DOI:
10.1039/D3QI02182C
(Research Article)
Inorg. Chem. Front., 2024,
11, 826-836
A highly Mn2+-doped narrowband green phosphor toward wide color-gamut display applications†
Received
24th October 2023
, Accepted 3rd December 2023
First published on 4th December 2023
Abstract
Weak blue light absorption severely limits the application of Mn2+-activated narrowband green phosphors. Herein, a strategy to improve the absorption of Mn2+-activated phosphors is proposed by selecting a suitable host to achieve a high doping concentration of Mn2+. Specifically, a magnetoplumbite-type compound La0.827Al11.9O19.09 with a unique layered structure is selected as the host to accommodate Mn2+ (La0.827Al11.9−xMnxO19.09,LAO:xMn2+) and a high doping concentration of x = 0.30 is obtained. Upon 450 nm excitation, the prepared phosphor LAO:0.30Mn2+ displays an intense narrowband green emission peaking at 517 nm with a full width at half-maximum (FWHM) of 28 nm, a color purity of 88% and an internal/external quantum yield of 90%/16%. Furthermore, it exhibits a low thermal quenching (6% at 423 K) and negligible color drifting (3.29‰ at 423 K). The LED device fabricated from the green phosphor LAO:0.30Mn2+, the commercial red phosphor K2TiF6:Mn4+ and a blue InGaN chip can emit bright white light with a corresponding correlated color temperature (CCT) of 5464 K and a color gamut value of 124% of the National Television Commission (NTSC) standard. These results indicate that the LAO:0.30Mn2+ phosphor has great potential for usage as a green component in wide-color gamut LCD backlight applications.
1. Introduction
Phosphor-converted white LEDs (pc-wLEDS) have been widely used in liquid-crystal display (LCD) backlight applications due to their low power consumption, high brightness, long lifetime, and eco-friendliness.1–3 For display applications, the color gamut is a key factor affecting display performance.4 Generally, phosphors for display applications need to have a narrow emission band, an appropriate emission peak position, a high photoluminescence quantum yield (PL QY), and outstanding PL thermal stability. In particular, the narrower full width at half maximum (FWHM) of the emission band for phosphors, the larger color gamut and vivid images will be displayed. Currently, the optimal commercial pc-wLED backlight is packaged by combining an InGaN-based blue chip (∼460 nm) with a narrowband emissive green phosphor β-SiALON:Eu2+ and a red phosphor K2SiF6:Mn4+ (KSF:Mn4+).5 The red phosphor KSF:Mn4+ has an extremely narrow FWHM (∼7 nm) and can well meet commercial expectations, while the green phosphor β-SiALON:Eu2+ has a considerably broader FWHM of ∼54 nm, which has plenty of room for optimization. Thus, the development of high-performance green phosphors with narrower FWHM is crucial for achieving further wider color gamut display devices.
To date, several types of narrowband green emitting materials have the potential for LCD backlight, including quantum dots, Eu2+-activated phosphors, Mn2+-activated phosphors, etc. Unfortunately, each of them has its own flaws that prevent them from their wide commercial use. The green-emitting quantum dots, typically CdSe/ZnS and perovskite CsPbBr3, have high PL QYs and ultra-narrow band emission (FWHM = ∼20 nm). However, their poor stability and toxicity of Cd/Pb content limit their practical applicability, especially in high-power density devices.6 The PL spectra of Eu2+-activated green phosphors generally have a broad FWHM, which is ascribed to the dipole-allowed 5d → 4f transition with strong electron–phonon coupling. For example, the nitride Ba[Li2(Al2Si2)N6]:Eu2+ has an FWHM of 57 nm,7 and NaBaB9O15:Eu2+ has an FWHM of 61 nm.8 Although several highly condensed narrowband green phosphors based on the unique structure of UCr4C4-type compounds have been reported, among which the RbNa[Li3SiO4]2:Eu2+ green phosphor has the narrowest emission FWHM of 41 nm,9 their poor chemical stability limits their application. In comparison, transition metal Mn2+-activated phosphors have a distinct advantage in narrowband green emission.10–14 Mn2+ ions commonly exhibit green emission in tetrahedral coordination with weak crystal field strength and red emission in octahedral coordination with strong crystal field strength, because the d–d transition of 3d electrons is extremely sensitive to the crystal field strength.15 Over the past few years, Mn2+-activated phosphors such as Zn2SiO4:Mn2+ and BaAl12O19:Mn2+ have been widely used as commercial green phosphors for PDP displays.16,17 Besides, Mn2+-activated narrowband green phosphors have been continuously discovered, and the FWHM has achieved a leap from 43 nm to 18 nm, such as those of the oxynitride γ-AlON (FWHM = ∼43 nm),10 MgAl2O4:Mn2+ (FWHM = ∼35 nm),11 BaZnAl10O17:Mn2+ (FWHM = ∼31 nm),12 Sr2MgAl22O36:Mn2+ (FWHM = ∼26 nm),13 and even 18 nm of ZnAl2O4:Mn2+.14 As a result, LEDs fabricated with Mn2+-activated green phosphors can theoretically achieve a large color gamut greater than the 110% NTSC standard. Nonetheless, the d–d parity-forbidden transition of Mn2+ severely limits its blue light absorption efficiency, resulting in significantly lower external PL QY than those of commercially available Eu2+- or Ce3+-activated phosphors. Generally, the introduction of a distorted crystal-field environment to loosen or break the selection rule limitation can effectively enhance the Mn2+ blue light absorption efficiency.18,19 In contrast, a more effective strategy is to introduce as many luminescent centers into the host as possible to increase the absorption efficiency while avoiding the occurrence of concentration quenching.
Hexaaluminates are a class of hexagonal aluminate compounds that are frequently utilized as high-temperature catalysts, thermal barrier coatings, and phosphor hosts due to their unique layered structure and excellent physicochemical stability.20–22 The structure of hexaaluminates consists of dense spinel blocks and mirror planes, stacked alternately along the c-axis. According to the difference in the charge and radius of the large cations positioned on the mirror planes, they can be classified as β-Al2O3 and magnetoplumbite (PbFe12O19) types.23,24 The commercial blue-emitting phosphor BaMgAl10O17:Eu2+ is a typical representation of the β-Al2O3 structure,25 whereas the magnetoplumbite structure is represented by the red-emitting phosphor CaAl12O19:Mn4+.26 In this work, we selected a La0.827Al11.9O19.09 (hereafter referred to as LAO) crystal as the host, which has a distorted magnetoplumbite structure that can also be viewed as a non-stoichiometric ratio compound related to La deficiency.27 In comparison, the LaMgAl11O19 crystal has an analogous magnetoplumbite structure, but it can be regarded as a perfect crystal structure.28 However, the LAO crystal can compensate for the additional charges induced by the partial lack of La on the mirror planes via structural rearrangements formed by a Frenkel defect mechanism.29 Interestingly, this particular defective structure has the benefit of giving free electrons to enable the spontaneous reduction of high-valent dopant ions in the crystal.30 Moreover, it has been established that Mn2+ preferentially occupies tetrahedral Al(2) sites in the spinel blocks of hexaaluminate structures, because the Al(2) sites have the longest Al–O bond length and the lowest bond-valence sum among all species of Al sites, making Al(2) much more suitable to accommodate the larger and less positive Mn2+ ions.31,32 Besides, the Al(2) sites have a high degree of symmetry and are placed in densely packed spinel blocks with a highly rigid structure, resulting in a narrow FWHM and low PL thermal quenching for doped Mn2+ ions. Most importantly, the Al(2) sites between the adjacent spinel blocks have a large distance of 9.84 Å due to the separating effect of the mirror planes, which is favorable for suppressing the concentration quenching effect caused by the energy migration between the Mn2+ luminescent centers.33,34
In this study, we prepared an Mn2+-activated green phosphor La0.827Al11.9−xMnxO19.09 (LAO:xMn2+) with a magnetoplumbite-type structure by a facile solid-state reaction method. We have meticulously investigated the impact of activator concentration on the luminescence properties. Under 450 nm excitation, the LAO:0.30Mn2+ sample exhibits a narrow green emission band peaking at 517 nm with an FWHM of 28 nm, a high PL QY and remarkable PL thermal stability. More significantly, using this phosphor as the green component, the fabricated LED achieves a high color gamut value of 124% NTSC, demonstrating the great potential of LAO:0.30Mn2+ as a green phosphor for LCD backlight applications.
2. Experimental
2.1. Materials and preparation
Mn2+-doped La0.827Al11.9O19.09 phosphors were synthesized by a conventional high-temperature solid-state method. The feed ratio was calculated according to the formula La0.827Al11.9−xMnxO19.09 in which Al3+ was partially replaced by Mn2+. The starting raw materials were La2O3 (99.99%, Aladdin), Al2O3 (A. R., Aladdin), MnCO3 (99.9%, Aladdin), and MnO2 (99.9%, Aladdin), with 2 wt% H3BO3 (A. R., aladdin) serving as a flux. These raw materials were weighed and ground thoroughly in an agate mortar for 30 minutes. Afterward, the mixtures were transferred to an alumina crucible and sintered at 1550 °C for 4 h in a horizontal tube furnace under an H2/N2 (5%/95%) reducing atmosphere. After the furnace was slowly cooled to room temperature, the sintered product was taken out and re-ground for further characterization.
2.2. Characterization
The phase purity and crystal structure were characterized using an X-ray diffractometer (Miniflex 600, Rigaku) with Cu Kα1 radiation (λ = 1.54187 Å) at an interval of 0.02°. The XRD data were collected in a 2θ range from 5° to 80° with a scanning speed of 8 °C min−1. The Rietveld method was employed for crystal structure refinement using the General Structure Analysis System (GSAS-II) program. EPR spectra were recorded on Bruker A300 at room temperature. The morphology and composition were characterized using a field-emission scanning electron microscope (Thermo Scientific, Apreo S) equipped with an energy dispersive spectroscopy (EDS) system. Diffuse reflection spectra were measured on a UV-Vis-NIR spectrophotometer (Agilent, Cary 5000) in the range of 200 to 800 nm and taking BaSO4 as the reference. The excitation and PL spectra were measured using a spectrometer (FLS 980, Edinburgh Instrument) equipped with 450 W xenon (Xe) lamps. The PL decay curves were measured with the same instrument by using an OPO laser (NT242-1K, EKSPLA, 195–2600 nm, 3–6 ns pulse duration, and 1000 Hz pulse repetition rate) as the excitation source. The temperature-dependent emission spectra were measured via a thermal heating stage (77–873 K, THMS 600, Linkam Scientific Instruments) which is coupled to the FLS 980 spectrometer. For PL QY measurements, the samples were put inside a barium sulfate coated integrating sphere coupled to a PG2000-Pro spectrophotometer (194–1119 nm, 50 um slit) and a standard tungsten halogen lamp was used to calibrate the optical response of the instrument.
2.3. LED performance
A white LED was fabricated by integrating the narrowband green phosphor LAO:0.30Mn2+, red phosphor K2TiF6:Mn4+, and InGaN blue chip. The phosphors were thoroughly mixed with silicone (part A + B, SHIN-ETSU Chemical Co. Ltd), and then coated on the blue InGaN LED chip. The weight ratio of silicone to LAO:0.30Mn2+ and to K2TiF6:Mn4+ was 0.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1. The packaged devices were further cured at 120 °C for 2 h in an oven, and then fixed in a 5054 PCB substrate to produce the desired LEDs. The photoelectric properties of the LEDs were measured using an integrating sphere spectroradiometer system (HASS-2000, 350–1100 nm, Everfine).
0.1. The packaged devices were further cured at 120 °C for 2 h in an oven, and then fixed in a 5054 PCB substrate to produce the desired LEDs. The photoelectric properties of the LEDs were measured using an integrating sphere spectroradiometer system (HASS-2000, 350–1100 nm, Everfine).
3. Results and discussion
The crystal structure of LAO is shown in Fig. 1a. It is derived from magnetoplumbite (PbFe12O19) with the space group of P63/mmc. Similar to other hexaaluminates, LAO is an orderly layered structure stacked along the c-axis and its structural units include a densely packed spinel block (Al11O16) and a so-called mirror plane (LaO + AlO).27,35 The spinel block (Al11O16) is composed of [Al(1)O6] octahedra, [Al(2)O4] tetrahedra, or [Al(3)O6] octahedra, respectively. Among these polyhedra, the [Al(2)O4] tetrahedra are located in the center of the spinel blocks and exhibit a corner-sharing with nearby [Al(1)O6] and [Al(3)O6] octahedra, while the [Al(1)O6] and [Al(3)O6] octahedra connect with each other by edge-sharing. In this way, a rigid 3D network structure is constructed from these polyhedra. Generally, the mirror plane will have large divalent and trivalent ions located inside it. Nevertheless, in contrast to most hexaaluminate structures, LAO is a non-stoichiometric compound with a portion of La deficiency on the mirror layer (LaO + AlO). To maintain charge balance, a series of point defects and vacancies spontaneously emerged and dispersed randomly across the mirror plane.30,36–38 In the hexaaluminate structure, Mn2+ prefers to occupy the Al(2) site in the [Al(2)O4] tetrahedron due to the lowest formation energy.29 Additionally, the Mn2+ doping sites in the host must be separated by a relatively large distance to attenuate the energy migration between Mn2+ ions, thus suppressing the PL concentration quenching effect and achieving a higher Mn2+ doping concentration. As shown in Fig. 1b, the closest distance between Al(2) sites in the [Al(2)O4] tetrahedron inside the spinel block is 3.43 Å. However, owing to the separating effect of mirror planes, the closest distance of Al(2) sites in [Al(2)O4] between the spinel layers rises to 9.84 Å. Benefiting from this, we achieve a high Mn2+ doping concentration of x = 0.30 for the LAO host in this study.
 |
| | Fig. 1 (a) Crystal structure of La0.827Al11.9O19.09 (LAO) and coordination polyhedra of Al(1)–Al(6) atoms. (b) The distance between the isolated [Al(2)O4] tetrahedron in the crystal structure. | |
The crystal structure and phase purity of the prepared LAO:xMn2+ samples were characterized by X-ray diffraction. The H3BO3 flux plays an important role in promoting the formation of a pure phase, which can only be obtained with the addition of 2 wt% H3BO3 (Fig. S1†). To investigate the impact of Mn2+ doping concentration on the luminescence properties, a series of LAO:xMn2+ (x = 0.00–0.40) samples were prepared and all of them were in the pure phase (Fig. S2†). A gradual shift of the diffraction peak corresponding to the (107) lattice plane towards a smaller angle is observed due to lattice expansion induced by the substitution of Mn2+ (r = 0.66 Å, CN = 4) for the smaller Al3+ (r = 0.53 Å, CN = 4).39Fig. 2a shows the XRD pattern of the LAO:0.30Mn2+ sample and the diffraction peaks match well with the standard pattern of the LAO crystal (ICSD NO.38371). To further investigate the microstructure of the LAO:xMn2+ sample, a structural Rietveld refinement of the LAO:0.30Mn2+ sample was performed and compared with that of the undoped LAO sample. The crystallographic data derived from ICSD NO.38371 were used as the initial structural model for the refinement. The refinement results converge well, showing a low residual coefficient of Rwp = 6.34 and χ2 = 1.34 (Fig. 2b). The corresponding cell parameters were calculated to be a = b = 5.5731(1) Å, c = 22.1039(3) Å, and V = 592.68(1) Å3 with a space group of P63/mmc. Compared with the undoped LAO sample (Fig. S3†), LAO:0.30Mn2+ displays considerable lattice expansion (Table S1†), which is consistent with the above observed XRD peak shift. Tables S2 and S3† show the detailed atomic coordinates of the undoped LAO and LAO:0.30Mn2+ samples, respectively, based on XRD Rietveld refinement. The valence state of the Mn element in the structure is determined to be divalent, because the EPR spectrum of the LAO:0.30Mn2+ sample exhibits sextet hyperfine peaks with a g factor of 2.03 (Fig. 2c), which are a result of a hyperfine coupling between the nuclear spin of Mn (I = +5/2) and the electrons in the 3d orbital of the Mn2+.40 SEM images of the LAO:0.30Mn2+ sample show an irregular particle morphology stacked with dozens of minor sheets and the particle size distribution is in the range of several dozen micrometers (Fig. 2d and e). In addition, the smooth surface suggests that the LAO:0.30Mn2+ sample has a high degree of crystallinity. The atomic and mass ratios of La, Al, O, and Mn are determined by EDS and are closer to the theoretical elemental ratio (Fig. 2f). The corresponding elemental mapping images exhibit a uniform distribution of the related elements (Fig. 2g–k). These results demonstrate the formation of well-crystallized samples and the successful doping of Mn2+ ions.
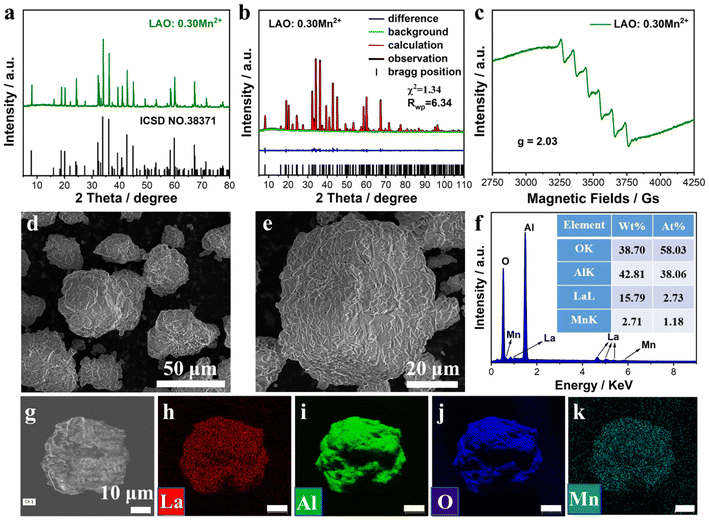 |
| | Fig. 2 (a) XRD patterns of the LAO:0.30Mn2+ sample and pure LAO crystals (ICSD NO. 38371) for comparison. (b) Rietveld refinement results of the LAO:0.30Mn2+ sample. (c) EPR spectrum of Mn2+ ions in the LAO:0.30Mn2+ sample. (d and e) SEM images of the LAO:0.30Mn2+ sample. (f) The mass and atomic percent of each element are determined by EDS analysis. (g–k) The mapping images of La, Al, O, and Mn elements. | |
The PL spectra of LAO:xMn2+ (x = 0.05–0.40) samples were measured and are shown in Fig. 3a. All samples exhibit a narrowband green emission peaking at 517 nm under 450 nm blue-light excitation, which is attributed to the 4T1(4G) → 6A1(6S) transition of Mn2+. The integrated PL intensity of the LAO:xMn2+ samples increased gradually as the doping concentration rose from 0.05 to 0.30, and then progressively declined as a result of the concentration quenching effect (Fig. 3b). Therefore, we obtain a high activator doping concentration of x = 0.30 in the LAO host. Generally, the nonradiative energy migration among Mn2+ ions followed by quenching by the structural defects leads to PL quenching, and the energy migration efficiency is highly dependent on the distance between the dopant Mn2+ ions. Specifically, when the distance is small enough, the energy migration is highly efficient and the excited electrons will migrate a longer distance in the lattice and have a larger probability of being captured by the structural defects, thus inducing strong PL quenching. To better analyze the mechanism, the critical distance (Rc), which is defined as the point at which the probabilities of nonradiative energy transfer and radiative emission are equal, is introduced. The value of Rc can be calculated using the equation below.41
| | 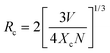 | (1) |
where
V is the volume per unit cell,
Xc is the critical concentration of Mn
2+, and
N is the number of cation sites that can be occupied by activator ions. In this case,
V = 592.681,
Xc = 0.30, and
N = 2, and the critical distance
Rc was determined to be 12.36 Å. Notably, this value exceeds 5 Å, indicating a greater tendency for the concentration quenching mechanism toward multipolar electron interactions. In view of Dexter's theory, the multipolar interactions can be further subdivided into an adjacent ion energy transfer mechanism, dipole–dipole interactions, dipole–quadrupole interactions, and quadrupole–quadrupole interactions, corresponding to
θ = 3,
θ = 6,
θ = 8 and
θ = 10, respectively. The type of interaction can be estimated by linear fitting according to the following equation.
42,43| | 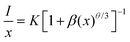 | (2) |
where
I represents the PL intensity,
x is the activator concentration over the critical concentration,
K and
β are constants, and
θ is used to describe the different modes of multipolar electron interactions. Fig. S4
† shows a plot of the relationship between log(
x)
versus log(
I/
x), which can be well-fitted with a slope of −3.14 (−
θ/3). Thus, the
θ was calculated to be 9.42 and close to 10, which suggests that quadrupole–quadrupole interactions dominate the PL concentration quenching mechanism in LAO:
xMn
2+.
 |
| | Fig. 3 (a) The PL spectra of the LAO:xMn2+ (x = 0.05–0.40) samples under excitation at 450 nm. (b) The evolution of PL peak intensity with Mn2+ doping concentration for the LAO:xMn2+ samples. (c) The PL and excitation spectra of the LAO:0.30Mn2+ sample and commercial green emitting β-SiALON:Eu2+ phosphor. The electroluminescence spectra of the InGaN chip are shown for comparison. (d) UV-vis diffuse reflectance spectra of the LAO:0.30Mn2+ sample. (e) The measured spectral data of the LAO:0.30Mn2+ sample under 450 nm excitation for calculating the internal and external PL QY. (f) The PL decay curve was measured by monitoring the emission at 517 nm of the LAO:0.30Mn2+ sample under excitation at 450 nm. | |
The emission peaks of the LAO:xMn2+ samples exhibit a subtle redshift of 4 nm and an imperceptible FWHM change with increasing Mn2+ concentration and the CIE chromaticity coordinates with a corresponding slight shift but remains stable in the green region (Fig. S5†). This redshift is probably due to the aggravated reabsorption with increasing dopant concentration, since there is an overlap between the emission and excitation spectra.44,45 For a more detailed investigation of LAO:xMn2+ green phosphors, we singled out the LAO:0.30Mn2+ sample and compared it with the commercial green phosphor β-SiALON:Eu2+. As shown in Fig. 3c, under 450 nm excitation, the LAO:0.30Mn2+ sample exhibits intense green emission with an FWHM of 28 nm, ranging from 480 nm to 600 nm. Its excitation spectrum measured by monitoring the emission at 517 nm contains five distinct peaks. The peak at 280 nm is a charge transfer band from O2− to Mn2+, while 361 nm, 384 nm, 426 nm, and 450 nm are attributed to the electronic transitions of Mn2+ from the ground state 6A1(6S) to the 4E(4D), 4T2(4D), [4A1(4G), 4E(4G)], and 4T2(4G) excited states, respectively. This is consistent with the results observed in the absorption spectra of the LAO:0.30Mn2+ sample (Fig. 3d). It is worth mentioning that the most intense absorption band is at 450 nm, which means that this phosphor can be excited by blue diode chips. In comparison, the commercial green phosphor β-SiALON:Eu2+ exhibits a broadband excitation in the range from 270 nm to 520 nm and a relatively narrow emission band peaking at 540 nm with an FWHM of 54 nm. It is obvious that the FWHM of the LAO:0.30Mn2+ sample is significantly narrower than that of β-SiALON:Eu2+. In addition, we also evaluated the color purity of the LAO:0.30Mn2+ phosphor since a narrower FWHM also implies a higher color purity. The CIE chromaticity coordinates of the LAO:0.30Mn2+ sample are (0.1798, 0.7442) determined by its PL spectra. The color purity is defined as the percentage of the linear distance between the chromaticity coordinates of the measured plots and the white light source as compared to the linear distance between the monochromatic light source and the white light source, which can be calculated using the following equation.46
| | 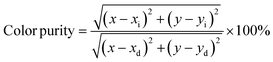 | (3) |
where (
x,
y) represent the CIE chromaticity coordinates of the LAO:0.30Mn
2+ sample, (
xi,
yi) represent the CIE chromaticity coordinates of the white light source, and (
xd,
yd) represent the color coordinates of the corresponding monochromatic light source. The color purity of the LAO:0.30Mn
2+ sample is determined to be 88%, which is promising to support its application in wide color gamut backlight displays. In addition, as shown in
Fig. 3e, the internal PL QY of the LAO:0.30Mn
2+ sample under 450 nm blue-light excitation at room temperature was found to be 90.0%, indicating high emission efficiency. The corresponding absorption efficiency is 17.9%. Fig. S6
† shows the variation of internal PL QYs, absorption efficiency, and external PL QYs with Mn
2+ doping concentration in LAO:
xMn
2+ (
x = 0.05–0.40) samples and the detailed data are listed in Table S4.
† The room-temperature PL decay curves of LAO:
xMn
2+ (
x = 0.05–0.40) samples measured by monitoring emission at 517 nm exhibit single exponential behavior (Fig. S7
†), and the fitted PL lifetime decreased from 6.05 to 4.58 ms with increasing
x values. The shorter PL lifetime with increasing Mn
2+ concentration in the range of
x = 0.05–0.40 may be due to the exchange-coupled interaction between the paramagnetic Mn
2+ ions, which enhances the radiative transition probability with increasing doping concentration.
47 The PL lifetime for the LAO:0.30Mn
2+ sample is determined to be 5.37 ms (
Fig. 3f).
The PL thermal quenching behavior of phosphors is a key factor in evaluating its potential device performance. Hence, the temperature-dependent PL spectra of the LAO:0.30Mn2+ sample were measured from 298 to 473 K with a temperature interval of 25 K under 450 nm excitation (Fig. 4a). With increasing temperature, the integrated PL intensity decreases from the initial value of 100% at 298 K to 94% at 423 K and 89% at 473 K, indicating excellent PL thermal stability. The decreased intensity is attributed to the accelerated lattice vibration and relaxation in the luminescent center at high temperatures, resulting in enhanced nonradiative transition.48 The activation of thermal quenching (ΔE) was estimated using the Arrhenius equation.
| |  | (4) |
where
I0 and
IT are the PL intensity at initial room temperature and a given temperature (
T), respectively, Δ
E represents the activation energy,
A is the constant for a given host, and
k is the Boltzmann constant. In general, a larger activation energy Δ
E implies better thermal stability. Based on ln(
I0/
IT − 1)
versus 1/
kT, the activation energy Δ
E was calculated to be 0.16 eV (Fig. S8
†). This value is comparable to those of previously reported hexaaluminate materials.
13Fig. 4b shows the normalized PL spectra of the LAO:0.30Mn
2+ sample with increasing temperature, and the emission-peak position remains unchanged at 517 nm, while the FWHM increases gradually from 28 nm (298 K) to 34 nm (473 K) (the inset in
Fig. 4b). The broadening of the FWHM with temperature can be explained by the configuration coordinate model and electronic Boltzmann distribution.
49| |  | (5) |
where
hv is the average phonon energy,
S is the Huang–Rhys factor, which represents the electron–phonon coupling strength, and
k is the Boltzmann constant. It is evident that temperature has a positive correlation with FWHM. As the temperature gradually increases, more excited state electrons diffuse to higher vibrational energy levels and then make a radiative transition from these different energy levels to the ground state, resulting in a broadened emission band. The variation of FWHM with temperature is well-fitted using
eqn (5) as shown in
Fig. 4c, and the values of
S and
hv are calculated to be 2.32 and 18.60 meV, respectively.
Fig. 4d gives the temperature-dependent PL decay curves of the LAO:0.30Mn
2+ sample and all curves can be well-fitted with a single exponential decay function. As the temperature increases from 298 K to 473 K, the PL lifetime decreases slightly from 5.37 to 5.22 ms due to the increase in nonradiative transition probability at high temperatures. These findings support that the LAO:0.30Mn
2+ sample has a low PL thermal quenching. Moreover, the chromaticity stability of the LAO:0.30Mn
2+ sample with increasing temperature is shown in
Fig. 4e. The CIE chromaticity coordinates of the LAO:0.30Mn
2+ sample shift from (0.1798, 0.7442) to (0.1823, 0.7290) and then to (0.1837, 0.7216) when the temperature changed from 298 K to 423 K and then to 473 K. Specifically, the chromaticity coordinate shift value (Δ
D) can be calculated using the following equation.
50,51| |  | (6) |
| | | u′ = 4x/12(3 − 2x + y) | (7) |
| | | v′ = 9y/(3 − 2x + 12y) | (8) |
where (
x,
y) represent the chromaticity coordinates in CIE 1931, (
u′,
v′) represent the chromaticity coordinates in
u′
v′ uniform color space, and 0 and
t are the chromaticity shifts at 298 K and given temperature conditions, respectively. As a result, the shifts of the chromaticity coordinates were determined to be 3.29‰ at 423 K and 4.86‰ at 473 K in comparison with the initial value of 298 K. This shift value is lower than those of most phosphors, demonstrating excellent chromaticity stability of the LAO:0.30Mn
2+ phosphor.
50–54Fig. 4f depicts the variation in the PL intensity of the LAO:0.30Mn
2+ sample after three heating–cooling cycles at the temperature range from 298 K to 473 K. As we can see, the emission intensity can recover its initial value well after each cycle, proving good stability of the LAO:0.30Mn
2+ sample. The first reason for the high thermal stability of the LAO:0.30Mn
2+ phosphor is its high structural rigidity, resulting in a relatively weak electron–phonon coupling strength as indicated by its narrow emission bandwidth (FWHM = 28 nm). This means that the lattice thermal variation can be effectively restricted, while simultaneously minimizing the horizontal offset Δ
R between two parabolas representing the lowest excited state
4T
1 and the ground state
6A
1 energy levels. Consequently, this leads to a larger thermal activation energy (Δ
E) required for nonradiative transition and reduces emission loss during the heating process.
48 In addition, our prepared LAO:0.30Mn
2+ phosphor exhibits excellent crystallinity, as evidenced by SEM analysis, along with an ultra-high internal PL QY of 90%. This implies a reduced presence of structural defects within the crystal lattice. With increasing temperature, the energy migration among Mn
2+ ions will become more efficient, thus facilitating easier trapping of excited electrons by structural defects. Therefore, the relatively low defect density in our samples also contributes to its remarkable thermal stability.
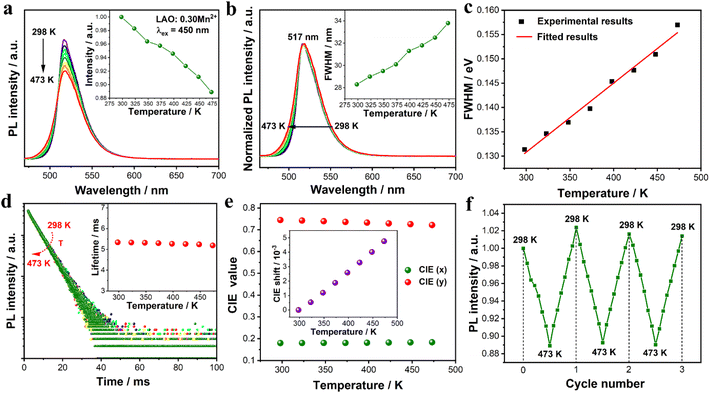 |
| | Fig. 4 (a) Temperature-dependent PL spectra of the LAO:0.30Mn2+ sample at 298–473 K. The inset shows the variation of normalized integrated emission intensities with temperature. (b) The normalized PL spectra of the LAO:0.30Mn2+ sample at 298–473 K. The inset shows the variation of FWHM with temperature. (c) Temperature-dependence of the FWHM (experimental and fitted results) in LAO:0.30Mn2+. (d) Temperature-dependent PL decay curves of the LAO:0.30Mn2+ sample at 298–473 K. All the curves exhibit a single exponential decay behavior, and the inset shows the fitted PL lifetime at various temperatures. (e) The CIE coordinates of the LAO:0.30Mn2+ sample at 298–473 K. The inset shows the CIE shift as a function of temperature. (f) The PL intensity corresponds to three heating and cooling cycles of the LAO:0.30Mn2+ sample. | |
To evaluate the practical application of the LAO:0.30Mn2+ phosphor in devices, white LED1 was fabricated by coating LAO:0.30Mn2+ green and KTF:Mn4+ red phosphors on 450 nm InGaN chips. Besides, white LED2 using a phosphor blend of green β-SiAlON:Eu2+ and red KTF:Mn4+ was also packaged for comparison. Fig. 5a and b show the electroluminescence (EL) spectra and chromaticity coordinates of these two LEDs, respectively. Driven by a voltage of 3.0 V and a current of 60 mA, LED1 shows white light emissions with chromaticity coordinates of (0.3329, 0.3160), a correlated color temperature (CCT) of 5464 K, and a luminous efficacy of 18 lm W−1, while LED2 shows white light emissions with chromaticity coordinates of (0.3393, 0.3307), a CCT of 5646 K, and a luminous efficacy of 89 lm W−1. The lower luminous efficacy of LED1 is attributed to the weak absorption of the LAO:0.30Mn2+ phosphor, which requires a large amount of phosphor to be coated on the LED chip, thus inducing serious light scattering and reabsorption losses. Noteworthily, the color gamut of LED1 reached 124% of the NTSC 1931 standard and 93% of the Recommendation 2020 (Rec. 2020) standard, whereas the corresponding color gamut of LED2 was only 94% and 70%, respectively (Fig. 5c and d). The significantly larger color gamut of LED1 comes mainly from the narrower green emission of the LAO:0.30Mn2+ phosphor. The EL spectra and the chromaticity coordinates of these two LEDs under various drive currents from 20 mA to 100 mA are shown in Fig. S9,† and the small variation in color coordinates proves that LED1 has similar excellent stability to LED2 under different input powers. Table S5† summarizes the main spectral parameters and device performances of various well-known narrowband green phosphors. It can be seen that the LAO:0.30Mn2+ phosphor has an ideal emission peak position, a narrow emission band, high PL thermal stability, and a wide color gamut. These results demonstrate that LAO:0.30Mn2+ has great potential for usage as a green phosphor to achieve high-quality wide-color gamut LCD backlight displays.
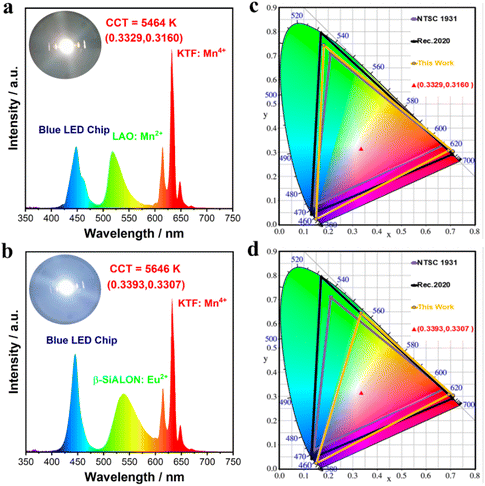 |
| | Fig. 5 Electroluminescence spectra of the LEDs fabricated using a blue InGaN chip, a red KTF:Mn4+ phosphor, along with (a) the green emitting LAO:0.30Mn2+ sample (LED1), and (b) the commercial green emitting β-SiALON:Eu2+ phosphor (LED2), respectively. The chromaticity coordinates of the fabricated (c) LED1 and (d) LED2 in CIE 1931, and the corresponding color space (yellow line) compared with the NTSC 1931 standard (purple line) and Rec. 2020 standard (black line). | |
4. Conclusions
In summary, we have successfully prepared a green phosphor LAO:xMn2+ (peak wavelength at ∼517 nm) with a narrow FWHM of 28 nm by the solid-state reaction method. Thanks to the separating effect of the mirror layer that makes the isolated Mn2+ stay far away from each other, we have achieved a high doping concentration of x = 0.30 in the LAO host. Under 450 nm excitation, the LAO:0.30Mn2+ phosphor shows a color purity of 88% and an internal/external PL QY of 90%/16%. Moreover, the phosphor shows excellent PL thermal stability with an emission intensity loss of 6% at 423 K, and 11% at 473 K compared to its initial value at 298 K. The corresponding temperature-dependent PL lifetime proves that LAO:0.30Mn2+ possesses a low nonradiative transition probability, probably due to its highly rigid structure and good crystal quality. Moreover, it has an excellent resistance to color drifting with chromaticity shifts of 3.39 ‰ at 423 K and 4.86 ‰ at 473 K. Finally, using the narrowband emitting LAO:0.30Mn2+ phosphor as the green component and the K2TiF6:Mn4+ phosphor as the red component, combined with a 450 nm InGaN blue chip, the fabricated white LED device can emit bright white light and achieve a wide color gamut of 124% NTSC 1931 standard with a CCT of 5464 K. These exciting results indicate that the prepared LAO:0.30Mn2+ green phosphor has promising applications in wide color gamut displays.
Conflicts of interest
There are no conflicts to declare.
Acknowledgements
This research was supported by the Natural Science Foundation of China (No. 11974351) and Fujian Science & Technology Innovation Laboratory for Optoelectronic Information of China (No. 2021ZZ114).
References
- M.-H. Fang, J. L. Leaño and R.-S. Liu, Control of Narrow-Band Emission in Phosphor Materials for Application in Light-Emitting Diodes, ACS Energy Lett., 2018, 3, 2573–2586 CrossRef CAS
 .
.
- M. Zhao, Q. Zhang and Z. Xia, Narrow-band emitters in LED backlights for liquid-crystal displays, Mater. Today, 2020, 40, 246–265 CrossRef CAS
 .
.
- Y. Zhang, L. Luo, G. Chen, Y. Liu, R. Liu and X. Chen, Green and red phosphor for LED backlight in wide color gamut LCD, J. Rare Earths, 2020, 38, 1–12 CrossRef CAS
 .
.
- I. Kim and K. Chung, Wide Color Gamut Backlight from three-band white LED, J. Opt. Soc. Korea, 2007, 11, 67–70 CrossRef
 .
.
- S. Li, L. Wang, D. Tang, Y. Cho, X. Liu, X. Zhou, L. Lu, L. Zhang, T. Takeda, N. Hirosaki and R.-J. Xie, Achieving High Quantum Efficiency Narrow-Band β-Sialon:Eu2+ Phosphors for High-Brightness LCD Backlights by Reducing the Eu3+ Luminescence Killer, Chem. Mater., 2017, 30, 494–505 CrossRef
 .
.
- X. Zhang, H.-C. Wang, A.-C. Tang, S.-Y. Lin, H.-C. Tong, C.-Y. Chen, Y.-C. Lee, T.-L. Tsai and R.-S. Liu, Robust and Stable Narrow-Band Green Emitter: An Option for Advanced Wide-Color-Gamut Backlight Display, Chem. Mater., 2016, 28, 8493–8497 CrossRef CAS
 .
.
- P. Strobel, S. Schmiechen, M. Siegert, A. Tücks, P. J. Schmidt and W. Schnick, Narrow-Band Green Emitting Nitridolithoalumosilicate Ba[Li2(Al2Si2)N6]:Eu2+ with Framework Topology whj for LED/LCD-Backlighting Applications, Chem. Mater., 2015, 27, 6109–6115 CrossRef CAS
 .
.
- Y. Zhuo, S. Hariyani, J. Zhong and J. Brgoch, Creating a Green-Emitting Phosphor through Selective Rare-Earth Site Preference in NaBaB9O15:Eu2+, Chem. Mater., 2021, 33, 3304–3311 CrossRef CAS
 .
.
- H. Liao, M. Zhao, Y. Zhou, M. S. Molokeev, Q. Liu, Q. Zhang and Z. Xia, Polyhedron Transformation toward Stable Narrow-Band Green Phosphors for Wide-Color-Gamut Liquid Crystal Display, Adv. Funct. Mater., 2019, 29, 1901988 CrossRef
 .
.
- Q. Dong, F. Yang, J. Cui, Y. Tian, S. Liu, F. Du, J. Peng and X. Ye, Enhanced narrow green emission and thermal stability in γ-AlON:Mn2+, Mg2+ phosphor via charge compensation, Ceram. Int., 2019, 45, 11868–11875 CrossRef CAS
 .
.
- E. H. Song, Y. Y. Zhou, Y. Wei, X. X. Han, Z. R. Tao, R. L. Qiu, Z. G. Xia and Q. Y. Zhang, A thermally stable narrow-band green-emitting phosphor MgAl2O4:Mn2+ for wide color gamut backlight display application, J. Mater. Chem. C, 2019, 7, 8192–8198 RSC
 .
.
- H. Li, Y. Liang, S. Liu, W. Zhang, Y. Bi, Y. Gong and W. Lei, Highly Efficient Green-Emitting Phosphor BaZnAl10O17:Mn2+ with Ultra-Narrow Band and Extremely Low Thermal Quenching for Wide Color Gamut LCD Backlights, Adv. Opt. Mater., 2021, 9, 2100799 CrossRef CAS
 .
.
- Y. Zhu, Y. Liang, S. Liu, H. Li and J. Chen, Narrow-Band Green-Emitting Sr2MgAl22O36:Mn2+ Phosphors with Superior Thermal Stability and Wide Color Gamut for Backlighting Display Applications, Adv. Opt. Mater., 2019, 7, 1801419 CrossRef
 .
.
- D. Q. Trung, N. Tu, N. V. Quang, M. T. Tran, N. V. Du and P. T. Huy, Non-rare-earth dual green and red-emitting Mn-doped ZnAl2O4 phosphors for potential application in plan-growth LEDs, J. Alloys Compd., 2020, 845, 156326 CrossRef CAS
 .
.
- Q. Zhou, L. Dolgov, A. M. Srivastava, L. Zhou, Z. Wang, J. Shi, M. D. Dramićanin, M. G. Brik and M. Wu, Mn2+ and Mn4+ red phosphors: synthesis, luminescence and applications in WLEDs. A review, J. Mater. Chem. C, 2018, 6, 2652–2671 RSC
 .
.
- C. Bertail, S. Maron, V. Buissette, T. Le Mercier, T. Gacoin and J.-P. Boilot, Structural and Photoluminescent Properties of Zn2SiO4:Mn2+ Nanoparticles Prepared by a Protected Annealing Process, Chem. Mater., 2011, 23, 2961–2967 CrossRef CAS
 .
.
- J. Zhou, Y. Wang, B. Liu and Y. Lu, Effect of H3BO3 on structure and photoluminescence of BaAl12O19:Mn2+ phosphor under VUV excitation, J. Alloys Compd., 2009, 484, 439–443 CrossRef CAS
 .
.
- L. Wu, B. Wang, Y. Zhang, L. Li, H. R. Wang, H. Yi, Y. F. Kong and J. J. Xu, Structure and photoluminescence properties of a rare-earth free red-emitting Mn(2+)-activated KMgBO3, Dalton Trans., 2014, 43, 13845–13851 RSC
 .
.
- L. J. Xu, C. Z. Sun, H. Xiao, Y. Wu and Z. N. Chen, Green-Light-Emitting Diodes based on Tetrabromide Manganese(II) Complex through Solution Process, Adv. Mater., 2017, 29, 1605739 CrossRef PubMed
 .
.
- C. Meisheng, W. Liangshi, Z. Na, L. Zhiqi, L. Dianqing and C. Aifan, La-Hexaaluminate Catalyst Preparation and Its Performance for Methane Catalytic Combustion, J. Rare Earths, 2006, 24, 690–694 CrossRef
 .
.
- X. Chen, Y. Zhao, W. Huang, H. Ma, B. Zou, Y. Wang and X. Cao, Thermal aging behavior of plasma sprayed LaMgAl11O19 thermal barrier coating, J. Eur. Ceram. Soc., 2011, 31, 2285–2294 CrossRef CAS
 .
.
- C. Huang, D. Huang, S. Xu, J. Zhang, S. Liang, J. Hu, K. Xu, D. Chen and H. Zhu, Highly Efficient and Thermally Stable Ba0.75Al11O17.25:Eu2+, Mn2+ Phosphor with Dual Narrowband Blue and Green Emissions: A Promising Candidate for Wide Gamut Displays, Adv. Photonics Res., 2022, 3, 2200069 CrossRef CAS
 .
.
- S. Liu, Z. Wang, H. Cai, Z. Song and Q. Liu, Highly efficient near-infrared phosphor LaMgGa11O19:Cr3+, Inorg. Chem. Front., 2020, 7, 1467–1473 RSC
 .
.
- C. Dou, F. Zhao, S. Liu, Z. Song and Q. Liu, Achieving efficient violet-light-excited blue phosphors by nitridation for violet-chip-based full-spectrum lighting, Inorg. Chem. Front., 2023, 10, 2430–2437 RSC
 .
.
- Z. Xiying, Z. Weidong, C. Xiangzhong, H. Huaqiang and H. Xiaowei, Preparation of BaMgAl10O17:Eu2+ and Its Phase Behavior in Microemulsion System, J. Rare Earths, 2006, 24, 736–739 CrossRef
 .
.
- T. Murata, T. Tanoue, M. Iwasaki, K. Morinaga and T. Hase, Fluorescence properties of Mn4+ in CaAl12O19 compounds as red-emitting phosphor for white LED, J. Lumin., 2005, 114, 207–212 CrossRef CAS
 .
.
- N. Iyi, Z. Inoue, S. Takekawa and S. Kimura, The crystal-structure of Lanthanum hexaaluminate, J. Solid State Chem., 1984, 54, 70–77 CrossRef CAS
 .
.
- J. Yu, X. Luo, Z. Wang, S. Yu, J. Wu, M. Li, X. Li, T. Tang, J. Wen and L. Zhao, A novel blue emitting europium doped lanthanum magnesium hexaaluminate long persistent phosphor and analysis of traps distribution, J. Lumin., 2022, 243, 118671 CrossRef CAS
 .
.
- S. R. Jansen, J. M. Migchels, H. T. Hintzen and R. Metselaar, Eu-Doped Barium Aluminum Oxynitride with the β-Alumina-Type Structure as New Blue-Emitting Phosphor, J. Electrochem. Soc., 1999, 146, 800 CrossRef CAS
 .
.
- J. Hu, E. Song, Y. Zhou, S. Zhang, S. Ye, Z. Xia and Q. Zhang, Non-stoichiometric defect-controlled reduction toward mixed-valence Mn-doped hexaaluminates and their optical applications, J. Mater. Chem. C, 2019, 7, 5716–5723 RSC
 .
.
- T. R. Wagner and M. O'Keeffe, Bond lengths and valences in aluminates with the magnetoplumbite and β-alumina structures, J. Solid State Chem., 1988, 73, 211–216 CrossRef CAS
 .
.
- M. Bellotto, G. Artioli, C. Cristiani, P. Forzatti and G. Groppi, On the Crystal Structure and Cation Valence of Mn in Mn-Substituted Ba-β-Al2O3, J. Catal., 1998, 179, 597–605 CrossRef CAS
 .
.
- F. Zhao, H. Cai, Z. Song and Q. Liu, Structural Confinement for Cr3+ Activators toward Efficient Near-Infrared Phosphors with Suppressed Concentration Quenching, Chem. Mater., 2021, 33, 3621–3630 CrossRef CAS
 .
.
- F. Zhao, H. Cai, Z. Song and Q. Liu, Structural Confinement toward Controlling Energy Transfer Path for Enhancing Near-Infrared Luminescence, Chem. Mater., 2021, 33, 8360–8366 CrossRef CAS
 .
.
- A. L. N. Stevels, Red Mn2+-luminescence in hexagonal aluminates, J. Lumin., 1979, 20, 99–109 CrossRef CAS
 .
.
- J.-G. Park and A. N. Cormack, Crystal/Defect Structures and Phase Stability in Ba Hexaaluminates, J. Solid State Chem., 1996, 121, 278–290 CrossRef CAS
 .
.
- Z. Wu and A. N. Cormack, Defects in BaMgAl10O17:Eu2+ blue phosphor, J. Electroceram., 2003, 10, 179–191 CrossRef CAS
 .
.
- K. C. Mishra, Theoretical Investigation of Structure and Stability of Reidinger Defects in Barium Magnesium Aluminate, J. Electrochem. Soc., 2005, 152, H84 CrossRef CAS
 .
.
- R. D. Shannon, Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr., 1976, 32, 751–767 CrossRef
 .
.
- V. Singh, R. P. S. Chakradhar, J. L. Rao, S. J. Dhoble and S. H. Kim, Electron Paramagnetic Resonance and Photoluminescence Studies of LaMgAl11O19:Mn2+ Green Phosphors, J. Electron. Mater., 2014, 43, 4041–4047 CrossRef CAS
 .
.
- S. Liang, P. Dang, G. Li, M. S. Molokeev, Y. Wei, Y. Wei, H. Lian, M. Shang, A. A. Al Kheraif and J. Lin, Controllable two-dimensional luminescence tuning in Eu2+, Mn2+ doped (Ca,Sr)9Sc(PO4)7 based on crystal field regulation and energy transfer, J. Mater. Chem. C, 2018, 6, 6714–6725 RSC
 .
.
- Z. Zhou, J. Zheng, R. Shi, N. Zhang, J. Chen, R. Zhang, H. Suo, E. M. Goldys and C. Guo, Ab Initio Site Occupancy and Far-Red Emission of Mn(4+) in Cubic-Phase La(MgTi)(1/2)O(3) for Plant Cultivation, ACS Appl. Mater. Interfaces, 2017, 9, 6177–6185 CrossRef CAS PubMed
 .
.
- K. Li, J. Fan, M. Shang, H. Lian and J. Lin, Sr2Y8(SiO4)6O2:Bi3+/Eu3+: a single-component white-emitting phosphor via energy transfer for UV w-LEDs, J. Mater. Chem. C, 2015, 3, 9989–9998 RSC
 .
.
- D. Zhang, X. Zhang, Z. An, R. Dong, Z. Shi, Y. Song and H. Zou, Photoluminescence and Color-Tunable Properties of Na(4)Ca(4)Mg(21)(PO(4))(18):Eu(2+),Tb(3+)/Mn(2+) Phosphors for Applications in White LEDs, Inorg. Chem., 2020, 59, 14193–14206 CrossRef CAS PubMed
 .
.
- H. Chen, T. Seto and Y. Wang, An efficient blue phosphor with high thermal stability for lighting and optical pressure sensor applications, Inorg. Chem. Front., 2022, 9, 1644–1654 RSC
 .
.
- X. Huang, H. Guo and B. Li, Eu3+-activated Na2Gd(PO4)(MoO4): A novel high-brightness red-emitting phosphor with high color purity and quantum efficiency for white light-emitting diodes, J. Alloys Compd., 2017, 720, 29–38 CrossRef CAS
 .
.
- A. P. Vink, M. A. de Bruin, S. Roke, P. S. Peijzel and A. Meijerink, Luminescence of
exchange coupled pairs of transition metal ions, J. Electrochem. Soc., 2001, 148, E313–E320 CrossRef CAS
 .
.
- P. Dang, W. Wang, H. Lian, G. Li and J. Lin, How to Obtain Anti-Thermal-Quenching Inorganic Luminescent Materials for Light-Emitting Diode Applications, Adv. Opt. Mater., 2022, 10, 2102287 CrossRef CAS
 .
.
- J. Yan, M. G. Brik, C. Liu, D. Hou, W. Zhou, B. Zhang, Y. Huang, Y. Tao and H. Liang, VUV–vis photoluminescence, low-voltage cathodoluminescence and electron-vibrational interaction of Mn2+ in Ba2MgSi2O7, Opt. Mater., 2015, 43, 59–65 CrossRef CAS
 .
.
- C. Li, H. Zheng, H. Wei, S. Qiu, L. Xu, X. Wang and H. Jiao, A color tunable and white light emitting Ca(2)Si(5)N(8):Ce(3+),Eu(2+) phosphor via efficient energy transfer for near-UV white LEDs, Dalton Trans., 2018, 47, 6860–6867 RSC
 .
.
- W. Wang, H. Yang, M. Fu, X. Zhang, M. Guan, Y. Wei, C. C. Lin and G. Li, Superior thermally-stable narrow-band green emitter from Mn2+-doped zero thermal expansion (ZTE) material, Chem. Eng. J., 2021, 415, 128979 CrossRef CAS
 .
.
- X. Zhang, L. Huang, F. Pan, M. Wu, J. Wang, Y. Chen and Q. Su, Highly thermally stable single-component white-emitting silicate glass for organic-resin-free white-light-emitting diodes, ACS Appl. Mater. Interfaces, 2014, 6, 2709–2717 CrossRef CAS PubMed
 .
.
- C. Li, X.-M. Wang, Z.-P. Yang and H. Jiao, Ca8Mg7Si9N22:Ce3+—A Yellow-Emitting Nitride Phosphor for White Light Emitting Diodes, ACS Appl. Electron. Mater., 2020, 2, 936–943 CrossRef CAS
 .
.
- Z. Leng, H. Bai, Q. Qing, H. He, J. Hou, B. Li, Z. Tang, F. Song and H. Wu, A Zero-Thermal-Quenching Blue Phosphor for Sustainable and Human-Centric WLED Lighting, ACS Sustainable Chem. Eng., 2022, 10, 10966–10977 CrossRef
 .
.
|
| This journal is © the Partner Organisations 2024 |
Click here to see how this site uses Cookies. View our privacy policy here.  *abcd,
Sisi
Liang
cd,
Yingping
Huang
cd,
Zihao
Wang
cd and
Maochun
Hong
*abcd,
Sisi
Liang
cd,
Yingping
Huang
cd,
Zihao
Wang
cd and
Maochun
Hong
 *abcd
*abcd
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1. The packaged devices were further cured at 120 °C for 2 h in an oven, and then fixed in a 5054 PCB substrate to produce the desired LEDs. The photoelectric properties of the LEDs were measured using an integrating sphere spectroradiometer system (HASS-2000, 350–1100 nm, Everfine).
0.1. The packaged devices were further cured at 120 °C for 2 h in an oven, and then fixed in a 5054 PCB substrate to produce the desired LEDs. The photoelectric properties of the LEDs were measured using an integrating sphere spectroradiometer system (HASS-2000, 350–1100 nm, Everfine).

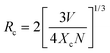
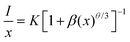
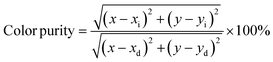



.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.




