paving the way for improved cell communication and advancements in tissue engineering applications.To further expand the MXenes’ connections with cell signaling, MXenes’ enhancement of cell signaling is primarily due to their unique structural and electrochemical properties. Their high electrical conductivity facilitates electrochemical interactions, modulating ion channel activity and influencing signaling pathways.37 Their mechanical properties also provide physical stimuli, triggering mechanical signaling pathways. The nanoscale size and surface chemistry of MXenes allow for effective interactions with cell membranes, leading to the activation of specific signaling pathways.38 Additionally, MXenes can alter the composition and mechanical properties of the extracellular matrix, further influencing cell behavior and signaling. While other conductive materials may also impact cell signaling, MXenes’ unique combination of properties makes them particularly promising for biomedical applications, including tissue engineering and regenerative medicine.
Beyond electrical stimulation and printability, MXenes play a key role in cell adhesion and growth by actively manipulating the cell–material interactions. This is achieved through enhanced surface chemistry and topography. Studies have revealed that MXenes can encourage cell adhesion and proliferation, which are key biological features.39 The inherent negative charge of MXenes facilitates strong electrostatic interactions with positively charged proteins in the cell culture environment. These adsorbed proteins often contain the RGD (arginine–glycine–aspartic acid) sequence, a well-known ligand for integrin receptors, which plays an important role in anchoring cells to the material surface.40 This combined effect of surface roughness and protein–integrin interaction creates a suitable microenvironment for cell attachment and subsequent proliferation.
Ti3C2 nanosheets encapsulated in hydrogels have emerged as promising bioinks with enhanced printability and shape preservation. These hybrid bioinks exhibit shear-thinning behavior, reducing viscosity under shear stress and allowing for easier extrusion. Despite this, MXene-based bioinks are more viscous at low shear rates compared to pure hydrogels.36 A power-law model can be used to quantify this shear-thinning behavior.41 Increased shear-thinning facilitates printing by reducing the required pressure and increasing the mass flow rate. While higher concentrations of Ti3C2 enhance rigidity, they can also lead to aggregation and clogging of the printing needle. Optimal MXene concentrations are typically between 1 mg ml−1 and 5 mg ml−1, with concentrations above 5 mg ml−1 increasing the risk of aggregation.36
Aside from their diverse functionalities, such as electrical stimulation, cell adhesion, and proliferation potential, MXenes offer a fascinating combination of biocompatibility and degradability, making them highly attractive for biomedical applications. Studies have shown that certain MXenes, like halogen-free Ti3C2Tx, exhibit excellent biocompatibility with no cytotoxicity even at high doses, unlike their fluorinated counterparts.42 This is particularly promising as it reduces the risk of cell death upon interaction with human cells. Furthermore, research suggests that MXenes can degrade within the body via enzymatic or reactive oxygen species (ROS), indicating their potential for safe clearance after serving their intended purpose. However, it is crucial to note that biocompatibility and degradability of MXenes are not one-size-fits-all properties. Factors like the specific composition of MXenes and any surface modifications they undergo can significantly influence these aspects.43 Therefore, ongoing research is essential to optimize these properties for each specific biomedical application, ensuring safety and efficacy.
MXenes’ biodegradability is a multifaceted issue influenced by their composition, structure, and environmental conditions.44 While carbon-based MXenes are generally biodegradable due to their layered structure and potential interaction with biological fluids, strong covalent bonds can hinder their degradation rates. Transition metal-based MXenes, on the other hand, are typically less biodegradable due to their stable metal–carbon bonds. However, the metal type and oxidation state can influence their biodegradability. Surface functionalization with hydrophilic groups can enhance MXenes’ biodegradability by promoting water interaction and facilitating enzymatic degradation.45 Environmental factors such as pH levels and temperature also play a crucial role, with acidic environments and higher temperatures generally accelerating degradation. Despite these advancements, further research is necessary to optimize MXenes’ properties for specific applications, particularly in biomedical fields.
A significant obstacle in 3D bioprinting with functional cell-laden hydrogels is achieving homogeneous nanoparticle dispersion within the bioink. MXenes offers a promising solution due to their ease of dispersion. Etching, followed by sonication in deionized (DI) water, effectively disperses MXene nanoparticles (NPs). This approach has been successfully implemented to create bioinks containing human stem cells using a 3D bioprinter, with GelMA/HAMA and MXenes as the primary components.46 Similarly, a novel electroconductive cell-laden bioink incorporated with Ti3C2 MXene nanosheets uniformly distributed throughout an HA/Alg hydrogel was developed in a study.36 The resulting bioinks exhibit excellent rheological properties, enabling the fabrication of high-resolution, structurally stable, and multilayered 3D constructs. Additionally, 3D-printed chitosan hydrogels embedded with uniformly dispersed Ti3C2 MXene quantum dots (QDs) have been shown to improve the physicochemical properties of the bioink while maintaining its printability.14 This paves the way for enhanced in vivo tissue regeneration due to the inherent advantages of Ti3C2 MXenes. Overall, the exceptional dispersibility and functional characteristics of MXenes position them as a promising candidate for developing multifunctional bioinks for 3D bioprinting applications.
Functionalization, chemical modification of MXenes’ surfaces with groups like hydroxyl (–OH) and carboxyl (–COOH), enhances their interaction with hydrogel components. These hydrophilic groups improve dispersion in water, leading to a more uniform distribution within the hydrogel matrix.47 This is especially beneficial for bioinks containing hydrophilic polymers like hyaluronic acid methacryloyl (HAMA) and gelatin methacryloyl (GelMA).46 Functionalized MXenes, due to their increased hydrophilicity, integrate more readily with these polymers, resulting in homogeneous and well-defined bioinks. The unique combination of MXenes and functional groups allows crosslink formation (covalent or hydrogen bonds), creating a highly interconnected 3D network within the hydrogel.48 For example, hydrogen bonds can form between GelMA's amine and carboxyl groups with functionalized MXenes, leading to a stronger and more flexible hydrogel. Furthermore, functionalization can improve biocompatibility by reducing MXenes’ inherent toxicity through surface modifications. Biocompatible polymers or peptides incorporated with functionalized MXenes can further enhance cell adhesion and proliferation and reduce cytotoxicity. Notably, functionalization also allows control over MXenes’ electrical conductivity, which is essential for electrically active tissues like cardiac or neural.49
Overall, the choice of MXenes for bioactive 3D bioprinting is driven by their exceptional properties. Their high surface area, excellent electrical conductivity, tunable surface chemistry, and impressive mechanical strength make them ideal for various biomedical applications. These characteristics facilitate cell adhesion, drug delivery, and electrical stimulation, crucial for tissue regeneration39 (Fig. 3). While cytotoxicity concerns exist, surface modifications can mitigate these issues. In contrast, other 2D nanomaterials like calcium phosphate and calcium silicate lack MXenes’ unique combination of electrical conductivity and mechanical strength, making MXenes a superior choice for effective tissue regeneration.
4. Applications of 3D bioprinted MXene scaffolds
3D bioprinting of MXene scaffolds represents a groundbreaking technology in biomedicine. This approach combines the exceptional properties of MXenes with the power of 3D printing, offering significant advantages for biomedicine.50 MXenes offer a unique combination of properties ideal for biomedical use, such as excellent biocompatibility, antibacterial activity, and a surface rich in Ca2+ binding sites that can be further functionalized.51 Their intriguing 2D nanomaterial structure with tunable chemistry has garnered significant scientific interest due to their potential in biomedical fields beyond bioprinting, such as drug delivery, magnetic resonance imaging (MRI), and cancer therapy.52 Furthermore, MXenes exhibit high photothermal conversion efficiency, making them promising candidates for photothermal ablation in cancer treatment.53 Moreover, certain MXenes, like Ti3C2, degrade in water and oxygen, releasing titanium ions that stimulate new bone growth, making them ideal for bone regeneration.Regenerating severely damaged or diseased skeletal muscle tissue remains a significant challenge. 3D bioprinting with MXenes offers a promising solution due to their unique properties. Studies have shown that MXenes possess excellent antioxidant, anti-inflammatory, and blood vessel growth (angiogenic) properties, all of which contribute to muscle repair by promoting muscle cell differentiation, regeneration, and blood vessel formation.54 Furthermore, 3D printed scaffolds incorporated with MXene nanoparticles have been demonstrated to stimulate the differentiation of muscle progenitor cells (C2C12 cells) into mature muscle cells without the need for additional growth factors (Fig. 4a).55 This approach also increased the expression of genes associated with both early and late stages of muscle development, ultimately enhancing muscle regeneration and reducing immune response in animal models with significant muscle loss. Additionally, bioinks can be optimized by combining GelMA with MXene nanosheets.56 This combination enhances the printability, conductivity, and flow characteristics of the bioink, resulting in better differentiation of encapsulated muscle cells and the creation of highly viable tissue constructs. These findings suggest that 3D bioprinted MXenes have the potential to revolutionize skeletal muscle regeneration strategies.
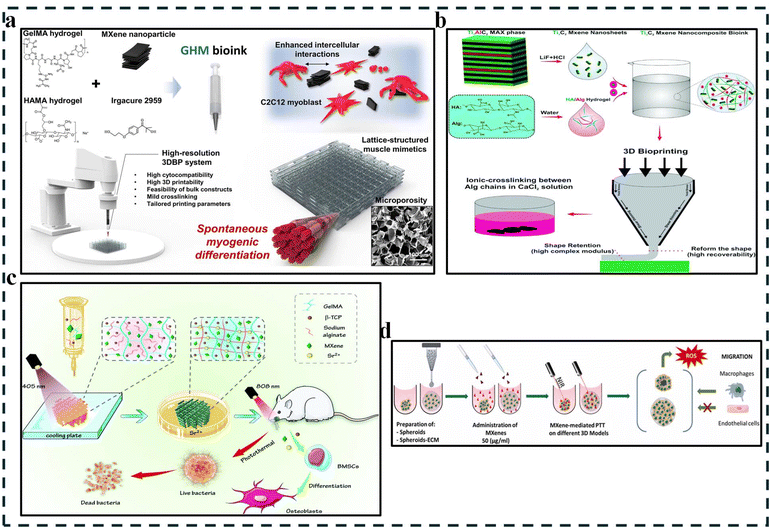 | ||
| Fig. 4 Typical illustration of 3D bioprinted MXenes in various applications in recent studies. (a) 3D bioprinted GelMA/HAMA–MXene (GHM) hydrogels for spontaneous muscle regeneration. This figure depicts the process of myogenesis (muscle cell growth) using the bioprinted hydrogel scaffold. Reproduced with permission from ref. 55, Elsevier, 2024. (b) MXene-based bioink synthesis. This figure showcases the preparation of the bioink. MXene nanosheets were obtained by etching a layered material (Ti3AlC2) and then incorporated with hydrogels for 3D printing applications. Reproduced with permission from ref. 36, the Royal Society of Chemistry, 2020. (c) 3D printing of MXene-based scaffolds for bone regeneration in infected mandibles. This figure illustrates the process of 3D printing of the bioink scaffolds and their implantation with subsequent light irradiation for bone regrowth and disinfection in a rat model. Reproduced with permission from ref. 57, the Royal Society of Chemistry, 2022. (d) Advanced applications of Ti3C2Tx MXenes for PTT in 3D breast cancer models. Reproduced with permission from ref. 58, under CC BY 4.0. | ||
Like other complex tissues, neural tissue regeneration faces difficulties in clinical settings due to its intricate structure and function. To address this challenge, researchers created a novel, electroconductive bioink (Fig. 4b). This bioink incorporates Ti3C2 MXene nanosheets into a base of hyaluronic acid/alginate hydrogels.36 This innovation offers excellent printability for fabricating intricate 3D neural structures and significantly enhances electrical conductivity due to the MXenes. Importantly, tests confirmed the biocompatibility of this MXene nanocomposite bioink through the high viability of HEK-293 cells, suggesting its potential for safe and effective use in neural tissue engineering.
Although 3D bioprinting has focused on soft tissue regeneration, its potential extends to hard tissues like bone. MXene-based scaffolds have emerged as a promising strategy to engineer bone tissue due to their unique properties that promote bone regrowth. Studies have investigated incorporating MXenes into various biomaterials. For example, one study demonstrated that hMSCs within GelMA–HAMA/MXene composites spontaneously differentiated into osteoblasts, highlighting the scaffold's ability to create favorable environments for bone formation.46 Additionally, MXenes can be integrated into personalized hydrogel scaffolds composed of GelMA/β-TCP/sodium alginate (Fig. 4c).57 These scaffolds exhibit excellent biocompatibility, promote cell adhesion and growth, and possess antibacterial properties crucial for infected bone defect repair. Incorporating MXenes in 3D bioprinted scaffolds enhances osteogenic effects and provides photothermal capabilities for antibacterial treatment, showcasing a multifunctional approach to address complex bone defects in another study. The accurate control over the electric potential and surface morphology of MXene-based scaffolds further contributes to creating a biomimetic microenvironment for optimal bone regeneration.59
Aside from tissue engineering applications, the in-vitro disease model is another interesting evolving biomedical application where the use of Mxenes has also been explored, especially for cancer. 3D bioprinting has revolutionized cancer modeling by enabling the creation of complex, multicellular constructs that mimic the tumor microenvironment (TME) with high precision.60,61 By incorporating MXenes in 3D bioprinted models, researchers can develop advanced cancer models replicating the TME and offering diagnostic and therapeutic potential through photodynamic therapy, photoacoustic imaging, and directed drug delivery.62 For example, studies have shown a substantial reduction in cancer cell viability and a rise in ROS after using photothermal therapy (PTT) on a 3D bioprinted hydrogel containing alginate and breast cancer cells with the help of MXenes (Fig. 4d).58 Interestingly, the impact of PTT on macrophage and endothelial cell migration towards cancerous regions within these 3D models suggests that PTT mediated by MXenes (both few and multi-layer) substantially modulates tumor progression via heat-induced cell death. This innovative approach paves the way for creating personalized cancer models that closely mirror the in vivo tumor environment. These models serve as invaluable tools for studying cancer biology and accelerating drug discovery through high-throughput testing of potential therapeutic compounds. Table 1 summarizes recent studies exploring 3D bioprinted MXene constructs for diverse biomedical applications.
| Bioink formulation | Printing technique | Key features | Applications | Ref. |
|---|---|---|---|---|
| GelMA, HAMA, and Ti3C2Tx | Extrusion-based | Excellent printability and structural integrity; microporosity; cytocompatibility; promotes muscle growth; enhanced regeneration; reduced immune response. | Muscle | 55 |
| (BioX Cellink) | ||||
| GelMA, HAMA, and Ti3C2Tx | Extrusion-based | GelMA/HAMA–MXene hydrogels exhibit exceptional properties, promoting hMSC growth, survival, and spontaneous differentiation into bone-forming cells (osteoblasts), making them ideal for bone regeneration applications. | Bone | 46 |
| (BioX Cellink) | ||||
| Alginate and Ti3C2Tx MXenes | Droplet printing | Reduced cell viability; increased ROS; temperature-mediated tumor control; versatility | Cancer model | 58 |
| (BIOX Cellink) | ||||
| GelMA/β-TCP/sodium alginate (Sr2+)/MXenes (Ti3C2) | Extrusion-based (Bio-Architect SR) | Anti-tumor potential; antibacterial properties; osteogenic potential; synergistic effects | Infected bone | 57 |
| GelMA, AuNPs and MXenes | Inkredible + bioprinter (Cellink) | Increased electrical conductivity; improved printability; enhanced cell differentiation; highly viable constructs | Muscle | 56 |
| Hyaluronic acid/alginate | Allevi 2 bioprinter (Philadelphia, USA) | Excellent rheological properties, high electrical conductivity, biocompatibility, and degradability | Neural | 36 |
| (HA/Alg) and Ti3C2 MXene hydrogels |
5. Challenges and future directions
Balancing the unique properties of MXenes with good printability in bioinks poses several challenges that researchers are actively addressing. Despite their superior dispersibility under physiological conditions compared to other common 2D nanomaterials, MXenes’ tendency to aggregate can lead to high viscosity in bioinks, causing issues with extrusion and filament formation. Achieving even dispersion of MXenes within the bioink is crucial for printability and desired properties, but interactions with other components can cause settling or clumping, impacting the final structure. Techniques that improve printability, such as high shear forces or specific solvents, may inadvertently damage MXene structures, affecting their conductivity and biocompatibility.63 However, machine learning (ML) can be leveraged for 3D bioprinting printability with MXenes to optimize processes and improve reliability. ML can analyze large past datasets to identify complex patterns and predict optimal parameters, reducing waste and enhancing component quality.64 Moreover, ensuring biocompatibility while enhancing printability is essential, as additives used to improve printability could introduce safety concerns for cells and tissues, necessitating careful selection of additives in bioink formulations. Strategies like surface modification, using biocompatible dispersants, and microfluidic printing techniques can be promising avenues for overcoming these challenges, enabling the full utilization of MXenes in bioprinting for biomedicine applications.MXenes, particularly in bioinks for biomedical applications, face challenges in terms of long-term biocompatibility and in vivo performance. The degradation behavior of MXenes within bioinks is not fully understood, potentially leading to the release of toxic byproducts. MXenes may trigger immune responses, necessitating tailored surface chemistry for enhanced biocompatibility and minimized negative interactions. Furthermore, in vivo stability issues like unexpected transformations or aggregation could compromise functionality. Long-term cell interaction effects of MXenes on encapsulated cells require further investigation to address potential alterations in cell behavior or viability. Overcoming these challenges involves developing controlled degradation mechanisms, surface modification strategies, and rigorous in vivo testing to ensure the long-term safety and efficacy of MXene-containing bioinks.
MXenes offer a promising avenue for advanced bioprinting bioinks, with potential future directions including tailored functionalities for tissue engineering by incorporating bioactive molecules or conductive elements. Further research is needed to enhance MXenes’ biocompatibility for in vivo applications, possibly through surface modifications or composite materials.65 Designing multi-material bioinks can lead to structures with tunable properties and improved printability. Exploring MXene bioinks for complex bioprinting techniques, such as multi-layered printing or embedding vasculature, can enhance tissue vascularization. Additionally, utilizing computational modeling tools to optimize MXene bioinks’ behavior and tissue properties represents a promising approach for the future of bioprinting and biomedicine.
Investigating vascularization in MXene bioprinted scaffolds is a promising advancement for tissue engineering. To optimize bioink design, incorporating pro-angiogenic factors or microfluidics to create channels for blood vessel growth within the scaffolds is crucial. Bio-printing MXene bioinks with endothelial cells can enhance vascularization post-implantation. Inspired by natural blood vessel structures, biomedical approaches can improve scaffold properties like porosity and degradability to facilitate blood vessel formation. Animal studies are critical to assess the efficacy of vascularized MXene scaffolds in real-world tissue regeneration applications.66 Long-term studies are necessary to evaluate the durability and functionality of the blood vessels within the scaffolds’ post-implantation, ensuring long-lasting tissue regeneration benefits.
Although carbon-based nanomaterials have raised concerns about biocompatibility, toxicity, and long-term effects, MXenes have shown promise in various studies. However, further research is crucial to fully comprehend their interactions with biological systems, such as proteins, nucleic acids, and cells, and to address these concerns. These interactions can significantly influence MXenes’ behavior and their impact on biological processes. Studies have demonstrated that MXenes can interact with proteins, leading to conformational changes that may affect biological functions.67 Although their intrinsic antibacterial properties offer potential benefits in medical applications, their long-term effects on human cells require further investigation. A comprehensive understanding of MXenes’ interactions with biological molecules is essential for designing safe and effective biomedical applications. To ensure safe integration into biomedical fields, it is imperative to thoroughly assess the potential risks and benefits associated with the use of MXenes.
In future with in vivo studies, researchers can focus on tailoring biocompatibility and degradation rates by fine-tuning MXenes’ composition and surface modifications. Exploring the engineering of complex tissues by incorporating multiple bioinks with different MXenes or biomaterials can mimic the intricate structures and functionalities of tissues like bones, muscles, or nerves. Delving into immunomodulation and inflammation control to understand how MXene bioinks interact with the immune system can lead to strategies for minimizing inflammation and enhancing tissue acceptance.68 Furthermore, integrating MXene bioinks with bioprinting technologies and exploring combination therapies with drug delivery or gene therapy can offer synergistic effects for tissue regeneration and disease treatment. By addressing these future directions, researchers can unlock the full prospect of MXene bioinks for groundbreaking advancements in tissue regeneration and other biomedical fields.
6. Conclusion
3D bioprinting with MXenes presents a promising future for biomedical applications due to MXenes’ large surface area, high electrical conductivity, biocompatibility, tailorable surface chemistry, and antibacterial properties. By incorporating MXenes into bioinks, tissue-engineered scaffolds can mimic native tissues, facilitating bone, cardiac, and neural tissue regeneration. MXenes also show potential in disease model applications, leveraging their specific binding abilities and electrical conductivity. Despite their promise, further advancements are needed to unlock the transformative potential of MXene-based 3D bioprinting fully. Optimizing bioink formulations to ensure proper printability and cell viability is crucial. Long-term biocompatibility of MXenes within the body needs thorough investigation. Finally, developing scalable production methods for MXenes will be essential for widespread adoption in clinical settings. Overcoming these hurdles will allow MXene-based 3D bioprinting to revolutionize various fields of biomedicine, ultimately leading to improved patient care and potentially groundbreaking medical advancements.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.References
- A. C. Daly, M. E. Prendergast, A. J. Hughes and J. A. Burdick, Bioprinting for the biologist, Cell, 2021, 184(1), 18–32 CrossRef CAS PubMed.
- Y. O. Waidi, I. Kariim and S. Datta, Bioprinting of gelatin-based materials for orthopedic application, Front. Bioeng. Biotechnol., 2024, 12, 1357460 CrossRef PubMed.
- S. Raees, F. Ullah, F. Javed, H. M. Akil, M. J. Khan, M. Safdar, I. U. Din, M. A. Alotaibi, A. I. Alharthi and M. A. Bakht, Classification, processing, and applications of bioink and 3D bioprinting: A detailed review, Int. J. Biol. Macromol., 2023, 232, 123476 CrossRef CAS PubMed.
- G. Huang, Y. Zhao, D. Chen, L. Wei, Z. Hu, J. Li, X. Zhou, B. Yang and Z. Chen, Applications, advancements, and challenges of 3D bioprinting in organ transplantation, Biomater. Sci., 2024, 12(6), 1425–1448 RSC.
- K. L. Miller, Y. Xiang, C. Yu, J. Pustelnik, J. Wu, X. Ma, T. Matsui, K. Imahashi and S. Chen, Rapid 3D BioPrinting of a human iPSC-derived cardiac micro-tissue for high-throughput drug testing, Organs Chip, 2021, 3, 100007 CrossRef CAS.
- X. Ma, J. Liu, W. Zhu, M. Tang, N. Lawrence, C. Yu, M. Gou and S. Chen, 3D bioprinting of functional tissue models for personalized drug screening and in vitro disease modeling, Adv. Drug Delivery Rev., 2018, 132, 235–251 CrossRef CAS PubMed.
- J. Halim, J. Zhou, M. Dahlqvist and J. Rosen, Synthesis of MXene Precursors–Tips and Tricks, Transition Metal Carbides and Nitrides (MXenes) Handbook: Synthesis, Process. Prop. Appl., 2024, 11–33 Search PubMed.
- X. Li, M. Zhan, Y. Liu, W. Tu and H. Li, MXene Synthesis and Carbon Capture Applications: Mini–Review, Chem. – Eur. J., 2024, e202400874 CrossRef CAS PubMed.
- B. Anasori and Û. G. Gogotsi, 2D metal carbides and nitrides (MXenes), Springer, 2019 Search PubMed.
- B. C. Wyatt, A. Rosenkranz and B. Anasori, 2D MXenes: tunable mechanical and tribological properties, Adv. Mater., 2021, 33(17), 2007973 CrossRef CAS PubMed.
- J. Zhou, Q. Tao, B. Ahmed, J. Palisaitis, I. Persson, J. Halim, M. W. Barsoum, P. O. Persson and J. Rosen, High-entropy laminate metal carbide (MAX phase) and its two-dimensional derivative MXene, Chem. Mater., 2022, 34(5), 2098–2106 CrossRef CAS.
- M. L. Matias, C. Pereira, H. V. Almeida, S. Jana, S. Panigrahi, U. D. Menda, D. Nunes, E. Fortunato, R. Martins and S. Nandy, 3D printed MXene architectures for a plethora of smart applications, Mater. Today Adv., 2024, 23, 100512 CrossRef CAS.
- V. Kamysbayev, A. S. Filatov, H. Hu, X. Rui, F. Lagunas, D. Wang, R. F. Klie and D. V. Talapin, Covalent surface modifications and superconductivity of two-dimensional metal carbide MXenes, Science, 2020, 369(6506), 979–983 CrossRef CAS PubMed.
- S. Pan, J. Yin, L. Yu, C. Zhang, Y. Zhu, Y. Gao and Y. Chen, 2D MXene–integrated 3D–printing scaffolds for augmented osteosarcoma phototherapy and accelerated tissue reconstruction, Adv. Sci., 2020, 7(2), 1901511 CrossRef CAS PubMed.
- H. Zheng, F. Cheng, D. Guo, X. He, L. Zhou and Q. Zhang, Nanoenzyme-Reinforced Multifunctional Scaffold Based on Ti3C2Tx MXene Nanosheets for Promoting Structure–Functional Skeletal Muscle Regeneration via Electroactivity and Microenvironment Management, Nano Lett., 2023, 23(16), 7379–7388 CrossRef CAS PubMed.
- J. Cai, H. Zhang, Y. Hu, Z. Huang, Y. Wang, Y. Xia, X. Chen, J. Guo, H. Cheng and L. Xia, GelMA-MXene hydrogel nerve conduits with microgrooves for spinal cord injury repair, J. Nanobiotechnol., 2022, 20(1), 460 CrossRef CAS PubMed.
- Y. Fang, M. Ji, Y. Yang, Y. Guo, R. Sun, T. Zhang, W. Sun and Z. Xiong, 3D printing of vascularized hepatic tissues with a high cell density and heterogeneous microenvironment, Biofabrication, 2023, 15(4), 045004 CrossRef PubMed.
- W. Fang, M. Yang, L. Wang, W. Li, M. Liu, Y. Jin, Y. Wang, R. Yang, Y. Wang and K. Zhang, Hydrogels for 3D bioprinting in tissue engineering and regenerative medicine: Current progress and challenges, Int. J. Bioprint., 2023, 9(5), 759 CrossRef PubMed.
- J. M. Lee, S. L. Sing, M. Zhou and W. Y. Yeong, 3D bioprinting processes: A perspective on classification and terminology, Int. J. Bioprint., 2018, 4(2), 151 CrossRef CAS PubMed.
- D. Szűcs, Z. Fekete, M. Guba, L. Kemény, K. Jemnitz, E. Kis and Z. Veréb, Toward better drug development: Three-dimensional bioprinting in toxicological research, Int. J. Bioprint., 2023, 9(2), 663 CrossRef PubMed.
- I. T. Ozbolat and M. Hospodiuk, Current advances and future perspectives in extrusion-based bioprinting, Biomaterials, 2016, 76, 321–343 CrossRef CAS PubMed.
- X. Chen, A. F. Anvari-Yazdi, X. Duan, A. Zimmerling, R. Gharraei, N. Sharma, S. Sweilem and L. Ning, Biomaterials/bioinks and extrusion bioprinting, Bioact. Mater., 2023, 28, 511–536 CAS.
- W. L. Ng and V. Shkolnikov, Jetting-based bioprinting: process, dispense physics, and applications, Bio-Des. Manuf., 2024, 1–29 Search PubMed.
- W. L. Ng, M. H. Goh, W. Y. Yeong and M. W. Naing, Applying macromolecular crowding to 3D bioprinting: fabrication of 3D hierarchical porous collagen-based hydrogel constructs, Biomater. Sci., 2018, 6(3), 562–574 RSC.
- W. L. Ng, J. M. Lee, M. Zhou, Y.-W. Chen, K.-X. A. Lee, W. Y. Yeong and Y.-F. Shen, Vat polymerization-based bioprinting—process, materials, applications and regulatory challenges, Biofabrication, 2020, 12(2), 022001 CrossRef CAS PubMed.
- A. Zennifer, S. Manivannan, S. Sethuraman, S. G. Kumbar and D. Sundaramurthi, 3D bioprinting and photocrosslinking: emerging strategies & future perspectives, Biomater. Adv., 2022, 134, 112576 CrossRef CAS PubMed.
- V. F. Paz, M. Emons, K. Obata, A. Ovsianikov, S. Peterhänsel, K. Frenner, C. Reinhardt, B. Chichkov, U. Morgner and W. Osten, Development of functional sub-100 nm structures with 3D two-photon polymerization technique and optical methods for characterization, J. Laser Appl., 2012, 24(4), 042004 CrossRef.
- J. M. Lee, W. L. Ng and W. Y. Yeong, Resolution and shape in bioprinting: Strategizing towards complex tissue and organ printing, Appl. Phys. Rev., 2019, 6(1), 011307 Search PubMed.
- S. Tian, H. Zhao and N. Lewinski, Key parameters and applications of extrusion-based bioprinting, Bioprinting, 2021, 23, e00156 CrossRef.
- W. L. Ng and V. Shkolnikov, Optimizing cell deposition for inkjet-based bioprinting, Int. J. Bioprint., 2024, 10(2), 2135 CrossRef CAS.
- E. S. Bishop, S. Mostafa, M. Pakvasa, H. H. Luu, M. J. Lee, J. M. Wolf, G. A. Ameer, T.-C. He and R. R. Reid, 3-D bioprinting technologies in tissue engineering and regenerative medicine: Current and future trends, Genes Dis., 2017, 4(4), 185–195 CrossRef CAS PubMed.
- A. Schwab, R. Levato, M. D'Este, S. Piluso, D. Eglin and J. Malda, Printability and shape fidelity of bioinks in 3D bioprinting, Chem. Rev., 2020, 120(19), 11028–11055 CrossRef CAS PubMed.
- M. Mehrali, A. Thakur, C. P. Pennisi, S. Talebian, A. Arpanaei, M. Nikkhah and A. Dolatshahi-Pirouz, Nanoreinforced hydrogels for tissue engineering: biomaterials that are compatible with load–bearing and electroactive tissues, Adv. Mater., 2017, 29(8), 1603612 CrossRef PubMed.
- S. Pei and H.-M. Cheng, The reduction of graphene oxide, Carbon, 2012, 50(9), 3210–3228 CrossRef CAS.
- L. Jia, S. Zhou, A. Ahmed, Z. Yang, S. Liu, H. Wang, F. Li, M. Zhang, Y. Zhang and L. Sun, Tuning MXene electrical conductivity towards multifunctionality, Chem. Eng. J., 2023, 146361 CrossRef CAS.
- H. Rastin, B. Zhang, A. Mazinani, K. Hassan, J. Bi, T. T. Tung and D. Losic, 3D bioprinting of cell-laden electroconductive MXene nanocomposite bioinks, Nanoscale, 2020, 12(30), 16069–16080 RSC.
- Y. Li, Y. Hu, H. Wei, W. Cao, Y. Qi, S. Zhou, P. Zhang, H. Li, G.-L. Li and R. Chai, Two-dimensional Ti3C2Tx MXene promotes electrophysiological maturation of neural circuits, J. Nanobiotechnol., 2022, 20(1), 398 CrossRef CAS PubMed.
- S. Sadashiva, I. Chakraborty, S. S. Havanagi, N. Mazumder and B. N. Singh, Recent Developments in MXene-Based Materials for Biomedical Applications in Regenerative Medicine, MXenes, 2025, 29–48 Search PubMed.
- J. Ma, L. Zhang and B. Lei, Multifunctional MXene-based bioactive materials for integrated regeneration therapy, ACS Nano, 2023, 17(20), 19526–19549 CrossRef CAS PubMed.
- Y. Zhong, S. Huang, Z. Feng, Y. Fu and A. Mo, Recent advances and trends in the applications of MXene nanomaterials for tissue engineering and regeneration, J. Biomed. Mater. Res., Part A, 2022, 110(11), 1840–1859 CrossRef CAS PubMed.
- J. Emmermacher, D. Spura, J. Cziommer, D. Kilian, T. Wollborn, U. Fritsching, J. Steingroewer, T. Walther, M. Gelinsky and A. Lode, Engineering Considerations on Extrusion-based Bioprinting: Interactions of Material Behavior, Mechanical Forces and Cells in the Printing Needle, Biofabrication, 2020, 12(2), 025022 CrossRef CAS PubMed.
- J. Yoon, S. Kim, K. H. Park, S. Lee, S. J. Kim, H. Lee, T. Oh and C. M. Koo, Biocompatible and oxidation–resistant Ti3C2Tx MXene with halogen–free surface terminations, Small Methods, 2023, 7(8), 2201579 CrossRef CAS PubMed.
- N. Chandrasekar, A. P. Steffi, B. Ramachandran, M. T. Hwang, V. Faramarzi and M. Govarthanan, MXenes–Versatile 2D materials for identification of biomarkers and contaminants in large scale environments–A review, Environ. Res., 2023, 228, 115900 CrossRef CAS PubMed.
- I. A. Vasyukova, O. V. Zakharova, D. V. Kuznetsov and A. A. Gusev, Synthesis, toxicity assessment, environmental and biomedical applications of MXenes: A review, Nanomaterials, 2022, 12(11), 1797 CrossRef CAS PubMed.
- B. Ma, C. Martín, R. Kurapati and A. Bianco, Degradation-by-design: how chemical functionalization enhances the biodegradability and safety of 2D materials, Chem. Soc. Rev., 2020, 49(17), 6224–6247 RSC.
- S. H. Lee, M. S. Kang, S. Jeon, H. J. Jo, S. W. Hong, B. Kim and D.-W. Han, 3D bioprinting of human mesenchymal stem cells-laden hydrogels incorporating MXene for spontaneous osteodifferentiation, Heliyon, 2023, 9(3), e14490 CrossRef CAS PubMed.
- S. Wu, H. Wang, L. Li, M. Guo, Z. Qi, Q. Zhang and Y. Zhou, Intercalated MXene-based layered composites: Preparation and application, Chin. Chem. Lett., 2020, 31(4), 961–968 CrossRef CAS.
- J. K. Wychowaniec, J. Litowczenko, K. Tadyszak, V. Natu, C. Aparicio, B. Peplińska, M. W. Barsoum, M. Otyepka and B. Scheibe, Unique cellular network formation guided by heterostructures based on reduced graphene oxide-Ti3C2Tx MXene hydrogels, Acta Biomater., 2020, 115, 104–115 CrossRef CAS PubMed.
- J. Zhou and S. Vijayavenkataraman, 3D-printable conductive materials for tissue engineering and biomedical applications, Bioprinting, 2021, 24, e00166 CrossRef.
- Z. Yu, G. Zhang, W. Li, Z. Yu, L. Ma, Z. Han, Y. Xi, M. Xiao, G. Li and L. Xu, 3D conductive scaffolds of MXene@ PCL with high conductivity and small line width fabricated by electric-field-driven jet 3D printing and electrostatic self-assembly, Mater. Today Commun., 2023, 35, 105704 CrossRef CAS.
- G. Yang, F. Liu, J. Zhao, L. Fu, Y. Gu, L. Qu, C. Zhu, J.-J. Zhu and Y. Lin, MXenes-based nanomaterials for biosensing and biomedicine, Coord. Chem. Rev., 2023, 479, 215002 CrossRef CAS.
- A. Tareen, K. Khan, M. Iqbal, S. Golovynskyi, Y. Zhang, A. Mahmood, N. Mahmood, J. Long, A. Al-Ghamdi and C. Li, Recent advances in MXenes: new horizons in biomedical technologies, Mater. Today Chem., 2022, 26, 101205 CrossRef CAS.
- Z. Li, H. Zhang, J. Han, Y. Chen, H. Lin and T. Yang, Surface nanopore engineering of 2D MXenes for targeted and synergistic multitherapies of hepatocellular carcinoma, Adv. Mater., 2018, 30(25), 1706981 CrossRef PubMed.
- T. Li, J. Ma, W. Wang and B. Lei, Bioactive MXene promoting angiogenesis and skeletal muscle regeneration through regulating M2 polarization and oxidation stress, Adv. Healthcare Mater., 2023, 12(4), 2201862 CrossRef CAS PubMed.
- H. J. Jo, M. S. Kang, H. J. Heo, H. J. Jang, R. Park, S. W. Hong, Y. H. Kim and D.-W. Han, Skeletal muscle regeneration with 3D bioprinted hyaluronate/gelatin hydrogels incorporating MXene nanoparticles, Int. J. Biol. Macromol., 2024, 265, 130696 CrossRef CAS PubMed.
- S. Boularaoui, A. Shanti, M. Lanotte, S. Luo, S. Bawazir, S. Lee, N. Christoforou, K. A. Khan and C. Stefanini, Nanocomposite conductive bioinks based on low-concentration GelMA and MXene nanosheets/gold nanoparticles providing enhanced printability of functional skeletal muscle tissues, ACS Biomater. Sci. Eng., 2021, 7(12), 5810–5822 CrossRef CAS PubMed.
- R. Nie, Y. Sun, H. Lv, M. Lu, H. Huangfu, Y. Li, Y. Zhang, D. Wang, L. Wang and Y. Zhou, 3D printing of MXene composite hydrogel scaffolds for photothermal antibacterial activity and bone regeneration in infected bone defect models, Nanoscale, 2022, 14(22), 8112–8129 RSC.
- G. Perini, A. Rosenkranz, G. Friggeri, D. Zambrano, E. Rosa, A. Augello, V. Palmieri, M. D. Spirito and M. Papi, Advanced usage of Ti3C2Tx MXenes for photothermal therapy on different 3D breast cancer models, Biomed. Pharmacother., 2022, 153, 113496 CrossRef CAS PubMed.
- Y. Fu, S. Huang, Z. Feng, L. Huang, X. Zhang, H. Lin and A. Mo, MXene-functionalized ferroelectric nanocomposite membranes with modulating surface potential enhance bone regeneration, ACS Biomater. Sci. Eng., 2023, 9(2), 900–917 CrossRef CAS PubMed.
- R. Sharma, M. R. Perez, V. A. da Silva, J. Thomsen, L. Bhardwaj, T. A. Andrade, A. Alhussan and S. M. Willerth, 3D bioprinting complex models of cancer, Biomater. Sci., 2023, 11(10), 3414–3430 RSC.
- N. Jain, B. L. Shashi Bhushan, M. Natarajan, R. Mehta, D. K. Saini and K. Chatterjee, Advanced 3D In Vitro Lung Fibrosis Models: Contemporary Status, Clinical Uptake, and Prospective Outlooks, ACS Biomater. Sci. Eng., 2024, 10(3), 1235–1261 CrossRef CAS PubMed.
- S. Iravani and R. S. Varma, MXenes in cancer nanotheranostics, Nanomaterials, 2022, 12(19), 3360 CrossRef CAS PubMed.
- K. Diedkova, A. D. Pogrebnjak, S. Kyrylenko, K. Smyrnova, V. V. Buranich, P. Horodek, P. Zukowski, T. N. Koltunowicz, P. Galaszkiewicz and K. Makashina, Polycaprolactone–MXene nanofibrous scaffolds for tissue engineering, ACS Appl. Mater. Interfaces, 2023, 15(11), 14033–14047 CAS.
- W. L. Ng, G. L. Goh, G. D. Goh, J. S. J. Ten and W. Y. Yeong, Progress and opportunities for machine learning in materials and processes of additive manufacturing, Adv. Mater., 2024, 2310006 CrossRef CAS PubMed.
- M. Pershaanaa, F. Kamarulazam, O. Gerard, Z. Goh, S. Bashir, K. Baruah, P. Deb, S. Ramesh and K. Ramesh, MXenes and their transformation to composites for potential applications, Mater. Today Commun., 2023, 35, 106143 CrossRef CAS.
- S. Chae, D.-H. Ha and H. Lee, 3D bioprinting strategy for engineering vascularized tissue models, Int. J. Bioprint., 2023, 9(5), 748 CrossRef PubMed.
- Y. S. Huh, M. Rethinasabapathy, D. Dhiman, D. K. S. Ranjith, S.-K. Hwang and P. Venkatesu. A Comprehensive Study on the Surface Property Changes of Antibodies on Interacting with Ti3c2tx, V2ctx, and Mo2tic2tx Mxenes, V2ctx, and Mo2tic2tx Mxenes.
- X. Qu, Y. Guo, C. Xie, S. Li, Z. Liu and B. Lei, Photoactivated MXene nanosheets for integrated bone–soft tissue therapy: effect and potential mechanism, ACS Nano, 2023, 17(8), 7229–7240 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |