Realizing efficient broadband near-infrared emission under blue light excitation in Sb3+-doped zero-dimensional organic tin(IV)-based metal halides via coordination structure modulation†
Bao
Ke
a,
Hui
Peng
 *b,
Yongqi
Yang
a,
Chengzhi
Yang
a,
Shangfei
Yao
a,
Arfan
Bukhtiar
*b,
Yongqi
Yang
a,
Chengzhi
Yang
a,
Shangfei
Yao
a,
Arfan
Bukhtiar
 a,
Qilin
Wei
*c,
Jialong
Zhao
ab and
Bingsuo
Zou
a,
Qilin
Wei
*c,
Jialong
Zhao
ab and
Bingsuo
Zou
 *ab
*ab
aSchool of Physical Science and Technology, Guangxi University, Nanning 530004, China. E-mail: zoubs@gxu.edu.cn
bState Key Laboratory of Featured Metal Materials and Life-cycle Safety for Composite Structures, and School of Resources, Environment and Materials, Guangxi University, Nanning 530004, China. E-mail: penghuimaterial@163.com
cSchool of Chemistry and Chemical Engineering, Shandong University, Jinan 250100, China. E-mail: qlwei@sdu.edu.cn
First published on 26th September 2024
Abstract
Realizing Sb3+-activated efficient broadband near-infrared (NIR) emission under blue light excitation remains a significant challenge in lead-free metal halides. To overcome the above difficulties, a coordination structure modulation strategy was adopted, and the broadband NIR emission under blue light excitation was achieved in Sb3+-doped zero-dimensional (0D) organic tin(IV) bromide. Compared to the weak visible light emission with a photoluminescence quantum yield (PLQY) of 2.4% for pure (TBP)2SbBr5 (TBP = tetrabutylphosphonium), Sb3+-doped (TBP)2SnBr6 exhibits an efficient broadband NIR emission band at 705 nm with a PLQY of 33.2% upon 452 nm excitation, which stems from self-trapped exciton emission. Combined with experiments and theoretical calculations, we find that the large excited-state lattice distortion degree compared to the ground state and the narrow bandgap are dominant reasons for Sb3+-doped (TBP)2SnBr6 showing efficient NIR emission under blue light excitation. Specifically, Sb3+-doped (TBP)2SnBr6 also has excellent anti-water stability, existing stably in water for more than 4 hours while maintaining a high luminous efficiency. Based on the excellent stability and unique optical properties of Sb3+-doped (TBP)2SnBr6, a high-performance NIR light-emitting diode (LED) was fabricated by combining Sb3+-doped (TBP)2SnBr6 with a commercial blue LED chip, and its application in night vision was demonstrated.
1. Introduction
Near-infrared (NIR) light sources are highly desired due to their low thermal effect, non-destructive nature, and deep tissue penetration, and are an ongoing pursuit for a variety of high-end applications such as night vision, food quality analysis, and biomedical imaging.1,2 In particular, broadband NIR sources in the 700–1100 nm range have received much attention because the interaction between infrared light and infrared-active molecular bonds in food and human tissues produces wide-response characteristic absorption signals in this spectral region.3 However, traditional NIR light sources, including halogen and tungsten lamps, are unsuitable for portable applications due to their large volumes and high working temperatures. Recently, NIR phosphor conversion LEDs (pc-LEDs), which combine broadband NIR phosphors with commercial blue LED chips, have sparked widespread attention because of their compact size, tunable broadband emission, and excellent efficiency. The NIR emitter, as an important component of NIR pc-LEDs, and its photophysical properties directly determine the performance of the device.4 Therefore, it is crucial to develop efficient broadband NIR luminescent materials with excellent stability under blue light excitation.Currently, many broadband NIR emitters have been reported, mainly focusing on the study of metal-ion doped oxides, such as Cr3+-doped Ga2−xScxO3,5 Eu2+-doped K3LuSi2O7,6 and Bi3+-doped BaAl12O19.7 However, these oxide phosphors have a high lattice energy and require high-temperature (>1000 °C) calcination to obtain the target products. Obviously, high-temperature synthesis not only results in serious energy consumption but also poses a series of safety risks in the preparation process. The above shortcomings drive us to develop efficient broadband NIR luminescent materials that can be synthesized at low temperatures. Low-dimensional lead-free metal halides have drawn much attention because of their simple preparation method, low toxicity, and adjustable optical properties. Parallelly, they generally exhibit broadband self-trapped exciton (STE) emission due to lattice distortion and strong electron–phonon coupling. Thus, this provides an opportunity for the development of a new generation of NIR luminescent materials.
Recently, some lead-free metal halides with broadband NIR emission have been developed. For instance, Wang et al. synthesized Sn2+-doped all-inorganic 0D Cs2ZnBr4, which exhibits broadband NIR emission at 700 nm with a photoluminescence quantum yield (PLQY) of 40%.8 Moreover, Yang et al. reported Cu(I)-based metal halides with highly efficient broadband NIR emission.9 However, the poor stability seriously limits their application in high-performance NIR light sources. In contrast, Sb3+-activated lead-free metal halides with NIR emission have attracted wide attention due to their high efficiency and remarkable stability. For example, Sb3+-doped Cs2ZnCl4 emits a broadband NIR emission band at 745 nm under 316 nm excitation,10 and Sb3+-doped (NH4)2SnCl6 exhibits an NIR emission band at 736 nm under 360 nm excitation.11 Although the aforementioned Sb3+-activated NIR emitters exhibit excellent optical properties, their excitation bands are limited to the ultraviolet (UV) region, which is difficult to match with commercial blue LED chips.
To address the above concerns, we report herein a new NIR emitter of Sb3+-doped tin(IV)-based metal halides via coordination structure modulation, which can exhibit bright broadband NIR emission under blue light excitation. Compared to the Sb(III)-based metal halide of (TBP)2SbBr5 with weak red emission (PLQY ∼ 2.4%), Sb3+-doped (TBP)2SnBr6 exhibits a broadband NIR emission band at 705 nm with a PLQY of 33.2% under blue light excitation. Combined with experiments and theoretical calculations, we find that the large excited-state lattice distortion degree compared to the ground state and the narrow bandgap are the dominant reasons for Sb3+-doped (TBP)2SnBr6 showing efficient NIR emission under blue light excitation. Finally, given the fascinating optical properties as well as the remarkable air and anti-water stability of Sb3+-doped (TBP)2SnBr6, a high-performance NIR pc-LED was fabricated by combining a commercial 460 nm blue LED chip with Sb3+-doped (TBP)2SnBr6 phosphors, and we further demonstrated its application in night vision. Therefore, this work provides a feasible approach for designing efficient broadband NIR light sources based on lead-free metal halides.
2. Experimental section
Materials
Tetrabutylphosphonium bromide (TBPBr, 98%), tin(IV) bromide (SnBr4, 99.0%), antimony(III) bromide (SbBr3, 99%), acetonitrile (99.5%), N,N-dimethylformamide (DMF, 99.9%), and ethyl ether (Et2O, 99.5%) were purchased from Aladdin Reagent and all materials were used without any purification.Growth of (TBP)2SbBr5 single crystals (SCs)
(TBP)2SbBr5 SCs were synthesized by the slow evaporation method. Typically, 0.3615 g of SbBr3 and 0.6786 g of TBPBr were dissolved in 5 mL of acetonitrile to form a transparent solution. Afterwards, the solution was evaporated in ambient air at 40 °C for about two days, and the bulk (TBP)2SbBr5 SCs can be obtained.Synthesis of undoped and Sb3+-doped (TBP)2SnBr6 single crystals
(TBP)2SnBr6 SCs were synthesized using the antisolvent recrystallisation method. Typically, 0.4383 g of SnBr4 and 0.6786 g of TBPBr were dissolved in DMF to form a transparent solution. The (TBP)2SnBr6 SCs can then be harvested by diffusing Et2O into the precursor solution at room temperature. In order to synthesize the Sb3+-doped (TBP)2SnBr6 SCs, only x mmol SbBr3 was used under otherwise identical conditions.Characterization
The crystal structures of the two compounds were determined through single-crystal X-ray diffraction (SCXRD, IPDS II-STOE). The crystallographic data of (TBP)2SbBr5 and (TBP)2SnBr6 can be obtained for free from the Cambridge Crystallographic Data Centre (CCDC) with accession numbers 2382097 and 2382098.† Powder XRD (PXRD) measurements were acquired via a Bruker D8 Discover X-ray diffractometer. The PL and PLE spectra, temperature-dependent PL spectra, time-resolved photoluminescence (TRPL) spectra and PLQY were recorded using a Horiba Jobin Yvon Fluorolog-4 spectrometer. The Raman and power-dependent PL spectra were obtained using a WITec alpha300R instrument with 532 and 405 nm lasers, respectively. UV-Vis absorption spectra were recorded using a Shimadzu UV3600 Plus instrument. The morphology was observed via a Hitachi SU8020 scanning electron microscope (SEM), and an energy-dispersive spectrometer (EDS, Oxford X-Max Aztec) was employed to determine the composition and distribution of elements. X-ray photoelectron spectroscopy (XPS) measurements were performed using a Thermo Fisher ESCALAB 250Xi. Thermal stability was measured using a Shimadzu DTG-60H instrument in a nitrogen environment with a heating rate of 15 °C min−1.Calculation methods
The Vienna ab initio simulation package (VASP) was used for all calculations.12 For the exchange and correlation functionals, the Perdew–Burke–Ernzerhof (PBE) parameterization was approximated using a generalized gradient method through the use of the projector-augmented wave method.13,14 For the wavefunction basis set, a 4 × 4 × 3 Monkhorst–Pack k-mesh for (TBP)2SbBr5 and 4 × 3 × 3 for (TBP)2SnBr6, and a 500 eV kinetic energy cutoff were used. For each element, ultrasoft pseudopotentials were employed. For structural relaxations, the energy convergence threshold was established at 1.0 × 10−5 eV. To calculate the lattice distortion of the excited state structure, the crystal structure of the excited state must first be obtained. The detailed process is as follows: first, the ground state structure is obtained through a standard VASP optimization and relaxation process. Then, the EIGENVAL file from the static calculation is read to examine the occupation of relevant electrons. By adding FERWE and FERDO to control the spin occupation of electrons, the excited state structure is relaxed. Finally, using eqn (1), the bond lengths of the excited state structure are analyzed to determine the degree of distortion in the excited state. | (1) |
NIR pc-LED fabrication
The as-synthesized Sb3+-doped (TBP)2SnBr6 SCs were ground into powders, then mixed in AB glue to form a uniform mixture, and finally coated onto a 460 nm LED chip. Subsequently, the performance parameters of the as-fabricated NIR pc-LED were measured using an Everfine HAAS-2000 instrument.3. Results and discussion
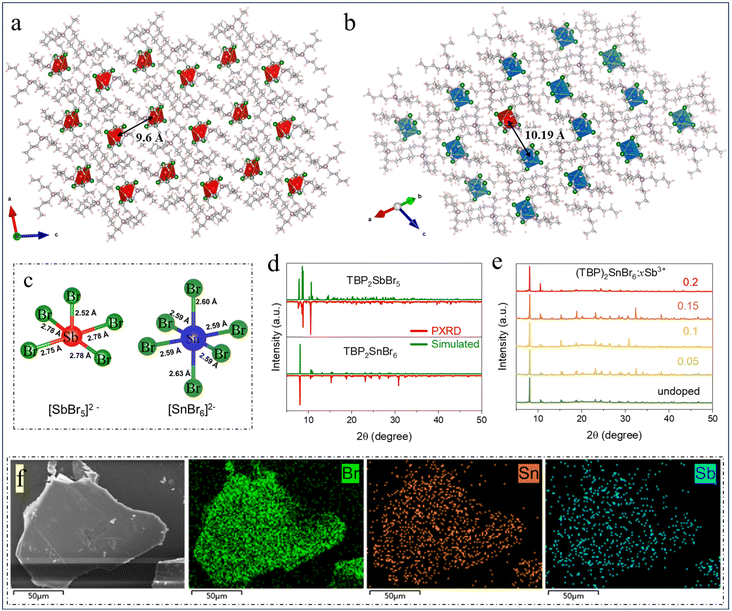
Two bulk assemblies of 0D organic metal halides of (TBP)2SbBr5 and (TBP)2SnBr6 with various coordination structures were synthesized via a simple solution method at room temperature (RT), and their crystal structures were determined by single-crystal X-ray diffraction (SCXRD). As shown in Fig. 1a and b, the crystallographic units of the two as-synthesized compounds both contain two TBP+ cations and a different inorganic polyhedron, that is, square-pyramidal [SbBr5]2− clusters for (TBP)2SbBr5 and octahedron-shaped [SnBr6]2− clusters for (TBP)2SnBr6. The isolated polyhedra are separated from each other and periodically embedded in the framework of organic cations in both compounds, thus constituting a typical 0D structure. Moreover, (TBP)2SbBr5 crystallizes in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , while (TBP)2SnBr6 adopts the monoclinic space group C2/c. As shown in Fig. 1c, the distance of Sb–Br drops in the range of 2.52–2.78 Å in the [SbBr5]2− unit of (TBP)2SbBr5, while that of Sn–Br ranges from 2.59 to 2.63 Å in the [SnBr6]2− octahedron of (TBP)2SnBr6. More detailed crystallographic data of (TBP)2SbBr5 and (TBP)2SnBr6 are summarized in Tables S1–S3,† and their different crystal structure will bring different optical properties. In particular, there are large M–M (M = Sn and Sb) distances >9.6 Å in both compounds, which indicates that there is a negligible interaction between adjacent inorganic polyhedra.15Fig. 1d shows the PXRD measurements of (TBP)2SbBr5 and (TBP)2SnBr6, and the diffraction patterns were consistent with the simulated ones, which demonstrates the dependability of SCXRD results.
, while (TBP)2SnBr6 adopts the monoclinic space group C2/c. As shown in Fig. 1c, the distance of Sb–Br drops in the range of 2.52–2.78 Å in the [SbBr5]2− unit of (TBP)2SbBr5, while that of Sn–Br ranges from 2.59 to 2.63 Å in the [SnBr6]2− octahedron of (TBP)2SnBr6. More detailed crystallographic data of (TBP)2SbBr5 and (TBP)2SnBr6 are summarized in Tables S1–S3,† and their different crystal structure will bring different optical properties. In particular, there are large M–M (M = Sn and Sb) distances >9.6 Å in both compounds, which indicates that there is a negligible interaction between adjacent inorganic polyhedra.15Fig. 1d shows the PXRD measurements of (TBP)2SbBr5 and (TBP)2SnBr6, and the diffraction patterns were consistent with the simulated ones, which demonstrates the dependability of SCXRD results.
Then, a series of Sb3+-doped (TBP)2SnBr6 with various doping concentrations were synthesized. The structures of Sb3+-doped (TBP)2SnBr6 are given in Fig. 1b. Clearly, Sb3+ ions can be substituted for Sn4+ sites due to their similar ion radii (CN = 6, rSb3+ = 76 pm and rSn4+ = 69 pm) and exist in the form of the [SbBr6]3− octahedron in the Sb3+-doped (TBP)2SnBr6 compound. The PXRD patterns of Sb3+-doped (TBP)2SnBr6 with various doping concentrations are similar to that of the pristine (TBP)2SnBr6, indicating that the doping of Sb3+ does not change the crystal structure of the host matrix (Fig. 1e). Table S4† shows the EDS results of Sb3+-doped (TBP)2SnBr6, and it can be seen that the doping concentration of Sb3+ is much lower than the feed content. Fig. S1† shows the high-resolution X-ray photoelectron spectroscopy (HRXPS) spectra of Sb 3d. Compared with (TBP)2SbBr5, the characteristic peaks of Sb3+ 3d5/2 and Sb3+ 3d3/2 in Sb3+-doped (TBP)2SnBr6 shift towards a lower binding energy (0.3 eV), which should be due to Sb3+ ions having different coordination chemical environments in the above two compounds. The weak signal-to-noise ratio of Sb3+ in HRXPS indicates the low Sb3+ content, which echoes the EDS results well. Moreover, the SEM images of these two compounds are given in Fig. 1f and S2,† and the elemental mapping shows the uniform distribution of Sn, Sb, and Br in (TBP)2SbBr5 and Sb3+-doped (TBP)2SnBr6. The morphology and size of single crystals are shown in Fig. S3;† both types of single crystals exhibit a yellow color under natural light, with sizes ranging from 2 to 10 mm. They display irregular block-like structures and have good transparency.
The optical properties of the as-synthesized compounds were measured at RT. For (TBP)2SnBr6, the UV-vis absorption spectrum showed an optical absorption edge at 468 nm, and the bandgap value was determined to be 2.78 eV through the Kubelka–Munk function (Fig. S4†). Moreover, (TBP)2SnBr6 is a yellow crystal in natural light and shows very weak PL emission under photoexcitation (Fig. S3a†). The PL and PLE spectra of (TBP)2SnBr6 are given in Fig. S5.† Clearly, this compound exhibits a broadband emission band at 480 nm with a full width at half-maximum (FWHM) of 78 nm. Normally, there are three mechanisms for the single PL emission in metal halides apart from the influence of organic molecules, namely, permanent defects, free excitons, and STEs.11 We analyzed the possibility of a respective mechanism that governs the emission in (TBP)2SnBr6 as follows: (i) the optical properties of TBPBr were also measured (Fig. S6†), which are greatly different from those of (TBP)2SnBr6, thus excluding the influence of the organic ligand on the optical properties of (TBP)2SnBr6; (ii) we measured the variation of emission with respect to the excitation power and found that the PL intensity exhibits linear dependence on the excitation power (Fig. S5a†). This rules out the permanent defect mechanism, otherwise the emission intensity should be saturated with an increase in the power density;16 (iii) the broad FWHM and large Stokes shift in (TBP)2SnBr6 are completely inconsistent with the free exciton mechanism since free excitons usually have smaller Stokes shifts and narrower FWHMs (Fig. S5b†);17 and (iv) the excitation wavelength-dependent PL spectra of (TBP)2SnBr6 show an identical profile, which illustrates that the observed PL stems from the same excitation state (Fig. S5c†). The average lifetime of the emission is 17.6 ns (Fig. S5d†), which is comparable to those of recently reported organic Sn(IV) halides with STE emission.16,18 Combining the unique photophysical properties discussed above, we can reasonably attribute the broadband emission observed in (TBP)2SnBr6 to STE emission.19
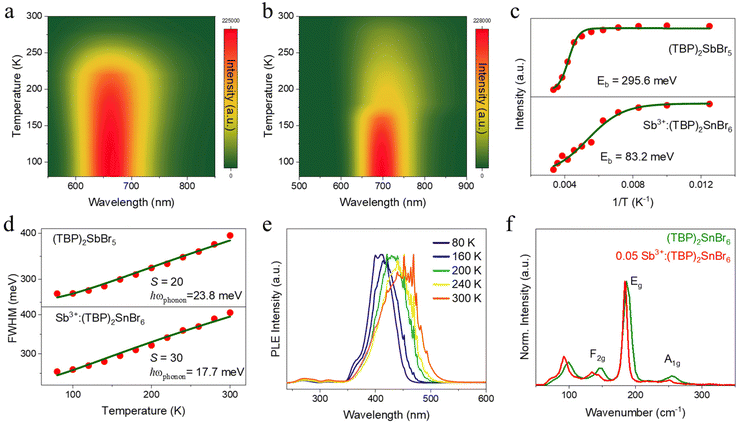
However, (TBP)2SnBr6 shows weak emission (PLQY < 1%), which severely restricts its further applications in photoelectric devices. Recently, Sb3+-activated low-dimensional metal halides have attracted extensive attention due to their fascinating optical properties.20–22 In order to improve the optical properties of (TBP)2SnBr6 and further understand the reasons for their efficient emission, we synthesized Sb3+-doped (TBP)2SnBr6 and the pure Sb(III)-based metal halide of (TBP)2SbBr5, respectively. Then, the PL and PLE spectra of (TBP)2SbBr5 and Sb3+-doped (TBP)2SnBr6 were recorded at RT, and the corresponding optical images are given in Fig. S3b and S3c.† As shown in Fig. 2a and b, both compounds have an intense excitation band within the blue light region (470 nm for (TBP)2SbBr5 and 452 nm for Sb3+-doped (TBP)2SnBr6). Upon blue light excitation, (TBP)2SbBr5 shows a broad visible emission band at 660 nm, while Sb3+-doped (TBP)2SnBr6 exhibits a NIR emission band at 705 nm. As a consequence, the two compounds have a large Stokes shift of 0.76 eV for (TBP)2SbBr5 and 0.98 eV for Sb3+-doped (TBP)2SnBr6. Moreover, the above two compounds also exhibit long PL decay lifetimes of 0.73 and 1.22 μs, respectively (Fig. S7†). Parallelly, the UV-vis absorption spectra of (TBP)2SbBr5 and 0.15Sb3+-doped (TBP)2SnBr6 are depicted in Fig. S8 and S9.† Clearly, both compounds show strong absorption in the visible light region, which corresponds to the yellow crystal in natural light (Fig. S3b and S3c†), but they have different optical absorption edges and bandgaps: 498 nm and 2.60 eV for (TBP)2SbBr5 and 475 nm and 2.76 eV for 0.15Sb3+-doped (TBP)2SnBr6. The greatly different optical properties of (TBP)2SbBr5 and Sb3+-doped (TBP)2SnBr6 should be attributed to their different crystal structures, which we will discuss in detail later.
Subsequently, the PL spectra of Sb3+-doped (TBP)2SnBr6 with various Sb3+ doping concentrations were recorded. As shown in Fig. 2b, the PL intensity increases first with the increase of Sb3+ doping concentration because more luminescence centers are generated. When the doping concentration of Sb3+ further increases, the emission intensity begins to decrease, which is caused by concentration quenching.22 The strongest NIR emission in Sb3+-doped (TBP)2SnBr6 can be witnessed when the feed content of Sb3+ is 15%, accompanied by an elevated PLQY of 33.2% (Fig. S10†). Furthermore, our Sb3+-doped (TBP)2SnBr6 also has the blue light excitation characteristic, which represents the most advanced NIR emitter in lead-free metal halides (Table S5†). In contrast, the PLQY of (TBP)2SbBr5 is only 2.4%, which is difficult to meet the practical application. To further confirm the nature of broadband emission, the varying PLE and PL spectra of (TBP)2SbBr5 and Sb3+-doped (TBP)2SnBr6 were recorded. As shown in Fig. 2c and d, both compounds show a broadband emission with a change in the excitation wavelength at RT, indicating that there are no other impurities or additional energy levels in the two samples. The power-dependent PL spectrum of Sb3+-doped (TBP)2SnBr6 is shown in Fig. S11,† and it can be seen that the PL intensity exhibits linear dependence on the excitation power, which rules out the assumption that the emission in Sb3+-doped (TBP)2SnBr6 stems from the permanent defect mechanism.23 For (TBP)2SbBr5, besides the broadband emission band observed in Fig. 2a, there is an additional weak emission band at 510 nm (band A) with a nanosecond lifetime (3.2 ns, Fig. S12 and S13†) under 309 nm excitation at 80 K, which is different from the broadband emission band at 660 nm (band B). Although Sb3+-doped (TBP)2SnBr6 exhibits a negligible change in broadband PL spectra (band B) at RT (Fig. 2d), an additional emission band at 528 nm (band A) (Fig. S14a†) with a decay lifetime of 3.18 ns can be observed under 368 nm excitation in the low-temperature PL spectra (80 K, Fig. S14b†). Moreover, the PLE spectra monitored at band A and band B exhibit different PLE spectra, which should correspond to 1S0 → 1P1 (360 nm) and 1S0 → 3P1 (410 nm) transition of Sb3+.21 Therefore, (TBP)2SbBr5 and Sb3+-doped (TBP)2SnBr6 exhibit different optical properties, and the dual-emission bands in both compounds should stem from different excited states. Generally, for Sb3+ ions with a 5s2 electron configuration, the ground state is 1S0, while the excited state is 1P1, 3P0, 3P1, and 3P2.22 In particular, 1S0–3P0 and 1S0–3P2 are forbidden transitions, while 1S0–3P1 and 1S0–1P1 are permitted transitions because of spin–orbit coupling.24,25 Under photoexcitation, the electrons are excited to 3P1 and 1P1 levels, and they will quickly be self-trapped to form two different STEs due to the large lattice distortion and strong electron–phonon coupling.23 In our studies, the band A emission shows a short lifetime (nanosecond level) and the band B emission exhibits a long lifetime (microsecond level), which should be attributed to the two emission bands that stem from different excited states. Generally, the decay lifetime of a singlet STE is significantly shorter than that of a triplet STE, and hence the observed band A and band B emissions come from singlet and triplet STEs, respectively.26
Recently, numerous lead-free metal halides with efficient broadband emission have been reported, making the relationship between the crystal structure and luminescence properties a key area of research. As we know, the crystal structure of Sb3+ has an important influence on its optical properties.27 In our findings, the Sb3+ ions have different coordination structures in (TBP)2SbBr5 and Sb3+-doped (TBP)2SnBr6, which enables them to exhibit different photophysical properties. To be more specific, (TBP)2SbBr5 exhibits visible light emission, while Sb3+-doped (TBP)2SnBr6 has a larger Stokes shift, resulting in efficient broadband NIR emission. Recent studies have shown that increasing the lattice distortion degree (η) of the excited state compared to the ground state is the key to yielding large Stokes shifts.28,29 Thus, the lattice distortion parameters (Δd) of [SbBr5]2− in (TBP)2SbBr5 and [SbBr6]3− in Sb3+-doped (TBP)2SnBr6 in the ground state (GS) and excited state (ES) were calculated using eqn (1). The calculated Δd values of (TBP)2SbBr5 and Sb3+-doped (TBP)2SnBr6 are given in Tables S6 and S7.† Although the Δd value of (TBP)2SbBr5 in GS (ΔdGS) and ES (ΔdES) is greater than that of Sb3+-doped (TBP)2SnBr6, the lattice distortion degree (η = (ΔdES − ΔdGS)/ΔdGS) of [SbBr6]3− is larger than that of [SbBr5]2−, which enables Sb3+-doped (TBP)2SnBr6 to exhibit a large Stokes shift and yield a broadband NIR emission. Parallelly, a large lattice distortion can increase nonradiative losses of excitation energy, thereby quenching the PL of low-dimensional metal halides with STE emission and vice versa.30,31 Therefore, Sb3+-doped (TBP)2SnBr6 with appropriate lattice distortion can emit efficient emission under photoexcitation.
To further understand the broadband emission mechanism of the as-synthesized samples, the band structures and projected density of states (PDOS) of (TBP)2SbBr5, (TBP)2SnBr6, and Sb3+-doped (TBP)2SnBr6 were calculated via density functional theory (DFT). All compounds show a direct bandgap with values of 1.15 eV for (TBP)2SbBr5 (Fig. 3a), 2.19 eV for (TBP)2SnBr6 (Fig. 3b), and 1.36 eV for Sb3+-doped (TBP)2SnBr6 (Fig. 3c), which are smaller than the experimental values (Fig. S3, S8, and S9†), and this should be attributed to the fact that DFT calculations typically underestimate the bandgap value. Compared with pure (TBP)2SnBr6, when Sb3+ is introduced into the lattice, the bandgap decreases significantly, which corresponds to the redshift of the absorption band after Sb3+ doping. In addition, all compounds exhibit a negligible dispersion in the valence band maximum (VBM) and conduction band minimum (CBM), which illustrates a strong quantum confinement effect and is further consistent with its long M–M (M = Sn4+, Sb3+) distance in our 0D structures.32 In particular, the calculated bandgaps of (TBP)2SbBr5 and Sb3+-doped (TBP)2SnBr6 were much smaller than those of other 0D lead-free metal halides,33–35 which makes them have strong absorption in the visible light region and yield bright emission under blue light excitation. Fig. 3d and g show the PDOS and partial charge density distribution of (TBP)2SbBr5. The VBM is composed of organic ligands, while Br p, Sb s, and Sb p states mainly dominate the CBM. Thus, a charge is transferred between the organic counterparts and inorganic clusters in (TBP)2SbBr5, which will weaken the PL intensity. In contrast to (TBP)2SbBr5, the VBM and CBM of (TBP)2SnBr6 are mostly contributed by an inorganic unit (Fig. 3e and h), that is, the VBM is mainly composed of the Br p state, while the CBM mainly consists of Br p and Sn s orbitals. After Sb3+ doping, there is little effect on the CBM, but a new impurity band appears in the VBM, which is contributed by Sb s and Br p characteristics (Fig. 3f and i). Moreover, the doping of Sb3+ will cause the downshift of Br p and Sn s states, which leads to the bandgap contraction. Although heterovalent doping introduces elements with different valence states, this does not necessarily lead to an increase in defects or a decrease in PLQY. In contrast, in some cases, appropriate heterovalent doping can improve the influence of defects by regulating the lattice structure and electronic environment of the material. For example, the incorporation of Sb3+ can form a stable local structure that passivates inherent defects in the material, thereby reducing non-radiative recombination and improving luminescence efficiency.18 This has been demonstrated in practical studies, where the lone pair electrons of Sb3+ can significantly enhance the optical performance of Sn4+ systems by modulating the electronic structure.22,36 In low-dimensional halide materials, Sb3+ doping is often accompanied by lattice distortion, which promotes the formation of STEs. STEs refer to electrons and holes that are confined to locally deformed regions of the material and release energy in the form of radiative recombination.37 This mechanism not only prevents a decrease in luminescence efficiency but also helps to generate broadband emission and enhance the PLQY of the material. Therefore, Sb3+ ion doping can effectively regulate the electronic properties of (TBP)2SnBr6, which makes Sb3+-doped (TBP)2SnBr6 exhibit fascinating optical properties compared with pure (TBP)2SnBr6.
To better understand the photophysical properties of (TBP)2SbBr5 and Sb3+-doped (TBP)2SnBr6, temperature-dependent PL spectra were investigated at 80 to 300 K. As shown in Fig. 4a and b, the PL intensities of both compounds gradually increase with a decrease in temperature, which should be attributed to the suppression of nonradiative transitions at low temperatures. Then, the thermal activation energy (Eb) values for the two compounds were calculated using the Arrhenius equation (eqn (2)):
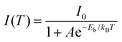 | (2) |
The Huang–Rhys factor (S) is another important physical parameter that can be used to evaluate the strength of electron–phonon coupling, which can be obtained using eqn (3):
 | (3) |
To gain further insight into the electron–phonon interaction, Raman spectra were obtained for pure and Sb3+-doped (TBP)2SnBr6 under 532 nm laser excitation (Fig. 4f). Clearly, a series of Raman peaks are observed, which should stem from inorganic units. Among them, the Raman bands at 254, 186, 147, and 93 cm−1 perfectly meet the following formula in the octahedron: 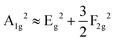 , where A1g, F2g, and Eg are the symmetric stretching, bending, and asymmetric stretching modes in [SnBr6]2− units, respectively.39 After Sb3+ doping, all the Raman modes shift to a lower wavenumber, which is caused by lattice expansion due to the larger ion radius of Sb3+ than that of Sn4+.40 In particular, Sb3+ doping can significantly narrow the FWHM of the Raman mode of the Eg mode and weaken the Raman intensity of the A1g mode, which indicates that Sb3+ doping can effectively regulate the symmetry and electron–phonon coupling of the host lattice, thus bringing rich photophysical properties. Furthermore, the Raman mode at the lowest energy should be the strongest in strong-confined metal halides in the small polaron (STE) system.41 However, the strongest Raman mode in this doped system is 186 cm−1, which is the overtone of the 93 cm−1. Their simultaneous presence means that their strong electron–phonon coupling Raman mode can be found in the just one octahedron with strong anharmonicity, resulting in an STE state within a cluster.
, where A1g, F2g, and Eg are the symmetric stretching, bending, and asymmetric stretching modes in [SnBr6]2− units, respectively.39 After Sb3+ doping, all the Raman modes shift to a lower wavenumber, which is caused by lattice expansion due to the larger ion radius of Sb3+ than that of Sn4+.40 In particular, Sb3+ doping can significantly narrow the FWHM of the Raman mode of the Eg mode and weaken the Raman intensity of the A1g mode, which indicates that Sb3+ doping can effectively regulate the symmetry and electron–phonon coupling of the host lattice, thus bringing rich photophysical properties. Furthermore, the Raman mode at the lowest energy should be the strongest in strong-confined metal halides in the small polaron (STE) system.41 However, the strongest Raman mode in this doped system is 186 cm−1, which is the overtone of the 93 cm−1. Their simultaneous presence means that their strong electron–phonon coupling Raman mode can be found in the just one octahedron with strong anharmonicity, resulting in an STE state within a cluster.
Based on the abovementioned results, Sb3+-doped (TBP)2SnBr6 exhibits strong NIR emission under blue light excitation. Moreover, the optical properties of Sb3+-doped (TBP)2SnBr6 are greatly different from those of (TBP)2SnBr6 and (TBP)2SbBr5. Combined with the crystal structure and DFT calculations, the NIR emission in Sb3+-doped (TBP)2SnBr6 should stem from [SbBr6]3− clusters. To identify the source of the luminescence, the Huang–Rhys factor of (TBP)2SbBr5 was calculated to be 30, which illustrates a strong electron–phonon interaction in this compound. Then, we recorded the power-dependent PL spectra of Sb3+-doped (TBP)2SnBr6, and the PL intensity exhibits a linear dependence on the excitation power, which rules out the assumption that the emission in Sb3+-doped (TBP)2SnBr6 stems from the permanent defect mechanism. Moreover, Sb3+-doped (TBP)2SnBr6 also exhibits a large lattice distortion, broad FWHM (152 nm), large Stokes shift (0.98 eV), and long decay lifetime (1.22 μs), and thus we can confirm that the broadband NIR emission in Sb3+-doped (TBP)2SnBr6 stems from STEs, and the photophysical mechanism is given in Fig. S16.† Under photoexcitation (e.g., 452 nm), the electrons in [SbBr6]3− clusters are excited from the ground state to the excited state. Subsequently, the excited electrons undergo quick intersystem crossing from the excited state to the triplet STE state, thus yielding a broad NIR emission peaking at 705 nm. In particular, compared with (TBP)2SbBr5 with visible light emission, Sb3+-doped (TBP)2SnBr6 exhibits a larger excited state lattice distortion degree compared to the ground state, and thus this compound exhibits NIR emission under blue light excitation.
Given the excellent NIR emission characteristics of Sb3+-doped (TBP)2SnBr6, we then investigated the stability. When Sb3+-doped (TBP)2SnBr6 was stored in an atmospheric environment for 3 months, the PXRD patterns (Fig. S17a†) and the emission spectra show a similar outline to the pristine one. In the meantime, the PL intensity remains basically unchanged (Fig. S17b†). The thermogravimetric analysis revealed that the decomposition temperature of Sb3+-doped (TBP)2SnBr6 is 333 °C (Fig. S17c†). Surprisingly, Sb3+-doped (TBP)2SnBr6 also exhibits remarkable anti-water stability. As shown in Fig. 5a, Sb3+-doped (TBP)2SnBr6 SCs can still maintain their original shape and have a high NIR emission intensity when soaked in water for 4 h. Moreover, the PL spectrum of the sample after water treatment shows a similar profile to the fresh sample (Fig. 5b), and the PLQY only dropped from 33.2 to 28.9% after soaking in water for 4 h (Fig. S18†). The PXRD patterns of Sb3+-doped (TBP)2SnBr6 after water treatment show an identical profile to that of the fresh sample, indicating that this compound did not undergo structural degradation after soaking in water (Fig. 5c). Hence, the above results illustrate that Sb3+-doped (TBP)2SnBr6 has excellent anti-water stability and further show that this compound emits bright NIR emission even when immersed in water. To explore the intrinsic mechanism of Sb3+-doped (TBP)2SnBr6 with excellent water stability, the HRXPS spectra were recorded (Fig. 5d). Compared with the fresh sample, the sample after water treatment has an additional satellite peak at 531.9 eV, which can be assigned to the O 1s. Therefore, we believe that after this compound has been treated with water, an amorphous tin layer will form on the surface, further protecting it from degradation.42 In the meantime, the absorption spectra exhibit a blue shift compared with the pristine sample, indicating that the formation of the oxide layer makes the optical bandgap larger (Fig. S19†). Therefore, when Sb3+-doped (TBP)2SnBr6 is treated with water, an amorphous tin oxide layer can be formed on its surface to protect it from further degradation, which is the dominant reason for its excellent anti-water stability.
Finally, a high-performance NIR pc-LED was prepared by combining an Sb3+-doped (TBP)2SnBr6 NIR phosphor with a blue LED chip (460 nm). Fig. 6a shows the electroluminescence (EL) spectra of the as-fabricated device, which shows a similar profile to the PL spectra. Moreover, the emission intensity of the NIR pc-LED increases gradually with an increase of driving currents (Fig. 6b), and the output power and photoelectric conversion efficiency of the device at different driving currents are given in Fig. 6c. The NIR output power increases gradually as the driving current increases, while the photoelectric conversion efficiency shows the opposite trend. The output power reaches a maximum of 9 mW at a current of 60 mA, at which point the photoelectric conversion efficiency drops to 5.4% due to the reduced efficiency of commercial LED chips.10 In particular, the device performance parameters of the Sb3+-doped (TBP)2SnBr6 based NIR pc-LED are much higher than those of the recently reported Sb3+-doped Cs2ZnCl4 based NIR pc-LED under the same driving current,10 which should be attributed to the fact that Sb3+-doped (TBP)2SnBr6 can be excited by a blue LED chip. In further experiments, we demonstrated the application of the Sb3+-doped (TBP)2SnBr6 based NIR pc-LED in night vision. In Fig. 6d, the image of a sunflower can be captured using a visible camera under sunlight, but nothing can be observed when the sunlight is turned off. In particular, the black-and-white image of a sunflower is captured using an NIR camera when the NIR pc-LED is turned on (Fig. 6f). Moreover, the operational stability of the NIR pc-LED device was measured, and the emission intensity remained high (Fig. S20†). These results demonstrate the great potential application of Sb3+-doped (TBP)2SnBr6 in night vision due to the efficient NIR emission and blue-light excitation characteristics.
4. Conclusions
In summary, we report a new NIR emitter of Sb3+-doped 0D (TBP)2SnBr6via coordination structure modulation. Compared with pure (TBP)2SbBr5 with [SbBr5]2− clusters, the coordination structure of Sb3+ ions in Sb3+-doped (TBP)2SnBr6 can be effectively regulated to the [SbBr6]3− octahedron. Combined with steady state/transient PL spectra and DFT calculations, it was found that [SbBr6]3− has a larger excited state lattice distortion compared to the ground state, which will cause a large Stokes shift in Sb3+-doped (TBP)2SnBr6. Moreover, Sb3+-doped (TBP)2SnBr6 exhibits a narrow bandgap and multi-level non-radiative relaxation. Thus, we can obtain the efficient broadband NIR emission under blue light excitation with a PLQY of 33.2%. The study of photophysical properties illustrates that the observed broadband NIR emission in Sb3+-doped (TBP)2SnBr6 stems from STE emission due to the strong electron–phonon coupling. Specifically, Sb3+-doped (TBP)2SnBr6 also exhibits remarkable air and anti-water stability, and we also demonstrated the application of Sb3+-doped (TBP)2SnBr6 in a high-performance NIR pc-LED. Consequently, this work not only presents a realistic proposal for designing low-dimensional lead-free metal halides with efficient broadband NIR emission under blue light excitation but also helps in understanding the relationship between the crystal structure and photophysical properties.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was supported by the Guangxi NSF project (no. 2020GXNSFDA238004), the “Guangxi Bagui Scholars” fund, the Guangxi Science and Technology Major Project (AA23073018), and the Scientific and Technological Bases and Talents of Guangxi (Guike AD21238027 and AD23026119) for financial support. The calculation was supported by the high-performance computing platform of Guangxi University.References
- Y. Wang, P. Dang, L. Qiu, G. Zhang, D. Liu, Y. Wei, H. Lian, G. Li, Z. Cheng and J. Lin, Multimode Luminescence Tailoring and Improvement of Cs2NaHoCl6 Cryolite Crystals via Sb3+/Yb3+ Alloying for Versatile Photoelectric Applications, Angew. Chem., Int. Ed., 2023, 62(45), e202311699 CrossRef CAS PubMed
.
- W. Gan, L. Cao, S. Gu, H. Lian, Z. Xia and J. Wang, Broad-Band Sensitization in Cr3+–Er3+ Co-Doped Cs2AgInCl6 Double Perovskites with 1.5 μm Near-Infrared Emission, Chem. Mater., 2023, 35(14), 5291–5299 CrossRef CAS
.
- S. Saikia, A. Ghosh and A. Nag, Broad Dual Emission by Codoping Cr3+ (d-d) and Bi3+ (s-p) in Cs2Ag0.6Na0.4InCl6 Double Perovskite, Angew. Chem., Int. Ed., 2023, 62(33), e202307689 CrossRef CAS PubMed
.
- Z. Wu, X. Han, Y. Zhou, K. Xing, S. Cao, L. Chen, R. Zeng, J. Zhao and B. Zou, Efficient Broadband Near-Infrared Luminescence of Cr3+ Doped Fluoride K2NaInF6 and Its NIR-LED Application Toward Veins Imaging, Chem. Eng. J., 2022, 427, 131740 CrossRef CAS
.
- M.-H. Fang, K.-C. Chen, N. Majewska, T. Leśniewski, S. Mahlik, G. Leniec, S. M. Kaczmarek, C.-W. Yang, K.-M. Lu, H.-S. Sheu and R.-S. Liu, Hidden Structural Evolution and Bond Valence Control in Near-Infrared Phosphors for Light-Emitting Diodes, ACS Energy Lett., 2021, 6(1), 109–114 CrossRef CAS
.
- J. Qiao, G. Zhou, Y. Zhou, Q. Zhang and Z. Xia, Divalent Europium-Doped Near-Infrared-Emitting Phosphor for Light-Emitting Diodes, Nat. Commun., 2019, 10(1), 5267 CrossRef PubMed
.
- Y. Wei, L. Cao, L. Lv, G. Li, J. Hao, J. Gao, C. Su, C. C. Lin, H. S. Jang, P. Dang and J. Lin, Highly Efficient Blue Emission and Superior Thermal Stability of BaAl12O19:Eu2+ Phosphors Based on Highly Symmetric Crystal Structure, Chem. Mater., 2018, 30(7), 2389–2399 CrossRef CAS
.
- G. Zhang, D. Wang, J. Ren, X. Zhou and Y. Wang, Highly Efficient Broadband Near-Infrared Emission from Sn2+ Alloyed Lead-Free Cesium Zinc Halides, Laser Photonics Rev., 2023, 17(10), 2300158 CrossRef CAS
.
- X. Yang, S. M. H. Qaid, B. Wang, W. Cai, Q. Qian and Z. Zang, Broadband Near-Infrared Emission from 0D Hybrid Copper Halides, Inorg. Chem., 2023, 62(45), 18591–18598 CrossRef CAS PubMed
.
- B. Su, M. Li, E. Song and Z. Xia, Sb3+ –Doping in Cesium Zinc Halides Single Crystals Enabling High–Efficiency Near–Infrared Emission, Adv. Funct. Mater., 2021, 31(40), 2105316 CrossRef CAS
.
- H. Lin, Q. Wei, B. Ke, W. Lin, H. Zhao and B. Zou, Excitation-Wavelength-Dependent Emission Behavior in (NH4)2SnCl6 via Sb3+ Dopant, J. Phys. Chem. Lett., 2023, 14(6), 1460–1469 CrossRef CAS PubMed
.
- J. P. Perdew and M. Levy, Physical Content of the Exact Kohn-Sham Orbital Energies: Band Gaps and Derivative Discontinuities, Phys. Rev. Lett., 1983, 51, 1884–1887 CrossRef CAS
.
- G. Kresse and J. Furthmüller, Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54(16), 11169–11186 CrossRef CAS PubMed
.
- P. E. Blöchl, Projector augmented-wave method, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50(24), 17953–17979 CrossRef PubMed
.
- C. Sun, Z. Deng, Z. Li, Z. Chen, X. Zhang, J. Chen, H. Lu, P. Canepa, R. Chen and L. Mao, Achieving Near-Unity Photoluminescence Quantum Yields in Organic-Inorganic Hybrid Antimony(III) Chlorides with the [SbCl5] Geometry, Angew. Chem., Int. Ed., 2023, 62(10), e202216720 CrossRef CAS PubMed
.
- G. Song, M. Li, Y. Yang, F. Liang, Q. Huang, X. Liu, P. Gong, Z. Xia and Z. Lin, Lead-Free Tin(IV)-Based Organic–Inorganic Metal Halide Hybrids with Excellent Stability and Blue-Broadband Emission, J. Phys. Chem. Lett., 2020, 11(5), 1808–1813 CrossRef CAS PubMed
.
- P. Klement, N. Dehnhardt, C. Dong, F. Dobener, S. Bayliff, J. Winkler, D. M. Hofmann, P. J. Klar, S. Schumacher, S. Chatterjee and J. Heine, Atomically Thin Sheets of Lead–Free 1D Hybrid Perovskites Feature Tunable White–Light Emission from Self–Trapped Excitons, Adv. Mater., 2021, 33(23), 2100518 CrossRef CAS PubMed
.
- L. Zhou, L. Zhang, H. Li, W. Shen, M. Li and R. He, Defect Passivation in Air-Stable Tin(IV)-Halide Single Crystal for Emissive Self-Trapped Excitons, Adv. Funct. Mater., 2021, 31(51), 2108561 CrossRef CAS
.
- X. Liu, K. Li, W. Shao, W. Shen, M. Li, L. Zhou and R. He, Revealing the Structure–Luminescence Relationship in Robust Sn(IV)-Based Metal Halides by Sb3+ Doping, Inorg. Chem., 2024, 63(11), 5158–5166 CrossRef CAS PubMed
.
- X. He, Q. Wei, H. Peng, Y. Li, X. Wang, B. Ke, J. Zhao and B. Zou, Large-Scale Room-Temperature Synthesis of the First Sb3+-Doped Organic Ge(IV)-Based Metal Halides with Efficient Yellow Emission for Solid-State Lighting and Latent Fingerprint Detection, Small Struct., 2024, 5(5), 2300472 CrossRef CAS
.
- Y. Jing, Y. Liu, M. Li and Z. Xia, Photoluminescence of Singlet/Triplet Self–Trapped Excitons in Sb3+ –Based Metal Halides, Adv. Opt. Mater., 2021, 9(8), 2002213 CrossRef CAS
.
- B. Ke, H. Peng, Q. Wei, C. Yang, X. Li, W. Huang, Z. Du, J. Zhao and B. Zou, Achieving Highly Efficient Orange Emission in Tin(IV)–Based Metal Halides with Outstanding Anti–Water Stability Through Antimony Doping and Reasonable Structural Modulation, Adv. Opt. Mater., 2024, 12(4), 2301665 CrossRef CAS
.
- J. Luo, X. Wang, S. Li, J. Liu, Y. Guo, G. Niu, L. Yao, Y. Fu, L. Gao, Q. Dong, C. Zhao, M. Leng, F. Ma, W. Liang, L. Wang, S. Jin, J. Han, L. Zhang, J. Etheridge, J. Wang, Y. Yan, E. H. Sargent and J. Tang, Efficient and Stable Emission of Warm-White Light from Lead-Free Halide Double Perovskites, Nature, 2018, 563(7732), 541–545 CrossRef CAS PubMed
.
- Y.-C. Peng, S.-H. Zhou, J.-C. Jin, T.-H. Zhuang, L.-K. Gong, H.-W. Lin, Z.-P. Wang, K.-Z. Du and X.-Y. Huang, [PPh3H]2[SbCl5]: A Zero-Dimensional Hybrid Metal Halide with a Supramolecular Framework and Stable Dual-Band Emission, J. Phys. Chem. C, 2022, 126(40), 17381–17389 CrossRef CAS
.
- J. Wu, X. Li, X. Lian, B. Su, J. Pang, M.-D. Li, Z. Xia, J. Z. Zhang, B. Luo and X.-C. Huang, Ultrafast Study of Exciton Transfer in Sb(III)-Doped Two-Dimensional [NH3(CH2)4NH3]CdBr4 Perovskite, ACS Nano, 2021, 15(9), 15354–15361 CrossRef CAS PubMed
.
- H. Peng, Y. Tian, Z. Yu, X. Wang, B. Ke, Y. Zhao, T. Dong, J. Wang and B. Zou, (C16H28N)2SbCl5: A New Lead-Free Zero-Dimensional Metal-Halide Hybrid with Bright Orange Emission, Sci. China Mater., 2022, 65(6), 1594–1600 CrossRef CAS
.
- J.-Q. Zhao, M.-F. Han, X.-J. Zhao, Y.-Y. Ma, C.-Q. Jing, H.-M. Pan, D.-Y. Li, C.-Y. Yue and X.-W. Lei, Structural Dimensionality Modulation toward Enhanced Photoluminescence Efficiencies of Hybrid Lead-Free Antimony Halides, Adv. Opt. Mater., 2021, 9(19), 2100556 CrossRef CAS
.
- B. Su, G. Song, M. S. Molokeev, Z. Lin and Z. Xia, Synthesis, Crystal Structure and Green Luminescence in Zero-Dimensional Tin Halide (C8H14N2)2SnBr6, Inorg. Chem., 2020, 59(14), 9962–9968 CrossRef CAS PubMed
.
- S. Yu, H. Peng, Q. Wei, T. Li, W. Huang, X. He, Z. Du, J. Zhao and B. Zou, Realizing Efficient Broadband Near-Infrared Emission and Multimode Photoluminescence Switching via Coordination Structure Modulation in Sb3+-Doped 0D Organic Metal Chlorides, Mater. Horiz., 2024, 11, 2230–2241 RSC
.
- X. Han, P. Cheng, R. Shi, Y. Zheng, S. Qi, J. Xu and X.-H. Bu, Linear Optical Afterglow and Nonlinear Optical Harmonic Generation from Chiral Tin(IV) Halides: The Role of Lattice Distortions, Mater. Horiz., 2023, 10(3), 1005–1011 RSC
.
- X. He, H. Peng, Q. Wei, Z. Zhou, G. Zhang, Z. Du, J. Zhao and B. Zou, Realizing Efficient Emission and Triple-Mode Photoluminescence Switching in Air-Stable Tin(IV)-Based Metal Halides via Antimony Doping and Rational Structural Modulation, Aggregate, 2024, 5(1), e407 CrossRef CAS
.
- D.-Y. Li, Y.-B. Shang, Q. Liu, H.-W. Zhang, X.-Y. Zhang, C.-Y. Yue and X.-W. Lei, 0D Hybrid Indium Halide as a Highly Efficient X-Ray Scintillation and Ultra-Sensitive Fluorescent Probe, Mater. Horiz., 2023, 10(11), 5004–5015 RSC
.
- V. Morad, Y. Shynkarenko, S. Yakunin, A. Brumberg, R. D. Schaller and M. V. Kovalenko, Disphenoidal Zero-Dimensional Lead, Tin, and Germanium Halides: Highly Emissive Singlet and Triplet Self-Trapped Excitons and X-Ray Scintillation, J. Am. Chem. Soc., 2019, 141(25), 9764–9768 CrossRef CAS PubMed
.
- M. Yin, B. Li, Z. Yi, Y. Zhang, Z. Xia and Y. Xu, Crystal-Glass Phase Transition Enabling Reversible Fluorescence Switching in Zero-Dimensional Antimony Halides, Chem. Commun., 2023, 59(76), 11361–11364 RSC
.
- Q. Wei, T. Chang, R. Zeng, S. Cao, J. Zhao, X. Han, L. Wang and B. Zou, Self-Trapped Exciton Emission in a Zero-Dimensional (TMA)2SbCl5·DMF Single Crystal and Molecular Dynamics Simulation of Structural Stability, J. Phys. Chem. Lett., 2021, 12(30), 7091–7099 CrossRef CAS PubMed
.
- J. Jin, Y. Wang, K. Han and Z. Xia, Rigid Phase Formation and Sb3+ Doping of Tin(IV) Halide Hybrids toward Photoluminescence Enhancement and Tuning for Anti–Counterfeiting and Information Encryption, Angew. Chem., Int. Ed., 2024, e202408653 CAS
.
- M. D. Smith and H. I. Karunadasa, White-Light Emission from Layered Halide Perovskites, Acc. Chem. Res., 2018, 51(3), 619–627 CrossRef CAS PubMed
.
- L. Zhou, J.-F. Liao, Y. Qin, X.-D. Wang, J.-H. Wei, M. Li, D.-B. Kuang and R. He, Activation of Self-Trapped Emission in Stable Bismuth-Halide Perovskite by Suppressing Strong Exciton–Phonon Coupling, Adv. Funct. Mater., 2021, 31(31), 2102654 CrossRef CAS
.
- H. Siddique, Z. Xu, X. Li, S. Saeed, W. Liang, X. Wang, C. Gao, R. Dai, Z. Wang and Z. Zhang, Anomalous Octahedron Distortion of Bi-Alloyed Cs2AgInCl6 Crystal via XRD, Raman, Huang–Rhys Factor, and Photoluminescence, J. Phys. Chem. Lett., 2020, 11(22), 9572–9578 CrossRef CAS PubMed
.
- H. Peng, T. Huang, B. Zou, Y. Tian, X. Wang, Y. Guo, T. Dong, Z. Yu, C. Ding, F. Yang and J. Wang, Organic-Inorganic Hybrid Manganese Bromine Single Crystal with Dual-Band Photoluminescence from Polaronic and Bipolaronic Excitons, Nano Energy, 2021, 87, 106166 CrossRef CAS
.
- D. Emin, Optical Properties of Large and Small Polarons and Bipolarons, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 48(18), 13691–13702 CrossRef CAS PubMed
.
- Z. Tan, Y. Chu, J. Chen, J. Li, G. Ji, G. Niu, L. Gao, Z. Xiao and J. Tang, Lead–Free Perovskite Variant Solid Solutions Cs2Sn1−xTexCl6: Bright Luminescence and High Anti–Water Stability, Adv. Mater., 2020, 32(32), 2002443 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2382097 and 2382098. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4qi01904k |
| This journal is © the Partner Organisations 2024 |