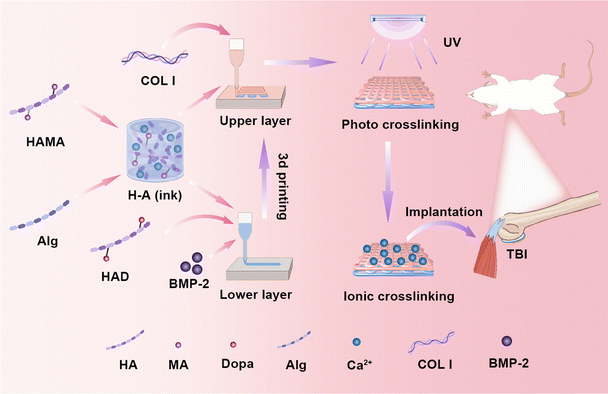
3D-printed biomimetic scaffolds loaded with ADSCs and BMP-2 for enhanced rotator cuff repair†
Zhonglian
Wu‡
 abcd,
Jian
Yang‡
bce,
Hui
Chong
abcd,
Jian
Yang‡
bce,
Hui
Chong
 *af,
Xiaomei
Dai
*af,
Xiaomei
Dai
 bc,
Haidi
Sun
a,
Junli
Shi
a,
Meijuan
Yuan
bc,
Dianwei
Liu
bcg,
Mengbo
Dang
bcg,
Hang
Yao
bc,
Haidi
Sun
a,
Junli
Shi
a,
Meijuan
Yuan
bc,
Dianwei
Liu
bcg,
Mengbo
Dang
bcg,
Hang
Yao
 *ad and
Wenyong
Fei
*bcd
*ad and
Wenyong
Fei
*bcd
aSchool of Chemistry and Chemical Engineering, Yangzhou University, Yangzhou 225009, P. R. China. E-mail: chonghui@yzu.edu.cn; yaohang@yzu.edu.cn
bDepartment of Sports Medicine, Northern Jiangsu People's Hospital, Yangzhou 225001, P. R. China. E-mail: sbydyx105@163.com
cDepartment of Orthopedics, Northern Jiangsu People's Hospital Affiliated to Yangzhou University/Clinical Medical College, Yangzhou University, Yangzhou 225001, P. R. China
dBasic and Clinical Research Center for Sports Medicine, Yangzhou University, Yangzhou 225002, P. R. China
eMedical College, Yangzhou University, Yangzhou 225001, P. R. China
fInstitute of Innovation Materials and Energy, Yangzhou University, Yangzhou 225002, China
gDalian Medical University, Dalian 116044, P. R. China
First published on 24th October 2024
Abstract
Rotator cuff tear repair poses significant challenges due to the complex gradient interface structure. In the face of disease-related disruptions in the tendon–bone interface (TBI), the strategy of constructing a biomimetic scaffold is a promising avenue. A novel 3D-printed rotator cuff scaffold loaded adipose stem cells (ADSCs), bone morphogenetic protein-2 (BMP-2), and collagen type I (COL I). The efficiency of the slow-release BMP-2 design depended on the dopamine-hyaluronic acid (HAD) and BMP-2 reaction. The cumulative release of BMP-2 was 44.97 ± 5.45% at 4 weeks. The 3D-printed bilayer scaffold, incorporating COL I and BMP-2, effectively promoted the differentiation of ADSCs into osteogenic, tenogenic, and chondrogenic lineages in vitro. The combination of 3D-printed bioactive scaffolds and ADSCs demonstrated a superior repair effect on rotator cuff injuries in vivo. Therefore, these findings indicates that the 3D-printed biomimetic scaffold loaded with ADSCs and BMP-2 holds potential as a promising graft for TBI healing.
1. Introduction
Rotator cuff injuries can cause pain and limit movement in the shoulder. Surgery can currently serve as an efficient treatment for Rotator cuff tears (RCT). However, it rarely restores full shoulder function due to the difficulty in accurately repositioning the torn rotator cuff. Even after surgery, patients typically undergo long periods of rehabilitation to regain near-normal function, and the rate of re-tears can be as high as 94%.1 One key challenge is accelerating the recovery of the structure and function of the tendon–bone interface (TBI) after injury.2 The TBI comprises four distinct layers: tendon, fibrocartilage, mineralized fibrocartilage, and bone. This arrangement helps distribute stress evenly and allows for a gradual load transfer from the tendon to the bone.3 Unfortunately, this complex structure is difficult to recreate surgically, making it hard to fully restore the mechanical properties of the repaired rotator cuff.4 Recent research into improving TBI healing has focused on three main strategies: stem cell therapies, growth factor regulation, and bio-scaffold development. Tissue engineering, which integrates these approaches, is a promising solution for enhancing TBI repair.5Biomimetic scaffolds made from different biomaterials are essential to mimic the complex structure of the TBI.6 Advances in 3D printing now allow for the creation of precisely engineered scaffolds that can replicate the structure of the TBI by using a variety of inks. These scaffolds can create microporous environments that support cell proliferation and migration, enhancing tissue regeneration.7,8 Biomaterials like hyaluronic acid (HA), collagen, and sodium alginate (Alg) are frequently used in scaffold fabrication and have demonstrated effectiveness in promoting TBI healing.9 Collagen type I (COL I), the primary component of the tendon's extracellular matrix, is an ideal biomimetic ink. HA, known for its biocompatibility and biodegradability, has been shown to support bone healing.10 HA can be chemically modified by introducing methacrylate groups to obtain methacrylated hyaluronic acid (HAMA), which can serve as 3D printing photo-crosslinking ink.11 Alg, a widely used natural polysaccharide, has been explored for various 3D printing techniques, such as pre-crosslinking and double crosslinking, and has been shown to aid in tendon repair and reduce inflammation.12–15
In addition to scaffolds, stem cell-based therapies are widely adopted to enhance TBI regeneration. Adipose-derived stem cells (ADSCs) are particularly promising for RCT treatment due to their self-renewal capacity and potential for differentiation into multiple cell types.16–18 Compared to the other mesenchymal stem cells, the advantages of ADSCs include readily accessible, rapid proliferation, and suitable for autologous transplantation.19 Importantly, ADSCs have been shown to reduce fat infiltration in chronic rotator cuff injuries.20
Bone morphogenetic protein-2 (BMP-2-) is vital for promoting chondrogenic and osteogenic differentiation in stem cells.21–23 While BMP-2 is crucial for ADSC differentiation, its clinical application is hindered by a short half-life and low localized concentrations.24 Additionally, the rapid release of BMP-2 can lead to heterotopic bone development, excessive bone growth, and nerve compression.25,26 To address these limitations, researchers have developed hydrogels made from HA and Alg that can deliver BMP-2 and stem cells to the target site, supporting bone formation.27,28 However, achieving precise control over BMP-2 release remains a challenge.
One promising approach involves the use of dopamine hydrochloride (Dopa), which exhibits strong adhesive properties.29,30 Hydrogels decorated with Dopa can immobilize BMP-2, allowing for controlled release and enhancing the scaffold's functionality. In this study, we developed 3D-printed slow-release BMP-2 hydrogel scaffolds that promote the repair of RCT by combining dopamine-hyaluronic acid (HAD) with BMP-2 (Fig. 1). These scaffolds, incorporating ADSCs, were engineered to mimic the TBI structure through gradient, multi-tissue interfaces. The study revealed that the functional hydrogel encapsulating BMP-2 and ADSCs showed excellent results in tendon–bone healing in vitro and in vivo.
2. Materials and methods
2.1. Materials
Dulbecco's modified Eagle's medium (DMEM) of low glucose was purchased from Gibco (Thermo Fisher Scientific, USA). Sodium alginate (Alg), 2-hydroxy-4′-(2-hydroxyethoxy)-2-methylpropiophenone (I 2959) were purchased from Sigma-Aldrich (USA). Methacrylic anhydride (MA), calcium chloride (CaCl2), hyaluronic acid (HA), dopamine hydrochloride (Dopa), EDTA, and urethane were purchased from Aladdin (China). Penicillin–streptomycin (PS), the cell counting kit-8 (CCK-8), and the calcein-AM/PI cell viability assay kit were provided by Beyotime Biotechnology (China). The enzyme-linked immunosorbent assay (ELISA) kit of human/murine/rat BMP-2 was provided by Thermo Fisher Scientific (USA). Collagen type I (COL I) was bought from Yuanye (China). Rat adipose-derived mesenchymal stem cells osteogenic induced differentiation Kit and chondrogenic induced differentiation kit were purchased from OriCell (Guangzhou, China). ADSCs were purchased from Servicebio (Wuhan, China). The recombinant human BMP-2 was bought from the Jiuyuan gene engineering (China).2.2. Synthesis of ink component
HA (1.00 g) was dissolved in 100 mL of deionized water. Then, 4 mL of MA was slowly added to the HA solution at 0 °C. The solution was adjusted to pH 8 and kept in an ice bath for 24-hour stirring. The solution was subsequently mixed with anhydrous ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 ratio, v/v) overnight at 4 °C to obtain the product as precipitation. Following, the precipitation was collected by a centrifuge at 10
5 ratio, v/v) overnight at 4 °C to obtain the product as precipitation. Following, the precipitation was collected by a centrifuge at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min. The product was collected and deionized in a dialysis bag (MWCO: 8000–14
000 rpm for 10 min. The product was collected and deionized in a dialysis bag (MWCO: 8000–14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da) for 5 days. The HAMA product was freeze-dried for 72 hours.
000 Da) for 5 days. The HAMA product was freeze-dried for 72 hours.
To synthesize HAD, 0.20 g of HA was dissolved in 20 ml of PBS, and the solution was kept under argon; 0.08 g of EDC and 0.08 g of NHS were added to the solution (pH5) and stirred until completely dissolved. 0.10 g of Dopa was slowly poured into the reaction vessel. The reaction then continued at 25 °C for four hours. The procedure for lyophilization and dialysis was the same as for HAMA. HAMA and HAD synthesized in this experiment were characterized using 1H-NMR (400 MHz, Agilent, USA).
2.3. Preparation of hydrogel ink
Alg (5.0% w/v) was dissolved in an aqueous solution of photoinitiator I2959 (0.5% w/v) and HAMA (2.0% w/v). COL I (2.0% w/v) was added to the HAMA and Alg mix solution to obtain the upper layer bioinks. The upper layer inks were defined as HAC inks. The HAD (0.5% w/v) and BMP-2 (10 μg ml−1) were also added to the mix solution of the HAMA and Alg to prepare the lower layer inks. The lower layer inks were defined as HAHB inks. The 0.02 mol L−1 CaCl2 was used to pre-crosslink the inks.2.4. 3D scaffold printing
The scaffolds were constructed utilizing a 3D printer (Allevi3, USA) with a 25G injection needle at 25 °C. The shape and size of the scaffold were set in the printing software. The printed path of the dual nozzle was converted to a g-code format for printing. The upper- and lower-layer inks were crosslinked by adding 0.02 M Ca2+ (pre-crosslinking) and then loaded into the printing syringe. The volume of the 3D-printed bilayer scaffold was 20 × 20 × 1 mm3. Finally, the scaffolds were cross-linked by UV light irradiation and a large amount of Ca2+ (0.2 M).2.5. The property analysis of the scaffolds
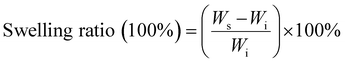 | (1) |
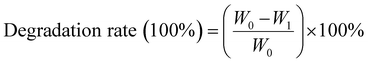 | (2) |
W 0 is the initial hydrogel dry weight, and W1 is the weight of the hydrogel remaining at various time points during degradation.
2.6. Release of BMP-2
10μg of BMP-2 was mixed in 1 mL of HAMA and Alg solution with HAD or without HAD and then cured to shape. Both scaffolds were immersed in 300 μL buffer solution and incubated at 37 °C. The supernatant was collected at predetermined times (days 1, 3, 5, 7, 14, 21, and 28) and replenished with an equal amount of buffer solution. The BMP-2 ELISA kit determined the release of BMP-2.2.7. In vitro experiments
2.8. Animal surgery
All animal experiments were approved by the Ethics Committee for Laboratory Animal Welfare of Yangzhou University (YZUDWLL-202303108). In this study, thirty-six 8-week-old Sprague–Dawley (SD) rats were used to establish the acute rotator cuff injury model. The rats were randomly divided into three groups based on treatment: no implantation scaffold (control), 3D-printed bilayer scaffold implantation (scaffold), and 3D-printed bilayer scaffold combined with ADSCs implantation (scaffold + ADSCs). SD rats were anesthetized via intraperitoneal injection of 20% urethane (1.0 g kg−1). Once anesthetized, the shoulder areas were shaved and sterilized. A longitudinal incision was made along the arm's axis at the acromion to bluntly separate the deltoid muscle and expose the supraspinatus tendon. The tendon was fully severed from the greater tuberosity, and any remaining tendon and fibrocartilaginous tissue in the footprint area were removed using a razor blade. A portion of the bone cortex tissue was also shaved. In the control group, the supraspinatus tendon was directly sutured and reattached to the original footprint. In the scaffold group, a double-layer 3D-printed hydrogel scaffold was cut to size, maintaining its microstructure, and implanted at the rotator cuff injury site. For the scaffold + ADSCs group, a suspension of ADSCs (1 × 105 cells per scaffold) was seeded into the bilayer scaffold and cultured for 24 hours before implantation. The scaffold with ADSCs was then fixed at the tendon–bone interface. Following surgery, the muscle and skin were sutured in layers, and penicillin sodium (2.2 × 105 U kg−1) was administered intramuscularly for 3 days postoperatively to prevent infection.2.9. Histologic and immunohistochemical examination
Rat rotator cuff specimens were collected at 4 and 8 weeks postoperatively and fixed in 4% paraformaldehyde for 48 hours. The specimens were decalcified using 10% ethylenediaminetetraacetic acid (EDTA) for 4 weeks. Tissue sections were stained with hematoxylin and eosin (HE), masson's trichrome (MT), safranin O/fast green (SO-FG), and sirius red (SR), and immunohistochemical (IHC) staining of COL I and COL II. Cell morphology and the degree of fibrovascular proliferation were scored according to the analysis of the HE staining results. Semi-quantification of stain results was calculated using ImageJ software.2.10. Micro-computed tomography (Micro-CT)
Repaired rotator cuff samples were collected at 8 weeks postoperatively and fixed using 4% paraformaldehyde for 24 hours. The humeral head of all samples was scanned and imaged by a small animal Micro-CT (SkyScan 1176, Bruker, Germany), and the TBI of the surgical area was set to the footprint area of the greater tuberosity of the humerus. The humeral head was reconstructed in three dimensions by CTAn and analyzed for bone mineral density (BMD) and bone volume fraction (BV/TV).2.11. Biomechanical testing
Samples were collected at 4 and 8 weeks postoperatively. The supraspinatus tendon and humerus were secured using a customized clamp in a modified 50 N sensor rheometer (MARS 60, Thermo Fisher Scientific, USA). The samples were loaded at a speed of 5 mm min−1 until the tendon ruptured at 25 °C. The load–displacement curves were recorded by the computer to obtain the maximum failure load.2.12. Statistical analysis
All data were expressed as mean ± standard deviation of at least three independent experiments. Unpaired t-tests were used for statistical comparisons between two groups, and one-way analysis of variance (ANOVA) was used for comparisons between more than two groups. Data are presented as mean ± SD, n = 3 per group. Statistically significant at *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. All analyses were performed using GraphPad Prism 8.0.3. Result
3.1. Characterization of the printing inks
The composition of the ink significantly affects both the printing process and the functional performance of the scaffold. The successful synthesis of HAMA and HAD was confirmed using 1H NMR analysis. As shown in Fig. S1A (ESI†), the proton signals at 6.1 ppm and 5.6 ppm (associated with methacrylate C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds) confirmed the successful synthesis of HAMA. Similarly, Fig. S1B (ESI†) displays the 1H NMR spectrum of HAD, where the peaks at 6.7 ppm and 7 ppm correspond to protons on the benzene ring, verifying the synthesis of HAD.
C bonds) confirmed the successful synthesis of HAMA. Similarly, Fig. S1B (ESI†) displays the 1H NMR spectrum of HAD, where the peaks at 6.7 ppm and 7 ppm correspond to protons on the benzene ring, verifying the synthesis of HAD.
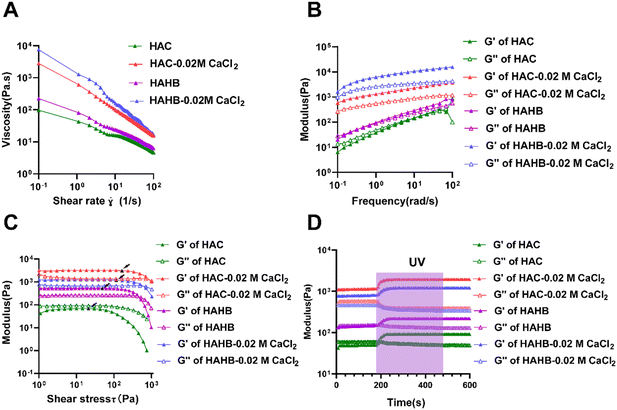
Fig. 2(A) illustrates the change in ink viscosity with increasing shear rate. The viscosity of both upper layer (HAC) and lower layer (HAHB) inks decreased with higher shear rates, indicating shear-thinning behavior. Adding 0.02 M CaCl2 increased the ink's viscosity while preserving its shear-thinning properties. Additionally, before Ca2+ cross-linking, the G′ was lower than the G′′, but the presence of Ca2+ reversed this relationship (G′ > G′′). A small amount of Ca2+ improved the viscosity and modulus, enhancing the scaffold's fidelity (Fig. 2(B)). The linear viscoelastic interval also showed that the addition of 0.02 M CaCl2 increased the yield stress of both the upper and lower layers of inks (Fig. 2(C)), indicating that pre-crosslinking with a small amount of Ca2+ optimized the extrusion process. Furthermore, photo-crosslink inks were tested for rheological properties under UV irradiation (Fig. 2(D)). The continuous increase in modulus during UV exposure further proved the effectiveness of HAMA.
3.2. Characterization of the hydrogel scaffolds
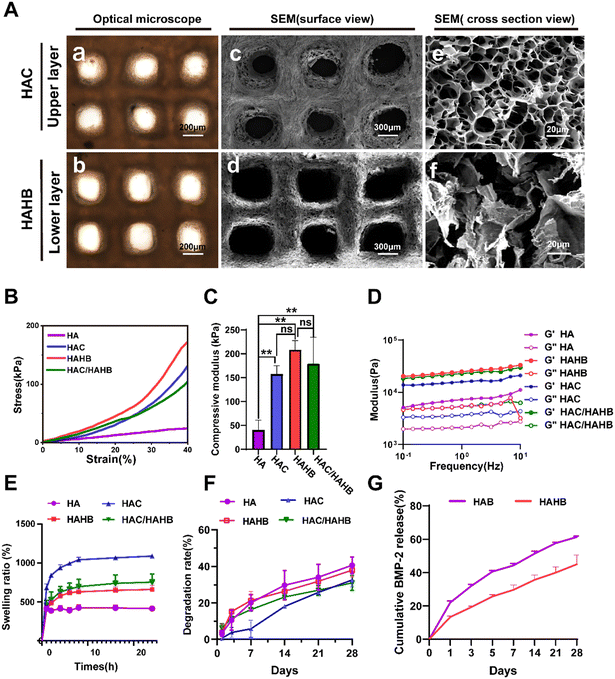
The pre-crosslinked inks were employed to fabricate porous hydrogel scaffolds successfully. Subsequently, the scaffolds underwent alternating exposure to ultraviolet light and were cross-linked with a high concentration of Ca2+ for 5 min each. Macro photographs of the scaffolds are depicted in Fig. S2 (ESI†). Surface morphology analysis showed that the pore size of the wet upper (HAC) and lower (HAHB) scaffold layers was approximately 200 μm (Fig. 3(A)-a and b), while the pore size of the dry scaffolds ranged from 300 to 500 μm (Fig. 3(A)-c and d). Although the freeze-drying process increased pore size, maintaining the scaffold's wet state is essential for practical applications. The wet pore size supports cell proliferation, migration, and nutrient transport. Additionally, the SEM images of the freeze-dried cross-section displayed a uniform network structure in both the upper and lower layers, indicating stability and suitability for biological applications (Fig. 3(A)-e and f).The hydrogel scaffolds were divided into groups to compare their mechanical, swelling, and degradation properties. The HA group consisted of HAMA and Alg, while the HAC group (upper layer) included HAMA, Alg, and COL I. The HAHB group (lower layer) comprised HAMA, Alg, HAD, and BMP-2. The HAC/HAHB group represented a bilayer scaffold. Mechanical properties were assessed using compression and oscillation tests. The stress–strain curves, shown in Fig. 3(B), revealed no rupture point within a 40% strain range across all groups. Compression modulus analysis indicated that scaffolds made of the HA scaffold alone had significantly lower compression modulus than HAC and HAHB scaffolds, suggesting that the addition of COL I and HAD effectively strengthened the hydrogel scaffolds. However, no significant difference was found between the bilayer scaffold and the individual upper or lower scaffolds (Fig. 3(C)). Oscillation resistance tests showed that the HAHB scaffolds (lower layer) had the highest shear strength, while the bilayer scaffold exhibited intermediate shear resistance between HAC and HAHB (Fig. 3(D)).
Regarding swelling properties, Fig. 3(E) illustrates that the wet weight of hydrogels in each group increased rapidly within the first 6 hours, reaching equilibrium by 24 hours. The bilayer scaffold's swelling ratio was between HAC and HAHB, with the HA hydrogel scaffolds showing the lowest swelling ratio. In terms of degradation, the HAC scaffold degraded slightly slower than the HAHB scaffold over 28 days, with the HA scaffold showing the highest degradation (Fig. 3(F)). The bilayer scaffold initially degraded quickly, providing sufficient space for early tissue regeneration. In summary, the data from the mechanical, swelling, and degradation tests is listed in Table S2 and Table S5 (ESI†).
The growth factors’ release behavior is essential for tissue regeneration. The adhesive function of dopamine in HAD was exploited to achieve the slow-release effect of BMP-2. To confirm a slow-release BMP-2 effect of the scaffold, the cumulative release of BMP-2 was compared between HAHB and HAB groups. Within the first 7 days, the initial BMP-2 release in HAHB was significantly lower than in the HAB group, where a burst release was observed (Fig. S3, ESI†). The cumulative BMP-2 release was lower in the HAHB group (44.97 ± 5.45%) compared to the HAB group (61.19 ± 0.61%), confirming the effectiveness of the slow-release system of the HAD and BMP-2 interaction (Fig. 3(G)).
3.3. Biocompatibility and biological function evaluation of the scaffolds
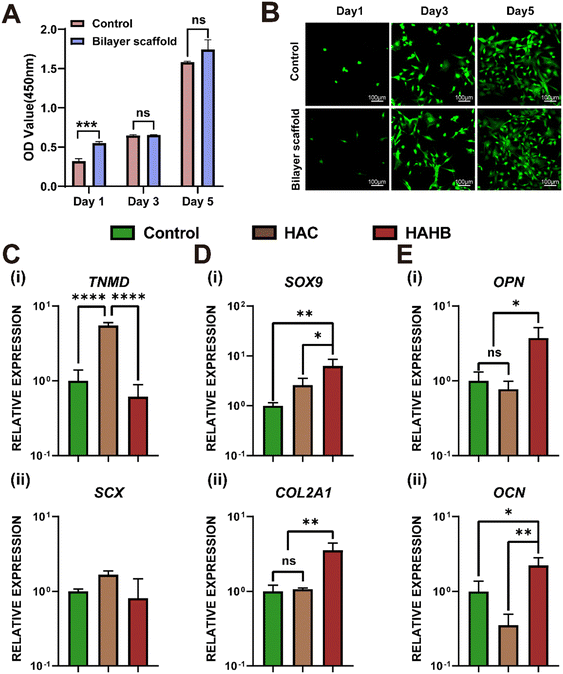
The toxicity of the double-layered scaffold on ADSCs was evaluated using the CCK8 assay kit and cell viability staining. As shown in Fig. 4(A), a significant difference in optical density (OD) value was observed between the scaffold group and the control group on the first day. However, no notable difference in cell proliferation was detected between the groups on the 3rd and 5th days, indicating similar growth rates at these later time points. The live/dead staining results demonstrated that the bilayer scaffold exhibited no apparent cytotoxic effects on ADSCs, as illustrated in Fig. 4(B).In the differentiation model in vitro, the RT-qPCR test was used to analyze gene expression related to tendon, bone, and cartilage differentiation in ADSCs within the upper layer (HAC) and lower layer (HAHB) of the 3D-printed hydrogel scaffold. After 14 days of culture, gene expression related to tendon differentiation was examined. Tendon-related gene (TNMD) expression revealed a significant upregulation in the HAC group compared to the HAHB and control groups (Fig. 4(C)-(i)). Although the expression of SCX, another tendon-related gene, did not differ significantly between groups, the HAC group displayed an upward trend in expression relative to the HAHB and control groups (Fig. 4(C)-(ii)). For cartilage differentiation, the HAHB group demonstrated a marked increase in the expression of cartilage-specific genes SOX9 and COL2 compared to the control group (Fig. 4(D)-(i) and (ii)). Regarding osteogenesis, the osteogenic markers OPN and OCN were significantly upregulated in the HAHB group compared to the HAC and control groups (Fig. 4(E)-(i) and (ii)). In conclusion, the upper scaffold (HAC) was effective in promoting tendon differentiation, while the lower scaffold (HAHB) was more conducive to osteogenesis, highlighting the specialized roles of the two scaffold layers in supporting different types of tissue regeneration.
3.4. Histologic staining and IHC staining
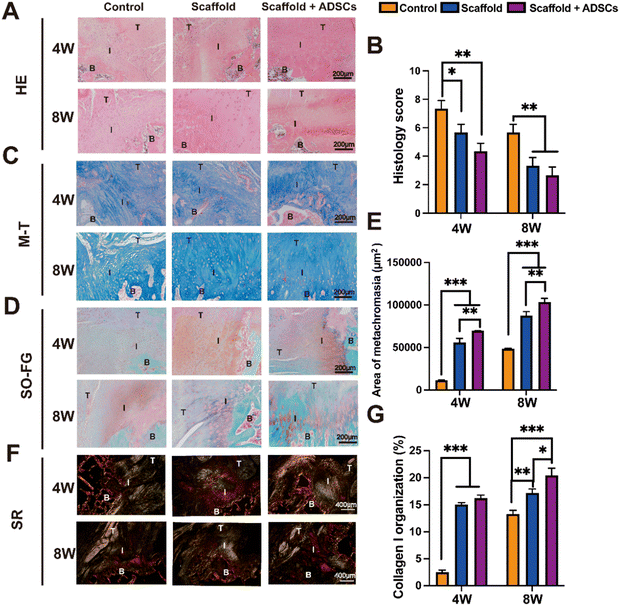
The promotion of rotator cuff repair by these two tissue engineering strategies (the scaffold group and the scaffold + ADSCs group) was evaluated in a rat model of acute rotator cuff injury. In contrast, the control group was not filled with scaffold. Immunofluorescence (IF) staining results demonstrated that inflammation persisted for 4 weeks post-operation. In both the control and scaffold groups, positive expression of the CD86 (M1 macrophage) marker was observed, whereas CD86 maker expression was nearly absent in the scaffold + ADSCs group. These findings indicated that the scaffold + ADSCs group effectively mitigated inflammation and promoted tissue repair (Fig. S4, ESI†). The criteria for evaluating tissue repair are summarized in Table S3 (ESI†), where a higher score indicates a poorer repair outcome. At 4 weeks post-surgery, the HE staining analysis revealed abnormal TBI cell morphology and significant fibrovascular proliferation in the control group compared to both the scaffold group and the scaffold + ADSCs group. While the scaffold and the scaffold + ADSCs group exhibited lower histological scores than the control group, there was no statistically significant difference between them. By 8 weeks, the scores showed a slight decrease in all groups, but the control group continued to have a significantly higher score than the scaffold group and the scaffold + ADSCs group (Fig. 5(A) and (B)).The SO-FG staining was used to assess glycosaminoglycan formation in fibrocartilage repair, with semi-quantification of the metachromasia area based on the SO-FG staining. The results indicated significant differences between the scaffold + ADSCs group and the control group at both 4 and 8 weeks (Fig. 5(D) and (E)). The M–T staining, commonly employed to evaluate collagen fiber regeneration, revealed limited collagen fiber formation in the control group compared to both the scaffold and scaffold + ADSCs groups at the same time points (Fig. 5(C)). Although M–T staining assesses collagen fiber regeneration, it cannot precisely determine collagen type or orientation.
The SR staining can evaluate preliminary insight into collagen type and orientation. The results of the SR staining showed a significantly higher amount of regenerated COL I in both the scaffold and scaffold + ADSCs groups compared to the control group at 4 weeks (Fig. 5(G)). Furthermore, the collagen fibers at the tendon–bone interface were denser and more orderly arranged in the scaffold and scaffold + ADSCs groups. By 8 weeks, collagen fiber regeneration had further progressed in all groups, but the TBI healing remained superior in the scaffold and scaffold + ADSCs groups compared to the surgical repair group (Fig. 5(F)).
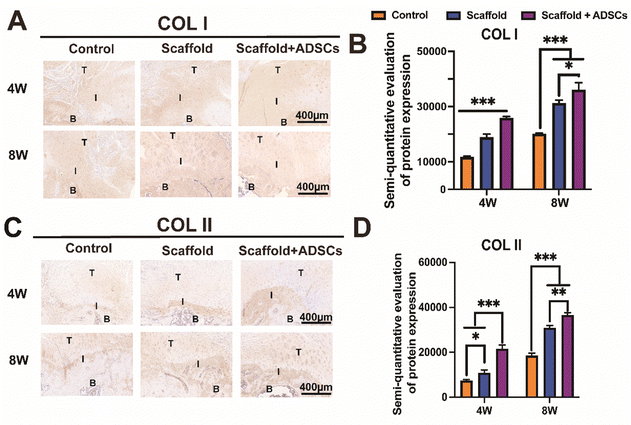
The IHC staining effectively identified collagen phenotypes, confirming accurate tissue characterization. The results for COL I indicated that its distribution at the TBI in each group at 4 and 8 weeks matched the normal collagen of tendons, cartilage, and bone (Fig. 6(A)). Semi-quantitative analysis revealed significantly higher COL I levels in the scaffold + ADSCs group compared to the control group (Fig. 6(B)). Similarly, COL II staining showed its distribution in the cartilage layer of the tendon–bone interface across all groups at both time points (Fig. 6(C)). The semi-quantitative data for COL II also demonstrated a significant increase in the scaffold + ADSCs group compared to the control group (Fig. 6D). These findings confirmed that the cartilage layer in all groups exhibited a mixed collagen phenotype, consistent with fibrocartilage characteristics. The scaffold + ADSCs group had the highest collagen content, indicating superior tendon–bone interface repair. In addition, all histological staining semi-quantitative data are listed in Table S4 (ESI†).
3.5. Micro-CT and biomechanical analysis
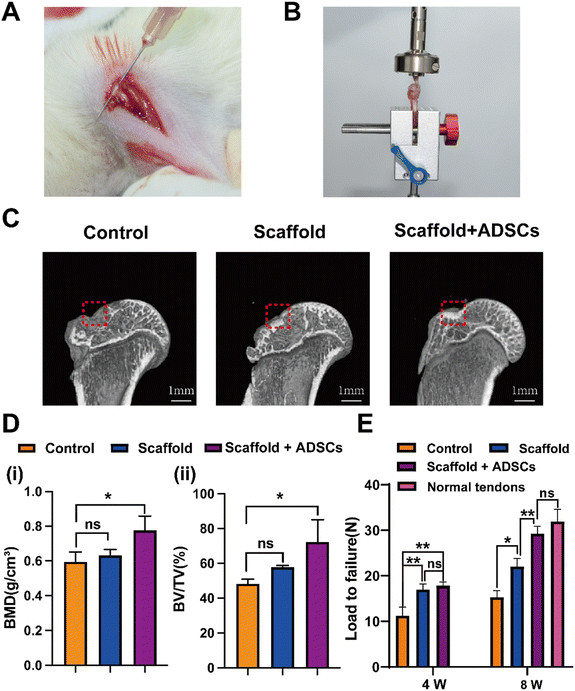
Eight weeks post-surgery, bone quality at the tendon–bone interface footprint was assessed using Micro-CT. During the establishment of the acute rotator cuff injury rat model, cortical bone damage was induced (Fig. 7(A)). Micro-CT imaging revealed a more intact bone cortex in the scaffold + ADSCs group compared to the other groups (Fig. 7(C)). In the control group, The BMD was measured at 0.596 ± 0.055 g cm−3, and BV/TV was 48.3 ± 2.6%. The scaffold group showed higher values, with a BV/TV of 57.9 ± 0.9% and a BMD of 0.633 ± 0.033 g cm−3, but these increases were not statistically significant compared to the control group. In contrast, the scaffold + ADSCs group demonstrated significantly enhanced bone parameters, with a BMD of 0.778 ± 0.082 g cm−3 and a BV/TV of 72.4 ± 12.7%, which were statistically significant compared to the other groups (Fig. 7(D)).Biomechanical testing of shoulder specimens was conducted at 4 and 8 weeks postoperatively (Fig. 7(B)). At 4 weeks, the maximum failure load was 11.21 ± 1.89 N in the control group, 16.98 ± 1.23 N in the scaffold group, and 17.83 ± 0.78 N in the scaffold + ADSCs group. At 8 weeks, the maximum failure load increased to 15.30 ± 1.44 N in the control group, 22.02 ± 1.76 N in the scaffold group, and 29.25 ± 1.62 N in the scaffold + ADSCs group. By 4 weeks, both the scaffold and scaffold + ADSCs groups exhibited significantly greater mechanical properties than the control group (Fig. 7(E)). At this stage, there was no significant difference between the scaffold and scaffold + ADSCs groups. By 8 weeks, all groups showed improved mechanical properties, with the scaffold + ADSCs group surpassing both the control and scaffold groups, exhibiting mechanical properties comparable to normal tendons.
4. Discussion
In recent years, tissue engineering therapeutic options have been optimized to restore the native rotator cuff structure after injury. These approaches include the development of improved scaffold materials, biomimetic structures, stem cell differentiation techniques, and the regulation of growth factors.37 In this study, we developed a 3D-printed biomimetic hydrogel scaffold with sustained BMP-2 release to facilitate rotator cuff repair. The scaffold's biomimetic components, such as proteoglycans and collagen, successfully directed the differentiation of ADSCs into osteogenic, chondrogenic, and tenogenic lineages, enabling effective tendon–bone interface (TBI) healing.The TBI of rotator cuff tissue is a complex structure with a gradient, which consists of tendon, cartilage, and bone tissues. Therefore, it is necessary to construct a bilayer scaffold loaded with biologically active substances, which can simultaneously induce the targeted differentiation of ADSCs to achieve tissue restoration with spatial gradient structure. The TBI in rotator cuff tissue is a complex, gradient structure composed of tendon, cartilage, and bone. Therefore, a bilayer scaffold loaded with bioactive substances is essential to induce targeted ADSCs differentiation and promote tissue regeneration with a spatial gradient. The precision of 3D printing allows the fabrication of personalized scaffolds with controlled spatial structures and the integration of bioactive components tailored to meet the specific needs of the injury site.38 For effective 3D printing, bioinks must exhibit specific rheological properties.39 In this study, we pre-crosslinked the bioinks by adding 0.02 M Ca2+, enhancing viscosity while maintaining shear-thinning behavior (Fig. 2(A)). After extrusion, the scaffolds underwent a secondary crosslinking step involving HAMA photocrosslinking and ionic crosslinking with 0.2 M Ca2+ to increase mechanical stiffness. Notably, the addition of HAD and COL I further improved scaffold stiffness, with the HAHB (lower layer) scaffold demonstrating greater rigidity than the HAC (upper layer) scaffold (Fig. 3(B)–(D)). The lower scaffold exhibits higher mechanical strength compared to the upper scaffold, which enhances the overall mechanical properties and stability of the construct. The 3D-printed scaffolds maintained their designed pore structures (Fig. 3(A) and Fig. S2, ESI†), which support cell growth, proliferation, differentiation, and the exchange of oxygen and nutrients.39,40 The bilayer porous scaffolds, each serving different functions, were designed to induce specific stem cell differentiation, thereby accelerating the healing of the multilayer TBI structure. ADSCs, with their multipotent differentiation capabilities, were used as seed cells in combination with the scaffolds to treat rotator cuff tears (RCTs) and promote TBI regeneration.
BMP-2 has demonstrated significant potential in promoting bone and cartilage repair in TBI healing.41,42 However, uncontrolled release of BMP-2 can lead to high local concentrations, resulting in complications such as heterotopic ossification and inflammation.43 Additionally, BMP-2 is unstable, making it challenging to sustain its therapeutic effects without adequate protection.44 To enhance the efficacy of BMP-2 in the prolonged process of tendon–bone healing, the adhesive properties of Dopa were utilized to facilitate the delivery and controlled release of BMP-2. The interaction between HAD and BMP-2 resulted in a hydrogel system designed for the sustained release of this growth factor.45,46 Release tests demonstrated that the incorporation of HAD effectively regulated the release rate of BMP-2, with a pronounced sustained release during the first week (Fig. S3, ESI†). In the HAHB group, the cumulative release of BMP-2 reached 44.97 ± 5.45%, indicating a continuous release throughout the tendon–bone healing process, which aids in accelerating tissue recovery (Fig. 3(G)).
In addressing the challenges of repairing the multilayered structures in RCT, single-layer scaffolds often fail to achieve spatially synchronized tissue regeneration. Previous studies have proved that multilayer scaffolds, designed with biomimetic structures and compositions, were more effective in promoting TBI regeneration.47 In this study, we developed a bilayer scaffold using two distinct functional bioinks, HAC and HAHB, to mimic the native structure and induce targeted differentiation. ADSCs cultured on the HAHB scaffold in the lower chamber of a transwell system exhibited increased osteogenic and chondrogenic differentiation after 14 days, while those exposed to the HAC scaffold showed enhanced expression of tendon-related genes (Fig. 4(C)–(E)). This effect was likely due to the different biomolecules in the two bioinks. Jiang et al. confirmed that COL I created a favorable microenvironment for cell adhesion, proliferation, and differentiation, particularly for fibroblasts and mesenchymal stem cells.48 Additionally, ADSCs interacted with the lower layer scaffold to enhance the expression of osteogenic and chondrogenic genes, primarily through BMP signaling pathways. Specifically, BMP-2 was released from the scaffold bound to the BMPR I and BMPR II receptors on the surface of the cells, leading to the phosphorylation of Smad1/5/8 proteins. The phosphorylated Smad1/5/8 then formed a complex with Smad4, which was translocated to the nucleus to initiate the expression of osteogenic genes essential for bone matrix formation. Moreover, some researches have proved that BMP-2 can activate Smad1/5/8 phosphorylation via the BMPR receptor, promoting cartilage matrix formation.49,50
Animal experiments provided compelling evidence for the efficacy of the biomimetic bilayer scaffold in enhancing TBI healing. Histological analysis demonstrated that the scaffold + ADSCs group exhibited the highest levels of collagen and glycosaminoglycan in the regenerated tissue. Fibrocartilage is a marker of tendon–bone interface regeneration.51 The SR and IHC staining revealed extensive COL I deposition in the Scaffold + ADSCs group, indicative of superior restoration of TBI structure and functionality. Additionally, the values of BMD and BV/TV in the footprint area were markedly highest in the scaffold + ADSCs group (Fig. 7(C) and (D)), which can be attributed to the role of BMP-2 in directing stem cell differentiation towards osteogenesis, facilitating new bone formation.52–54 Moreover, the HAC/HAHB bilayer scaffold not only accelerated the regeneration of the gradient structure of the TBI but also enhanced its mechanical properties. This is particularly evident in the scaffold + ADSCs group, which demonstrated the highest maximum failure load at 8 weeks post-surgery (Fig. 7(E)). The enhanced mechanical strength in this group was strongly correlated with the successful restoration of the gradient architecture of the TBI.
This study has several limitations. First, it utilized an acute rotator cuff injury model with an 8-week postoperative observation period. However, chronic rotator cuff injuries are more prevalent in clinical settings and are often accompanied by fat infiltration, tendon retraction, and more complex pathologies. These conditions also require longer recovery periods following surgery. Second, while ADSCs were employed as seed cells, only the final therapeutic outcomes were evaluated. The migration, proliferation, differentiation, and paracrine effects of stem cells during the healing process were not monitored or analyzed. Lastly, the tissue-engineered scaffold used in this study consisted of only a two-layer structure. Future efforts should introduce additional biomolecules to enhance region-specific differentiation and achieve more comprehensive biomimetic reconstruction.
5. Conclusion
In this study, a 3D-printed biomimetic scaffold with slow-release BMP-2 was successfully constructed to promote TBI repair. The sustained-release system was achieved through the interaction between HAD and BMP-2, effectively reducing the initial rapid-release BMP-2 behavior, as confirmed by ELISA results. The qPCR analysis demonstrated that the bilayer scaffold exhibited excellent osteogenic, chondrogenic, and tenogenic potential in vitro. This was primarily attributed to the proteoglycan (HAMA and Alg), and COL I created a favorable microenvironment for cell activity. At the same time, the sustained release of BMP-2 from the lower scaffold facilitated osteogenic induction. Combining the bilayer scaffold and ADSCs successfully promoted organized structural regeneration and mechanical recovery of the TBI in vivo. In conclusion, 3D-printed biomimetic scaffolds with sustained BMP-2 release, in conjunction with ADSCs, present a promising therapeutic approach for improving the structural and functional recovery of the tendon–bone interface, making them a valuable adjunctive treatment option for rotator cuff tear (RCT) surgery.Data availability
The data supporting this article have been included as part of the ESl.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank The National Orthopaedic and Sports Rehabilitation Clinical Medical Research Center Project (grant 2021-NCRC-CXJJ-PY-07), The Scientific research project of Jiangsu Provincial Health and Health Commission (grant M2021042), The 2023 Major Sports Research Projects in Jiangsu Province (grant ST231209), The Multi-discipline Scientific Research Foundation of Northern Jiangsu People's Hospital (grant SBJC22005) and the Young scientists lifting project of Jiangsu Province, China (TJ-2022-072). Part of the drawing materials in Fig. 1 were drawn through the Figdraw platform.References
- B. S. Miller, B. K. Downie, R. B. Kohen, T. Kijek, B. Lesniak, J. A. Jacobson, R. E. Hughes and J. E. Carpenter, Am. J. Sports Med., 2011, 39, 2064–2070 CrossRef PubMed.
- N. Saveh-Shemshaki, L. S. Nair and C. T. Laurencin, Acta Biomater., 2019, 94, 64–81 CrossRef CAS PubMed.
- K. L. Moffat, W. H. Sun, P. E. Pena, N. O. Chahine, S. B. Doty, G. A. Ateshian, C. T. Hung and H. H. Lu, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 7947–7952 CrossRef CAS PubMed.
- S. Patel, J. M. Caldwell, S. B. Doty, W. N. Levine, S. Rodeo, L. J. Soslowsky, S. Thomopoulos and H. H. Lu, J. Orthop. Res., 2018, 36, 1069–1077 CrossRef PubMed.
- J. Shi, H. Yao, B. Wang, J. Yang, D. Liu, X. Shang, H. Chong, W. Fei and D.-A. Wang, Macromol. Rapid Commun., 2024, 45, 2300508 CrossRef CAS PubMed.
- Q. Zhang, C. Qian, W. Xiao, H. Zhu, J. Guo, Z. Ge and W. Cui, RSC Adv., 2019, 9, 18344–18352 RSC.
- Z. Wu, H. Yao, H. Sun, Z. Gu, X. Hu, J. Yang, J. Shi, H. Yang, J. Dai, H. Chong, D.-A. Wang, L. Lin and W. Zhang, Mater. Today Bio, 2024, 26, 101080 CrossRef CAS PubMed.
- C. Zhang, H. Wu, J. Chen, P. Zhu and C. Gao, J. Appl. Polym. Sci., 2021, 138, 50978 CrossRef CAS.
- H. Honda, M. Gotoh, T. Kanazawa, H. Ohzono, H. Nakamura, K. Ohta, K. I. Nakamura, K. Fukuda, T. Teramura, T. Hashimoto, S. Shichijo and N. Shiba, Am. J. Sports Med., 2017, 45, 3322–3330 CrossRef PubMed.
- Z. Wu, H. Sun, X. Hu, J. Yang, Z. Gu, C. Wang, H. Chong, J. Dai, H. Yao and D.-A. Wang, Eur. Polym. J., 2024, 218, 113343 CrossRef CAS.
- W. Wu, Q. Ni, Y. Xiang, Y. Dai, S. Jiang, L. Wan, X. Liu and W. Cui, RSC Adv., 2016, 6, 92449–92453 RSC.
- J. Guo, J. Jiang, X. Gu, X. Li and T. Liu, Colloids Surf., A, 2021, 608, 125548 CrossRef CAS.
- H. Yao, X. Yuan, Z. Wu, S. Park, W. Zhang, H. Chong, L. Lin and Y. Piao, Int. J. Mol. Sci., 2023, 24, 1240–1256 CrossRef CAS PubMed.
- G. Falcone, P. Mazzei, A. Piccolo, T. Esposito, T. Mencherini, R. P. Aquino, P. Del Gaudio and P. Russo, Carbohydr. Polym., 2022, 276, 118746 CrossRef CAS PubMed.
- J. Namba, K. Shimada, M. Saito, T. Murase, H. Yamada and H. Yoshikawa, J. Biomed. Mater. Res., Part B, 2007, 80, 273–279 CrossRef PubMed.
- Y. Qin, G. Ge, P. Yang, L. Wang, Y. Qiao, G. Pan, H. Yang, J. Bai, W. Cui and D. Geng, Adv. Sci., 2023, 10, 2207334 CrossRef CAS PubMed.
- S. K. Madhurakkat Perikamana, J. Lee, T. Ahmad, E. M. Kim, H. Byun, S. Lee and H. Shin, Biomaterials, 2018, 165, 79–93 CrossRef CAS PubMed.
- I. Calejo, R. L. Reis, R. M. A. Domingues and M. E. Gomes, Adv. Healthcare Mater., 2022, 11, 2102076 CrossRef CAS PubMed.
- Q. Huang, Y. Zou, M. C. Arno, S. Chen, T. Wang, J. Gao, A. P. Dove and J. Du, Chem. Soc. Rev., 2017, 46, 6255–6275 RSC.
- J. H. Oh, S. W. Chung, S. H. Kim, J. Y. Chung and J. Y. Kim, J. Shoulder Elbow Surg., 2014, 23, 445–455 CrossRef PubMed.
- J. Lee, W. I. Choi, G. Tae, Y. H. Kim, S. S. Kang, S. E. Kim, S.-H. Kim, Y. Jung and S. H. Kim, Acta Biomater., 2011, 7, 244–257 CrossRef CAS PubMed.
- C. H. Chen, C. H. Chang, K. C. Wang, C. I. Su, H. T. Liu, C. M. Yu, C. B. Wong, I. C. Wang, S. W. Whu and H. W. Liu, Knee Surg. Sports Traumatol. Arthrosc., 2011, 19, 1597–1607 CrossRef PubMed.
- Y. Hirakawa, T. Manaka, K. Orita, Y. Ito, K. Ichikawa and H. Nakamura, J. Shoulder Elbow Surg., 2018, 27, 894–902 CrossRef PubMed.
- B. Wang, Y. Guo, X. Chen, C. Zeng, Q. Hu, W. Yin, W. Li, H. Xie, B. Zhang, X. Huang and F. Yu, Int. J. Nanomed., 2018, 13, 7395–7408 CrossRef CAS PubMed.
- C. L. Anderson and M. C. Whitaker, Spine, 2012, 37, E502–506 CrossRef PubMed.
- K. Miyazawa, T. Kawai and M. R. Urist, Clin. Orthop. Relat. Res., 1996, 259–268, DOI:10.1097/00003086-199603000-00032.
- J. Kim, I. S. Kim, T. H. Cho, K. B. Lee, S. J. Hwang, G. Tae, I. Noh, S. H. Lee, Y. Park and K. Sun, Biomaterials, 2007, 28, 1830–1837 CrossRef CAS PubMed.
- T. Gonzalez-Fernandez, E. G. Tierney, G. M. Cunniffe, F. J. O’Brien and D. J. Kelly, Tissue Eng., Part A, 2016, 22, 776–787 CrossRef CAS PubMed.
- H. Lee, S. M. Dellatore, W. M. Miller and P. B. Messersmith, Science, 2007, 318, 426–430 CrossRef CAS PubMed.
- J. Kang, S. Tada, T. Kitajima, T. I. Son, T. Aigaki and Y. Ito, BioMed Res. Int., 2013, 2013, 265980 Search PubMed.
- J. Y. Lee, M. H. Kang, J. E. Jang, J. E. Lee, Y. Yang, J. Y. Choi, H. S. Kang, U. Lee, J. W. Choung, H. Jung, Y. C. Yoon, K. H. Jung, S. S. Hong, E. C. Yi and S. G. Park, Sci. Rep., 2022, 12, 8620 CrossRef CAS PubMed.
- Y. Gao, N. Mi, Y. Zhang, X. Li, W. Guan and C. Bai, J. Nanobiotechnol., 2022, 20, 487 CrossRef CAS PubMed.
- I. C. Iser, L. R. Beckenkamp, J. H. Azambuja, F. L. Rahmeier, P. A. Bracco, A. P. S. Bertoni, R. de Cássia Sant’Anna Alves, E. Braganhol, L. L. Xavier, M. da Cruz Fernandes, G. Lenz and M. R. Wink, Stem Cell Rev. Rep., 2022, 18, 1495–1509 CrossRef CAS PubMed.
- F. Zhao, J. Cheng, J. Zhang, H. Yu, W. Dai, W. Yan, M. Sun, G. Ding, Q. Li, Q. Meng, Q. Liu, X. Duan, X. Hu and Y. Ao, Acta Biomater., 2021, 131, 262–275 CrossRef CAS PubMed.
- S. C. Chen, T. Jiang, Q. Y. Liu, Z. T. Liu, Y. F. Su and H. T. Su, Stem Cell Res. Ther., 2022, 13, 453 CrossRef CAS PubMed.
- X. Duan, Y. Luo, R. Zhang, H. Zhou, W. Xiong, R. Li, Z. Huang, L. Luo, S. Rong, M. Li, Y. He and Q. Ye, Mater. Today Adv., 2023, 17, 100336 CrossRef CAS.
- D. L. Butler, Tissue Eng. Regener. Med., 2022, 16, 1091–1108 CrossRef CAS PubMed.
- S. Wang, S. Zhao, J. Yu, Z. Gu and Y. Zhang, Small, 2022, 18, 2201869 CrossRef CAS PubMed.
- A. Schwab, R. Levato, M. D’Este, S. Piluso, D. Eglin and J. Malda, Chem. Rev., 2020, 120, 11028–11055 CrossRef CAS PubMed.
- Y. Zhang, N. Sun, M. Zhu, Q. Qiu, P. Zhao, C. Zheng, Q. Bai, Q. Zeng and T. Lu, Biomater. Adv., 2022, 133, 112651 CrossRef CAS PubMed.
- C. Chen, Q. Shi, M. Li, Y. Chen, T. Zhang, Y. Xu, Y. Liao, S. Ding, Z. Wang, X. Li, C. Zhao, L. Sun, J. Hu and H. Lu, Bioact. Mater., 2022, 16, 451–471 CAS.
- S. Qian, Z. Wang, Z. Zheng, J. Ran, J. Zhu and W. Chen, Med. Sci. Monit., 2019, 25, 269–278 CrossRef CAS PubMed.
- A. W. James, G. LaChaud, J. Shen, G. Asatrian, V. Nguyen, X. Zhang, K. Ting and C. Soo, Tissue Eng., Part B, 2016, 22, 284–297 CrossRef CAS PubMed.
- H. K. Min, S. H. Oh, J. M. Lee, G. I. Im and J. H. Lee, Acta Biomater., 2014, 10, 1272–1279 CrossRef CAS PubMed.
- S. J. Lee, D. Lee, T. R. Yoon, H. K. Kim, H. H. Jo, J. S. Park, J. H. Lee, W. D. Kim, I. K. Kwon and S. A. Park, Acta Biomater., 2016, 40, 182–191 CrossRef CAS PubMed.
- K. W. Lee, J. S. Lee, J. W. Jang, Y. B. Shim and K. I. Lee, Tissue Eng. Regener. Med., 2017, 11, 1435–1441 CrossRef CAS PubMed.
- S. Chae, Y. Sun, Y.-J. Choi, D.-H. Ha, I. Jeon and D.-W. Cho, Biofabrication, 2021, 13, 035005 CrossRef CAS PubMed.
- Q. Jiang, L. Wang, Z. Liu, J. Su, Y. Tang, P. Tan, X. Zhu, K. Zhang, X. Ma, J. Jiang, J. Zhao, H. Lin and X. Zhang, Bioact. Mater., 2023, 20, 1–15 CrossRef CAS PubMed.
- M. Wu, S. Wu, W. Chen and Y. P. Li, Cell Res., 2024, 34, 101–123 CrossRef CAS PubMed.
- V. S. Salazar, L. W. Gamer and V. Rosen, Nat. Rev. Endocrinol., 2016, 12, 203–221 CrossRef CAS PubMed.
- C. Ye, W. Zhang, S. Wang, S. Jiang, Y. Yu, E. Chen, D. Xue, J. Chen and R. He, Int. J. Mol. Sci., 2016, 17, 1780 CrossRef PubMed.
- S. A. Rodeo, K. Suzuki, X. H. Deng, J. Wozney and R. F. Warren, Am. J. Sports Med., 1999, 27, 476–488 CrossRef CAS PubMed.
- Y. Kawakami, K. Takayama, T. Matsumoto, Y. Tang, B. Wang, Y. Mifune, J. H. Cummins, R. J. Warth, R. Kuroda, M. Kurosaka, F. H. Fu and J. Huard, Am. J. Sports Med., 2017, 45, 584–597 CrossRef PubMed.
- D. M. Kim, I. K. Shim, M. J. Shin, J. H. Choi, Y. N. Lee, I. H. Jeon, H. Kim, D. Park, E. Kholinne and K. H. Koh, Am. J. Sports Med., 2020, 48, 2161–2169 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4tb01073f |
| ‡ The authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |