Long-wavelength near-infrared emission in chromium-activated LiZnNbO4 spinel crystals and valence-converting enhancement via Er3+ ion heterotopic doping†
Wen
Song
a,
Kaiwen
Zhang
a,
Xiaoyi
Dong
a,
Liang
Xu
a,
Yongjin
Li
 *ab,
Rui
Hu
*c,
Zhaoyi
Yin
ab,
Zhengwen
Yang
ab,
Jianbei
Qiu
*ab,
Rui
Hu
*c,
Zhaoyi
Yin
ab,
Zhengwen
Yang
ab,
Jianbei
Qiu
 ab and
Zhiguo
Song
ab and
Zhiguo
Song
 *ab
*ab
aFaculty of Materials Science and Engineering, Kunming University of Science and Technology, Kunming 650093, PR China. E-mail: liyongjin@kust.edu.cn; songzg@kmust.edu.cn
bKey Lab. of Advanced Materials of Yunnan Province, Kunming 650093, PR China
cCollage of Chemistry and Chemical Engineering, China West Normal University, Nanchong 637000, PR China. E-mail: hr2022@cwnu.edu.cn
First published on 9th August 2024
Abstract
Realizing efficient long-wavelength near-infrared (NIR) emission of Cr3+ ions is still a challenge in spinel-based phosphors due to the limitations of strong crystal fields. Here, we propose three design strategies for obtaining weak crystal fields in spinel-type crystals, and report for the first time designed Cr-activated LiZnNbO4 (LZNO) crystals with unique weak crystal fields and application of an NIR enhancement strategy via heterotopic and heterovalent doping with trivalent rare earth ions. Under irradiation with 468 nm blue light, the phosphor presents ultra-wideband NIR emission centered at 800 nm covering the region of 650–1300 nm, which is attributed to the larger radius, high valence state of cations and the low symmetry of octahedral sites in the LZNO spinel. Supported by XRD refinement, XPS analysis, and density functional theory (DFT) calculation results, it was shown that when Er3+ ions are designedly doped with the Zn2+ sites of the spinel crystals, effective promotion of the valence state transformation of Cr4+ to Cr3+ in the LZNO:Cr3+ system is achieved by defect charge compensation, and this enhances the NIR emission by nearly 3 times. The results of this work not only enrich the material family of Cr3+-activated NIR emitting phosphors, but also offer a novel and simple method for improving the luminescence efficiency of Cr3+-activated phosphors.
1 Introduction
NIR light sources have received increasing attention due to their wide range of applications in night vision, plant growth, biosensing, quantitative composition analysis and nondestructive testing.1–4 In recent years, the emergence of NIR-emitting phosphor-converted light-emitting diodes (NIR pc-LEDs) has greatly expanded the development and utilization of NIR technology in practical applications due to their low cost, long lifespan, miniaturization, highly efficient luminosity and fast response characteristics.5,6 The key to the production of efficient NIR pc-LEDs is the preparation of NIR luminescent phosphors that can be effectively pumped by blue light. Therefore, researchers have made significant efforts to explore a large number of NIR inorganic luminescent phosphors doped with active ions, including transition metal ions (e.g., Cr3+, Cr4+, Fe3+, Ni2+, and Mn4+), Bi2+ ions and lanthanide ions (e.g., Pr3+, Nd3+, Eu2+, Tm3+, Yb3+ and Er3+).7–13 Among them, the transition metal ion Cr3+ can demonstrate broad absorption capacity that is well-aligned with blue LED chips and it can emit photons ranging from 650 to 1300 nm when located in a weak octahedral crystal field.14–17 This enhances its potential for use in the creation of NIR light source devices, addressing the crucial issue of emerging phosphorus powder-coated NIR pc-LEDs. However, just like every coin has two sides, long-wavelength emission is frequently associated with low luminous efficiency. Many reported Cr3+ phosphors still exhibit low photoluminescence efficiency and insufficient full width at half maximum (FWHM). This greatly hinders the practical application development of NIR pc-LEDs.18–20 Therefore, research work on developing new Cr3+-activated materials, as well as methods that can effectively improve their luminescence efficiency, are still urgently needed.Spinel materials are considered as excellent hosts for photoluminescence because of their ability to adjust crystal structures and their suitability for ion doping.21 However, most of the octahedral sites of spinel phosphors typically exhibit strong crystal fields, leading to the emission of the doped Cr3+ ion being located at about 700 nm (several Cr3+-activated spinel-type NIR phosphors are listed in Table S1†).22 This NIR emission property greatly limits the practical application of spinel phosphors in some scenarios like food analysis23,24 and biological imaging.25 However, until now, from the perspective of materials selection and design, there is little understanding of obtaining long-wavelength broadband NIR in Cr3+-activated spinel phosphors.
The long wavelength emission of Cr3+ ions in octahedral sites is most likely due to weak crystal fields. This phenomenon is frequently observed in Cr3+-doped garnet-structured phosphors featuring local low-symmetry unit cells.26–29 On the basis of these findings, we recognize that this feature is possibly applicable in achieving Cr ion long-wavelength broadband emission in spinel phorhpors. Based on controlling the redshift of Cr ion emission wavelength in garnets, we propose the following design principles for achieving Cr ion long-wavelength broadband emission: 1. (high valence state) the higher the valence state of the cation, the larger the volume of its coordination polyhedron, which can weaken the crystal field around Cr; 2. (large radius) the larger radius of the cation always corresponds with longer bond lengths and greater metal–ligand distances, which can decrease the crystal field; and 3. (low symmetry) the large spatial structure and low symmetry are more likely to cause polyhedral distortion, thereby causing uneven distribution of electric fields and intensifying the splitting of Cr3+ sub-energy levels.
According to the above given criteria, we initially considered designing phosphors with niobate materials, as they contain Nb5+ ions with high valence and larger ionic radius. Then the LiZnNbO4 compound was selected because it can manifest the behavior of a superstructure or existence of empty interstitial space.30 This feature can easily result in local distortion in the doping process, as well as low local symmetry. In addition, it is also expected to provide suitable octahedral sites for Cr3+. Therefore, these structural characteristics indicate that the compound has significant potential for producing long-wavelength broadband NIR emission activated by Cr3+.
On the other hand, it is important to mention the dominant factor that affects the luminescence of Cr3+-doped phosphors. It is common in the high-temperature synthesis of phosphors that the self-oxidation process of Cr3+-doped materials sintered in air will, in general, partially form Cr4+ ions.31 Due to the low and unsatisfactory actual formation rate of Cr3+, a significant amount of luminescence “killer” Cr4+ with intense absorption in the NIR region can function as a non-radiative relaxation center, capturing energy and significantly impacting the luminescence efficiency of the phosphor.32 Although it can be partially prevented by the reducing atmosphere, this method is not suitable for all hosts. As a result, to address the issue of the low luminescence efficiency of Cr3+ due to these mentioned factors, researchers have carried out thorough research and suggested several effective methods. One uses energy transfer33–35 of suitable sensitizers, such as Er3+ ions, to boost their luminescence effectiveness. However, due to the limitation of forbidden f–f transitions, the enhancement effect of rare earth ions is still far from satisfactory in practical applications. Therefore, exploring a suitable method that can inhibit the generation of Cr4+ simply and effectively will provide new insight into studying Cr3+-activated NIR phosphors.
In this work, to address the challenges associated with exploiting new Cr3+-activated spinel materials with long-wavelength NIR emission while devising a novel strategy to improve the luminescence efficiency, we exploited the NIR emission of a Cr-doped LiZnNbO4 spinel system, as well as enhancing the formation of Cr3+ ions by heterotopic charge compensation of Er3+ ions for the first time. Based on optical and structural analyses and DFT calculations, it is shown that the octahedral sites of the LZNO spinel exhibit special weak crystal fields, and Cr3+-ion dopants present NIR emission centered at 800 nm as supposed. Moreover, when the Er3+ ion is selected for designed heterotopic doping at the Zn2+ site, Cr4+ luminescence assassins, which occupy the octahedral sites of Nb5+ ions, are transformed into beneficial Cr3+ emission centers by defect charge compensation. The universality experiments indicated that the strategy of heterotopic and heterovalent doping of trivalent rare earth ions offers a simple but efficient way to enhance the NIR luminescence efficiency of Cr3+ phosphors. Finally, under the excitation of a 470 nm blue laser, the quantum efficiency of LiZn(Er)NbO4:Cr3+ is enhanced from 8.3% to 17.6%. The use of pc-LEDs in combination with commercial blue-light chips demonstrated significant potential in biological organ imaging and night-vision applications.
2 Experimental section
2.1 Materials and preparation
Powder samples containing LZNO:xCr3+ (x = 0–0.03) and LiZn1−y(Er)yNbO4:0.01Cr3+ (y = 0–0.02) compositions were synthesized by high-temperature solid-phase reactions. Raw materials of Li2CO3 (99.9%), ZnO (99.99%), Nb2O5 (99.99%), Cr2O3 (99.99%) and Er2O3 (99.99%) were weighed stoichiometrically (all raw materials are purchased from Aladdin reagents, https://www.aladdinsci.com), mixed and ground in an agate mortar for at least 30 min to create a well-homogenized powder. Then, the mixture was placed into an alumina crucible and sintered under an air atmosphere at 1100 °C for 4 h until it naturally cooled to room temperature. After cooling to room temperature naturally, the samples were ground into powders, which are the final target phosphors for further measurements. NIR pc-LEDs were fabricated using the optimal composition of LiZn0.986Er0.014NbO4:0.01Cr3+ (λem = 800 nm) with high-power blue-LED chips (5 W, 470 nm, San'an Optoelectronics Co., Ltd). The phosphor was evenly mixed with epoxy resin in a weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and then applied to the chips.
1 and then applied to the chips.
2.2 Characterization
X-ray diffraction (XRD) patterns were obtained using a Bruker D8 X-ray diffractometer with Cu Kα radiation (λ = 1.54056 Å) at 40 kV and 40 mA. The general structure analysis system (GSAS) program was used to perform the Rietveld refinements of XRD profiles. The morphological properties were characterized using a scanning electron microscope (SEM, SU8000, Hitachi) equipped with an energy dispersive X-ray spectroscopy (EDS) system. A UV-3600 spectrometer was utilized for recording the diffuse reflectance (DR) spectra. Photoluminescence spectra at room temperature (RT) and different temperatures were recorded using an FLS-980 spectrometer. The NIR photoluminescence quantum yield (PLQY) values were measured using a Quantaurus-QY Plus instrument (C13534-12, Hamamatsu Photonics) and the luminescence decay time was measured using an integrated sphere on an Edinburgh FLS1000 spectrophotometer. X-ray photoelectron spectra (XPS) were recorded using an ESCALAB 250 photoelectron spectrometer (ThermoFisher Scientific, USA) with Al Kα (1486.6 eV) as the X-ray source. Electroluminescence (EL) spectra and the performances of the fabricated NIR pc-LED devices were measured using an integrating sphere (Labsphere) and the data were collected using a high accuracy array spectroradiometer (HAAS-2000, Everfine). Visible images and NIR images were obtained using a modified visible/infrared camera (Canon M100).2.3 Calculation details
The crystal structures of LZNO, LZNO-Cr and LZNO-Cr-Er were optimized using the Vienna ab initio simulation package, which is a plane-wave pseudopotential total energy package based on density functional theory (DFT).36 The atomic positions and cell parameters were relaxed with an electronic convergence criterion of 1 × 10–5 eV and an atomic convergence criterion of 0.01 eV Å−1. A cutoff energy of 520 eV was used for the basis set of plane waves and a 2 × 2 × 1 Γ-centered Monkhorst–Pack k-point grid was used to sample the first Brillouin zone. The Perdew–Burke–Ernzerhof (PBE) exchange–correlation functional was employed for structural optimization, density of states and charge density calculations, whereas the subsequent electronic properties were determined using the HSE06 hybrid functional.373 Results and discussion
3.1 Phase, crystal structure and morphology
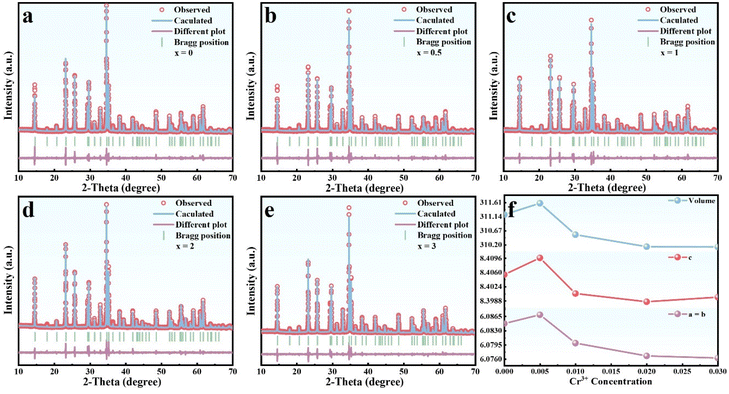
Fig. 1a shows the components of LZNO where it adopts a non-centrosymmetric tetragonal crystal structure with the P4122 space group. Usually, asymmetric crystals can result in an uneven distribution of electric fields, which leads to smaller crystal fields.38 In addition, the structure comprises two crystallographically independent octahedral units, LiO6 and NbO6, which are linked through shared edges and angles. The ZnO4 tetrahedra are linked to two octahedra only through shared angles, resulting in a large spatial gap. The Li–O and Nb–O bond lengths in the two octahedra are 2.173 and 2.016 Å, respectively, indicating a greater distance between the metal and the ligand. On the other hand, as the radius of Cr3+ (CN = 6, r = 0.615 Å) is closer to that of Nb5+ (CN = 6, r = 0.64 Å), when the Cr3+ ion is doped with LZNO, it is easier to enter the Nb5+ position with octahedral coordination as a luminescent center. Therefore, the analysis results indicated that the LZNO crystal structure is in line with our proposed three criteria, namely 1. high valence state, 2. large radius, and 3. low symmetry.Fig. 1b shows the diffraction profiles of all the samples and they agree with those of the tetragonal spinel LiZnNbO4 phase [ICDD no. 23-1206], indicating that the high-purity target product was successfully prepared. The card number was matched using Jade software. Additionally, it should be noted that a previous article reported the ICSD number of the LiZnNbO4 compound as 85735.39 To further determine the target phase and crystal structure, the XRD data were analyzed by adopting the Rietveld refinement, as shown in Fig. 2. The confidence factors Rwp and χ2 are shown in Table S3† and they indicate reliable refinement results for all the samples. The obtained data indicate good phase purity of the synthesized products and yield their structural parameters.
The SEM images of the two representative LZNO:1%Cr3+ and LZNO:1%Cr3+,1.4%Er3+ samples are displayed in Fig. 1c and d. Both samples exhibit good crystallinity and a dense microstructure. It can be seen that the samples contain individual irregular block structures with smooth surfaces, and the particle size is in the range of 20–30 μm. Co-doping with Er3+ did not cause significant morphological changes. Moreover, the EDS mapping of the LZNO:1%Cr3+,1.4%Er3+ sample was also conducted on a randomly selected plate, as shown in Fig. 1e. This further proves the homogeneous distribution of constituent elements in the grain. Notably, the Li element was ionized by the electron beam in the chamber and could not be observed in the EDS mapping spectrum due to the experimental limitations.
3.2 Site occupation and theoretical calculations
In order to clearly determine the site occupancy of Cr ions in the LZNO crystals, the refined cell parameters with increasing doping concentration of Cr3+ are plotted, as shown in Fig. 2f. This shows that parameters a, b, c and V initially increase and then decrease, as the concentration of Cr3+ increases. This non-linear trend indicates that Cr ions have successively occupied different sites. As is well known, Cr3+ prefers to occupy the octahedrally coordinated environment due to its luminescence nature. Therefore, the luminescence properties of Cr3+ are typically observed and discussed in the context of a six-coordinated environment, specifically the octahedral site.40 Considering the crystal structure of LZNO containing two crystallographically independent octahedral sites (NbO6 and LiO6 octahedra), it is meaningful to determine the site occupation preference of Cr3+. As stated above, the radius of Cr3+ is closer to that of Nb5+, and it is easier to enter the Nb5+ sites with octahedral coordination. Nevertheless, taking into account that the substitution of Nb5+ with Cr3+ will result in an excess of free electrons, it probably leads to the formation of defects and charge imbalance. Thus, the Cr3+ ion can easily be oxidized into the higher valence state of Cr4+ (CN = 6, r = 0.55 Å). This is because that when Cr4+ replaces Nb5+, it is beneficial for maintaining the stability of the system towards charge balance.According to the above analysis and taking into account the characteristic emission of Cr3+ in the PL spectrum (Fig. 3a and b), we can consider that, at a low Cr3+ doping level, Cr3+ primarily replaces Zn2+ (CN = 4, r = 0.6 Å), and any remaining Cr3+ will enter the Nb5+ site simultaneously to maintain the electrical neutrality of the system. Then, as the Cr-doping content increases, it becomes easier to form Cr4+ to stably exist at Nb5+ sites while maintaining the system's electrical neutrality. Undoubtedly, replacing Nb5+ with Cr4+ will cause lattice contraction, resulting in a decrease in cell parameters. Therefore, although we only considered replacing Nb5+ with Cr3+/4+ from the initial experimental observation, we believe that in the actual synthesis process, there is a portion of Cr3+ ions that will actively replace the Zn2+ ions to maintain a certain charge balance. This could result in a reduction in the creation of efficient luminescent sites. As is well known, the electronic structure of host materials is closely related to the luminescence performance of phosphors. However, the electronic structure of LZNO has never been reported in previous studies. Therefore, to better understand LZNO crystals, their electronic structure was calculated and analyzed using the DFT method and VASP software. The detailed information and descriptions are presented in the ESI (Fig. S1 and S2†).
3.3 Photoluminescence
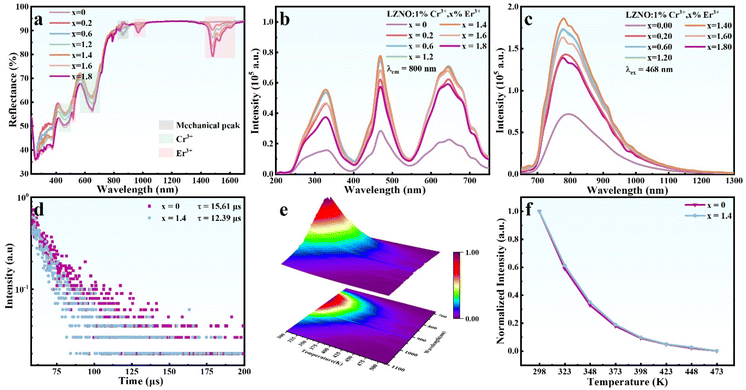
The DR spectra of LZNO:x%Cr3+ (x = 0.5, 1, 2 and 3) were recorded as shown in Fig. 3a. The absorption spectrum of the undoped sample is consistent with the calculated electronic structure of LZNO crystals (Fig. S2†). This indicates an indirect bandgap with allowed transitions, which results in an optical bandgap of 2.81 eV. The Cr3+-substituted samples exhibit intense absorption at around 463 and 653 nm in the DR spectrum, corresponding to the 4A2 → 4T1 (4F) and 4A2 → 4T2 (4F) Cr3+ electronic transitions, respectively. The optical bandgap (Eg) of LZNO can be evaluated by fitting the data with eqn (1):41,42| [hνα]1/n = A(hν − Eg) | (1) |
Significantly, we did not detect any distinctive absorption peaks associated with Cr4+, as mentioned earlier. This is an intriguing phenomenon, as Cr4+ seems to be playing a game of “hide and seek” with us. To coax out this “naughty mouse,” we conducted tests on the DR spectrum of LZNO highly doped with Cr3+ (Fig. S3†). When the Cr3+ doping concentration reaches or exceeds 4%, a new absorption peak emerges at 575 nm, unmistakably associated with the characteristic 3A2 → 3T1 (3F) absorption transition of Cr4+.46 Cr4+ typically undergoes specific energy level transitions only in a tetrahedral coordination environment, whereas the Cr4+ in LZNO remains stable in an NbO6 octahedral coordination environment, making it undetectable. As the doping level of Cr3+ increases, a larger amount of Cr4+ is more readily produced in the system. Consequently, to maintain charge balance, Cr4+ will actively occupy the ZnO4 tetrahedral site, leaving it with no means of escape.
Then, to verify Cr3+ as the luminescent ion in the LZNO host, both the excitation spectra (Fig. 3b) and the emission spectra (Fig. 3c) of LZNO with Cr concentrations from 0.5% to 3% were investigated. The excitation spectrum covers a wide region of 250–750 nm, and the peaks centered at 325, 468 and 650 nm correspond to the 4A2 → 4T1 (4P), 4A2 → 4T1 (4F) and 4A2 → 4T2 (4F) spin-allowed transitions of Cr3+ in a weak octahedral crystal field.47,48 Under 468 nm excitation, the phosphor exhibits an ultra-broadband emission spectrum centered at 800 nm with an FWHM of about 200 nm, which is attributed to the spin-allowed 4T2 → 4A2 (4F) transition of the octahedrally coordinated Cr3+ ions.49 Concurrently, the emission intensity of Cr3+ is less affected by the doping concentration, which may also be related to the limited luminescence efficiency of Cr3+ owing to the increased content of non-emission Cr4+.
In order to confirm whether the crystal field environment of Cr3+ in the LZNO host meets our standards as a weak field, we performed quantitative calculations. The crystal field strength can be characterized by crystal field splitting (Dq) and the Racah parameter (B) calculated using eqn (2)–(4):50
| 10Dq = E(4T2) = E(4A2 → 4T2) | (2) |
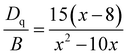 | (3) |
 | (4) |
The calculated Dq and B parameters equal to 1459.8 and 722.7 cm−1 yield a Dq/B ratio of 2.02 upon examination of the data. The corresponding Tanabe–Sugano energy level diagram of the Cr3+ ion in octahedral coordination is shown in Fig. S4.† This indicates that the [NbO6] octahedra in the LZNO host indeed provide a relatively weak crystal field environment for Cr3+. The principles we proposed in this work may offer practical solutions for designing long-wavelength broadband NIR emitting spinel materials and even other material systems activated by Cr3+ in weak crystal fields.
In addition, we observed that the Cr3+ emission peak in the LZNO host is asymmetric. Since the LiO6 octahedral site is also suitable as a luminescent site for Cr3+, it is necessary to analyze and determine the actual luminescent center of Cr3+. The excitation spectra, which were normalized and monitored at different wavelengths (λem = 750, 810, 890, and 990 nm, Fig. 3d), show a significant overlap, clearly demonstrating that there are no multiple crystallographically independent emission sites. Meanwhile, the low-temperature-dependent spectrum of LZNO:1%Cr3+ is displayed in Fig. 3e. As the temperature gradually decreases, the emission peak undergoes a slight blue shift and becomes narrower, which is attributed to the reduced electroacoustic coupling between Cr3+ and the environment. However, no new emission peaks appeared, further indicating that the transition emission process of Cr3+ in the host is independent and singular with no multiple luminescent centers. Conversely, the asymmetric emission spectra may be attributed to local structural distortion around the Cr3+ cation, which exacerbates the low symmetry of the crystal field and results in uneven peak broadening.
Fig. 3f shows the RT photoluminescence decay curve of LZNO:Cr3+ obtained by exciting the phosphor at 468 nm while monitoring at 800 nm. All the decay curves can be accurately fitted by a single-exponential function. These findings further corroborate the dominance of a single type of emission center in this phosphor. As the concentration of Cr3+ increases from 0.5% to 3%, the decay curves exhibit a reduction in lifetime, dropping from 16.7 to 10.1 μs. This is due to the concentration quenching effect of Cr3+ and the combined effect of Cr4+ as the energy transfer center. Hence, in LZNO:1%Cr3+ under 468 nm excitation, the QY value is determined to be 8.3% (Fig. S5†) and it is relatively low. Therefore, it is imperative to suppress the generation of Cr4+ and stabilize the valence state of Cr3+.
3.4 NIR enhancement by heterotopic doping of Er3+
As we know, the luminescence properties of Cr3+-activated phosphors will be suppressed because of the formation of Cr4+, especially during inequivalent substitution of Cr3+ for Nb5+ in the doping process. Doping charge compensator ions into phosphors is a common method to improve inequivalent substitution. In order to avoid competition with Cr ions entering the LZNO host lattice, we designed charge compensation by doping rare earth ions of Er3+ at the Zn2+ sites. The XRD patterns and Rietveld refinement results of co-doped Er3+ are shown in Fig. S6 and S7.† While maintaining purity, the trend of the overall expansion of crystal cell parameters indicates the successful replacement of Zn2+ by Er3+. It should be noted that the radius difference between Er3+ (CN = 6, r = 0.89 Å) and Zn2+ (CN = 4, r = 0.6 Å) is significant, but this is a comparison under different coordination environments. We know that when Er3+ enters the four-coordinated Zn site, its radius will inevitably decrease due to the change in the coordination environment from six to four. This process will also force the Cr ions originally intended to enter the Zn2+ sites to enter the Nb5+ sites instead as potentially effective luminescent centers.To confirm the role of heterotopic doping of Er3+ in the increase of Cr3+ ions, the DR spectra and NIR luminescence performances of 1%Cr doped samples with varying concentrations of Er3+ were investigated. The introduction of Er3+ significantly enhances the light absorption of Cr3+ ions, indicating that the content of Cr3+ in the system may be increased (Fig. 4a). The additional absorption peaks at 520, 970 and 1455–1640 nm are attributed to electronic transitions from the ground state 4I15/2 of Er3+ to its 2H11/2, 4I11/2 and 4I13/2 excited states, respectively. The PL and PLE spectra of the Li1−xErxZnNbO4:0.01Cr3+ samples are shown in Fig. 4b and c. The peak shapes of the PLE and PL spectra are similar to those of single-doped Cr3+, and there is no significant positional deviation. As the doping concentration of Er3+ reaches 1.4%, the integrated intensity of the Cr3+ emission band (650–1300 nm) is 2.9 times that of the original sample. The subsequent decrease in intensity is attributed to concentration quenching. The IQE (17.6%) of the optimal 1.4%Er3+ co-doped sample is more than two times that compared to the sample doped solely with 1%Cr3+ (8.3%). We also noticed that as the concentration of Er3+ increases, a new concave peak appears at 808 nm in the PL spectrum. Fig. S9† shows the low-temperature-dependent spectrum of the 1.4%Er3+ co-doped sample, analyzing the reason for the formation of this concave peak. At lower temperatures, the concave peak becomes more noticeable. When Er3+ is in an excited state, there is a higher likelihood of reabsorbing the photons it emits, particularly at lower temperatures. Therefore, the concave peak at 808 nm is due to the self-absorption of the 4I9/2 level of Er3+. The decay curve of co-doped Er3+ is presented in Fig. 4d. Co-doping with Er3+ leads to a reduction in the lifetime of Cr3+, suggesting that the localized defect energy level created by Er3+ at the bottom of the conduction band has a trapping effect for electrons.
Due to the significant substitution radius of Er3+, it may lead to a modification in the rigidity of the crystal structure, subsequently impacting the near-infrared luminescence of Cr3+. The fluorescence thermal stability, which can indirectly reflect the structural rigidity of LiZn1−xErxNbO4:0.01Cr3+ (x = 0 and 0.014), was examined through temperature-dependent emission spectra recorded from 298 to 473 K under 468 nm excitation, as shown in Fig. S10.† The 3D mapping surface for the emission spectrum of LiZn99.86Er0.014NbO4:0.01Cr3+ is plotted in Fig. 4e. When heated to 423 K, its integrated emission intensity remains at about 12% of the RT (298 K) value. Fig. 4f shows the temperature variation curves of LZNO:0.01Cr3+ and LiZn0.986Er0.014NbO4:0.01Cr3+ phosphors, indicating that Er3+ doping has almost no effect on the structural rigidity of LZNO crystals, and thus, the enhancement of Cr3+ luminescence is independent of crystal rigidity. More detailed analysis regarding the fluorescence thermal stability can be found in Fig. S11–S13 in the ESI.†
3.5 Underlying mechanism of NIR enhancement by trivalent rare earth ions
Spectral analysis and thermal stability studies have indicated that the increase in the NIR emission of Cr3+ in the LZNO system through co-doping with Er3+ is not attributed to energy transfer behavior or structural changes, suggesting a different underlying mechanism. As mentioned above, Cr3+ and Cr4+ can coexist in the LZNO:Cr system due to the matching of the radius and valence state. Therefore, our initial focus was on observing the changes in the Cr valence state in the system before and after Er3+ doping. As shown in Fig. 5a and b, the high-resolution XPS spectra of the Cr 2p doublet in LZNO:0.01Cr3+ before and after Er3+ doping were analyzed. Four Gaussian components can be resolved in the two peaks of Cr. Specifically, the peaks at 586.17 and 577.65 eV are attributed to Cr 2p1/2 and Cr 2p3/2 components related to Cr4+ and they can be used to determine the Cr4+/Cr3+ ratio. Obviously, the XPS peaks at 585.07and 575.58 eV are attributed to the Cr 2p1/2 and Cr 2p3/2 of Cr3+.It is found that, on co-doping with Er3+, the peak area associated with Cr4+ is notably decreased and the peak area related to Cr3+ is significantly increased. This observation contrasts with the behavior of the LZNO phosphor doped solely with Cr3+. It is evident that the addition of Er3+ effectively enhances the valence state transition from Cr4+ to Cr3+ in the LZNO system. Fig. S3b† shows the band gap variation of LZNO after Er3+ co-doping. Compared to Cr3+ single doping, 1.4%Er3+ co-doping resulted in a decrease in the band gap of LZNO from 2.62 eV to 2.45 eV. According to Fig. S1,† Er3+ doping will form impurity energy levels near the bottom of the conduction band in the LZNO host, thereby reducing the bandgap width. Moreover, due to Er3+ co-doping, it promotes the conversion of Cr4+ to Cr3+ in the system. Therefore, the change in the valence state of Cr leads to the broadening of the Cr3+ impurity energy level, as shown in Fig. S1c,† ultimately resulting in a decrease in the bandgap width. The decrease in the bandgap is consistent with the behavior of Cr3+ single doping, which may indicate that the concentration of Cr3+ ions in the system indeed increased.
Furthermore, to clarify the specific impact of Er3+ on promoting the conversion of Cr4+ to Cr3+, the change in charge density after Er3+ doping to LZNO:Cr was calculated. As depicted in Fig. 5c, Cr3+ tends to shift electrons towards the surrounding O2− anions. However, when Er3+ replaces the Zn2+ cation, Er3+ significantly redistributes the charge density around Cr and this results in an increase in its electron cloud, indicating that Cr4+ in the system will gain electrons and transform into Cr3+. Therefore, we can conclude that the underlying mechanism of doping Er3+ that promotes the conversion of Cr4+ to Cr3+ is mainly through charge compensation. Then, a model is proposed that the heterotopic and heterovalent doping of Er3+ leads to defect charge compensation. This process can be explained by the following equation:
 | (5) |
| V··O → 2h+ + Oox | (6) |
| Cr3+ + h+ → Cr4+ | (7) |
 | (8) |
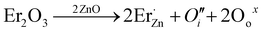 | (9) |
| V′′Zn → 2e+ + VZnx | (10) |
| O′′i → 2e+ + Oox | (11) |
| Cr4+ + h+ → Cr3+ | (12) |
When Cr3+ is doped into the LZNO host at high temperature (1100 °C), Cr3+ replaces Nb5+ to form Cr′′Nb defects because of valence mismatch. At the same time, to maintain charge balance, an equal amount of  is formed (eqn (5)). Positive charges in oxygen vacancies can be released under thermal disturbance, causing Cr3+ to self-oxidize to Cr4+ (eqn (6) and (7)). Similarly, when Er3+ is introduced to replace Zn2+, negative zinc vacancy defects or interstitial oxygen defects will be generated to balance the charge (eqn (8) and (9)). Afterwards, electrons in these negatively charged defects can be released through thermal stimulation (eqn (10) and (11)). Due to the appropriate electronegativity of Er3+, the constructed defect positions are suitable to form efficient electron transfer channels with Cr4+ within adjacent Nb5+ sites, ultimately allowing Cr4+ to capture electrons and reduce to Cr3+ (eqn (12)). Fig. S14a, b and c† show the high-resolution XPS spectra of the Zn 2p and O 1s core energy levels of LZNO:1%Cr3+ before and after Er3+ compensation. The diminished Zn signal, indicating an increase in Zn vacancies,51 and the increased interstitial oxygen signal (chemically adsorbed oxygen peaks at 532 eV with adsorbed states that can be molecular, atomic, or interstitial oxygen52–55) in Er3+-doped phosphors provide reliable evidence for our model.
is formed (eqn (5)). Positive charges in oxygen vacancies can be released under thermal disturbance, causing Cr3+ to self-oxidize to Cr4+ (eqn (6) and (7)). Similarly, when Er3+ is introduced to replace Zn2+, negative zinc vacancy defects or interstitial oxygen defects will be generated to balance the charge (eqn (8) and (9)). Afterwards, electrons in these negatively charged defects can be released through thermal stimulation (eqn (10) and (11)). Due to the appropriate electronegativity of Er3+, the constructed defect positions are suitable to form efficient electron transfer channels with Cr4+ within adjacent Nb5+ sites, ultimately allowing Cr4+ to capture electrons and reduce to Cr3+ (eqn (12)). Fig. S14a, b and c† show the high-resolution XPS spectra of the Zn 2p and O 1s core energy levels of LZNO:1%Cr3+ before and after Er3+ compensation. The diminished Zn signal, indicating an increase in Zn vacancies,51 and the increased interstitial oxygen signal (chemically adsorbed oxygen peaks at 532 eV with adsorbed states that can be molecular, atomic, or interstitial oxygen52–55) in Er3+-doped phosphors provide reliable evidence for our model.
The schematic diagram of the defect charge compensation mechanism model for Er3+ heterotopic and heterovalent doping is depicted in Fig. 6, and it can help us to vividly understand the valence conversion process of Cr ions. In the depicted scene, a mischievous demon is using fireball magic to attack Cr3+ ions. This process occurs during high-temperature sintering, where O2 provides Cr3+ ions with positive charges to facilitate the formation of Cr4+. In order to effectively suppress the transformation process of Cr ions, we introduced Er3+ ions into the Zn2+ sites. This action is akin to embedding an energetic magic stone in the core defense center, enabling the release of lightning magic through its own mechanism (zinc vacancy) and attracting elf fairies (interstitial oxygen) capable of employing frost magic. Together, these measures safeguard Cr3+ ions from the fireball assaults of the mischievous demon.
 | ||
| Fig. 6 Schematic diagram of the Cr valence-converting mechanism model via defect charge compensation for Er3+ heterotopic and heterovalent doping. | ||
In order to confirm the reliability and universality of the proposed strategy, namely, the valence conversion of Cr4+ to Cr3+ induced by co-doped lanthanum ions of appropriate electronegativity, the relationship between the electronegativity of trivalent rare earth ions and the NIR luminescence of Cr3+ ions is further investigated. Fig. 5d shows the variation of the optical electronegativity of trivalent lanthanide ions with the number of 4f orbital electrons. As shown in Fig. S15,† besides Er3+, both Dy3+ and Tm3+ can boost the NIR luminescence of Cr3+. However, Nd3+ co-doping decreases the luminescence of Cr3+ ions. We proposed that this may be attributed to the variation in electronegativity among different rare earth ions, resulting in different defect constructions and charge compensation abilities. The electronegativity values of Dy3+, Er3+, and Tm3+ are very similar and the number of 4f electrons is also close. Although the electronegativity of Nd3+ is similar to that of Dy3+, Er3+, and Tm3+, the difference in the number of 4f electrons is too large. Electronegativity indicates the ion's ability to attract electrons, while the variation in the number of 4f electrons indicates the difference in the chemical stability of lanthanide ions. Based on the above analysis, we predict that when Eu3+/Yb3+ is doped into LZNO, the ion will not stabilize the Cr3+ ion due to partial/full 4f orbitals, as it is not possible to construct suitable defects to effectively compensate for electrons to Cr4+. The luminescence quenching of Cr3+, as shown in Fig. S16,† indeed proves our idea. In summary, the fundamental reason for co-doping trivalent rare earth ions to facilitate the conversion of Cr4+ to Cr3+ is closely linked to the electronegativity values of the lanthanide ions and the number of their 4f electrons.
3.6 Performance of NIR pc-LEDs
Finally, the actual application of the LiZn(Er)NbO4:Cr3+ phosphor was evaluated. Fig. 7a shows the NIR pc-LED device prototype fabricated by combining the as-prepared optimal LiZn0.986Er0.014NbO4:0.01Cr3+ phosphor and a 470 nm blue InGaN chip. Fig. 7b shows the electroluminescence (EL) spectra of the NIR pc-LED device under different forward bias currents (20–280 mA), while the NIR output power and photoelectric efficiency as a function of current are shown in Fig. S17.† With the increase in driving current, the NIR output power increased gradually from 0.21 to 1.66 mW, while the NIR photoelectric conversion efficiency decreased gradually from 0.41% to 0.21%. The continuous decrease in the current-dependent photoelectric efficiency may be attributed to the low absorption of the phosphor and thermal quenching resulting from the high-temperature working environment. Under strong currents, an apple can be clearly captured by an NIR camera under the light of the NIR pc-LEDs, as shown in Fig. 7c. Fig. 7d shows the particular use of our NIR phosphor in imaging a biological object. Placing the palm in front of the NIR pc-LEDs enabled the light to pass through easily, capturing the detailed blood vessel distribution. Under visible light, an ordinary camera was unable to reveal any clear details. While its photoelectric conversion efficiency properties still require improvement, these findings still emphasize the potential for real-world applications in night-vision and bio-tissue imaging technology.4 Conclusion
In summary, according to the possible standards (1. high valence state; 2. large radius; and 3. low symmetry) proposed for achieving long-wavelength broadband emission of Cr ions in spinel-type crystals, we have successfully developed novel Cr-activated LiZnNbO4 spinel phosphors with excellent long-wavelength NIR emission performance. Under the excitation of 468 nm blue light, LZNO:Cr3+ shows an ultra-wide and long-wavelength NIR emission at 800 nm and the designed LiZnNbO4:Cr3+ was comprehensively investigated. In addition, a compensation strategy for defect charges involving the heterotopic doping of trivalent rare earth ions has been implemented to enhance the NIR luminescence of Cr3+ ions for the first time, which is closely linked to the electronegativity of lanthanide ions and the number of 4f electrons. Experimental evidence indicates that this strategy effectively promotes the valence state transformation of Cr4+ to Cr3+ in the LZNO:Cr3+ system, where 1.4%Er3+ co-doping increases the IQE from 8.3% to 17.8%. Finally, the LiZn(Er)NbO4:Cr3+ phosphor was incorporated into a prototype NIR pc-LED device to demonstrate its practical application. This study not only broadens the range of spinel NIR luminescent materials activated by Cr3+, but also explores the inherent relationship between rare earth ion heterotopic doping and Cr valence conversion. The design principles of Cr3+-activated long-wavelength spinel phosphors and the NIR emission enhancement strategy proposed in this study may offer a roadmap for the efficient creation of long-wavelength NIR emitting phosphors activated by Cr3+, and also presents a new approach for enhancing the luminescence efficiency.Author contributions
Wen Song: conceptualization, methodology, data curation, and writing – original draft. Kaiwen Zhang: software and investigation. Xiaoyi Dong: data curation and resources. Liang Xu: data curation and resources. Yongjin Li, Rui Hu, and Zhiguo Song: project administration, funding acquisition, writing – review and editing, and supervision. Zhaoyi Yin: data curation and resources. Zhengwen Yang: data curation and resources. Jianbei Qiu: data curation and resources.Data availability
All data supporting the findings of this study are available within the paper and its ESI.†Conflicts of interest
The authors declare no competing interests.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 12264023), the National Natural Science Foundation of High and Foreign Experts Introduction Plan (No. G2022039008L), the Yunnan Major Scientific and Technological Projects (No. 202202AG050016), the Yunnan Fundamental Research Projects (No. 202301AT070459), the Analysis and Testing Foundation of Kunming University of Science and Technology (No. 2021T20200124) and the Yunnan Xing Dian Youth Talent Support Program (No. XDYC-QNRC-2022-0591). We also thank the Shiyanjia Lab (https://www.shiyanjia.com) for the XRD and XPS analyses and the Shenzhen Shuidi Scientific Research Service Co., Ltd for the testing of the PLQYs and NIR pc-LEDs.References
- A. T. Eggebrecht, S. L. Ferradal, A. Robichaux-Viehoever, M. S. Hassanpour, H. Dehghani, A. Z. Snyder, T. Hershey and J. P. Culver, Mapping distributed brain function and networks with diffuse optical tomography, Nat. Photonics, 2014, 8, 448–454 CrossRef CAS PubMed
.
- K. T. Ly, R. W. Chen-Cheng, H. W. Lin, Y. J. Shiau, S. H. Liu, P. T. Chou, C. S. Tsao, Y. C. Huang and Y. Chi, Near-infrared organic light-emitting diodes with very high external quantum efficiency and radiance, Nat. Photonics, 2017, 11, 63–68 CrossRef
.
- G. C. Liu, M. S. Molokeev and Z. G. Xia, Structural Rigidity Control toward Cr3+-Based Broadband Near-Infrared Luminescence with Enhanced Thermal Stability, Chem. Mater., 2022, 34, 1376–1384 CrossRef CAS
.
- L. Huang, Z. J. Li, Y. Zhao, J. Y. Yang, Y. C. Yang, A. I. Pendharkar, Y. W. Zhang, S. Kelmar, L. Y. Chen, W. T. Wu, J. Z. Zhao and G. Han, Enhancing Photodynamic Therapy through Resonance Energy Transfer Constructed Near-Infrared Photosensitized Nanoparticles, Adv. Mater., 2017, 29, 7 Search PubMed
.
- Q. Y. Shao, H. Ding, L. Q. Yao, J. F. Xu, C. Liang and J. Q. Jiang, Photoluminescence properties of a ScBO3:Cr3+ phosphor and its applications for broadband near-infrared LEDs, RSC Adv., 2018, 8, 12035–12042 RSC
.
- W. T. Huang, C. L. Cheng, Z. Bao, C. W. Yang, K. M. Lu, C. Y. Kang, C. M. Lin and R. S. Liu, Broadband Cr3+, Sn4+-Doped Oxide Nanophosphors for Infrared Mini Light-Emitting Diodes, Angew. Chem., Int. Ed., 2019, 58, 2069–2072 CrossRef CAS PubMed
.
- Z. W. Pan, Y. Y. Lu and F. Liu, Sunlight-activated long-persistent luminescence in the near-infrared from Cr3+-doped zinc gallogermanates, Nat. Mater., 2012, 11, 58–63 CrossRef CAS PubMed
.
- L. F. Yuan, Y. H. Jin, H. Y. Wu, K. Y. Deng, B. Y. Qu, L. Chen, Y. H. Hu and R. S. Liu, Ni2+-Doped Garnet Solid-Solution Phosphor-Converted Broadband Shortwave Infrared Light-Emitting Diodes toward Spectroscopy Application, ACS Appl. Mater. Interfaces, 2022, 14, 4265–4275 CrossRef CAS PubMed
.
- Z. Liao, H. Xu, W. Zhao, H. Yang, J. Zhong, H. Zhang, Z. Nie and Z.-K. Zhou, Energy transfer from Mn4+ to Mn5+ and near infrared emission with wide excitation band in Ca14Zn6Ga10O35:Mn phosphors, Chem. Eng. J., 2020, 395, 125060 CrossRef CAS
.
- X. Z. Chen, Y. Li, K. Huang, L. Huang, X. M. Tian, H. F. Dong, R. Kang, Y. H. Hu, J. M. Nie, J. R. Qiu and G. Han, Trap Energy Upconversion-Like Near-Infrared to Near-Infrared Light Rejuvenateable Persistent Luminescence, Adv. Mater., 2021, 33, 7 Search PubMed
.
- F. Ren, L. H. Ding, H. H. Liu, Q. Huang, H. Zhang, L. J. Zhang, J. F. Zeng, Q. Sun, Z. Li and M. Y. Gao, Ultra-small nanocluster mediated synthesis of Nd3+-doped core-shell nanocrystals with emission in the second near-infrared window for multimodal imaging of tumor vasculature, Biomaterials, 2018, 175, 30–43 CrossRef CAS PubMed
.
- J. W. Qiao, G. J. Zhou, Y. Y. Zhou, Q. Y. Zhang and Z. G. Xia, Divalent europium-doped near-infrared-emitting phosphor for light-emitting diodes, Nat. Commun., 2019, 10, 7 CrossRef PubMed
.
- P. Kaithrikkovil Varriam and S. Ganesanpotti, Harnessing the Dual–Mode Luminescence of Er/Yb Co–Doped SrLaLiTeO6 Double Perovskite Phosphors for Remarkably Wide Range Temperature Sensing and NIR pc–LEDs, Laser Photonics Rev., 2024 DOI:10.1002/lpor.202400245
.
- P. P. Dang, Y. Wei, D. J. Liu, G. G. Li and J. Lin, Recent Advances in Chromium-Doped Near-Infrared Luminescent Materials: Fundamentals, Optimization Strategies, and Applications, Adv. Opt. Mater., 2023, 11, 27 Search PubMed
.
- S. H. Miao, Y. J. Liang, Y. Zhang, D. X. Chen and X. J. Wang, Broadband Short-Wave Infrared Light-Emitting Diodes Based on Cr3+-Doped LiScGeO4 Phosphor, ACS Appl. Mater. Interfaces, 2021, 13, 36011–36019 CrossRef CAS PubMed
.
- M. U. Dumesso, W. G. Xiao, G. J. Zheng, E. T. Basore, M. X. Tang, X. F. Liu and J. R. Qiu, Efficient, Stable, and Ultra-Broadband Near-Infrared Garnet Phosphors for Miniaturized Optical Applications, Adv. Opt. Mater., 2022, 10, 8 Search PubMed
.
- X. K. Zou, X. J. Wang, H. R. Zhang, Y. Y. Kang, X. Yang, X. J. Zhang, M. S. Molokeev and B. F. Lei, A highly efficient and suitable spectral profile Cr3+-doped garnet near-infrared emitting phosphor for regulating photomorphogenesis of plants, Chem. Eng. J., 2022, 428, 9 CrossRef
.
- T. Y. Liu, H. Cai, N. Mao, Z. Song and Q. L. Liu, Efficient near-infrared pyroxene phosphor LiInGe2O6:Cr3+ for NIR spectroscopy application, J. Am. Ceram. Soc., 2021, 104, 4577–4584 CrossRef CAS
.
- X. X. Xu, Q. Y. Shao, L. Q. Yao, Y. Dong and J. Q. Jiang, Highly efficient and thermally stable Cr3+-activated silicate phosphors for broadband near-infrared LED applications, Chem. Eng. J., 2020, 383, 8 Search PubMed
.
- Q. M. Lin, Q. Wang, M. Liao, M. X. Xiong, X. Feng, X. Zhang, H. F. Dong, D. Y. Zhu, F. G. Wu and Z. F. Mu, Trivalent Chromium Ions Doped Fluorides with Both Broad Emission Bandwidth and Excellent Luminescence Thermal Stability, ACS Appl. Mater. Interfaces, 2021, 13, 18274–18282 CrossRef CAS PubMed
.
- T. Q. Zhao, R. Abdurahman, R. Aiwaili, S. Q. Wu and X. B. Yin, Spinel-type persistent luminescence nanoparticles: From mechanisms, compositions to applications, Coord. Chem. Rev., 2023, 488, 25 CrossRef
.
- W. T. Huang, K. C. Chen, M. H. Huang and R. S. Liu, Tunable Spinel Structure Phosphors: Dynamic Change in Near-Infrared Windows and Their Applications, Adv. Opt. Mater., 2023, 22, DOI:10.1002/adom.202301166
.
- H. J. Jiang, L. Y. Chen, G. J. Zheng, Z. H. Luo, X. H. Wu, Z. H. Liu, R. Y. Li, Y. F. Liu, P. Sun and J. Jiang, Ultra-Efficient GAGG:Cr3+ Ceramic Phosphor-Converted Laser Diode: A Promising High-Power Compact Near-Infrared Light Source Enabling Clear Imaging, Adv. Opt. Mater., 2022, 10, 8 Search PubMed
.
- L. P. Jiang, X. Jiang, C. X. Wang, P. Liu, Y. Zhang, G. C. Lv, T. Lookman and Y. J. Su, Rapid Discovery of Efficient Long-Wavelength Emission Garnet:Cr NIR Phosphors via Multi-Objective Optimization, ACS Appl. Mater. Interfaces, 2022, 14, 52124–52133 CrossRef CAS PubMed
.
- V. Rajendran, M. H. Fang, G. N. De Guzman, T. Lesniewski, S. Mahlik, M. Grinberg, G. Leniec, S. M. Kaczmarek, Y. S. Lin, K. M. Lu, C. M. Lin, H. Chang, S. F. Hu and R. S. Liu, Super Broadband Near-Infrared Phosphors with High Radiant Flux as Future Light Sources for Spectroscopy Applications, ACS Energy Lett., 2018, 3, 2679–2684 CrossRef CAS
.
- V. Rajendran, W. T. Huang, K. C. Chen, H. Chang and R. S. Liu, Energy-saving chromium-activated garnet-structured phosphor-converted near-infrared light-emitting diodes, J. Mater. Chem. C, 2022, 10, 14367–14378 RSC
.
- J. Q. Fan, W. Y. Zhou, J. L. Zhang, P. C. Chen, Q. Pang, L. Y. Zhou, C. Y. Zhou and X. G. Zhang, A novel efficient broadband near-infrared phosphor LiGaGe2O6:Cr3+ with EQE enhancement and spectral tuning by Sc3+-Ga3+ substitution for NIR pc-LED application, Inorg. Chem. Front., 2023, 10, 511–521 RSC
.
- F. Y. Zhao, H. Cai, Z. Song and Q. L. Liu, Structural Confinement for Cr3+ Activators toward Efficient Near-Infrared Phosphors with Suppressed Concentration Quenching, Chem. Mater., 2021, 33, 3621–3630 CrossRef CAS
.
- Y. Wang, Z. Wang, G. Wei, Y. Yang, S. He, J. Li, Y. Shi, R. Li, J. Zhang and P. Li, Ultra-Broadband and high efficiency Near-Infrared Gd3ZnxGa5-2xGexO12:Cr3+ (x = 0–2.0) garnet phosphors via crystal field engineering, Chem. Eng. J., 2022, 437, 135346 CrossRef CAS
.
- M. Ferriol and S. Lecocq, Structural characterization of ZnLiNbO4, Eur. J. Solid State Inorg. Chem., 1998, 35, 707–714 CrossRef CAS
.
- H. Cai, H. Chen, H. Zhou, J. Zhao, Z. Song and Q. L. Liu, Controlling Cr3+/Cr4+ concentration in single-phase host toward tailored super-broad near-infrared luminescence for multifunctional applications, Mater. Today Chem., 2021, 22, 7 Search PubMed
.
- Z. W. Jia, C. X. Yuan, Y. F. Liu, X. J. Wang, P. Sun, L. Wang, H. C. Jiang and J. Jiang, Strategies to approach high performance in Cr3+-doped phosphors for high-power NIR-LED light sources, Light: Sci. Appl., 2020, 9, 9 CrossRef PubMed
.
- T. Y. Gao, W. D. Zhuang, R. H. Liu, Y. H. Liu, C. P. Yan, J. Tian, G. Chen, X. Chen, Y. H. Zheng and L. G. Wang, Site occupancy and enhanced luminescence of broadband NIR gallogermanate phosphors by energy transfer, J. Am. Ceram. Soc., 2020, 103, 202–213 CrossRef CAS
.
- G. Merkininkaite, A. Zabiliute-Karaliune, T. Jüstel, V. Klimkevicius, S. Sakirzanovas and A. Katelnikovas, Ce3+ → Cr3+ energy transfer in Y3Al3MgSiO12 garnet host and application in horticultural lighting, Ceram. Int., 2023, 49, 16796–16808 CrossRef CAS
.
- S. C. Lal, I. N. Jawahar and S. Ganesanpotti, Enhancing the inherent NIR photoluminescence in SrLaLiTeO6 through Cr3+-Yb3+ co-substitution for high performance pc-LEDs, Dalton Trans., 2024, 53, 1230–1244 RSC
.
- J. Hafner,
Ab initio simulations of materials using VASP:: Density-functional theory and beyond, J. Comput. Chem., 2008, 29, 2044–2078 CrossRef CAS PubMed
.
- J. Heyd, G. E. Scuseria and M. Ernzerhof, Hybrid functionals based on a screened Coulomb potential, J. Chem. Phys., 2003, 118, 8207–8215 CrossRef CAS
.
-
N. W. Ashcroft and N. D. Mermin, Solid State Physics, Cengage Learning Press, 1976 Search PubMed
.
- G. B. M. Melo, S. S. Pedro, A. López, G. K. B. Costa and L. P. Sosman, Unexpected photoluminescence from Mn2+ in LiZnNbO4, Opt. Mater., 2020, 99, 5 CrossRef
.
- S. Adachi, Photoluminescence Properties of Cr3+-Activated Oxide Phosphors, ECS J. Solid State Sci. Technol., 2021, 10, 20 Search PubMed
.
- P. Makula, M. Pacia and W. Macyk, How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV-Vis Spectra, J. Phys. Chem. Lett., 2018, 9, 6814–6817 CrossRef CAS PubMed
.
- R. G. J. Tauc and A. Vancu, Optical Properties and Electronic Structure of Amorphous Germanium, Phys. Status Solidi, 1996, 627–637 Search PubMed
.
- V. V. Atuchin, L. I. Isaenko, V. G. Kesler, Z. S. Lin, M. S. Molokeev, A. P. Yelisseyev and S. A. Zhurkov, Exploration on anion ordering, optical properties and electronic structure in K3WO3F3 elpasolite, J. Solid State Chem., 2012, 187, 159–164 CrossRef CAS
.
- Z. Xia, Y. Zhang, M. S. Molokeev and V. V. Atuchin, Structural and Luminescence Properties of Yellow-Emitting NaScSi2O6:Eu2+ Phosphors: Eu2+ Site Preference Analysis and Generation of Red Emission by Codoping Mn2+ for White-Light-Emitting Diode Applications, J. Phys. Chem. C, 2013, 117, 20847–20854 CrossRef CAS
.
- H. Ji, Z. Huang, Z. Xia, M. S. Molokeev, X. Jiang, Z. Lin and V. V. Atuchin, Comparative investigations of the crystal structure and photoluminescence property of eulytite-type Ba3Eu(PO4)3and Sr3Eu(PO4)3, Dalton Trans., 2015, 44, 7679–7686 RSC
.
- F. Y. Zhao, Z. Song and Q. L. Liu, Advances in Chromium-Activated Phosphors for Near-Infrared Light Sources, Laser Photonics Rev., 2022, 16, 42 Search PubMed
.
- B. Bai, P. P. Dang, D. Y. Huang, H. Z. Lian and J. Lin, Broadband Near-Infrared Emitting Ca2LuScGa2Ge2O12:Cr3+ Phosphors: Luminescence Properties and Application in Light-Emitting Diodes, Inorg. Chem., 2020, 59, 13481–13488 CrossRef CAS PubMed
.
- G. C. Liu, M. S. Molokeev, B. F. Lei and Z. G. Xia, Two-site Cr3+ occupation in the MgTa2O6:Cr3+ phosphor toward broad-band near-infrared emission for vessel visualization, J. Mater. Chem. C, 2020, 8, 9322–9328 RSC
.
- J. Y. Zhong, Y. Zhuo, F. Du, H. S. Zhang, W. R. Zhao, S. H. You and J. Brgoch, Efficient Broadband Near-Infrared Emission in the GaTaO4:Cr3+ Phosphor, Adv. Opt. Mater., 2022, 10, 8 RSC
.
- B. Struve and G. Huber, The effect of the crystal field strength on the optical spectra of Cr3+ in gallium garnet laser crystals, Appl. Phys. B: Photophys. Laser Chem., 1985, 195–201 CrossRef CAS
.
- V. V. Atuchin, E. N. Galashov, O. Y. Khyzhun, A. S. Kozhukhov, L. D. Pokrovsky and V. N. Shlegel, Structural and Electronic Properties of ZnWO4 (010) Cleaved Surface, Cryst. Growth Des., 2011, 11, 2479–2484 CrossRef CAS
.
- V. V. Atuchin, J. C. Grivel, A. S. Korotkov and Z. Zhang, Electronic parameters of Sr2Nb2O7 and chemical bonding, J. Solid State Chem., 2008, 181, 1285–1291 CrossRef CAS
.
- V. V. Atuchin, D. A. Vinnik, T. A. Gavrilova, S. A. Gudkova, L. I. Isaenko, X. Jiang, L. D. Pokrovsky, I. P. Prosvirin, L. S. Mashkovtseva and Z. Lin, Flux Crystal Growth and the Electronic Structure of BaFe12O19 Hexaferrite, J. Phys. Chem. C, 2016, 120, 5114–5123 CrossRef CAS
.
- Z. Xing, P. Li, D. Dai, X. Li, C. Liu, L. Zhang and Z. Wang, Self-Luminescence of Perovskite-Like LaSrGaO4 via Intrinsic Defects and Anomalous Luminescence Analysis of LaSrGaO4:Mn2+, Inorg. Chem., 2019, 58, 4869–4879 CrossRef CAS PubMed
.
- X. Kang, W. Lü, Z. Zhu and Q. Pan, Multiple defects induced near-infrared self-luminescence of (Ca,Sr)LaMgTaO6 double perovskite phosphor, Ceram. Int., 2023, 49, 32719–32726 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4qi01168f |
| This journal is © the Partner Organisations 2024 |