Rational design for broad near-infrared emission from a two-sited Rb2LiAlF6:Cr3+ phosphor with high efficiency and thermal stability for spectroscopic applications†
Song
Qing
a,
Jing
Wan
a,
Tao
Yang
a,
Qiang
Zhou
 *a,
Yayun
Zhou
*a,
Yayun
Zhou
 b,
Zhengliang
Wang
b,
Zhengliang
Wang
 *a,
Dawei
Wen
c and
Mingmei
Wu
*a,
Dawei
Wen
c and
Mingmei
Wu
 *d
*d
aKey Laboratory of Green Chemistry Materials in University of Yunnan Province, Yunnan Key Laboratory of Chiral Functional Substance Research and Application, School of Chemistry and Environment, Yunnan Minzu University, Kunming 650500, P. R. China. E-mail: q-zhou@ymu.edu.cn; wangzhengliang@foxmail.com
bGuangdong-Hong Kong-Macao Joint Laboratory for Intelligent Micro-Nano Optoelectronic Technology, School of Physics and Optoelectronic Engineering, Foshan University, Foshan 528225, China
cSchool of Applied Physics and Materials, Wuyi University, Jiangmen 529020, P. R. China
dSchool of Chemical Engineering and Technology, Sun Yat-Sen University, Zhuhai 519082, P. R. China. E-mail: ceswmm@mail.sysu.edu.cn
First published on 26th March 2024
Abstract
The exploration of high-performance near-infrared phosphors has attracted widespread attention. In this work, a brand new Rb2LiAlF6:Cr3+ (denoted as RLAF:Cr) phosphor has been constructed by the substitution of Al3+ ions with Cr3+ ions. Evidence shows that two sets of near-infrared emission bands, which originated from two types of Cr3+ sites, were observed upon blue light excitation. These emission bands merged into a wide emission band locating in the region of 650 nm–1050 nm, with a full width at half maximum (FWHM) of 125 nm. In addition, a high quantum efficiency of 77.7% and an excellent thermal stability at 417 K, with a retention rate of 90.5% of that at room temperature (RT), were witnessed. Profiting from the luminescence properties of the NIR phosphor, clear images of biological tissues and human palm veins were obtained using a light-emitting diode (LED) as a lighting source, which was constructed using an RLAF:Cr phosphor and a blue InGaN chip. These images showed the large potential of the RLAF:Cr phosphor for night vision and bioimaging in LED devices.
1. Introduction
Near-infrared (NIR) light has been widely used as an energy source in several application fields, such as biological imaging, night vision, food quality analysis, and plant lighting. The reasons for these applications are the favourable benign features of the NIR light, such as low energy, minimum damage, and deep penetration.1–3 Among the latest technologies, the NIR phosphor-converted light-emitting diode (pc-LED) is an emerging lighting source that is based on the combination of an NIR phosphor and a blue LED chip, and it is regarded as the best technical solution for a quick and portable application of the NIR light. This is due to its attractive properties, such as small size, high efficiency, and long lifetime.4–6 Although it is a crucial component of the NIR pc-LED device, the high-performance NIR phosphor, with its high efficiency and excellent thermal stability, is still a challenge to explore and has consequently attracted worldwide research interest.7–9A number of NIR phosphors have been realized by the incorporation of different metal ions, including lanthanide ions and transition-metal ions, typically Eu2+ and Cr3+, into suitable host structures.10–12 In particular, the trivalent Cr3+ ion has been considered a preferable NIR activator since its 3d3 electronic configuration enables tuneable emissions, from narrowband R-line emission (2E → 4A2) to wideband emission (4T2 → 4A2).13,14 Recently, fluorides have gained increasing attention, which is due to the fact that their low phonon energies favour weak electron–phonon coupling (EPC) effects.15 A Cr3+-doped fluoride-induced high efficiency and excellent thermal stability are then expected. For example, K2NaAlF6:Cr3+ shows a quantum efficiency (QE) of 68.08% and an excellent anti-thermal quenching of 96.5% at 150 °C but has a small NIR spectral range (FWHM = 96 nm).16 As is known, emission bandwidth is an important parameter for the evaluation of NIR phosphors since a wider NIR spectrum is beneficial for the detection of more substances.17 Experimentally, different crystal field strengths give various emission bandwidths. This means that only one type of occupied Cr3+ site will result in a narrow NIR emission band. In other words, a wide NIR emission band can be achieved when the Cr3+ ions occupy several types of lattice sites. According to this observation, substitution of different lattice sites with Cr3+ ions is an effective way to widen the spectral range of the phosphor.18,19 For fluorides, LiMgGaF6:Cr3+ has a two-site Cr3+ ion occupation with an ultra-broad NIR emission (FWHM = 189.9 nm); however, the unsatisfactory QE value (42.3%) and thermal stability (50% at 400 K) limit its further applications.20 That is to say, the design of a high-performance fluoride phosphor with wideband NIR emission, an enhanced QE value, and low thermal quenching is still difficult to achieve.21,22 Therefore, to address this issue, a newly developed Cr3+-doped fluoride NIR phosphor with a wide emission band, high efficiency, and excellent luminescence thermal stability is of great interest to explore.
In this work, a highly efficient and thermally stable RLAF:Cr phosphor has been designed by the substitution of two different types of Al3+ ions with Cr3+ ions. A broad NIR emission band composed of two NIR emission bands was then observed upon blue light excitation. An NIR pc-LED was also fabricated using the RLAF:Cr phosphor and a blue LED chip and used as an NIR lighting source to show the potential use of the RLAF:Cr phosphor.
2. Experimental
2.1 Materials and preparation
The starting materials, including aluminium hydroxide (Al(OH)3, AR), rubidium fluoride (RbF, AR), lithium carbonate (Li2CO3, AR), chromium fluoride (CrF3, AR), ammonium fluorohydride (NH4HF2, AR), hydrofluoric acid (HF, 40 wt%), and absolute ethanol, were purchased from Shanghai Aladdin Biochemical Technology Co. Ltd, China. All chemicals were used as supplied without further purification.(NH4)3CrF6 was prepared in advance according to the method described in the literature.23 The synthesis of the RLAF:Cr phosphor was performed in a plastic tube at ambient temperature using a co-precipitation method. For the typical RLAF:0.02Cr sample, 5.0 mmol of Al(OH)3, 15.0 mmol of RbF, 2.8 mmol of Li2CO3, and 0.1 mmol of (NH4)3CrF6 were weighed and added to 4.5 mL of HF solution. This mixture was magnetically stirred for 8.0 h, which was followed by centrifugation. Subsequently, the precipitate was washed five times with absolute ethanol and finally dried at 80 °C for 10 h for further use. A schematic diagram of the preparation process is shown in Fig. S1 in the ESI.†
The LED device was fabricated by coating the surface of a blue InGaN chip (3 W, 445 nm–450 nm; Guhoon Optoelectronics Co., Ltd, China) with the as-prepared RLAF:Cr phosphor, using epoxy resin (OE-6550A/B, Dow Corning, USA) as a binder. The device was dried at 150 °C for 60 min, which was followed by photoelectric tests.
2.2 Characterization and calculations
The X-ray diffraction (XRD) patterns were obtained using a Bruker D8 Advance powder diffractometer with Cu Kα radiation (λ = 0.15406 nm) and a graphite monochromator that operated at 40 mA and 40 kV from 10° to 70°, with a step size of 0.02°. The scanning rate was 10° min−1. The same diffractometer was used for the Rietveld refinements, at a scanning rate of 1° min−1 in the range of 5°–120°. Scanning electron microscopy (SEM) was performed with a FEI Nova NanoSEM-450 microscope that was equipped with an energy-dispersive X-ray spectrometer (EDS). It was used for the analyses of the morphologies and elemental compositions of the as-prepared samples. The diffuse reflectance spectra (DRS) were recorded using a UV-Vis-NIR spectrophotometer (UV-3600i Plus, Shimadzu, Japan), using BaSO4 as a reference. The photoluminescence excitation (PLE), photoluminescence (PL) spectra, and QEs were recorded using a steady-state fluorescence spectrophotometer (FLS1000, Edinburgh Instruments, UK) with an additional integrating sphere. The aging test was performed in a constant temperature and humidity chamber (BPS-50CL, Blue pard) maintaining a high temperature (85 °C) and high humidity (85%) atmosphere. In addition, the thermal quenching behaviour was analyzed using a fiber spectrophotometer (AVANTES Avaspec Mini 2048CL-SHB3), with a 460 nm laser diode as the excitation source and a constant temperature heating instrument (JF-956S). The electron paramagnetic resonance (EPR) spectra of the samples were recorded using a Bruker EMXplus-6/1 spectrometer. Furthermore, a fast-scan spectrophotometer (OHSP-350M, Hangzhou Hopoo Light & Color Technology Co., Ltd, China) was used to record the electroluminescence spectra of the as-assembled LED devices. An infrared thermal imager (FLIR E8) was used to capture the thermographs of the operational pc-LED. Also, an industrial NIR camera (XA10E, Canon, Japan) was used for the demonstration images.Rietveld XRD refinement was performed with the Fullprof software and the crystallographic data of the RLAF host (ICSD no. 22115) was used as the initial structural model. The crystalline structure was constructed by using the Visualization for Electronic and Structural Analysis (Ver. 3.4.4) software. The electronic structure was obtained using a CASTEP module of the Materials Studio package on the basis of the density functional theory (DFT). Specifically, the Perdew–Burke–Ernzerhof (PBE) functional and the generalized gradient approximation (GGA) were used in these calculations, in addition to the electronic configurations of Rb (4p65s1), Li (2s1), Al (3s23p1), and F (2s22p5) for the electronic structural determinations. Furthermore, the distribution of Cr3+ ions in the RLAF host was evaluated by using a 2 × 2 × 2 supercell and a cutoff energy of 520 eV in the theoretical calculations.
3. Results and discussion
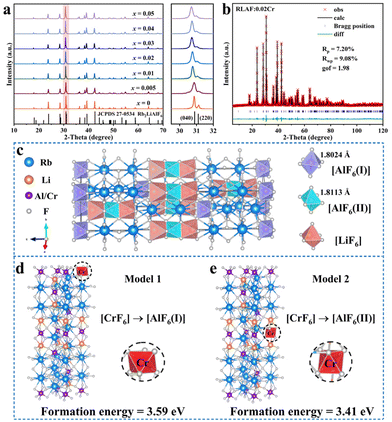
Fig. 1a shows the XRD patterns of the RLAF:xCr products and the standard diffraction card of the RLAF host. The enhanced diffraction peaks of each sample matched well with the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m (166) space group of trigonal RLAF (JCPDS no. 27-0534) in the standard powder diffraction file. The impurity-free patterns and the sharp diffraction peaks showed single phases and high crystallinities of the products. They also indicated that the crystalline structures were stable after the incorporation of Cr3+ ions into RLAF, even for the highest doping contents.24 With the same coordination number of 6, the ionic charges and radii of Rb+ (1.52 Å), Li+ (0.76 Å), Al3+ (0.535 Å), and Cr3+ (0.615 Å) indicated that the lattice sites of Al3+ ions in RLAF were preferable sites for Cr3+ doping. As can be seen in Fig. 1a, the right panel presents some parts of the magnified XRD patterns of the RLAF:xCr products. The (040) peak that was located at ∼31° slowly moved towards a smaller diffraction angle with an increasing value of x (from 0.00 to 0.05). This was a result of the gradual expansions of the cell volumes and the increase in lattice spacings.25
m (166) space group of trigonal RLAF (JCPDS no. 27-0534) in the standard powder diffraction file. The impurity-free patterns and the sharp diffraction peaks showed single phases and high crystallinities of the products. They also indicated that the crystalline structures were stable after the incorporation of Cr3+ ions into RLAF, even for the highest doping contents.24 With the same coordination number of 6, the ionic charges and radii of Rb+ (1.52 Å), Li+ (0.76 Å), Al3+ (0.535 Å), and Cr3+ (0.615 Å) indicated that the lattice sites of Al3+ ions in RLAF were preferable sites for Cr3+ doping. As can be seen in Fig. 1a, the right panel presents some parts of the magnified XRD patterns of the RLAF:xCr products. The (040) peak that was located at ∼31° slowly moved towards a smaller diffraction angle with an increasing value of x (from 0.00 to 0.05). This was a result of the gradual expansions of the cell volumes and the increase in lattice spacings.25
The Rietveld refinement results of the RLAF:0.02Cr sample are presented in Fig. 1b and Table S1 in the ESI.† The R factors and goodness-of-fit values indicated a high correspondence between the calculated results and observed data (Fig. 1b), which proved the high reliability of the refinement.26 The refined parameters (a = b = 5.8092(3) Å, c = 28.0803(9) Å, and V = 851.5471 Å3) were higher than those of the RLAF host, which is due to the larger ionic radius of Cr3+ compared to that of Al3+ (Table S2†). The crystalline structure of RLAF:0.02Cr is presented in Fig. 1c, where each Al3+ or Li+ ion was coordinated with 6 F− ions and located at the centre of an octahedron. On the other hand, each Rb+ ion was coordinated by 12 F− ions to form a [RbF12] polyhedron. Thus, there were three different types of octahedra (i.e., [LiF6], [AlF6(I)], and [AlF6(II)]) and one type of [RbF12] polyhedron in the RLAF structural network. The Al(I)3+ ions predominantly occupied the corner sites of the unit cells to form the [AlF6(I)] octahedra that were connected with [RbF12] polyhedra through face-sharing F− anions. In addition, each of the [AlF6(II)] octahedra was connected with two neighbouring [LiF6] octahedra through face-sharing. The average bond lengths of Al–F in [AlF6(I)] and [AlF6(II)] were 1.8024 Å and 1.8113 Å, respectively.
By considering the same valence states and similar ionic radii of the dopant and acceptor ions and the quite similar coordination environments of the Al(I)3+ and Al(II)3+ ions, the possibility for two different types of occupation sites for Cr3+ in the RLAF host has been investigated here. To achieve more information about the occupation of Cr3+ ions in the RLAF host, a 2 × 2 × 2 supercell with 48 formula units of RLAF was selected for DFT calculations. This supercell contained in total 480 atoms. There were two possible types of substitutions: M1 (Fig. 1d) and M2 (Fig. 1e). For the substitution of the [AlF6(I)] (substitution type M1) or [AlF6(II)] (substitution type M2) octahedra in RLAF with [CrF6] octahedra, the formation energies were calculated according to the equation Ef = E(doped) − E(pure) + μAl − μCr, where E(doped) and E(pure) are the total energies of the Cr3+-doped system and the perfect system, respectively. In addition, μAl and μCr are the chemical potentials for the Al and Cr atoms, respectively. The formation energies of the models for the two substitution types M1 and M2 were determined to be 3.59 eV and 3.41 eV, respectively. The comparable formation energies indicated that both [AlF6(I)] and [AlF6(II)] octahedra tended to be replaced by the [CrF6] octahedra.
The SEM images, elemental mappings, and EDS spectra showed that the RLAF:Cr sample adopted a regular hexagonal prism shape with the presence of Rb, Al, F, and Cr elements (Fig. S2†). However, the absence of the Li signal could be explained by its light element nature. The EPR spectrum was used to verify the presence and the valence state of chromium in the as-prepared RLAF:0.02Cr phosphor (Fig. S3†). The calculated g-factor of 1.997 ensured the presence of trivalent chromium ions with an electron spin S = 3/2 in this case, and the intense EPR signal originated from the transition of Cr3+ from the ms = −1/2 to 1/2 state located in the octahedral crystal field.16,27 The band structure that can be seen in Fig. S4a† showed that the host material had a direct bandgap, with the conduction band minimum (CBM) and the valence band maximum (VBM) located at the same point.28 The resulting bandgap (Eq = 6.292 eV) was wide enough to accommodate the excited and ground states that originated from the crystal field splitting of the Cr3+ 3d state, implying that the generation of NIR luminescence can be expected from the Cr3+-doped RLAF phosphor.28,29 Besides, the CBM was composed of Al 3p states, and the VBM was composed of F 2p states (Fig. S4b†), which implied that the luminescence properties induced by the Cr3+ ions were highly dependent on the [AlF6] group in the RLAF host.30
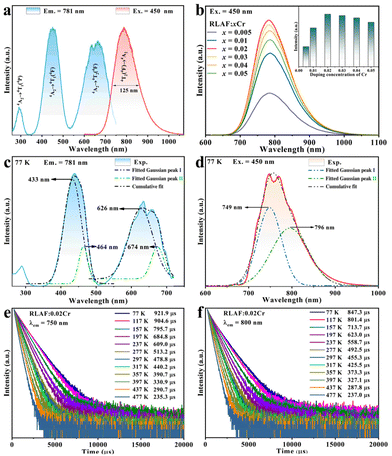
Fig. 2a shows the RT PLE and PL spectra of the RLAF:0.02Cr phosphor, in which three excitation bands were observed at ∼291 nm, 450 nm, and 637 nm in the UV, blue, and red regions, respectively. These bands correspond to the spin-allowed transitions of Cr3+ from the 4A2 state to the 4T1 (4P), 4T1 (4F), and 4T2 (4F) states, respectively.31 The three d–d transitions of Cr3+ from the ground state to the excited states were consistent with the three noticeable DRS absorption bands (Fig. S5†).32 The broad blue excitation band that ranged from 350 nm to 540 nm, with a FWHM of 82 nm, was much wider than that of the blue InGaN chip, implying that the RLAF:Cr phosphor could be used together with the commercial blue LED chip.33 Upon 450 nm blue light excitation, a wide NIR emission band that peaked at 781 nm in the range of 650 nm–1050 nm, with a FWHM of 125 nm, was observed. It originated from the spin-allowed 4T2 (4F) → 4A2 transition. The concentration-dependent PL spectra, which were recorded at RT, showed that the RLAF:0.02Cr phosphor had the strongest PL intensity (Fig. 2b). A further increase in the x value resulted in a concentration quenching effect of the degeneration on the PL intensity (inset of Fig. 2b).34
The two different types of Al3+ sites indicated the possibility of two types of Cr3+ activators in the RLAF crystalline structure. To experimentally verify the Cr3+ occupancy in the RLAF matrix, low temperature PLE and PL spectra of the RLAF:0.02Cr sample were recorded at 77 K. Each of the spin-allowed transitions consisted of two wide bands according to the fitted Gaussian peak splitting results (Fig. 2c and d).20,35 Specifically, the 4A2 → 4T1 transition was composed of two blue excitation bands that peaked at 433 nm and 464 nm. Also, the 4A2 → 4T2 transition was composed of two excitation bands that peaked at 626 nm and 674 nm in the red region. The 4T2 → 4A2 transition could, therefore, be divided into two NIR emission bands with peak locations at 749 nm and 796 nm. That is to say, the occupations of Cr3+ ions in two different types of sites in the RLAF facilitated a wider emission band as compared with a single site substitution. Generally, the longer emission wavelength is always associated with a weaker crystal field.36 According to the averaged bond lengths of Al–F in the [AlF6(I)] and [AlF6(II)] octahedra, when a Cr3+ ion replaced the Al3+ ion in the [AlF6(II)] octahedron, it resulted in a longer emission at 796 nm. Therefore, the two types of six-coordinated Al3+ lattice sites in the RLAF crystalline structure, which were available occupation sites for the Cr3+ activators, could be further verified by analysing the decay trends of the Cr3+ ions at the different luminescent sites. The PL decay curves of the RLAF:0.02Cr phosphor, which were obtained by different λem values of 750 nm, 800 nm, and 770 nm at various temperatures, are displayed in Fig. 2e, f, and S8,† respectively. The double-site occupancies of the Cr3+ ions were then verified by fitting the PL decay curves, monitored with a different detection wavelength for both luminescent centres, to a double-exponential function (eqn (S1) and (S2) in the ESI†).37 The listed average lifetimes presented enhanced luminescence decay trends as the temperature increased from 77 K to 477 K. This was attributed to the enhanced thermal vibrations and the increased nonradiative transition probabilities among the Cr3+–Cr3+ pairs at higher temperatures. This was also the reason why the dual NIR emissions, which originated from the two types of Cr3+ lattice sites, slowly decreased and gradually merged into a wider single NIR emission band at an increased temperature (Fig. S6†).38 In addition, based on the fitted Gaussian profiles with two groups of peak energies for the 4T1 and 4T2 states, the local crystal field strengths of the two types of Cr3+ lattice sites were estimated by using the 4T1 and 4T2 peak energies at the PLE maxima.39 As can be seen in the Tanabe–Sugano diagram (Fig. S7†), the Dq/B ratios that were calculated from eqn (S3)–(S5)† were 2.15 and 2.10, respectively. This implied that the RLAF host offered weak crystal fields for the incorporation of Cr3+ activators into two different types of occupation sites.28 Two sets of wide emission bands in the NIR region were, thereby, generated.
Luminescence always declines at higher temperatures, which is due to nonradiative transitions. To determine the thermal quenching properties of the RLAF:0.02Cr phosphor, temperature-dependent PL spectra were recorded from 297 K to 477 K under blue light excitation (Fig. 3a). Obviously, only one emission band could be observed at 297 K. As the temperature increased, the PL intensity gradually declined. This was attributed to the increasing number of nonradiative transitions at higher temperatures.40 The PL intensity preserved 93.5% (of the PL intensity at 297 K) at 377 K and 90.5% at 417 K (Fig. 3b). These intensities were much better than most of the intensities reported for Cr3+-doped fluoride NIR phosphors (Table S3†), thereby proving the outstanding PL thermal stability of the as-prepared RLAF:Cr phosphor.
The configurational coordinate diagram presented in Fig. 3c could be used to explain the low thermal-quenching behavior of the RLAF:Cr phosphor. Upon blue light excitation, the Cr3+ electrons in the ground state 4A2 absorbed energy and became excited to the 4T2 energy level via pathway I. At RT, most of the electrons in the 4T2 excited state returned to their ground state via pathway II, which was a radiative transition. Nevertheless, as the temperature increased, the electrons in the 4T2 state could be excited to the intersection point of the 4T2 and 4A2 energy levels and, thereafter, return to the ground state via the nonradiative pathway III. The energy difference between the lowest position of the 4T2 excited state and the intersection point of the 4T2 and 4A2 states was defined as the activation energy ΔE (Fig. 3c), which was an important parameter for the description of the thermal-quenching behaviour of luminescence materials.41 In this case, the ΔE value could be calculated using the modified Arrhenius equation (eqn (S6)†). By plotting the relationship between ln[(I0/IT) − 1] and 1/kT, a straight fitting line with a correlation coefficient of 0.988 and a slope of −0.228 was obtained. This indicated that the fitting result was trustworthy and that ΔE was 0.228 eV (Fig. 3g). This value was much larger than the thermal disturbance energy (0.026 eV) at RT, indicating that the energy barrier for the nonradiative relaxation was hard to overcome and therefore, the RLAF:Cr phosphor had excellent thermal stability.42
Fig. 3d–f show the normalized temperature-dependent PL spectra of the RLAF:0.02Cr sample in the temperature region of 297 K to 477 K, in addition to the relationships between the PL peak positions, FWHMs, and temperatures. Apparently, as the working temperature increased, the PL peak position became instantly redshifted towards a longer wavelength in the region of 781 nm to 805 nm (Fig. 3e). This was due to the unit cell expansion in the hot environment.43 In addition, the emission band became gradually wider, with an increase in FWHM from 125 nm to 158 nm (Fig. 3f), which originated from the stronger EPC effect at higher temperatures.44 This spectral broadening phenomenon could be explained by the Huang–Rhys factor with different electron-vibrational interactions, which could be estimated using eqn (S7) and (S8).†![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 Based on the temperature-dependent PL spectra, a fitting line with a high correlation coefficient of 0.998 was obtained by plotting FWHM2versus 2kT (Fig. 3h), indicating that the fitting results were highly reliable. According to the slope of the line, the hω and S values were determined to be 14.9 meV and 2.64, respectively, which indicated that Cr3+ activators experienced a weak EPC effect in RLAF host.46 This was also assumed to be the reason for the remarkable PL thermal stability of RLAF:Cr. Moreover, the QE of RLAF:0.02Cr upon excitation with a wavelength of 450 nm was found to be 77.7%. The integral PL intensity of RLAF:0.02Cr was maintained at 88.1% of the initial value after being aged at 85 °C and 85% relative humidity for 24 h and still maintained at 83.7% for 168 h (Fig. S9†), indicating the high reliability of the RLAF:0.02Cr phosphor in a high temperature and high humidity environment when it is used as a colour converter in NIR pc-LED devices. It is at present difficult to achieve both high efficiency and superb thermal stability of a Cr3+-doped fluoride (Table S3†). Therefore, in comparison with other one- or two-sited Cr3+-doped fluorides, a high-performance near-infrared two-sited Cr3+-doped RLAF phosphor with outstanding luminescence, thermal stability and high efficiency has rarely been reported.
45 Based on the temperature-dependent PL spectra, a fitting line with a high correlation coefficient of 0.998 was obtained by plotting FWHM2versus 2kT (Fig. 3h), indicating that the fitting results were highly reliable. According to the slope of the line, the hω and S values were determined to be 14.9 meV and 2.64, respectively, which indicated that Cr3+ activators experienced a weak EPC effect in RLAF host.46 This was also assumed to be the reason for the remarkable PL thermal stability of RLAF:Cr. Moreover, the QE of RLAF:0.02Cr upon excitation with a wavelength of 450 nm was found to be 77.7%. The integral PL intensity of RLAF:0.02Cr was maintained at 88.1% of the initial value after being aged at 85 °C and 85% relative humidity for 24 h and still maintained at 83.7% for 168 h (Fig. S9†), indicating the high reliability of the RLAF:0.02Cr phosphor in a high temperature and high humidity environment when it is used as a colour converter in NIR pc-LED devices. It is at present difficult to achieve both high efficiency and superb thermal stability of a Cr3+-doped fluoride (Table S3†). Therefore, in comparison with other one- or two-sited Cr3+-doped fluorides, a high-performance near-infrared two-sited Cr3+-doped RLAF phosphor with outstanding luminescence, thermal stability and high efficiency has rarely been reported.
Due to the different types of occupation sites for the Cr3+ activators, the low thermal-quenching, high-efficiency RLAF:Cr NIR phosphor was successfully used in the construction of NIR pc-LEDs with excellent optical performances. The NIR pc-LEDs were assembled by coating blue InGaN chips with RLAF:Cr powders. Fig. 4a shows the LED photos and the current-dependent PL spectra of a packaged LED device. The weak blue emission band in the region of 425 nm–475 nm originated from the LED chip, and the dominant broad NIR emission band in the region of 650 nm–1050 nm originated from RLAF:Cr. With a continuous increase in the drive current from 20 mA to 320 mA, both of the blue and NIR PL intensities gradually increased with stable spectral shapes. Thus, these results showed that RLAF:Cr was promising for applications in high-power NIR LED devices. As monitored using an NIR imaging camera, the temperature and thermographs of the working LED device for four different drive currents are shown in Fig. 4b. As induced by the accumulated heat at higher drive currents, the working temperature of the LED device increased from 17.0 °C to 90.9 °C. Thus, it was concluded that the RLAF:Cr phosphor could be stably used in an LED, which was due to its high PL thermal stability. Moreover, as the drive current increased from 20 mA to 320 mA, the NIR output power of the LED device continuously increased from 2.52 mW to 37.25 mW, and the photoelectric conversion efficiency changed from 4.84% to 3.69% (Fig. S10†), in which the latter one is mainly attributed to the decreasing external QE of the blue LED chip at higher temperatures.44 Subsequently, to validate the role of the RLAF:Cr phosphor in the LED device and to verify the non-invasive and penetration properties of the NIR light, the potential use of the NIR pc-LED for various applications was investigated. Fig. 4c–e present a series of photos of some objects (i.e., flowers, an apple and a doll), which were taken with an NIR camera with its natural and near-infrared modes and by using the assembled NIR LED device as a lighting source. Very clear photos of the objects are presented in Fig. 4c1 and d1. These photos were taken with the camera in its natural mode and under natural light irradiation. The colourful photos were then visible to the human eye. On the other hand, nothing was captured when the natural light was missing (Fig. 4c2 and d2), while the camera took clear photos of the objects when the NIR pc-LED was working (Fig. 4c3 and d3). Additionally, the NIR light (700 nm–1100 nm) showed strong penetration characteristics for biological tissues. The blood vessel distribution in a palm can be clearly observed in Fig. 4e, with the packaged LED as a lighting source. These results have strongly illustrated that the highly efficient and thermally stable RLAF:Cr phosphor has great potential for night vision and biological imaging applications in NIR pc-LEDs.
4. Conclusions
In this work, a highly efficient and thermally stable RLAF:Cr phosphor with two occupation sites has been presented by incorporating Cr3+ as luminescent centres into the RLAF host. The structural analysis and calculated formation energies disclose that both Al3+ sites are presumed to be substituted by Cr3+ activators. The spectral features confirm that two groups of Cr3+:4T2 (4F) → 4A2 transitions with two sets of NIR emission bands are witnessed, which merge into a broad NIR emission band (FWHM = 125 nm) spanning the range of 650–1050 nm. Upon blue light excitation, RLAF:Cr demonstrates the capability to produce an excellent thermal stability at 417 K, with a ratio of 90.5% of that at RT, which originates from the subdued EPC effect around Cr3+ activators and the high activation energy of the RLAF:Cr phosphor. Due to the high thermal stability and high QE (77.7%) of the NIR phosphor, clearly visible photographs of actual objects and human hand veins were obtained using the fabricated NIR pc-LED as a lighting source. These results showed the potential applications of the RLAF:Cr phosphor in NIR pc-LED devices.Author contributions
S. Qing: investigation, methodology, data curation, and writing – original draft. J. Wan: writing – review and editing. T. Yang: writing – review and editing. Q. Zhou: conceptualization, supervision, and writing – review and editing. Y. Y. Zhou: writing – review and editing. Z. L. Wang: supervision and writing – review and editing. D. W. Wen: writing – review and editing. M. M. Wu: project administration, resources, and writing – review and editing.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (grant nos. 22365034, 22065039, 52272174 and U22A20135) and the Yunnan Fundamental Research Projects (202101AT070072).References
- P. M. Pattison, J. Y. Tsao, G. C. Brainard and B. Bugbee, LEDs for photons, physiology and food, Nature, 2018, 563, 493–500 CrossRef CAS PubMed
.
- D. J. Liu, G. G. Li, P. P. Dang, Q. Q. Zhang, Y. Wei, H. Z. Lian, M. M. Shang, C. C. Lin and J. Lin, Simultaneous broadening and enhancement of Cr3+ photoluminescence in LiIn2SbO6 by chemical unit cosubstitution: night-vision and near-infrared spectroscopy detection applications, Angew. Chem., Int. Ed., 2021, 60, 14644–14649 CrossRef CAS PubMed
.
- V. Rajendran, M. H. Fang, W. T. Huang, N. Majewska, T. Lesniewski, S. Mahlik, G. Leniec, S. M. Kaczmarek, W. Pang, V. K. Peterson, K. M. Lu, H. Chang and R. S. Liu, Chromium ion pair luminescence: a strategy in broadband near-infrared light-emitting diode design, J. Am. Chem. Soc., 2021, 143, 19058–19066 CrossRef CAS PubMed
.
- M. Vasilopoulou, A. Fakharuddin, F. P. G. de Arquer, D. G. Georgiadou, H. Kim, A. R. bin, M. Yusoff, F. Gao, M. K. Nazeeruddin, H. J. Bolink and E. H. Sargent, Advances in solution-processed near-infrared light-emitting diodes, Nat. Photonics, 2021, 15, 656–669 CrossRef CAS
.
- J. W. Qiao, G. J. Zhou, Y. Y. Zhou, Q. Y. Zhang and Z. G. Xia, Divalent europium-doped near-infrared-emitting phosphor for light-emitting diodes, Nat. Commun., 2019, 10, 5267 CrossRef PubMed
.
- Z. W. Jia, C. X. Yuan, Y. F. Liu, X. J. Wang, P. Sun, L. Wang, H. C. Jiang and J. Jiang, Strategies to approach high performance in Cr3+-doped phosphors for high-power NIR-LED light sources, Light: Sci. Appl., 2020, 9, 86 CrossRef CAS PubMed
.
- G. H. Wei, P. L. Li, R. Li, Y. Wang, S. X. He, J. H. Li, Y. W. Shi, H. Suo, Y. B. Yang and Z. J. Wang, How to achieve excellent luminescence properties of Cr ion-doped near-infrared phosphors, Adv. Opt. Mater., 2023, 11, 2301794 CrossRef CAS
.
- S. W. Wang, R. Pang, T. Tan, H. Y. Wu, Q. Wang, C. Y. Li, S. Zhang, T. X. Tan, H. P. You and H. J. Zhang, Achieving high quantum efficiency broadband NIR Mg4Ta2O9:Cr3+ Phosphor through lithium-ion compensation, Adv. Mater., 2023, 35, 2300124 CrossRef CAS PubMed
.
- L. You, R. Tian, T. Zhou and R. J. Xie, Broadband near-infrared phosphor BaMgAl10O17:Cr3+ realized by crystallographic site engineering, Chem. Eng. J., 2021, 417, 129224 CrossRef CAS
.
- J. W. Qiao, S. Zhang, X. Q. Zhou, W. B. Chen, R. Gautier and Z. G. Xia, Near-infrared light-emitting diodes utilizing a europium-activated calcium oxide phosphor with external quantum efficiency of up to 54.7%, Adv. Mater., 2022, 34, 2201887 CrossRef CAS PubMed
.
- X. Zhou, J. M. Xiang, J. M. Zheng, X. Q. Zhao, H. Suo and C. F. Guo, Ab initio two-sites occupancy and broadband near-infrared emission of Cr3+ in Li2MgZrO4, Mater. Chem. Front., 2021, 5, 4334–4342 RSC
.
- H. H. Lin, G. X. Bai, T. Yu, M. K. Tsang, Q. Y. Zhang and J. H. Hao, Site occupancy and near-infrared luminescence in Ca3Ga2Ge3O12:Cr3+ persistent phosphor, Adv. Opt. Mater., 2017, 5, 1700227 CrossRef
.
- F. Y. Zhao, Z. Song and Q. L. Liu, Advances in chromium-activated phosphors for near-infrared light sources, Laser Photonics Rev., 2022, 16, 2200380 CrossRef CAS
.
- Y. Y. Liang, Q. T. Cao, Y. Q. Zhou, W. L. Zhou, J. L. Zhang, L. P. Yu, S. X. Lian and Z. X. Qiu, Towards control facilities for mimicking plant growth optimum action spectrum: efficient near-ultraviolet to far-red light-conversion in Cr3+-doped rare-earth aluminate phosphors, Chem. Eng. J., 2023, 454, 140235 CrossRef CAS
.
- Y. J. Jia, Y. X. Pan, Y. Q. Li, L. J. Zhang, H. Z. Lian and J. Lin, Improved moisture-resistant and luminescence properties of a red phosphor based on dodec-fluoride K3RbGe2F12:Mn4+ through surface modification, Inorg. Chem., 2021, 60, 231–238 CrossRef CAS PubMed
.
- J. Li, H. F. Zhe, H. Ming, Y. Y. Zhou, Y. Q. Ye, Q. Zhou and Z. L. Wang, A near-infrared phosphor doped with Cr3+ towards zero-thermal-quenching for high-power LEDs, Mater. Today Chem., 2022, 24, 100839 CrossRef CAS
.
- P. P. Dang, Y. Wei, D. J. Liu, G. G. Li and J. Lin, Recent advances in chromium-doped near-infrared luminescent materials: fundamentals, optimization strategies, and applications, Adv. Opt. Mater., 2023, 11, 2201739 CrossRef CAS
.
- S. H. Miao, Y. J. Liang, D. X. Chen, R. Q. Shi, X. H. Shan, Y. Zhang, F. Xie and X. J. Wang, Site-selective occupancy control of Cr ions toward ultrabroad-band infrared luminescence with a spectral width up to 419 nm, ACS Appl. Mater. Interfaces, 2022, 14, 53101–53110 CrossRef CAS PubMed
.
- Q. Q. Zhang, D. J. Liu, P. P. Dang, H. Z. Lian, G. G. Li and J. Lin, Two selective sites control of Cr3+-doped ABO4 phosphors for tuning ultra-broadband near-infrared photoluminescence and multi-applications, Laser Photonics Rev., 2022, 16, 2100459 CrossRef CAS
.
- W. D. Nie, J. X. Zuo, S. Yang, Y. Li, W. F. Zou, G. Chen, S. H. Wu, D. Wu, S. B. Liu, J. Q. Peng, L. Han and X. Y. Ye, Ultra-broad NIR emission from two-site Cr3+ occupation in fluoride phosphor via composition-driven structural transformation, Mater. Today Chem., 2022, 26, 101164 CrossRef CAS
.
- J. Q. Fan, W. Y. Zhou, J. L. Zhang, P. C. Chen, Q. Pang, L. Y. Zhou, C. Y. Zhou and X. G. Zhang, A novel efficient broadband near-infrared phosphor LiGaGe2O6:Cr3+ with EQE enhancement and spectral tuning by Sc3+-Ga3+ substitution for NIR pc-LED application, Inorg. Chem. Front., 2023, 10, 511–521 RSC
.
- X. K. Zou, X. J. Wang, H. R. Zhang, Y. Y. Kang, X. Yang, X. J. Zhang, M. S. Molokeev and B. F. Lei, A highly efficient and suitable spectral profile Cr3+-doped garnet near-infrared emitting phosphor for regulating photomorphogenesis of plants, Chem. Eng. J., 2022, 428, 132003 CrossRef CAS
.
- F. Q. He, E. H. Song, Y. Y. Zhou, H. Ming, Z. T. Chen, J. C. Wu, P. S. Shao, X. F. Yang, Z. G. Xia and Q. Y. Zhang, A general ammonium salt assisted synthesis strategy for Cr3+-Doped hexafluorides with highly efficient near infrared emissions, Adv. Funct. Mater., 2021, 31, 2103743 CrossRef CAS
.
- G. H. Wei, P. L. Li, R. Li, J. H. Li, Y. W. Shi, Y. Wang, S. X. He, Y. B. Yang, H. Suo and Z. J. Wang, Chromium(III)-doped phosphors of high-efficiency two-site occupation broadband infrared emission for vessel visualization applications, Inorg. Chem., 2022, 61, 5665–5671 CrossRef CAS PubMed
.
- T. Yang, L. X. Chu, Y. Qin, Q. Zhou, J. Wan, H. J. Tang, Y. Q. Ye and Z. L. Wang, An efficient and thermally stable source with Cr3+ near-infrared luminescence for non-destructive testing applications, Mater. Today Chem., 2024, 35, 101918 CrossRef CAS
.
- T. Tan, S. W. Wang, J. Y. Su, W. H. Yuan, H. Y. Wu, R. Pang, J. T. Wang, C. Y. Li and H. J. Zhang, Design of a novel near-infrared luminescence material Li2Mg3TiO6:Cr3+ with an ultrawide tuning range applied to near-infrared light-emitting diodes, ACS Sustainable Chem. Eng., 2022, 10, 3839–3850 CrossRef CAS
.
- Q. M. Lin, Q. Wang, M. Liao, M. X. Xiong, X. Feng, X. Zhang, H. F. Dong, D. Y. Zhu, F. G. Wu and Z. F. Mu, Trivalent chromium ions doped fluorides with both broad emission bandwidth and excellent luminescence thermal stability, ACS Appl. Mater. Interfaces, 2021, 13, 18274–18282 CrossRef CAS PubMed
.
- E. H. Song, H. Ming, Y. Y. Zhou, F. Q. He, J. C. Wu, Z. G. Xia and Q. Y. Zhang, Cr3+-doped Sc-based fluoride enabling highly efficient near infrared luminescence: a case study of K2NaScF6:Cr3+, Laser Photonics Rev., 2021, 15, 2000410 CrossRef CAS
.
- H. S. Zhang, J. Y. Zhong, F. Du, L. Chen, X. L. Zhang, Z. F. Mu and W. R. Zhao, Efficient and thermally stable broad-band near-infrared emission in a KAlP2O7:Cr3+ phosphor for nondestructive examination, ACS Appl. Mater. Interfaces, 2022, 14, 11663–11671 CrossRef CAS PubMed
.
- D. C. Huang, S. S. Liang, D. J. Chen, J. Hu, K. Y. Xu and H. M. Zhu, An efficient garnet-structured Na3Al2Li3F12:Cr3+ phosphor with excellent photoluminescence thermal stability for near-infrared LEDs, Chem. Eng. J., 2021, 426, 131332 CrossRef CAS
.
- Y. W. Zhu, Y. F. Yang, Y. Q. Zhu, Y. F. Chen, Z. Y. Liu, F. M. Lei, H. Yu, Q. N. Mao, J. S. Zhong and J. Wang, Enhanced photoluminescence of a HF free novel NIR phosphor of K2ZnF4:Cr3+ by lithium ion doping, J. Mater. Chem. C, 2023, 11, 10694–10702 RSC
.
- F. Y. Zhao, Z. Song, J. Zhao and Q. L. Liu, Double perovskite Cs2AgInCl6:Cr3+: broadband and near-infrared luminescent materials, Inorg. Chem. Front., 2019, 6, 3621–3628 RSC
.
- Q. Zhou, L. Dolgov, A. M. Srivastava, L. Zhou, Z. L. Wang, J. X. Shi, M. D. Dramicanin, M. G. Brik and M. M. Wu, Mn2+ and Mn4+ red phosphors: synthesis, luminescence and applications in WLEDs. A review, J. Mater. Chem. C, 2018, 6, 2652–2671 RSC
.
- H. J. Yu, J. Chen, R. Y. Mi, J. Y. Yang and Y. G. Liu, Broadband near-infrared emission of K3ScF6:Cr3+ phosphors for night vision imaging system sources, Chem. Eng. J., 2021, 417, 129271 CrossRef CAS
.
- S. S. Ding, H. J. Guo, P. Feng, Q. F. Ye and Y. H. Wang, A new near-infrared long persistent luminescence material with its outstanding persistent luminescence performance and promising multifunctional application prospects, Adv. Opt. Mater., 2020, 8, 2000097 CrossRef CAS
.
- S. W. Yuan, F. G. Wu, J. Wang, L. L. Lou, S. Zhao, D. Y. Zhu and Z. F. Mu, Ultra-broadband near infrared phosphor with wide spectral range and long peak wavelength achieved by double-site occupation, J. Rare Earths, 2023, 41, 1670–1677 CrossRef CAS
.
- H. Li, J. K. Jiao, X. F. Xiang, J. Z. Wu, W. B. Hu, J. Y. Xie, S. P. Huang, H. Z. Zhang and J. Zhu, Directly Identifying Multiple Cr3+ Emitting Centers for Broad Near-Infrared Emission in an Efficient and Near-Zero Thermal Quenching Garnet-Type Phosphor, Adv. Opt. Mater., 2024, 12, 2302391 CrossRef
.
- H. T. Zeng, T. L. Zhou, L. Wang and R. J. Xie, Two-site occupation for exploring ultra-broadband near-infrared phosphor—double-perovskite La2MgZrO6:Cr3+, Chem. Mater., 2019, 31, 5245–5253 CrossRef CAS
.
- Q. X. Pang, Y. C. Wang, L. Q. Yan, G. Zhu, S. Xu, J. S. Zhang, X. Z. Zhang, Y. Z. Cao and B. J. Chen, Cr3+-Cr3+ ion pair induced fast energy migration in Cr3+ doped Na-β-Al2O3 ultra-wide near-infrared phosphors for NIR spectroscopy application, Laser Photonics Rev., 2024, 18, 2301039 CrossRef CAS
.
- D. C. Huang, X. G. He, J. R. Zhang, J. Hu, S. S. Liang, D. J. Chen, K. Y. Xu and H. M. Zhu, Efficient and thermally stable broadband near-infrared emission from near zero thermal expansion AlP3O9:Cr3+ phosphors, Inorg. Chem. Front., 2022, 9, 1692–1700 RSC
.
- Q. Zhou, J. Wan, Y. Y. Zhou, S. Zhang, D. X. Shi, X. L. Xie, H. Q. Pu, Y. Q. Ye and Z. L. Wang, Ultraintense zero-phonon line from a Mn4+ red-emitting phosphor for high-quality backlight display applications, Inorg. Chem., 2021, 60, 19197–19205 CrossRef CAS PubMed
.
- Z. X. Wu, X. X. Han, J. Wang, Y. Y. Zhou, K. Xing, S. Cao, J. L. Zhao, B. S. Zou and R. S. Zeng, Highly efficient and thermally stable broadband near-infrared emitting fluoride Cs2KGaF6:Cr3+ for multiple LED applications, J. Mater. Chem. C, 2022, 10, 10292–10301 RSC
.
- S. Y. Guo, L. G. Ma, M. Abudureyimu, R. F. Wei, F. M. Lu, F. F. Hu and H. Guo, Improving and broadening luminescence in Gd2−xAlxGaSbO7:Cr3+ phosphors for NIR LED applications, Inorg. Chem. Front., 2023, 10, 2197–2205 RSC
.
- Z. X. Wu, X. X. Han, Y. Y. Zhou, K. Xing, S. Cao, L. Chen, R. S. Zeng, J. L. Zhao and B. S. Zou, Efficient broadband near-infrared luminescence of Cr3+ doped fluoride K2NaInF6 and its NIR-LED application toward veins imaging, Chem. Eng. J., 2022, 427, 131740 CrossRef CAS
.
- D. Wu, L. L. Liu, H. Liang, H. B. Duan, W. D. Nie, J. R. Wang, J. Q. Peng and X. Y. Ye, LiBAlF6:Cr3+ (B = Ca, Sr) fluoride phosphors with ultra-broad near-infrared emission for NIR pc-LEDs, Ceram. Int., 2022, 48, 387–396 CrossRef CAS
.
- J. H. Xie, J. H. Tian and W. D. Zhuang, Near-Infrared LuCa2ScZrGa2GeO12:Cr3+ garnet phosphor with ultra-broadband emission for NIR LED applications, Inorg. Chem., 2023, 62, 10772–10779 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4qi00429a |
| This journal is © the Partner Organisations 2024 |