Enhanced VIS-NIR emission of Re4+ doped Cs2ZrCl6 for optical thermometry and near-infrared illumination applications†
Boya
Li
a,
Zheng
Huang
a,
Peiqing
Cai
 *a,
Yuxin
Zhan
a,
Xuhui
Feng
a,
Xipeng
Pu
*a,
Yuxin
Zhan
a,
Xuhui
Feng
a,
Xipeng
Pu
 b,
Shala
Bi
*c,
Guanghua
Wang
d,
Jie
Zhang
d and
Zugang
Liu
*a
b,
Shala
Bi
*c,
Guanghua
Wang
d,
Jie
Zhang
d and
Zugang
Liu
*a
aCollege of Optical and Electronic Technology, China Jiliang University, Hangzhou, Zhejiang 310018, China. E-mail: pqcai@cjlu.edu.cn; zgliu78@cjlu.edu.cn
bSchool of Materials Science and Engineering, Liaocheng University, Liaocheng, Shandong 252000, China
cDepartment of Physics and Interdisciplinary Program of Biomedical, Mechanical and Electrical Engineering, Pukyong National University, Busan 608-737, Republic of Korea. E-mail: BI1425820017@outlook.com
dYunnan Olightek Opto-electronic Technology Co., Ltd., Kunming 650223, China
First published on 28th June 2024
Abstract
The luminescence properties of transition metal d3 ions have exhibited widespread application potential in optical thermometry, while the emissive characteristics of self-trapped excitons in lead-free metal halides have garnered significant attention in the field of optoelectronics. Nonetheless, a comprehensive investigation into the temperature-dependent luminescence features of d3 ion-doped lead-free metal halides remains an area demanding in-depth exploration. Herein, we report on the visible and near-infrared (NIR) photoluminescence of 5d3 Re4+ doped vacancy-ordered metal halide Cs2ZrCl6 synthesized via hydrothermal methods. The undoped Cs2ZrCl6 host exhibits broad band self-trapped exciton (STE) emission. PL measurements reveal that its anti-thermal quenching PL under 270 nm excitation is predominantly due to the enhancement of the hot absorption band tail. Upon UV excitation, Re4+ doped Cs2ZrCl6 concurrently emits STE emission and features a narrow-band NIR emission at 730 nm with vibronic sideband details from the ReCl62− octahedra, alongside a narrow-band emission at 1340 nm. By harnessing the luminescence intensity ratio (LIR) technique, combined with the anti-thermal quenching of STEs and the thermal quenching of Re4+ emission, optical thermometry with a relative sensitivity as high as 2.76% K−1 is achieved. Our findings suggest that Re4+-doped Cs2ZrCl6 represents a multifunctional optoelectronic platform with promising applications in NIR illumination and temperature sensing.
1. Introduction
In recent years, all-inorganic metal halides have emerged as a novel class of optoelectronic materials, attracting considerable research attention in fields such as solar cells, LEDs, and sensors.1–4 For example, solar cells employing lead-based perovskite materials have attracted substantial commercial investment.5 Nevertheless, concerns persist regarding the toxicity and stability of lead-based metal halides in optoelectronic applications, posing significant challenges to the practical feasibility of these materials.6As a contender of lead metal halides in illumination and detection, the vacancy-ordered lead-free metal halide Cs2ZrCl6 has garnered increasing attention from researchers. Its appeal lies in its reduced heavy metal toxicity and outstanding luminescence performance. Recent application reports have showcased its distinct advantages, including solution processability,7 nanoparticle to nanoscale dimensions,8 high-efficiency light yield,9 white light emission,10 and high-entropy functionalized solid solutions.11 The dynamic luminescence mechanisms within Cs2ZrCl6 matrices have also sparked widespread interest.12,13 With a vacancy-ordered perovskite structure, Cs+ ions with larger ionic radii separate ZrCl62− groups in the crystal lattice, forming discrete zero-dimensional soft lattice frameworks. Under external high-energy ultraviolet excitation, localized confined self-trapped exciton (STE) recombination occurs, leading to efficient long-lived triplet state emission. Ultrafast femtosecond transient absorption studies have revealed the presence of two STE energy levels with a small energy gap. Additionally, preliminary examinations have indicated an anti-thermal quenching phenomenon of emission within a temperature range from low to room temperature, attributed to thermally activated delayed fluorescence effects.8 Therefore, the unique luminescence properties of the matrix hold significant theoretical and practical research value.
On the other hand, ion doping represents a crucial method for enhancing and modulating the luminescence of the Cs2ZrCl6 host. Trivalent rare earth Ln3+ ions possess abundant energy levels that can potentially engage in resonance energy transfer or multi-phonon relaxation with the exciton levels of Cs2ZrCl6, facilitating effective energy transfer processes for the narrow-band luminescence of rare earth ions.14,15 Nevertheless, the unequal substitution of trivalent rare earth ions Ln3+ and Zr4+ may introduce new defects or trap levels, thereby impeding the occurrence of efficient luminescence.16 Moreover, main group ns2 metal ions (Bi3+, Sb3+, and Te4+) can also serve as effective dopants for Cs2ZrCl6, yielding broad emission with a significant Stokes shift.17–20 This characteristic holds promising prospects in fields such as temperature sensing, white light generation, and near-infrared illumination, underscoring the potential of non-rare earth ion doping to effectively enhance the luminescence performance of the Cs2ZrCl6 system.
Transition metal (TM) ions, especially ions with 3d3 electron configurations such as Cr3+ and Mn4+, have been widely studied in recent years and are of significant research value in display, optical temperature sensing, and near-infrared detection fields.21,22 Taking Cr3+ TM ions as an example, efficient red or near-infrared emission can be achieved in crystals with octahedral lattice positions, forming anionic groups such as CrO69−, CrF63−, or CrCl63−.23 The ZrCl62− anionic groups in Cs2ZrCl6 also have octahedral configurations, so it can be assumed that d3 ions will also exhibit good luminescence performance when doped into Cs2ZrCl6, although there are relatively few reports in the literature.24,25 The metal rhenium Re4+ ions, which have similar 3d3 configurations to 5d3 ions, mainly emit narrow-band near-infrared light due to allowed magnetic dipole transitions of 2T2g → 4A2g and 2T1g → 4A2g. Early reports by Dorain26 and Schmidtke27et al. showed that the ReCl62− ion groups doped in hosts such as K2PtCl6 and Cs2ZrCl6 exhibited narrow-band near-infrared luminescence characteristics. Güdel further discovered that Re4+ in the Cs2GeF6 lattice could produce visible orange emission at 588 nm when excited with an infrared laser at 910 nm from 15 K to room temperature, indicating an energy-transfer up-conversion mechanism.28,29 These findings underscore the rich luminescence properties and application potential of metal halide systems doped with Re4+ ions featuring a 5d3 configuration.
In this work, pure Cs2ZrCl6 microcrystals and Cs2ZrCl6 microcrystals doped with varying concentrations of Re4+ were synthesized using a hydrothermal method. The phase structures were confirmed using X-ray powder diffraction, demonstrating that low concentrations of Re4+ doping did not affect the crystal lattice of the host. Under excitation with ultraviolet light and X-rays, the substrate material emitted efficient blue light, while the Re4+-doped material extended the spectrum into the near-infrared region. Temperature-dependent photoluminescence spectra indicated a negative thermal quenching effect in the host material highly correlated with the excitation wavelength. Effective energy transfer processes between the STE emissions and Re4+ were observed, with Re4+ emissions significantly affected by electron–phonon coupling and displaying notable thermal quenching effects. By coupling the negative thermal quenching of the host and the thermal quenching of the Re4+ emission, a highly sensitive thermometer could be designed. Additionally, Re4+-doped Cs2ZrCl6, when encapsulated with ultraviolet chips, could be fabricated into near-infrared (NIR) LED devices, highlighting its multifunctionality as an optoelectronic lighting and sensing material.
2. Experimental section
2.1 Materials and synthesis process
Cesium chloride (99.9%) and zirconium tetrachloride (99.9%) were purchased from Adamas, while potassium hexachlororhenate K2ReCl6 (99.99%) was obtained from Rhawn Co., Ltd, and 37% HCl acid was purchased from Sinopharm Chemical Reagent Co., Ltd. All reagents and solvents were used without further purification.Pure Cs2ZrCl6 and samples doped with x Re4+ (x = 0.5%, 1%, 3%, 5%, 7%) were prepared via hydrothermal reactions. Initially, accurately weighed amounts of CsCl (2 mmol) and ZrCl4 (1 mmol) and varying doping concentrations of K2ReCl6 (0.005 mmol, 0.01 mmol, 0.03 mmol, 0.05 mmol, 0.07 mmol) were added to five 25 ml polytetrafluoroethylene (PTFE) liners. Subsequently, 5 ml of 37% HCl saturated aqueous solution was added to each liner, and the mixture was stirred at room temperature for 30 minutes to ensure thorough mixing. The PTFE containers were then placed into a high-pressure reactor, transferred to a heating oven, and heated to 180 °C. The reaction was maintained at this temperature for 720 minutes. Subsequently, the mixture was cooled to room temperature at a rate of 0.05 °C min−1, resulting in the collection of white crystals at the bottom of the containers. After washing three times with ethanol and filtering, the final product was obtained by overnight storage in a vacuum oven at 120 °C.
2.2 Characterization
A Rigaku X-ray diffractometer (Japan) with Cu-Kα radiation was used to characterize the phase compositions and the purity of the compounds. The XRD patterns were limited to the range of 5°–90° (2θ) with a scanning speed of 10° min−1. A field-emission scanning electron microscope (SEM) attached with an Energy Dispersive X-Ray spectrometer (EDX) (ZEISS Sigma, Germany) was used to determine the morphology and the elemental composition of the compound. The elemental ratios of the doped samples were measured with an Agilent 720ES inductively coupled plasma-optical emission spectrometer (ICP-OES). The photoluminescence quantum efficiency (PLQY), photoluminescence excitation (PLE), and photoluminescence (PL) emission spectra were recorded at room temperature using an Edinburgh FLS1000 photoluminescence spectrometer coupled with a Teflon integrating sphere. The NIR emission was detected using a NIR-sensitive PMT housed in a nitrogen-cooled environment, covering the spectral range from 300 nm to 1700 nm. The PLQY values of the Cs2ZrCl6 microcrystals were calculated under 260 nm excitation with a PVDF blank pallet as the reference. The PLQY value is calculated as follows: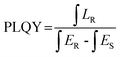 | (1) |
3. Results and discussion
3.1 Phase structure and analysis
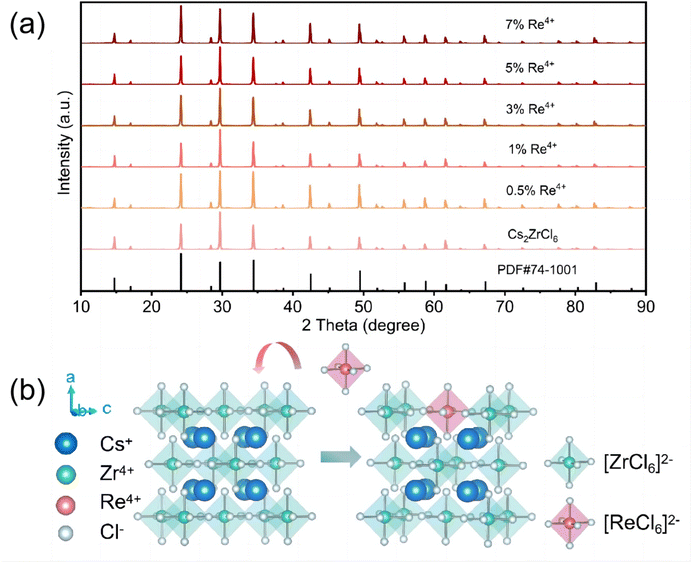
The phase information of a series of as-prepared Cs2ZrCl6:Re4+ samples with different Re4+ concentrations was obtained by XRD analysis. Fig. 1(a) illustrates the XRD patterns of the synthesized Cs2ZrCl6:xRe4+ (x = 0, 0.5%, 1.0%, 3.0%, 5.0%, and 7.0%, respectively). Compared to the standard PDF card information of pure Cs2ZrCl6, no residual impurity phases such as K2ReCl6 and ZrCl4 were detected, even at a high Re4+ doping concentration of 7%. This suggests that the introduction of the dopant ion Re4+ had no significant impact on the crystallinity of the crystal. The Cs2ZrCl6 host adopts a typical vacancy-ordered double perovskite structure, crystallizing into a K2PtCl6-type cubic structure (space group Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m), where Zr atoms coordinate with the remaining six Cl atoms to form [ZrCl6]2− octahedra. The structural units of [ZrCl6]2− octahedra and vacancies are alternately arranged and separated by Cs+ cations (Fig. 1(b)).13 Re4+ (0.63 Å, CN = 6) doped Cs2ZrCl6 microcrystals were synthesized via a hydrothermal method at 180 °C. Due to the similar ionic radius and valence state to Zr4+ (0.79 Å, CN = 6), Re4+ tends to replace Zr4+ in the octahedral position.30 By analyzing the XRD patterns of the series of samples with different Re4+ concentrations in the range of 23.5°–25°, a significant angular shift of the diffraction peaks is observed with the increase in Re4+ doping levels (see Fig. S1(a)†). The lattice shrinks when smaller ions are doped into the lattice, causing the diffraction peaks to shift to higher angles. However, Rietveld refinement data of the xRe4+-doped sample, processed using the General Structural Analysis System (GSAS) program, show that the cell volume increases with increasing Re4+ concentration (Fig. S1(b)†), which may be due to the stronger covalency of Re–Cl compared to Zr–Cl.31
m), where Zr atoms coordinate with the remaining six Cl atoms to form [ZrCl6]2− octahedra. The structural units of [ZrCl6]2− octahedra and vacancies are alternately arranged and separated by Cs+ cations (Fig. 1(b)).13 Re4+ (0.63 Å, CN = 6) doped Cs2ZrCl6 microcrystals were synthesized via a hydrothermal method at 180 °C. Due to the similar ionic radius and valence state to Zr4+ (0.79 Å, CN = 6), Re4+ tends to replace Zr4+ in the octahedral position.30 By analyzing the XRD patterns of the series of samples with different Re4+ concentrations in the range of 23.5°–25°, a significant angular shift of the diffraction peaks is observed with the increase in Re4+ doping levels (see Fig. S1(a)†). The lattice shrinks when smaller ions are doped into the lattice, causing the diffraction peaks to shift to higher angles. However, Rietveld refinement data of the xRe4+-doped sample, processed using the General Structural Analysis System (GSAS) program, show that the cell volume increases with increasing Re4+ concentration (Fig. S1(b)†), which may be due to the stronger covalency of Re–Cl compared to Zr–Cl.31
Fig. 2(a) presents the EDX spectrum of Re4+ doped Cs2ZrCl6 collected at the labeling position of the particle, revealing that all the elements of the samples were detected. Fig. 2(b)–(g) illustrate the morphology and elemental mapping results of 0.1% Re4+ doped Cs2ZrCl6. The atomic ratios corresponding to Cs, Zr, Cl, and Re were 26.47%, 11.9%, 61.93%, and 0.11%, respectively. The measured values approximately corresponded to the stoichiometric content of Cs2ZrCl6. The elements are distinguished by different colors, indicating the uniform distribution of Cs, Zr, Cl, and Re elements in Cs2ZrCl6:Re4+ microcrystals. In addition, ICP-OES measurement was also performed to determine the nominal and actual doping ratios between Zr4+ and Re4+ in the Cs2ZrCl6 host with varying Re4+ concentrations. These ratio values are listed in Table S1.† The results indicate that the actual Re4+ doping levels are approximately equal to the nominal values achieved through the hydrothermal method. These elemental analyses provide solid evidence for the successful doping of Re4+ into the Cs2ZrCl6 host lattice.
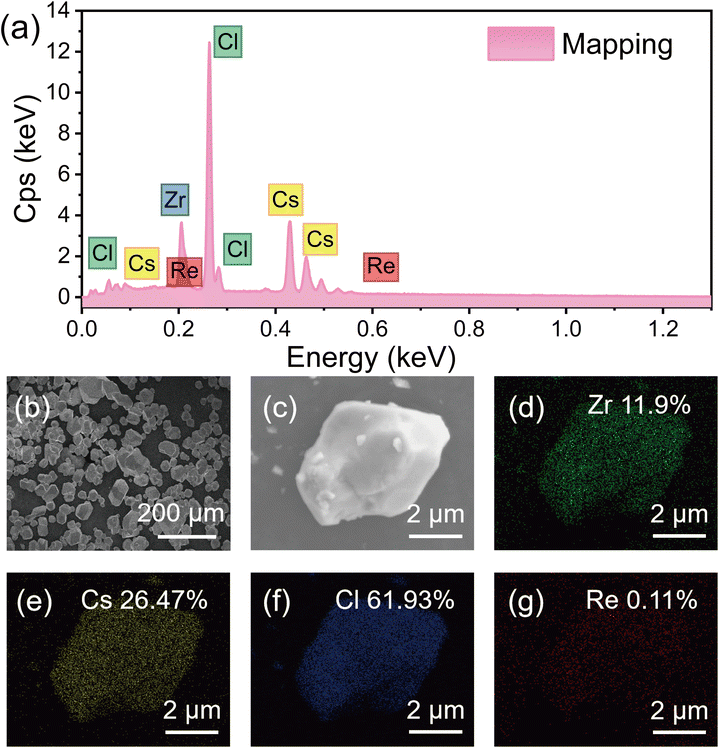 | ||
| Fig. 2 (a) The EDS spectrum and (b)–(g) the elemental mapping analysis of the Cs2ZrCl6:0.1% Re4+ sample. | ||
3.2 Photoluminescence studies
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 822 cm−1) and a triplet-type microsecond lifetime (14.62 μs) as shown in Fig. S2(a).†
822 cm−1) and a triplet-type microsecond lifetime (14.62 μs) as shown in Fig. S2(a).†
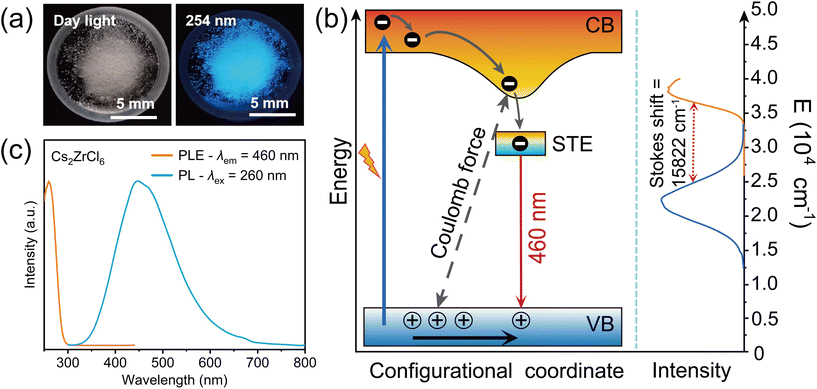
In contrast to the thermal quenching effects of common luminescent materials, the PL results of this phosphor exhibit characteristics different from those of common thermally quenched materials. The STE emission with spin singlet and triplet features is strongly influenced by the surrounding lattices and temperature. For example, in the temperature-dependent PL spectra of Cs2SnCl6:Sb3+,32 as the temperature increases from 80 K to room temperature, the emission band at 490 nm broadens due to spin–orbit coupling (SOC) perturbation and ISC processes and quenched at higher temperature. In this work, as shown in Fig. 4(a) and (b), the temperature-dependent PL spectra demonstrate that under excitation at 250 nm and 255 nm, the emission peak intensity decreases with increasing temperature; however, under excitation at 270 nm and 275 nm, Cs2ZrCl6 exhibits unusual anti-thermal quenching phenomena, wherein the emission peak intensity increases with temperature (as shown in Fig. 4(c) and (d)). Similar characteristics are also reflected in the temperature-dependent luminescence spectra of Cs2ZrCl6 nanocrystals reported by Han et al., attributed to the thermally activated delayed fluorescence mechanism, partially consistent with the experimental phenomena of this work.8,9 However, the anomalous anti-thermal quenching characteristics cannot be attributed solely to the thermally activated up-conversion process from the triplet to the singlet state, although the blue shift and shortening of the PL spectra from low to high temperatures reflect the presence of re-population processes induced by thermal activation.33,34 It can be observed from Fig. S2(a)–(c)† that the temperature-dependent lifetimes decrease with increasing temperature, and the quantum efficiency of 63.4% at room temperature indicates that the role of non-radiative channels induced by temperature tends to be enhanced, which often leads to luminescence quenching, contrary to the results of anti-thermal quenching of the measured results in Fig. 4(c) and (d). Therefore, the strong electron–phonon coupling effect on the temperature-sensitive spectral characteristics deserves more attention.35
Fig. 4(e) depicts the trend of PL intensity and full width at half maximum (FWHM) with temperature variation at different excitation wavelengths. It can be observed that the FWHM does not change significantly at different excitation wavelengths, indicating a relatively consistent luminescence origin. However, with increasing temperature, the FWHM significantly broadens, attributed to enhanced lattice vibrations with increasing temperature, resulting in spectral broadening. The relationship between the FWHM and temperature can be well fitted using the Huang–Rhys factor:36
 | (2) |
The temperature-dependent PLE spectra shown in Fig. 4(f) further reflect the results of this strong electron–phonon coupling. Prior to the excitation wavelength of 255 nm, the intensity of the PLE spectrum decreases with increasing temperature, while within the effective excitation range from 255 nm to 280 nm, the intensity of the PLE increases. This reflects the phenomenon of spectral broadening caused by enhanced anti-Stokes thermal repopulation near the absorption tail.25,38 Therefore, the temperature-dependent excitation spectrum provides solid and primary experimental evidence for the temperature dependent PL characteristics determined by the excitation wavelength in this case, precisely due to the effective excitation at the low-energy tail with the broadening of hot-band absorption due to thermal vibration, resulting in the enhancement of anti-thermal quenching of the PL intensity.
 | ||
| Fig. 6 (a) DSR spectra of Cs2ZrCl6:xRe4+. (b) The selected PL spectra of Cs2ZrCl6:3%Re4+ at 80 K, 200K and 300 K, respectively. | ||
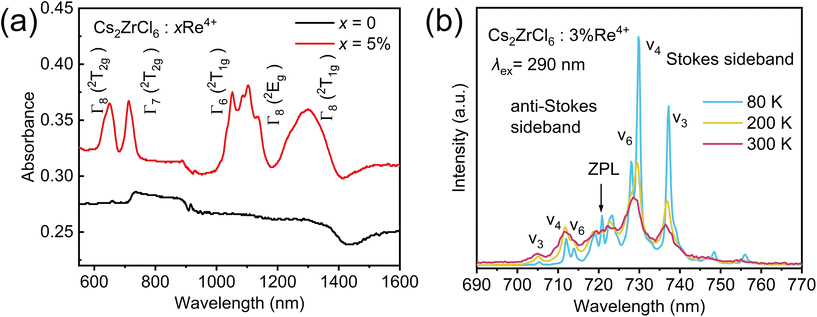
The temperature-dependent PL spectra of the Re4+ Γ7 (2T2g) → 4A2g transition shown in Fig. 6(b) further illustrats the vibrational transition details of the sub-excited states. From the shape of the lines, this transition exhibits remarkable similarity to the characteristic 2Eg → 4A2g transition of the well-known K2SiF6:Mn4+46 or the R-lines of ruby47 with abundant vibrational features. At low temperature (80 K), very clear details of the vibrational transition can be observed, mainly characterized by sharp multiple emission peaks formed by sharp anti-Stokes vibration bands at 723 nm (ZPL) and Stokes vibration bands at 729 nm (ν6), 737 nm (ν4), and 748 nm (ν3), as well as 704 nm (ν3), 712 nm (ν4), and 720 nm (ν6).48 As the temperature increases, the anti-Stokes vibrational band increases in intensity with increasing temperature, while the Stokes vibration band decreases in intensity with increasing temperature, accompanied by broadening of the lines. The anti-Stokes and Stokes vibrations can be regarded as two coupled vibration levels, and their luminescence intensities exhibit temperature-dependent relationships consistent with the Boltzmann distribution, aligning with the PL–temperature relationship of the 2Eg → 4A2g transition of Mn4+ doped Cs2WO2F4, indicating its potential application value in the field of temperature sensing.49
As shown in Fig. 7(a) and Fig. S4,† the PLE and PL intensity of pure Cs2ZrCl6 gradually decrease with increasing Re4+ doping concentration. Observing the PLE spectra of Cs2ZrCl6 STE (Fig. S4(a)†) and Re4+ as a function of concentration (Fig. 7(b)), it can be noted that with increasing Re4+ concentration, the PLE spectrum of Re4+ widens to some extent, while the peak position of the PL spectra remains relatively unchanged, but the PL intensity of Re4+ gradually increases. Cs2ZrCl6:3%Re4+ microcrystals achieve the optimal near-infrared PL emission intensity (Fig. S4(b)†), indicating the existence of an energy transfer process between the STE excited state level and the Γ7 (2T2g) level of Re4+. Benefiting from the enhanced effect of energy transfer, the test results in Fig. 7(c) show that the PLQY value obtained for Cs2ZrCl6 doped with 0.5% Re4+ (76.53%) under 260 nm excitation is higher than that of the pure host (63.4%) and the Re4+ emission under 290 nm excitation (Fig. S5†). The schematic diagram of energy transfer between the STE and 5d3 electron levels in Fig. 7(d) briefly describes such a process. Further increasing the doping level of Re4+ ions will result in a reduction in the concentration quenching effect due to strong dipole–dipole interactions. The decay curve obtained by monitoring the 729 nm emission under excitation of a 290 nm pulsed xenon lamp is shown in Fig. S6,† and the lifetime fitting can be performed using the following formula:
| I(t) = Be−t/τ | (3) |
The energy transfer efficiency ηT from STE to Cs2ZrCl6:Re4+ phosphors can be expressed through the following equation:
 | (4) |
 | (5) |
LIR = A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−ΔE/kT) + C exp(−ΔE/kT) + C | (6) |
 | (7) |
 | (8) |
 | ||
| Fig. 8 (a) The normalized temperature dependent PL spectra in the range of 80 K to 290 K. (b) The decay curves of Cs2ZrCl6:3%Re4+ at 80 K–290 K. | ||
Within the temperature range of 80–290 K, the fitting results of the LIR and sensitivity are shown in Fig. 9(b), with a maximum Sa max of 0.268% K−1 at 125 K and a maximum Sr max of 0.713% K−1 at 95 K. The sensitivity decreases with increasing temperature, demonstrating that the LIR mode of the Ianti-S/IS value based on Re4+ emission exhibits high sensitivity at low temperatures.
Furthermore, Fig. 8(b) shows the significant thermal quenching of the lifetime of Re4+ emission; thus an optical thermometer based on the lifetime of Re4+ can be obtained, with a temperature range between 80 K and 290 K. As shown in Fig. 9(c), at 80 K, the lifetime of Cs2ZrCl6:3%Re4+ is 104.94 μs, which shortens to 46.12 μs at 290 K. The relationship between lifetime and temperature can be expressed as follows:54
 | (9) |
 | (10) |
 | (11) |
The simulated results in Fig. 9(d) show that the Sa max reaches 0.42% K−1 at 123 K and the Sr max reaches 0.47% K−1 at 110 K, which is comparable to the LIR model of Ianti-S/IS.
To delve deeper into enhancing the sensitivity of optical thermometry, as depicted in Fig. 10(a), within the temperature range of 80–350 K under 260 nm excitation, both the STE and Γ7(2T2g) → 4A2g transitions exhibit comparable intensities. The STE emission band of the host Cs2ZrCl6 (350 nm–680 nm) gradually amplifies with rising temperature, yet experiencing a decline above 350 K due to thermal quenching. Additionally, the integrated intensity of Re4+ undergoes thermal quenching with increasing temperature, resulting in a reduction in PL emission intensity. This combines the negative thermal quenching effect in STE induced by the hot absorption band of Cs2ZrCl6 with the significant thermal quenching phenomenon of Re4+ itself. By considering the contrasting optical temperature behaviors of the two non-thermal coupling levels: ISTE/IRe4+, the optical thermometer manifests an optimum performance that can be approximately fitted using the LIR model (eqn (5) and Fig. 10(b)). The fitting parameter ΔE is determined to be 101.34 meV, with Sa max reaching 6.59% K−1 at 300 K and Sr max reaching 2.76% K−1 at 217 K (Fig. 10(c)), significantly enhanced compared with the LIR model of Ianti-S/IS and the decay model. In general, Sr max serves as a conventional parameter for comparing the temperature sensing capabilities of various luminescent materials. Table 1 illustrates the sensing performance of several dual-mode luminescent temperature sensors based on LIR and decay models. Notably, Cs2ZrCl6:Re4+ demonstrates outstanding dual-mode temperature sensing performance, boasting a high Sr max of 2.76% K−1 at 217 K. In addition, as a material for temperature sensing, the stability of Cs2ZrCl6:Re4+ should be further characterized. PL spectral thermal stability experiments were conducted using liquid nitrogen and a heating device to monitor the PL intensity variations of Cs2ZrCl6:3%Re4+ during heating–cooling cycles, as depicted in Fig. S8.† The samples exhibited stable PL emission across three cycles, indicating excellent thermal stability.
 | ||
| Fig. 10 (a) PL spectra and the LIR (ISTE/IRe4+) fitting result (b). (c) The calculated Sr and Sa as a function of temperature. | ||
| Optical thermometer | Model | S r max, % K−1 | S a max, % K−1 | Ref. |
|---|---|---|---|---|
| [N(NH3)4]2TiF6:Mn4+ | LIR | 2.47@100 K | — | 56 |
| Lifetime | 0.41@133 K | — | ||
| Ca2MgWO6:Er3+/Yb3+ | LIR | 0.92 @ 303 K | 0.82@453 K | 57 |
| Lifetime | 0.11@573 K | — | ||
| Cs2NaInCl6:Sb3+/Er3+ | LIR | 1.33@460 K | 2.27@434 K | 58 |
| Lifetime | 0.44@300 K | — | ||
| ZrO2:Eu3+ | LIR | 1.8@293 K | — | 59 |
| Lifetime | 0.33@573 K | — | ||
| Na2NbOF5:Mn4+ | LIR | 0.19@348 K | 0.0041@423 K | 60 |
| Lifetime | 5.36@448 K | — | ||
| Cs2NaErCl6:Yb3+ | LIR | 1.48@280 K | — | 61 |
| Lifetime | 0.72@440 K | — | ||
| Cs2ZrCl6:Re4+ | LIR@STE/Re4+ | 2.76@217 K | 6.59@300 K | This work |
| LIR@Re4+ | 0.71@95 K | 0.268@125 K | ||
| Lifetime@Re4+ | 0.47@123 K | 0.43@110 K |
Fig. 11(a) displays the X-ray radiation luminescence (RL) spectra of Cs2ZrCl6, Cs2ZrCl6:3%Re4+, and Cs2ZrCl6:5%Re4+ under high-energy excitation. All three samples exhibit an effective response to high-energy X-rays, suggesting their potential applications in X-ray radiation detection. Fig. 11(b) showcases the PL spectra of Cs2ZrCl6:3%Re4+ under excitation from a commercial 280 nm LED chip. Utilizing the excellent near-infrared emission characteristics of Re4+, the NIR-LED chips prepared using PDMS gel and a curing agent are depicted in Fig. 11(c), revealing a near-infrared emission spectrum consistent with the characteristic peaks of Re4+. Driven with 8 V and 20 mA, the LED emits bright near-infrared light. Under low-light conditions, the LED incorporating Cs2ZrCl6:Re4+ on a commercial chip effectively illuminates objects in a dark room, distinctly revealing object patterns and details. Additionally, Cs2ZrCl6:Re4+ exhibits significant potential in near-infrared imaging for penetrating the human body. Fig. 11(c) illustrates the visualization of blood vessel distribution on the human upper arm captured from a distance of 20 cm, clearly discernible under NIR-LED illumination. These experiments underscore the advantages of Cs2ZrCl6:Re4+ in X-ray detection and near-infrared imaging applications.
4. Conclusion
In this study, successful doping of Re4+ ions into a pure-phase Cs2ZrCl6 perovskite with ordered vacancies was achieved using a low-temperature hydrothermal synthesis method. Temperature-dependent PL and PLE studies on the Cs2ZrCl6 matrix revealed that different excitation wavelengths in the UV range (250–300 nm) can induce both thermal quenching and anti-thermal quenching effects on the STE PL of the Cs2ZrCl6 matrix. These effects are attributed to the enhancement of non-radiative relaxation with increasing temperature and the strengthening of hot absorption bands induced by thermal activation, respectively. Re4+ doped Cs2ZrCl6 exhibits dual peak emissions at near-infrared wavelengths (729 nm and 1340 nm) corresponding to Γ7 (2T2g) → 4A2g (729 nm) and Γ8 (2T1g) → 4A2g (1340 nm), respectively. Analysis using the Tanabe–Sugano diagram suggests a 5d3 electron configuration for ReCl62− under octahedral coordination. Energy transfer processes between the highly excited states of Re4+ ions and STE levels lead to a significant enhancement in PLQY for Cs2ZrCl6:0.5% Re4+ (76.53%) compared to the pure host PLQY (63.4%). By exploiting the anti-thermal quenching and thermal quenching relationships between STE and Γ7 (2T2g) levels, an optical thermometer with high sensitivity was developed, exhibiting a high Sr max of 2.76% K−1 at 217 K. Additionally, the efficient near-infrared emission properties of Re4+ can be excited by X-rays and 280 nm UV LEDs, enabling clear near-infrared vascular imaging and darkroom imaging, demonstrating the wide practical applicability of Re4+-doped Cs2ZrCl6 as an optoelectrical sensing and detection material.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The work was supported by the National Natural Science Foundation of China (11904345 and 52072355) and the Liu Zugang Expert Workstation of Yunnan Province under Grant No. 202205AF150076.References
- Q. Cui, D. Zhang, Y. Gao, C. Fan, Q. Cai, H. Li, X. Wu, M. Zhu, J. Si, X. Dai, H. He and Z. Ye, Controlling interfacial amidation reaction rate to regulate crystal growth toward High-Performance FAPbBr3-based inverted light-emitting diodes, ACS Nano, 2024, 18, 10609–10617, DOI:10.1021/acsnano.4c00639
.
- R. Xu, D. Zhang, J. Si, Y. Du, Q. Hu, X. Hao, H. Zhao, P. Cai, Q. Ai, X. Yao, Y. Gao, M. Zhu, Z. Zhang, M. Cai, H. W. Mo, K. Harada, Z. Ye, X. Dai, C. Adachi and Z. Liu, Low-Voltage driving copper Iodide-Based broadband electroluminescence, ACS Energy Lett., 2022, 7, 4408–4416, DOI:10.1021/acsenergylett.2c02471
.
- J. Song, T. Kong, Y. Zhang, X. Liu, M. Saliba and D. Bi, Synergistic effect of sodium cyanoborohydride in Pb-Sn perovskite solar cells, Adv. Funct. Mater., 2023, 33, 2304201, DOI:10.1002/adfm.202304201
.
- Y. Liu, C. Gao, D. Li, X. Zhang, J. Zhu, M. Wu, W. Liu, T. Shi, X. He and J. Wang, Dynamic X-ray imaging with screen-printed perovskite CMOS array, Nat. Commun., 2024, 15, 1588, DOI:10.1038/s41467-024-45871-2
.
- N. Li, X. Niu, Q. Chen and H. Zhou, Towards commercialization: the operational stability of perovskite solar cells, Chem. Soc. Rev., 2020, 49, 8235–8286, 10.1039/d0cs00573h
.
- M. Ren, X. Qian, Y. Chen, T. Wang and Y. Zhao, Potential lead toxicity and leakage issues on lead halide perovskite photovoltaics, J. Hazard. Mater., 2022, 426, 127848, DOI:10.1016/j.jhazmat.2021.127848
.
- H. Wei, Q. Yang, G. Li, X. Liu, J. Huang, C. Wang, X. Li and G. Cai, InCl3-Assisted surface defects restoring to enhance lead–free Cs2ZrCl6 nanocrystals for X–ray imaging and blue LED applications, Small, 2024, 2309926, DOI:10.1002/smll.202309926
.
- S. Liu, B. Yang, J. Chen, D. Wei, D. Zheng, Q. Kong, W. Deng and K. Han, Efficient thermally activated delayed fluorescence from all–inorganic cesium zirconium halide perovskite nanocrystals, Angew. Chem., 2020, 132, 22109–22113, DOI:10.1002/ange.202009101
.
- F. Zhang, Y. Zhou, Z. Chen, M. Wang, Z. Ma, X. Chen, M. Jia, D. Wu, J. Xiao and X. Li, Thermally activated delayed fluorescence zirconium-based perovskites for large-area and ultraflexible X-ray scintillator screens, Adv. Mater., 2022, 34, 2204801, DOI:10.1002/adma.202204801
.
- Y. Liu, Y. Wu, Z. Juan, X. Sun, W. Zhang, H. Zeng and X. Li, Efficient, stable, and tunable cold/warm white light from lead-free halide double perovskites Cs2Zr1-xTexCl6, Adv. Opt. Mater., 2021, 9, 2100815, DOI:10.1002/adom.202100815
.
- M. C. Folgueras, Y. Jiang, J. Jin and P. Yang, High-entropy halide perovskite single crystals stabilized by mild chemistry, Nature, 2023, 621, 282–288, DOI:10.1038/s41586-023-06396-8
.
- M. Liu, C.-K. Duan, P. A. Tanner, C.-G. Ma, X. Wei and M. Yin, Understanding photoluminescence of Cs2 ZrCl6 doped with post-transition-metal ions using first-principles calculations, Phys. Rev. B, 2022, 105, 195137, DOI:10.1103/PhysRevB.105.195137
.
- V. Mykhaylyk, S. Nagorny, V. Nahorna, P. Wang, M. Frogley, L. Swiderski, V. Kolomiets and L. Vasylechko, Growth, structure, and temperature dependent emission processes in emerging metal hexachloride scintillators Cs2HfCl6 and Cs2ZrCl6, Dalton Trans., 2022, 51, 6944–6954, 10.1039/D2DT00223J
.
- X. Jia, B. Wang, M. Zhu, Y. Zhang, X. Chong, J. Li and J. Wang, Growth process and optical properties of Cs2ZrCl6 doped with Ce3+ and Li+ crystals, Cryst. Growth Des., 2023, 23, 3589–3594, DOI:10.1021/acs.cgd.3c00073
.
- C. Fang, J. Yang, G. Zhou, Z. Zhang, Y. Mao, X. Yun, L. Liu, D. Xu, X. Li and J. Zhou, Energy transfer from self-trapped excitons to rare earth ions in Cs2ZrCl6 perovskite variants, J. Mater. Chem. C, 2023, 11, 1095–1102, 10.1039/D2TC04657A
.
- S. Han, D. Tu, Z. Xie, Y. Zhang, J. Li, Y. Pei, J. Xu, Z. Gong and X. Chen, Unveiling local electronic structure of lanthanide-doped Cs2NaInCl6 double perovskites for realizing efficient near-infrared luminescence, Adv. Sci., 2022, 9, 2203735, DOI:10.1002/advs.202203735
.
- G. Li, X. Chen, M. Wang, S. Cheng, D. Yang, D. Wu, Y. Han, M. Jia, X. Li, Y. Zhang, C. Shan and Z. Shi, Regulating exciton de-trapping of Te4+-doped zero-dimensional scandium-halide perovskite for fluorescence thermometry with record high time-resolved thermal sensitivity, Adv. Mater., 2023, 35, 2305495, DOI:10.1002/adma.202305495
.
- J. Liu, Q. Hu, H. Xu, H. Yu, B. Du, Q. Han and W. Wu, Vacancy-ordered Te4+-doped Rb2ZrCl6 double perovskite microcrystals for solid-state lighting and non-contact optical thermometry, Appl. Phys. Lett., 2023, 123, 111903, DOI:10.1063/5.0165439
.
- B. Chen, Y. Guo, Y. Wang, Z. Liu, Q. Wei, S. Wang, A. L. Rogach, G. Xing, P. Shi and F. Wang, Multiexcitonic emission in zero-dimensional Cs2ZrCl6:Sb3+ perovskite crystals, J. Am. Chem. Soc., 2021, 143, 17599–17606, DOI:10.1021/jacs.1c07537
.
- Y. Tang, M. Deng, Z. Zhou, C. Kang, J. Wang and Q. Liu, Recent advances in lead-free Cs2ZrCl6 metal halide perovskites and their derivatives: from fundamentals to advanced applications, Coord. Chem. Rev., 2024, 499, 215490, DOI:10.1016/j.ccr.2023.215490
.
- X. Liu, P. Cai, Y. Huang, X. Pu, Q. Ai, J. Si, X. Yao, G. Bai and Z. Liu, Mn4+-doped organometallic hafnium hexafluoride phosphors: Achieving near-unity quantum yield to elevate LED performance, Ceram. Int., 2024, 50, 1239–1247, DOI:10.1016/j.ceramint.2023.10.217
.
- T. Wang, Y. Wang, W. Chen and Z. Xia, Crystallization of Na2SrGe6O14: Cr3+, Yb3+ glass ceramics enabling a watt-level output power NIR-I/NIR-II lighting source, Laser Photonics Rev., 2024, 18, 2300784, DOI:10.1002/lpor.202300784
.
- E. Song, H. Ming, Y. Zhou, F. He, J. Wu, Z. Xia and Q. Zhang, Cr3+-doped Sc-based fluoride enabling highly efficient near infrared luminescence: A case study of K2NaScF6: Cr3+, Laser Photonics Rev., 2021, 15, 2000410, DOI:10.1002/lpor.202000410
.
- S. Zhao, K. Sang, Y. Yang, J. Zhang, Y. Hu, M. Chen, X. Zhang, Q. Pang, L. Zhou and P. Chen, Visible TADF and NIR quenching emission of chromium activated zirconium-halide perovskite toward ultra-high-sensitive ratiometric fluorescent thermometer, Adv. Opt. Mater., 2024, 2302979, DOI:10.1002/adom.202302979
.
- D. R. Gamelin and H. U. Güdel, Spectroscopy and dynamics of Re4+ near-IR-to-visible luminescence upconversion, Inorg. Chem., 1999, 38, 5154–5164 CrossRef CAS PubMed
.
- P. B. Dorain and R. G. Wheeler, Optical Spectrum of Re4+ in single crystals of K2PtCl6 and Cs2ZrCl6 at 4.2° K, J. Chem. Phys., 1966, 45, 1172–1181, DOI:10.1063/1.1727734
.
- R. Wernicke and H.-H. Schmidtke, The luminescence spectrum of ReCl62− doped in various host lattices with antifluorite-type structure, Mol. Phys., 1979, 37, 607–622, DOI:10.1080/00268977900100481
.
- D. R. Gamelin and H. U. Güdel, Design of luminescent inorganic materials: new photophysical processes studied by optical spectroscopy, Acc. Chem. Res., 2000, 33, 235–242, DOI:10.1021/ar990102y
.
- W. Markus and U. G. Hans, Luminescence spectroscopy and NIR to VIS upconversion of Cs2GeF6: 2% Re4+, J. Phys.: Condens. Matter, 2001, 13, 9583, DOI:10.1088/0953-8984/13/42/317
.
- R. T. Shannon and C. T. Prewitt, Effective ionic radii in oxides and fluorides, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1969, 25, 925–946, DOI:10.1107/S0567740869003220
.
- J. Zhou, X. Rong, M. S. Molokeev, Y. Wang, X. Yun, D. Xu and X. Li, Alloying Cs+ into Rb2ZrCl6:Te 4+ toward highly efficient and stable perovskite variants, Mater. Chem. Front., 2021, 5, 4997–5003, 10.1039/D1QM00302J
.
- Y. Jing, Y. Liu, J. Zhao and Z. Xia, Sb3+ doping-induced triplet self-trapped excitons emission in lead-free Cs2SnCl6 nanocrystals, J. Phys. Chem. Lett., 2019, 10, 7439–7444, DOI:10.1021/acs.jpclett.9b03035
.
- H. Uoyama, K. Goushi, K. Shizu, H. Nomura and C. Adachi, Highly efficient organic light-emitting diodes from delayed fluorescence, Nature, 2012, 492, 234–238, DOI:10.1038/nature11687
.
- G. Blasse and D. R. McMillin, On the luminescence of bis (triphenylphosphine) phenanthroline copper(I), Chem. Phys. Lett., 1980, 70, 1–3, DOI:10.1016/0009-2614(80)80047-9
.
- S. Yan, Negative Thermal quenching of photoluminescence: an evaluation from the macroscopic viewpoint, Materials, 2024, 17, 586, DOI:10.3390/ma17030586
.
- Y. Zhan, P. Cai, X. Pu, Q. Ai, J. Si, X. Yao, G. Bai and Z. Liu, Exceptional optical performance of the zero-dimensional hybrid cuprous halide ETPA2Cu2I4 as an X-ray scintillator, Inorg. Chem. Front., 2024, 11, 579–588, 10.1039/D3QI02169F
.
- H. Zhao and H. Kalt, Energy-dependent huang-rhys factor of free excitons, Phys. Rev. B: Condens. Matter Mater. Phys., 2003, 68, 125309, DOI:10.1103/PhysRevB.68.125309
.
- O. Dimitriev, R. Yamakado, A. Nagaoka, M. Giancaspro, F. Rizzi, N. Depalo, E. Fanizza, L. Yoshida, A. Kosar and T. Yoshida, Plasmon enhancement of the hot-band absorption-assisted anti-stokes photoluminescence of near-infrared dyes, J. Phys. Chem. C, 2023, 127, 20620–20631, DOI:10.1021/acs.jpcc.3c05695
.
- J. C. Collingwood, S. Piepho, R. Schwartz, P. Dobosh, J. Dickinson and P. Schatz, The optical absorption and magnetic circular dichroism spectra of Re4+ in the cubic hosts, Cs2ZrCl6, Cs2NaYCl6, and Cs2ZrBr6, Mol. Phys., 1975, 29, 793–814, DOI:10.1080/00268977500100711
.
- A. Reinberg and S. Parker, Sharp-line luminescence of Re4+ in cubic single crystals of Cs2ZrCl6 and Cs2HfCl6, Phys. Rev. B: Solid State, 1970, 1, 2085, DOI:10.1103/PhysRevB.1.2085
.
- D. R. Gamelin and H. U. Güdel, Two-photon spectroscopy of d3 transition metals: near-IR-to-visible upconversion luminescence by Re4+ and Mo3+, J. Am. Chem. Soc., 1998, 120, 12143–12144, DOI:10.1021/ja982742m
.
- P. Cai, L. Qin, C. Chen, J. Wang, S. Bi, S. I. Kim, Y. Huang and H. J. Seo, Optical thermometry based on vibration sidebands in Y2MgTiO6:Mn4+ double perovskite, Inorg. Chem., 2018, 57, 3073–3081, DOI:10.1021/acs.inorgchem.7b02938
.
- S. Adachi, Racah parameter ratio C/B for the 3d3-configuration ions like Mn4+ and Cr3+ in the tanabe-sugano diagram, ECS J. Solid State Sci. Technol., 2020, 9, 066003, DOI:10.1149/2162-8777/aba679
.
- B. N. Figgis, J. Lewis, R. S. Nyholm and R. D. Peacock, The magnetic properties of some d, d and d configurations, Discuss. Faraday Soc., 1958, 26, 103–109, 10.1039/DF9582600103
.
- M. Kasha, Characterization of electronic transitions in complex molecules, Discuss. Faraday Soc., 1950, 9, 14–19, 10.1039/DF9500900014
.
- R. Kasa and S. Adachi, Red and deep red emissions from cubic K2SiF6: Mn4+ and hexagonal K2MnF6 synthesized in HF/KMnO4/KHF2/Si solutions, J. Electrochem. Soc., 2012, 159, J89, DOI:10.1149/2.005204jes
.
- S. P. Feofilov and A. B. Kulinkin, On the possibility of laser cooling of Cr3+ ions doped crystals, Opt. Mater., 2018, 75, 554–560, DOI:10.1016/j.optmat.2017.11.002
.
- R. Wernicke and H. H. Schmidtke, Low symmetry splittings due to phase transitions in the vibronic spectrum of ReCl62− and ReBr62− doped in K2PtCl6-type crystals, J. Chem. Phys., 1980, 72, 1938–1944, DOI:10.1063/1.439339
.
- P. Cai, L. Qin, C. Chen, J. Wang and H. J. Seo, Luminescence, energy transfer and optical thermometry of a narrow red emitting phosphor: Cs2WO2F4: Mn4+, Dalton Trans., 2017, 14331–14340, 10.1039/C7DT02751F
.
- J. Zhou, X. Yun, R. Wang, D. Xu and X. Li, Self-trapped exciton to dopant energy transfer in Sb3+-doped Cs2ZrCl6 perovskite variants, Mater. Chem. Front., 2021, 5, 6133–6138, 10.1039/D1QM00697E
.
- P. A. Tanner, L. Zhou, C. Duan and K.-L. Wong, Misconceptions in electronic energy transfer: bridging the gap between chemistry and physics, Chem. Soc. Rev., 2018, 47, 5234–5265, 10.1039/C8CS00002F
.
- Q. Zeng, M. Runowski, J. Xue, L. Luo, L. Marciniak, V. Lavín and P. Du, Pressure-induced remarkable spectral red-shift in Mn2+-activated NaY9(SiO4)6O2 red-emitting phosphors for high-sensitive optical manometry, Adv. Sci., 2024, 11, 2308221, DOI:10.1002/advs.202308221
.
- L. Li, G. Tian, Y. Deng, Y. Wang, Z. Cao, F. Ling, Y. Li, S. Jiang, G. Xiang and X. Zhou, Constructing ultra-sensitive dual-mode optical thermometers: utilizing FIR of Mn4+/Eu3+ and lifetime of Mn4+ based on double perovskite tellurite phosphor, Opt. Express, 2020, 28, 33747–33757, DOI:10.1364/OE.409242
.
- P. Cai, R. Teng, D. Zhang, S. Wang, Y. Zhan, X. Wang, J. Si, X. Yao, Q. Ai and Z. Liu, Self-trapped exciton luminescence and light-emitting-diodes based on zero-dimensional organic-inorganic hybrid antimony chlorideJ, Chin. J. Lumin., 2022, 43, 94–102, DOI:10.37188/CJL.20210318
.
- G. Li, G. Li, Q. Mao, L. Pei, H. Yu, M. Liu, L. Chu and J. Zhong, Efficient luminescence lifetime thermometry with enhanced Mn4+-activated BaLaCa1−xMgxSbO6 red phosphors, Chem. Eng. J., 2022, 430, 132923, DOI:10.1016/j.cej.2021.132923
.
- P. Cai, S. Wang, T. Xu, Y. Tang, X. Yuan, M. Wan, Q. Ai, J. Si, X. Yao and Y. Cao, Mn4+ doped zero-dimensional organic-inorganic hybrid material with narrow-red emission, J. Lumin., 2020, 228, 117661, DOI:10.1016/j.jlumin.2020.117661
.
- Y. Jiang, Y. Tong, S. Chen, W. Zhang, F. Hu, R. Wei and H. Guo, A three-mode self-referenced optical thermometry based on up-conversion luminescence of Ca2MgWO6: Er3+, Yb3+ phosphors, Chem. Eng. J., 2021, 413, 127470, DOI:10.1016/j.cej.2020.127470
.
- R. Song, S. Xu, Y. Li, Y. Gao, H. Yu, Y. Cao, X. Zhang and B. Chen, Designing multi-mode optical thermometers via Sb3+/Er3+ co-doped Cs2NaInCl6 lead-free double perovskite microcrystals, J. Alloys Compd., 2023, 961, 171126, DOI:10.1016/j.jallcom.2023.171126
.
- J. Zhou, R. Lei, H. Wang, Y. Hua, D. Li, Q. Yang, D. Deng and S. Xu, A new generation of dual-mode optical thermometry based on ZrO2: Eu3+ nanocrystals, Nanophotonics, 2019, 8, 2347–2358, DOI:10.1515/nanoph-2019-0359
.
- K. Chen, Z. Shao, C. Zhang, S. Jia, T. Deng, R. Zhou, Y. Zhou and E. Song, Illuminating the path to high-precision dual-mode optical thermometry: a Mn4+-activated oxyfluoride single crystal for a portable optical fiber thermometric platform, Chem. Eng. J., 2023, 477, 147165, DOI:10.1016/j.cej.2023.147165
.
- W. Chen, M. Jin, J. Tang, Y. Li, C. Chen, J. Xiang, Z. Li and C. Guo, Self-calibrated multi-mode optical thermometry in up-converting phosphor Cs2NaErCl6: Yb3+, J. Lumin., 2023, 263, 120132, DOI:10.1016/j.jlumin.2023.120132
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4qi01336k |
| This journal is © the Partner Organisations 2024 |