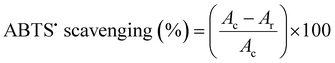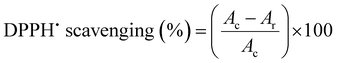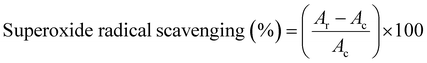Post-therapy via integrated curcumin and doxorubicin modified cerium-based UiO-66 MOFs using an antioxidant and anticancer therapeutic strategy†
Chao-Jan
Liu
a,
Jung-Hua
Lin
a,
Man-Tzu
Li
b,
Er-Chieh
Cho
*bcde and
Kuen-Chan
Lee
 *af
*af
aDepartment of Science Education, National Taipei University of Education, No. 134, Sect. 2, Heping E. Rd., Da’an District, Taipei City 106, Taiwan. E-mail: kclee@tea.ntue.edu.tw
bMaster Program in Clinical Genomics and Proteomics, College of Pharmacy, Taipei Medical University, 250 Wuxing Street, Taipei City, 110, Taiwan. E-mail: echo@tmu.edu.tw
cSchool of Pharmacy, College of Pharmacy, Taipei Medical University, 250 Wuxing Street, Taipei City, 110, Taiwan
dCancer Center, Wan Fang Hospital, Taipei Medical University, 110, Taiwan
eTMU Research Center of Cancer Translational Medicine, Taiwan
fPh.D. Program in Medical Neuroscience, College of Medical Science and Technology, Taipei Medical University, Taipei City, 110, Taiwan
First published on 16th October 2024
Abstract
The quest for effective cancer treatment methodologies underpins numerous research endeavors. Despite the therapeutic efficacy of conventional chemotherapy against malignant tumors, tumor recurrence post-therapy remains a formidable challenge. Addressing this, we developed a dual drug delivery system, rooted in a modified metal–organic framework (MOF), specifically by substituting the metal nodes of Uio-66 with cerium to augment its anti-oxidative potential. This engineered system, pyrene-modified hyaluronic acid, functions as a linker, enabling the self-assembly and encapsulation of both the material and the therapeutic agents, and encompasses both doxorubicin and curcumin, aimed at targeting cancer cell eradication and tumorigenesis inhibition. This system demonstrated significant antioxidant capacity through free radical scavenging assays, positioning it as a potential agent in mitigating tumor recurrence. Enhanced anti-tumor activity was distinctly evidenced in human colon cancer cell lines. Additionally, in vitro drug release assessments revealed slow-release kinetics and acid-responsive traits, attributed to the incorporation of pyrenylated hyaluronic acid. Within the xenograft nude mouse model, this system contained a lower amount of doxorubicin, yet, exhibited tumor inhibition capability comparable to the free doxorubicin group. Moreover, it delivered anticancer efficiency under conditions of enhanced antioxidative capacity, underscoring its prospective utility in clinical cancer therapeutics. This dual drug delivery platform not only advances cancer treatment and prophylaxis but also extends novel insights into the therapeutic implications of simultaneous dual drug delivery systems.
1. Introduction
Cancer is universally acknowledged as a significant global public health challenge characterized by limited effective treatment options, dismal prognoses, and high mortality rates.1–3 Oxidative stress, induced by the excessive generation of free radicals or reactive oxygen species (ROS), is recognized as a pivotal pathogenic mechanism contributing to the initiation and progression of cancer.4 Consequently, the exploration of polyphenols in cancer treatment has gained prominence in recent years.5 Polyphenols exert their antioxidant effects by capitalizing on benzene ring-bound hydroxyl groups, which confer the capability to donate hydrogen atoms or electrons to neutralize free radicals.6 One noteworthy polyphenolic compound is curcumin, extracted from turmeric, which has been employed in cancer and inflammation treatment. While curcumin boasts a wide array of pharmacological potential, its bioavailability remains limited, thus constraining its therapeutic utility upon administration.7 To surmount this limitation, researchers have investigated various nanocarriers encapsulating curcumin, such as metal nanoparticles, polymers and metal–organic frameworks.8–13Metal–organic frameworks (MOFs) are structures composed of metal ions or clusters linked by organic molecules.14 Recently, nanoscale MOFs have gained attention for their potential in biomedical fields, especially drug delivery.15 Their large surface areas and tunable pore sizes and compositions make them ideal for drug encapsulation. UiO-66-BDC (UiO = Universitetet i Oslo) is a sub-class of MOFs used as organic ligands by 1,4-benzenedicarboxylic acid (H2BDC) or its derivatives to connect metal ions (Zr4+).16 Given its robust chemical, mechanical, and thermal properties, UiO-66-BDC is a prime candidate for drug encapsulation, notably in antioxidant and anticancer therapies.15,17–19 Its ability to degrade under acidic conditions makes it effective for targeting cancer cells, which exhibit a lower pH due to elevated glycolysis.17 However, in the realm of research on metal–organic frameworks (MOFs) as drug delivery platforms, the synergistic effects of substituting different metal ions have been seldom discussed by scientists. A review of the literature reveals that cerium-based materials exhibit promising results in both antioxidant and anti-cancer applications.20,21 While the majority of rare earth elements predominantly exist in a trivalent state (+3), cerium is unique in that it can also be found in a tetravalent (+4) state.22 This distinction is attributed to the relatively low reduction potential (∼1.52 V) of the Ce4+/Ce3+ redox couple, allowing cerium to readily oscillate between these two oxidation states.23,24 This inherent redox capability enables cerium ions to act as exceptional oxygen buffers. As the cerium oxidation state shifts, cerium-based materials undergo structural changes, forming oxygen vacancies or defects in their lattice.25 This is a result of the loss of oxygen and/or its electrons. These structural alterations endow cerium-based materials with a remarkable regenerative capacity, making it an effective antioxidant material. Therefore, in our study, we substituted the prevalent zirconium moiety in UiO-66 with a cerium group. This modification aims to synergistically enhance the antioxidant capacity when utilizing the MOF as a carrier for curcumin.
From the perspectives outlined above, and in our proactive approach to combat the detrimental effects of cancer, we have developed an innovative drug delivery system. This system collaboratively delivers two prominent anti-cancer agents: curcumin and doxorubicin. Central to our approach is cerium-based UiO-66, which acts as a carrier. With its inherent antioxidant potential, UiO-66 collaborates with the drugs to manifest potent antioxidant and anti-cancer properties. Augmenting this system, pyrene-modified hyaluronic acid functions as a linker, enabling the self-assembly encapsulation of both the material and the therapeutic agents. Crucially, the integration capitalizes on the free radical scavenging abilities of cerium-based materials, boosting the system's antioxidant capacity. This, in turn, preserves the tumor suppressive impact of the anticancer drugs. Collectively, this strategy offers a compelling new avenue for cancer treatment and anti-oxidant therapy (Scheme 1).
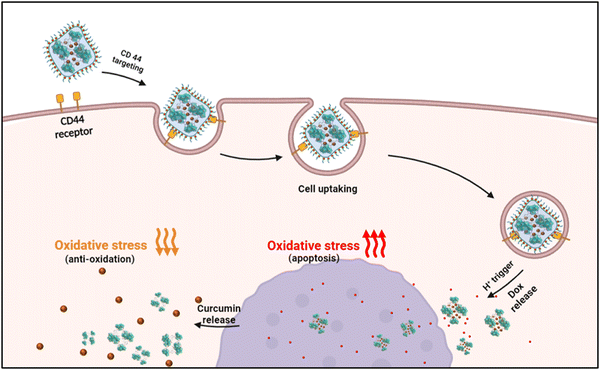 | ||
| Scheme 1 Schematic diagram of the cellular uptake and chemotherapeutic applications of dox/cur@Ce-Uio-66. | ||
2. Materials and methods
2.1 Materials
Diammonium cerium(IV) nitrate (Ce(NH4)2(NO3)6) and N,N-dimethylformamide (DMF) were purchased from Showa Kako corporation. Curcumin was purchased from Cayman Chemical Company. Hyaluronic acid (MW: 600–800 kDa) was purchased from Kikkoman. AzBTS (ABTS) was obtained from Tokyo Chemical Industry Co., Ltd. 1-Pyrenebutyric acid N-hydroxysuccinimide ester, N-hydroxysuccinimide (NHS) and 3-(ethyliminomethyleneamino)-N,N-dimethyl-propan-1-amine (EDC) were provided by Thermo Fisher Scientific. Dimethyl sulfoxide (DMSO) and ethylenediamine (C2H4(NH2)2) were purchased from Echochemical. Terephthalic acid (BDC), 2,2-diphenyl-1-picrylhydrazyl (DPPH), nitroblue tetrazolium chloride (NBT), titanium(IV) oxide (TiO2), and doxorubicin (hydrochloride) were all purchased from Sigma-Aldrich.2.2 Synthesis of Ce-Uio-66
Ce-Uio-66 was synthesized according to a previously published protocol.26 In brief, 1,4-benzendicarboxylic acid (H2BDC, 35.4 mg, 213 μmol) was dissolved in N,N-dimethylformamide (DMF, 1.2 ml). After that, an aqueous solution of diammonium cerium(IV) nitrate (400 μL, 0.5333 M) was added. The mixtures were stirred and heated at 100 °C for 15 min. Then, the formed light-yellow precipitate was collected by centrifugation. To remove unreacted H2BDC, the solid was washed and centrifuged with DMF two times. Then washed and centrifuged with ethanol four times to remove DMF. The resulting solid was dried in air at 60 °C for 24 h.2.3 Curcumin loading
To prepare cur@Ce-Uio-66, the co-precipitation method was used for curcumin loading. After collecting the dried Ce-Uio-66, 8 mg of Ce-Uio-66 and 20 mg of curcumin were soaked in 2 mL of ethanol. The mixtures were allowed to stand overnight in the dark while stirring in sealed glass bottles. Next, the curcumin loaded MOF (cur@Ce-Uio-66) was washed three times using pure ethanol to remove curcumin from the outer surface of the cur@Ce-Uio-66 composites. The sample was collected by suction filtration and dried in air at 60 °C for 24 h. To calculate the drug loading efficiency, the supernatant solution of cur@ Ce-Uio-66 after centrifugation (1089 × g for 10 min) was collected. The concentration of curcumin was determined by absorption at 422 nm, which was detected using a UV-Vis spectrophotometer.2.4 Curcumin release
In vitro release of curcumin from the cur@Ce-Uio-66 composite was measured using a UV-Vis spectrophotometer. In a typical experiment, the drug release study was performed at physiological pH (pH ∼7.5) and lysosomal pH (pH ∼5.0) at 37 °C to evaluate the drug release efficiency of the composite. In brief, 0.5 mg of cur@Ce-Uio-66 was dispersed in 10 ml of PBS at pH 7.5 and 5.0 at 37 °C, respectively. After incubation, 1 ml of mixture was obtained and centrifuged. The supernatant was taken out to test the concentration of the released curcumin using a UV-Vis spectrophotometer.2.5 Hyaluronic acid-coating
First, 17.4 mg of amino-modified 1-pyrenebutyric acid N-hydroxysuccinimide was suspended in 17.4 ml of dimethyl sulfoxide (DMSO) under sonication for 10 min. Then, the solution was stirred overnight after 3 μl of ethylenediamine (EDA) was added to form amino-modified pyrene. The hyaluronic acid-coated cur@Ce-Uio-66 was prepared by soaking 5 mg of cur@Ce-Uio-66 and 20 mg of HA into 4 ml of DI water, and followed by sonication for at least 30 min and stirring overnight to gain the HA modified cur@Ce-Uio-66 solution. Subsequently, 2 ml of amino-modified pyrene was mixed with HA modified cur@Ce-Uio-66 solution. 100 μl of 3-(ethyliminomethyleneamino)-N,N-dimethyl-propan-1-amine (EDC) and N-hydroxysuccinimide (NHS). The mixture was maintained under constant stirring for 12 hours and then purified by dialysis (MWCO: 3.5–5 kD) against DI water.2.6 Doxorubicin loading
To load doxorubicin into MOF particles, 0.5 milligrams per milliliter of dox was added into the previously obtained mixture under sonication for 30 min. After stirring overnight, the mixture was centrifuged (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 min) and washed 3 times with DI water and ethanol. Purified dox/cur@Ce-Uio-66 powder was collected by lyophilization. To calculate the doxorubicin encapsulation efficiency and drug loading efficiency, the supernatant solution of cur@Ce-Uio-66 after centrifugation (1089 × g for 10 min) was collected. The concentration of dox was determined by absorption at 484 nm, which was detected using a UV-Vis spectrophotometer (Hitachi U-5100 UV-Vis spectrophotometer).
000 rpm for 5 min) and washed 3 times with DI water and ethanol. Purified dox/cur@Ce-Uio-66 powder was collected by lyophilization. To calculate the doxorubicin encapsulation efficiency and drug loading efficiency, the supernatant solution of cur@Ce-Uio-66 after centrifugation (1089 × g for 10 min) was collected. The concentration of dox was determined by absorption at 484 nm, which was detected using a UV-Vis spectrophotometer (Hitachi U-5100 UV-Vis spectrophotometer).
2.7 Doxorubicin release
Dox release studies were performed at physiological pH and lysosomal pH as follows: 1 mg of dox/cur@Ce-Uio-66 was dispersed in 10 mL of pH = 7.5 medium at 37 °C. In another group, a little 1.0 M HCl was added into the solution until the pH value of the mixture reached pH = 5.0. After incubation, 1 ml of the mixture was obtained and centrifuged. The supernatant was taken out to test the concentration of released doxorubicin using a UV-Vis spectrophotometer.2.8 Characterization
Crystal structures of MOFs were analyzed by X-ray powder diffraction (XRD) using a Bruker D8-Advance diffractometer, with a scan range of 2θ from 5°–50° and a step size of 0.035484°. X-ray Photoelectron Spectra (XPS) were acquired with a JEOL JPS-9030 using MgKα radiation (1253.6 eV), referencing the C 1s core level at 284.8 eV. Fourier transform infrared (FT-IR) spectra were captured between 500 and 4000 cm−1 using a JASCO FT/IR-4600 with a JASCO ATR PRO ONE accessory. Nitrogen sorption experiments, conducted at 77 K on a Micromeritics 3FLEX analyzer, used the Brunauer–Emmett–Teller (BET) equation to determine the surface areas. The morphology and microstructure of cur@Ce-Uio-66 were observed with a HitachiS4800 FE-SEM. Samples for testing were prepared by depositing a drop of the cur@Ce-Uio-66 suspension onto carbon-coated copper grids and drying it at room temperature post excess liquid removal. Particle ξ potential was measured using an ELS-2000ZS Zeta Sizer before and after drug loading, and dynamic light scattering (DLS) was used to assess average hydrodynamic particle sizes.2.9 ABTS free radical scavenging assay
60 μg ml−1 free curcumin, Uio-66, Ce-Uio-66, and cur@Ce-Uio-66 were mixed with ABTS˙+ radical solution by different methods, and then the absorbance at 734 nm within 60 min was examined using a cell imaging multi-mode reader. The analysis of ABTS˙+ scavenging activity was determined according to the method of Re et al.27 Briefly, ABTS˙+ was produced by reacting 2 mM ABTS in ddwater with 2.45 mM potassium persulfate (K2S2O8), and then the mixture was stored in the dark at room temperature for 4 hours. The ABTS˙+ solution was diluted to give an absorbance of 0.750 ± 0.025 at 734 nm in sodium phosphate buffer (PBS buffer, pH 7.5). After that, different amounts (50 μL, 100 μL, and 200 μL) of antioxidant (600 μg ml−1) were added to 800 μL of ABTS˙+ solution and topped up to 1 ml with dd-water. The remaining ABTS˙+ was quantified spectrophotometrically at 734 nm.28 After a fixed time period, the absorbance was recorded after mixing and the percentage of radical scavenging was calculated for each concentration 11 relative to a blank containing no scavenger. The scavenging capability of the test compounds was calculated using the following equation:where Ac is the absorbance of the control (blank) without any radical scavenger and Ar is the absorbance of the remaining ABTS˙+ after reacting with a radical scavenger.
2.10 DPPH free radical scavenging assay
This assay makes use of 2,2-diphenyl-1-picrylhydrazyl (DPPH˙) which is a relatively stable radical as a reagent in order to assess the DPPH˙ free radical scavenging capacity of cur-Ce-Uio-66. The method was slightly modified from that previously described by Gülçin.29 The DPPH radical absorbs at 517 nm, and after reduction by an antioxidant or a radical scavenger, its absorption decreases due to a hydrogen atom or electron being transferred to the odd electron in DPPH˙.30 Briefly, a 0.1 mM solution of DPPH˙ was prepared in methanol and antioxidants were also prepared in methanol with different concentrations. After that, 1 mL of antioxidant was added to 1 mL of DPPH˙ solution. These solutions were vortexed thoroughly and incubated in the dark for 60 min. The absorbance was measured at 517 nm using a UV-Vis spectrophotometer against blank samples without a scavenger to calculate the percentage of radical scavenging. The capability to scavenge the DPPH˙ radical was calculated using the 12 following equation:where Ac is the absorbance of the control (blank) without any radical scavenger and Ar is the absorbance of the remaining DPPH˙ after reacting with a radical scavenger.
2.11 Superoxide radical scavenging assay
Nitroblue tetrazolium chloride (NBT) staining was used to detect the production of superoxide radicals in situ and was carried out by titanium(IV) oxide (TiO2) which was exposed to UV light radiation. In brief, 1 mg of TiO2 was dissolved in 1 ml of ddwater and the reaction was initiated by exposure to UV light radiation for different times (10 min, 30 min, and 60 min). After that, 1 ml of different concentrations (30 μg ml−1, 60 μg ml−1, and 120 μg ml−1) of antioxidants was added and 100 μl of 0.05 mM NBT was added to the mixture as a detector of superoxide. The reduction of NBT in the reaction mixture was followed by an absorbance increase at 560 nm in UV-Vis spectra under aerobic conditions with time. The capability to scavenge the superoxide radical was calculated using the following equation:where Ac is the absorbance of the control (blank) without any radical scavenger and Ar is the absorbance of the NBT which has been reduced after being exposed to radiation by UV light.
2.12 Cell viability assay
In this study, an MTT assay was used to detect the anticancer efficiency of dox/cur@Ce-Uio-66. With the classical method, 6 × 103 cells per well were seeded in 96 wells for 24 h. Next, the cells were treated with indicated concentrations of dox/cur@Ce-Uio-66 for indicated times and then analyzed using the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide) (Golodbio) assay. In brief, the MTT reagent was added to each well after the treatment. After 4 h of incubation in a 37 °C and 5% CO2 incubator, the liquid mixture in the well was removed, and then dimethyl sulfoxide (DMSO) was added. Finally, the concentration of the obtained purple formazan crystals was read through absorbance at 570 nm using an Epoch microplate spectrophotometer. The morphology of the cells was inspected using an IX73 inverted microscope and the pictures of the cell morphology with different concentrations of drug feeding were taken under white light and fluorescence light at different magnifications (100×).2.13 Xenograft animal model experiment
The animal study was conducted following the guidelines of the Animal Care and Use Committee of Taipei Medical University (protocol code LAC-2020-0402). Six-week-old nude mice were subcutaneously injected with 1 × 106 HCT116 cells into the right leg and were randomly grouped with N ≥ 3 in each group. The animals’ body weights and tumor sizes were measured three times a week. When the tumor size reached 100 mm3, 1.5 mg kg−1 dox or 100 μl of 10-fold dox/cur@Ce-Uio-66 dilutions (containing 1 mg kg−1 dox) were administered to the mice by intraperitoneal injection three times a week, ten times in total. At the end of the experiment, the animals were sacrificed, the tumors were collected and measured, and mean ± SEM was used for body weight and tumor size analyses.3. Results and discussion
As depicted in Scheme 2, we constructed dox/cur@Ce-Uio-66 through a sequence of reactions. Specifically, Ce-Uio-66 was initially synthesized using the hydrothermal method, leveraging the unique capability of cerium to self-assemble into hexanuclear metal clusters. This process endows the material with a precursor platform for drug-loading, distinguished by its high specific surface area and inherent antioxidant potential. After loading a substantial quantity of the antioxidant drug curcumin, we applied a coating of hyaluronic acid (HA) to cur@Ce-Uio-66 to augment its stability. Subsequently, ethylenediamine pyrene was grafted onto HA using an EDC/NHS cross-linking reaction, establishing a π-resonance layer. Eventually, through π–π stacking, doxorubicin self-assembled with cur@Ce-Uio-66, culminating in the formation of a novel drug delivery and treatment system: dox/cur@Ce-Uio-66.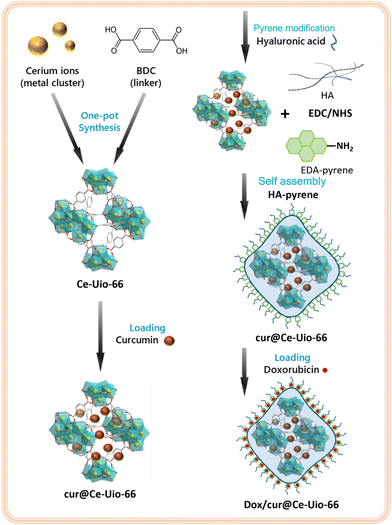 | ||
| Scheme 2 Schematic illustration of Ce-Uio-66, encapsulation of curcumin, coating of hyaluronic acid and loading of doxorubicin. | ||
3.1 Characterization of Ce-Uio-66
Uio-66 is renowned for its hexanuclear [M6O4(OH)4]12+ cluster, which is characteristic of various metal(IV) cations. During the synthesis of the archetypal zirconium-based UiO-66, Zr4+ cations naturally form hexanuclear [Zr6O4(OH)4]12+ clusters. These clusters are interconnected by 12 dicarboxylate linkers, resulting in a framework punctuated by open octahedral and tetrahedral cavities.26 Given cerium's ability to similarly self-assemble into hexanuclear metal clusters, there have been reports of successful full substitution of zirconium with cerium in the structure, leading to the synthesis of Ce-based UiO-66. The structural integrity of these Ce-Uio-66 particles was confirmed through PXRD characterization, as shown in Fig. 1(a). The observed PXRD peaks of cerium-based UiO-66 (Ce-Uio-66) closely aligned with those simulated from zirconium-based UiO-66 single-crystal data, showcasing characteristic peaks at 2θ values of 7.37°, 8.51°, 12.56°, and 25.72°, among others. These findings indicate that even with the substitution of the zirconium metal cluster by the cerium counterpart, the morphology of Ce-Uio-66 remains strikingly similar to the archetypal UiO-66. This suggests that Ce-Uio-66 retains the high specific surface areas characteristic of the original UiO-66. Therefore, to assess the porosity and surface area of Ce-Uio-66, we measured the specific surface areas using nitrogen adsorption–desorption isotherms at 77 K. The resulting type-I isotherms, depicted in Fig. 1(b), are characteristic of microporous materials. Further calculations using the Brunauer–Emmett–Teller (BET) method revealed that the surface area and total pore volume of Ce-Uio-66 were approximately 996.48 m2 g−1 and 0.57 cm3 g−1, respectively. These findings not only underscore the porosity of Ce-Uio-66 but also validate its suitability for loading diverse cargos, highlighting its significant potential as a platform for drug delivery systems.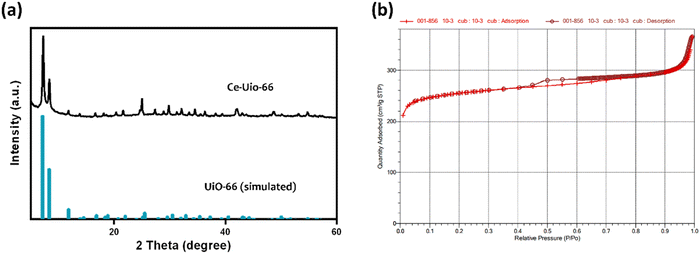 | ||
| Fig. 1 Characterization of Ce-Uio-66. (a) XRD patterns of Ce-Uio-66 and (b) N2 adsorption–desorption isotherms. | ||
Further analysis using X-ray photoelectron spectra, as illustrated in Fig. 2, reveals the presence of divalent states of cerium in Ce-Uio-66. The XPS spectrum of cerium ions in Ce-Uio-66 prominently displays peaks for Ce3+ 3d5/2 (at binding energies of 886.8 and 889.1 eV) and Ce3+ 3d3/2 (at 899.4 and 885.9 eV). These findings confirm the successful incorporation of cerium into the Ce-Uio-66 structure. Additionally, as highlighted in the literature, cerium's unique redox properties can significantly amplify its antioxidant and anti-cancer capabilities. This is further evidenced by the XPS spectrum, which indicates the presence of both Ce4+ and Ce3+ ions in the structure, potentially enhancing the antioxidant efficiency of Ce-Uio-66.31,32
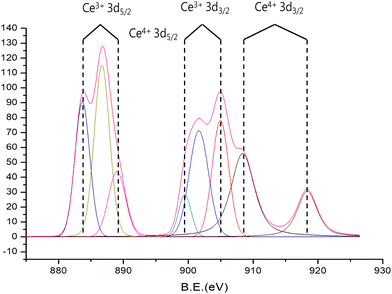 | ||
| Fig. 2 X-ray photoelectron spectroscopy (XPS) survey scan of Ce-Uio-66. Peaks of electronic levels of Ce3+ and Ce4+. | ||
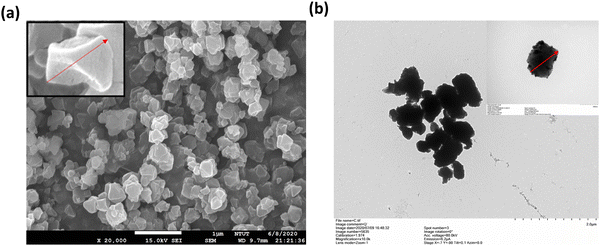
In general, UiO-66 crystals display a distinct octahedral shape.33 The surface morphology and size of Ce-Uio-66 were examined using field emission scanning electron microscopy (FE-SEM) and high-resolution transmission electron microscopy (HR-TEM), as shown in Fig. 3. The SEM (Fig. 3(a)) images highlight the nanoparticle morphology of Ce-Uio-66, showcasing a uniform MOF-like conformation with no significant structural defects. The TEM images (Fig. 3(b)) of Ce-Uio-66 reveal a UiO-66-like nanoparticle with an average particle size of 312.37 ± 79.4 nm, consistent with the SEM observations. Based on these findings, we can confidently conclude that Ce-Uio-66 indeed exhibits the anticipated MOF structure following the synthesis process.
3.2 Characterization of cur@Ce-Uio-66
Typically, curcumin can be incorporated into the cavities of UiO-66 to create a drug delivery system. In this study, the curcumin loading capacity of cur@Ce-Uio-66 composites was evaluated using UV-Vis spectra of the supernatant solution before and after curcumin encapsulation. To provide basic drug-loading characterization, the UV-Vis absorption spectrum of diluted cur@Ce-Uio-66 is presented in Fig. S1 (ESI†). Apparently, a characteristic peak is observed at 422 nm, attributed to the conjugation between the π-electron clouds of the two vinylguaiacol units.34 The absorbance in correlation with concentration can be utilized to construct a calibration curve. Using the equation of calibration, we determined the amount of loaded curcumin, enabling us to evaluate both the encapsulation efficiency (EE) and drug loading efficiency (DLE) of cur@Ce-Uio-66.In our investigation, we explored optimal methods for maximizing curcumin loading. Drawing from prior research, we adopted a one-pot synthesis approach, mixing varying concentrations of curcumin with H2BDC and diammonium cerium(IV) nitrate, resulting in products named cur0.25-Ce-UiO-66, cur0.5-Ce-UiO-66, and cur-Ce-UiO-66.35 An alternative post-synthesis method involved adding curcumin after MOF synthesis, capitalizing on pore absorption, yielding products named cur0.25@Ce-UiO-66, cur0.5@Ce-UiO-66, and cur@Ce-UiO-66.36 As detailed in Table S1 (ESI†), both methods exhibited a dose-dependent increase in Ce-UiO-66 EE for curcumin. However, the EEs for one-pot synthesized samples were consistently lower than their post-synthesis counterparts at equivalent curcumin concentrations. XRD analysis further confirmed the crystal structure of Ce-UiO-66 post-curcumin loading for both methods, as shown in Fig. 4(a) and (b), respectively. Notably, when curcumin concentration reached 4 mg ml−1, the structure of Ce-Uio-66 was compromised, limiting further curcumin loading in one-pot synthesis. These findings suggest that post-synthesis is the more suitable method for curcumin loading into Ce-UiO-66, achieving a maximum EE of approximately 52.80%, which is relatively higher than traditional drug delivery systems.
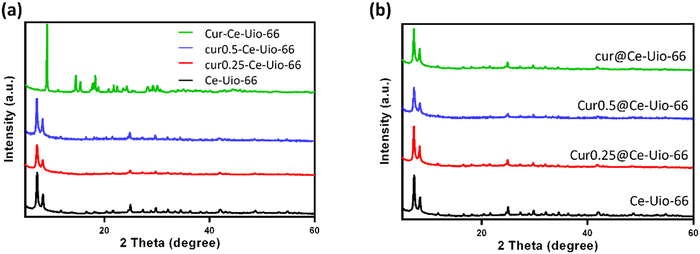 | ||
| Fig. 4 XRD patterns of samples synthesized by (a) the one-pot synthesis method and (b) the post synthesis method. | ||
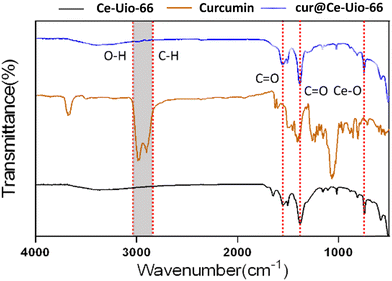
Additionally, the interaction between Ce-UiO-66 and curcumin was characterized using Fourier transform infrared (FT-IR) spectroscopy, with spectra presented in Fig. 5. The pronounced bands between 2840 and 3035 cm−1, attributed to C–O and C–H bond stretching,37 displayed minor peaks in cur@Ce-Uio-66 compared to Ce-UiO-66. Another band, ranging from 1370 to 1570 cm−1, corresponded to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C vibration mode of H2BDC ligands,38,39 which also exhibited a slight shift due to curcumin loading. Peaks in the 733–753 cm−1 region are likely associated with Ce–O bonds in Ce-Uio-66.40 In brief, these peak positions align with the known absorption regions of cerium-based UiO-66, indicating that the Ce-UiO-66 structure remained intact during the curcumin encapsulation process. Furthermore, FTIR results suggest that curcumin loading primarily occurs through post-synthesis absorption and coordination of covalent bonding with Ce-Uio-66. In conclusion, as a carrier, Ce-Uio-66 demonstrates exceptional efficiency and stability for curcumin loading, positioning cur@Ce-Uio-66 as an ideal drug delivery system for antioxidants.
C vibration mode of H2BDC ligands,38,39 which also exhibited a slight shift due to curcumin loading. Peaks in the 733–753 cm−1 region are likely associated with Ce–O bonds in Ce-Uio-66.40 In brief, these peak positions align with the known absorption regions of cerium-based UiO-66, indicating that the Ce-UiO-66 structure remained intact during the curcumin encapsulation process. Furthermore, FTIR results suggest that curcumin loading primarily occurs through post-synthesis absorption and coordination of covalent bonding with Ce-Uio-66. In conclusion, as a carrier, Ce-Uio-66 demonstrates exceptional efficiency and stability for curcumin loading, positioning cur@Ce-Uio-66 as an ideal drug delivery system for antioxidants.
3.3 Characterization of dox/cur@Ce-Uio-66
In numerous studies on metal–organic frameworks (MOFs), hydrophobicity has been identified as a significant limitation for drug delivery applications. Additionally, the open pores of MOFs can lead to reduced structural stability and premature drug release. To address these challenges, in this study, curcumin-loaded Ce-Uio-66 was coated with a layer of hyaluronic acid (HA) biopolymer through a self-assembly process. Furthermore, HA was cross-linked with amino-modified 1-pyrenebutyric acid N-hydroxysuccinimide to facilitate the loading of doxorubicin through π–π stacking (aromatic) interactions. To verify the morphological changes in Ce-Uio-66 after the HA coating and doxorubicin loading, we utilized field emission scanning electron microscopy (FESEM), as depicted in Fig. 6. Compared to the original Ce-Uio-66, the surface morphology of cur@Ce-Uio-66 remained largely unchanged, confirming that curcumin could be incorporated into Ce-Uio-66 without compromising its structural integrity. However, the SEM images of dox/cur@Ce-Uio-66 revealed a fiber-like structure with a smooth surface, likely due to the polymer structure of the HA coating.41 In summary, these observations confirm the successful integration of HA and doxorubicin with Ce-Uio-66. Importantly, the structural integrity of Ce-Uio-66 remained intact after the HA coating and doxorubicin loading.Complementing the curcumin and doxorubicin loading studies, the surface charges of Ce-Uio-66, cur@ Ce-Uio-66, and dox/cur@ Ce-Uio-66 nanoparticles were analyzed using zeta potential measurements. As shown in Fig. 7(a), the surface zeta potentials of Ce-Uio-66, cur@ Ce-Uio-66, and dox/cur@ Ce-Uio-66 in a pH 7.0 environment were approximately 25.4 mV, −0.87 mV, and −27.24 mV, respectively. These shifts in surface zeta potential further substantiate the successful cargo loading. Additionally, the size of dox/cur@ Ce-Uio-66 was determined using dynamic light scattering (DLS) (Fig. 7(b)). The average hydrodynamic particle size of dox/cur@Ce-Uio-66 was found to be about 488.7 nm, suggesting an increase in size after the HA coating and doxorubicin loading. Additionally, the UV-Vis absorption spectrum of dox/cur@Ce-Uio-66 is also recorded in Fig. S1 (ESI†). The intense absorption peak contributed by doxorubicin appears around 480–490 nm, and due to its significant height and width, it overlaps with part of the absorption peaks of curcumin. In summary, as a dual-drug delivery system for future therapeutic applications, dox/cur@Ce-Uio-66 was successfully synthesized and qualitatively characterized.
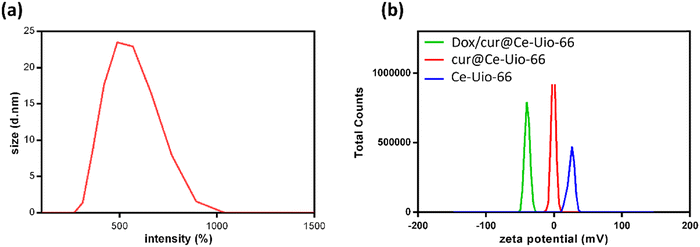 | ||
| Fig. 7 (a) The DLS intensity distribution and (b) surface ξ potential from Ce-Uio-66, cur@Ce-Uio-66 and dox/cur@Ce-Uio-66 in pH 7.0 DI water. | ||
3.4 Characterization of the free radical scavenging ability of cur@Ce-Uio-66
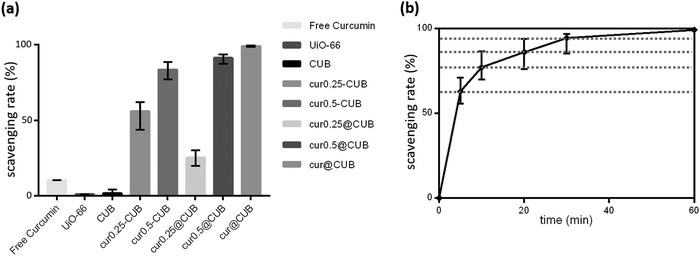
To assess the enhanced antioxidant properties of our constructed system, we conducted a series of free radical scavenging experiments. The first assay employs the decolorization of the ABTS˙+ radical cation, measured spectrophotometrically. To further ascertain the antioxidant efficiency of cerium-ion-doped and curcumin-loaded UiO-66, the antioxidant potential was evaluated using UV-Vis spectral absorbance. As illustrated in Fig. 8(a), free curcumin, archetypal UiO-66, and Ce-Uio-66 exhibited modest scavenging efficiency. However, upon loading curcumin into Ce-Uio-66, the majority of the samples demonstrated ABTS˙+ scavenging capacity. Notably, the scavenging efficiency augmented with the increasing ratio of curcumin to Ce-Uio-66. The cur@Ce-Uio-66 sample exhibited peak efficiency, scavenging approximately 99.1% of ABTS˙+ within 60 minutes. On the whole, samples synthesized via the post-synthesis method displayed superior ABTS˙+ scavenging capabilities, aligning with the encapsulation efficiency (EE) observed in the curcumin loading tests. Furthermore, the data suggest that the antioxidant capacity of cur@Ce-Uio-66 surpasses that of free curcumin. This is likely because free curcumin molecules are more susceptible to oxidation by other agents, leading to structural alterations. It can be inferred that Ce-Uio-66 serves as an innovative carrier that not only safeguards against degradation but also enhances the solubility of curcumin in aqueous solvents. Additionally, we conducted a time-dependent study on the ABTS˙+ scavenging efficiency of cur@Ce-Uio-66. As depicted in Fig. 8(b), the scavenging percentage steadily increased, reaching 77.15% within the initial 10 minutes. By the 30-minute mark, a substantial 94.35% of ABTS˙+ radicals had been scavenged. Collectively, these findings underscore the exceptional ABTS˙+ radical scavenging prowess of curcumin-loaded Ce-Uio-66, which attains its zenith with specific synthesis techniques and ratios.DPPH radicals serve as stable model free radicals suitable for laboratory assays evaluating the radical scavenging activity of compounds. DPPH radicals exhibit a characteristic absorption peak at 517 nm. When these radicals interact with scavengers, their absorption value diminishes. As detailed in Table S2 (ESI†), the initial antioxidant capacity of all groups was modest during the first hour. However, a discernible difference in DPPH concentration was observed between Ce-Uio-66 and cur@Ce-Uio-66, with the latter demonstrating superior DPPH efficiency compared to UiO-66. Nonetheless, the variations in antioxidant activity between Ce-Uio-66 and cur@Ce-Uio-66 were not statistically significant. This observation was further supported by color changes: while samples containing UiO-66 retained a purple hue, both Ce-Uio-66 and cur@Ce-Uio-66 transitioned to a light-yellow color, indicating a higher degree of DPPH reduction. Consistent with the data in Table S2 (ESI†), Fig. 9 suggests that cerium-ion-doped Ce-Uio-66 offers enhanced DPPH free radical scavenging efficiency, a trait not evident in UiO-66. Furthermore, the scavenging rate peaked after curcumin loading. In conclusion, due to the incorporation of cerium ions, cur@Ce-Uio-66 can effectively scavenge both ABTS and DPPH free radicals.
 | ||
| Fig. 9 Color change of groups containing UiO-66, Ce-Uio-66 and cur@Ce-Uio-66 in the DPPH scavenging assay after incubation for 24 h. | ||
To verify the multifaceted free radical scavenging capabilities of cur@Ce-Uio-66, we further employed the superoxide radical scavenging assay. In this test, titanium(IV) oxide (TiO2) generated the superoxide radical. The concentration of nitroblue tetrazolium chloride (NBT) post-reaction served as an indicator of the sample's superoxide radical scavenging efficiency. As depicted in Fig. 10, the control group's absorbance rapidly decreased at 259 nm when exposed to UV light for the initial 30 minutes. In contrast, the cerium-doped UiO-66 sample, labeled as Ce-Uio-66, displayed a significantly higher absorbance, suggesting that cerium could scavenge a portion of the superoxide radicals. Furthermore, cur@Ce-Uio-66 maintained a marginally higher absorbance than Ce-Uio-66 after 50 minutes. However, cur@Ce-Uio-66-HA did not exhibit any significant scavenging efficiency throughout the test. Based on these findings, both Ce-Uio-66 and cur@Ce-Uio-66, with cerium doping and curcumin loading, demonstrated potential superoxide radical scavenging capabilities. The slightly enhanced radical scavenging efficiency of cur@Ce-Uio-66 compared to Ce-Uio-66 after 50 minutes might result from the release of curcumin situated on the external layer, which subsequently reacts with the superoxide radicals. On the other hand, the diminished radical scavenging efficiency of cur@Ce-Uio-66 could be attributed to the hyaluronic acid (HA) coating. This coating potentially inhibits curcumin leakage, preserving the structure of cur@Ce-Uio-66 in neutral solutions and acting as a barrier between the superoxide radicals and the cerium/curcumin complex during the test. In summary, these results not only highlight the potential superoxide radical scavenging capabilities but also suggest that the HA coating can protect the structural integrity of cur@Ce-Uio-66.
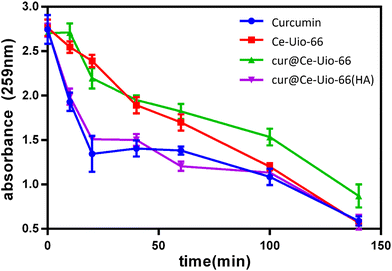 | ||
| Fig. 10 Superoxide radical scavenging activity of curcumin, Ce-Uio-66, cur@Ce-Uio-66 and cur@Ce-Uio-66 (HA) with time. | ||
3.5 In vitro drug release
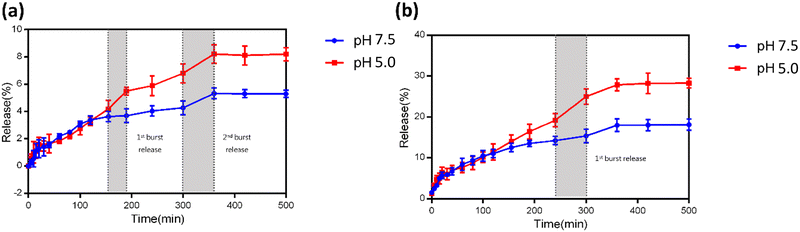
The release profile of curcumin was examined to validate its sustained and pH-sensitive release from the designed platform. The release profiles of doxorubicin and curcumin from dox/cur@Ce-Uio-66 were evaluated under simulated physiological conditions at pH values of 5.0 and 7.5, and a temperature of 37 °C. As depicted in Fig. 11(a) and (b), the release of curcumin and doxorubicin under acidic conditions reached 8.1% and 28.2%, respectively, after 7 hours. These release rates were higher than those observed under standard physiological conditions, where they were 5.3% for curcumin and 18% for doxorubicin. This observed behavior is in line with our hypothesis. The enhanced release can be attributed to protonation under acidic conditions, which disrupts the coordination linkage between the Ce ions and H2BDC, leading to an increase in the release of curcumin from dox/cur@Ce-Uio-66. Simultaneously, the elevated release of doxorubicin is likely due to the gradual cleavage of amide bonds between HA and pyrene. Additionally, the data indicated a single burst release for doxorubicin and two burst releases for curcumin. Considering the structure of dox/cur@Ce-Uio-66, the initial burst release of doxorubicin in an acidic environment may arise from the erosion of HA and doxorubicin on the external layer. The primary burst release of curcumin could be attributed to its leakage from the external surface of Ce-Uio-66, whereas the secondary burst release might stem from curcumin release from the internal structure. Overall, these findings demonstrate that dox/cur@Ce-Uio-66 can achieve pH-dependent drug release, highlighting its potential as a delivery platform for anticancer therapy.3.6 In vitro cytotoxicity assay
To assess potential in vitro toxicity towards human cancer cells, we employed the widely-used MTT cell toxicity assay. The anticancer capacity was evaluated using HT29 and HCT116 human colorectal carcinoma cell lines. According to the results presented in Fig. 12(a), both HT29 and HCT116 cells were treated with dox/cur@Ce-Uio-66 at the indicated concentrations, and then cell viability was measured at 24 h and 48 h time points. The viability of both cancer cells was significantly suppressed after the treatment, suggesting the powerful cancer killing potential of dox/cur@Ce-Uio-66. These findings also demonstrate that dox/cur@Ce-Uio-66 exhibits potent inhibitory effects on both cell lines in a dose-dependent manner. Overall, the data underscore the potential anticancer efficacy of the dox/cur@Ce-Uio-66 drug delivery system.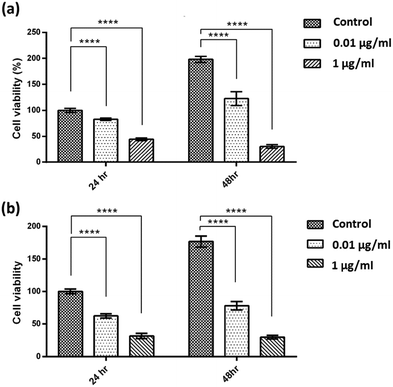 | ||
| Fig. 12 In vitro cell viability of (a) HT29 and (b) HCT116 at 0.01 μg ml−1 and 1 μg ml−1 of dox/cur@Ce-Uio-66. | ||
3.7 In vivo assessment of the antitumor ability
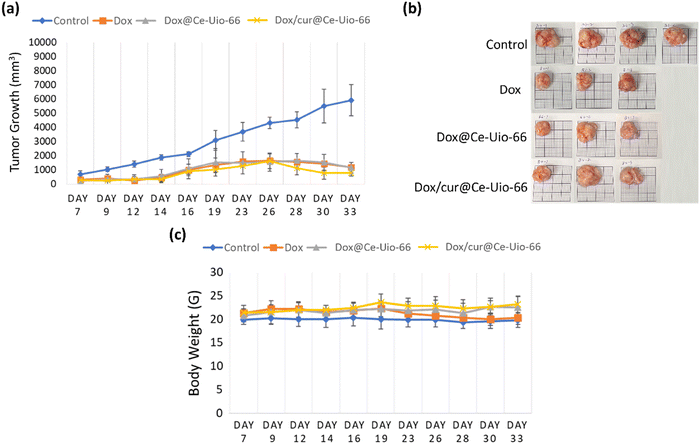
Building on the encouraging in vitro results of dox/cur@Ce-Uio-66, we proceeded to assess its efficacy in a mouse model. The primary aim of this experiment was to determine if the dox/cur@Ce-Uio-66 system, designed as a drug delivery mechanism with antioxidant activity, could match the anti-cancer effects of doxorubicin alone in vivo. For this evaluation, we employed a xenograft mouse model with HCT116 cancer cells. The subjects were methodically divided into four groups to assess the treatment outcomes. The groups were: (1) control group (control), (2) free doxorubicin at a concentration of 1.5 mg kg−1 (dox), (3) Ce-Uio-66 containing 1 mg kg−1 doxorubicin (dox@Ce-Uio-66), and (4) Ce-Uio-66 combined with curcumin, containing 1 mg kg−1 doxorubicin (dox/cur@Ce-Uio-66). The study was conducted for over 33 days in total. Throughout the treatment period, we monitored changes in both the tumor volume and the body weight in each animal (as depicted in Fig. 13(a)–(c)). Fig. 13(a) presents the tumor growth rate, expressed as the average tumor volume (mm3). In comparison to the control group, mice treated with any of the doxorubicin encapsulation groups (dox, dox@Ce-Uio-66, and dox/cur@Ce-Uio-66) exhibited significant reductions in tumor size (Fig. 13(a) and Fig. S2a, ESI†). This indicates that doxorubicin, when delivered through the carrier, retains its potency in targeting cancer cells. The tumor images in Fig. 13(b) further corroborate this finding, suggesting that our designed system with antioxidant potential allows doxorubicin to effectively exert its anti-cancer properties. Such findings offer fresh perspectives on prognosis and potential recurrence prevention. Moreover, time-dependent changes in body weight within the treatment groups serve as a reliable metric for toxicity evaluations. As per Fig. 13(c), none of the treatment groups displayed excessive weight loss, underscoring the biosafety of dox/cur@Ce-Uio-66. Organs of mice in each group were also collected for further safety evaluation and demonstration (Fig. S2b, ESI†). Collectively, the in vivo results consistently highlight the anti-cancer potential of dox/cur@Ce-Uio-66 as a versatile drug delivery system.4. Conclusion
We successfully designed and synthesized a novel UiO-66 metal–organic framework (MOF) as a pH-triggered drug delivery system capable of multidrug loading, showcasing its potential in both antioxidant and anticancer applications. In our approach, zirconium ions were effectively substituted with cerium ions, resulting in a Ce-based UiO-66. XRD and SEM analyses further confirmed the preservation of the UiO-66 crystalline lattice structure, akin to Zr-based UiO-66, highlighting its potential as a drug carrier. Our methodology also yielded a high curcumin-loaded Ce-Uio-66. Notably, in vitro free radical scavenging assays revealed enhanced antioxidant activity against multiple free radicals, such as DPPH˙, ABTS˙+, and O2˙−, an improvement not observed with free curcumin or Zr-based UiO-66 alone. This suggests that Ce-based UiO-66 is a promising carrier for cerium and curcumin in antioxidant applications. Additionally, the effective hyaluronic acid coating and significant doxorubicin loading (∼77.01 wt%) augmented the stability and anticancer efficacy of Ce-Uio-66. The observed results effectively showcase the anti-cancer effects both in vitro (using the MTT assay) and in vivo (using the xenograft mouse model). Fundamentally, the development of this multifunctional nanosystem, which can accommodate multi-drug loading, provides crucial insights for anti-cancer strategies and antioxidant relapse prevention using Ce-based MOFs.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This project was partly supported by the National Science and Technology Council (NSTC 112-2628-B-038-009-MY3, 112-2221-E-152-001, and 113-2221-E-152-001), Taiwan. We also appreciate the support from the Core Facility Center and the Animal Center at Taipei Medical University and the National Taipei University of Education (NTUE), Taiwan, for the instrumental analysis and technical support.References
- W. Chen, et al., Annual report on status of cancer in China, 2011, Chin. J. Cancer Res., 2015, 27(1), 2 CrossRef PubMed.
- W. Lear, E. Dahlke and C. A. Murray, Basal cell carcinoma: review of epidemiology, pathogenesis, and associated risk factors, J. Cutaneous Med. Surg., 2007, 11(1), 19–30 CrossRef PubMed.
- G.-L. Deng, S. Zeng and H. Shen, Chemotherapy and target therapy for hepatocellular carcinoma: New advances and challenges, World J. Hepatol., 2015, 7(5), 787 CrossRef PubMed.
- L. Alili, et al., Downregulation of tumor growth and invasion by redox-active nanoparticles, Antioxid. Redox Signaling, 2013, 19(8), 765–778 CrossRef CAS PubMed.
- Y. Zhou, et al., Natural polyphenols for prevention and treatment of cancer, Nutrients, 2016, 8(8), 515 CrossRef PubMed.
- P. Maleki Dana, F. Sadoughi, Z. Asemi and B. Yousefi, The role of polyphenols in overcoming cancer drug resistance: a comprehensive review, Cell. Mol. Biol. Lett., 2022, 27(1), 1 CrossRef CAS PubMed.
- P. Anand, A. B. Kunnumakkara, R. A. Newman and B. B. Aggarwal, Bioavailability of Curcumin: Problems and Promises, Mol. Pharmaceutics, 2007, 4(6), 807–818 CrossRef CAS.
- S. Maleki Dizaj, et al., Curcumin nanoformulations: Beneficial nanomedicine against cancer, Phytother. Res., 2022, 36(3), 1156–1181 CrossRef CAS PubMed.
- M. Ramezani Farani, et al., Folic Acid-Adorned Curcumin-Loaded Iron Oxide Nanoparticles for Cervical Cancer, ACS Appl. Bio Mater., 2022, 5(3), 1305–1318 CrossRef CAS PubMed.
- S. Hafez Ghoran, et al., Curcumin-Based Nanoformulations: A Promising Adjuvant towards Cancer Treatment, Molecules, 2022, 27(16), 5236 CrossRef CAS PubMed.
- J. Li, et al., Flexible Curcumin-Loaded Zn-MOF Hydrogel for Long-Term Drug Release and Antibacterial Activities, Int. J. Mol. Sci., 2023, 24(14), 11439 CrossRef CAS.
- Q. Li, et al., Symphony of nanomaterials and immunotherapy based on the cancer–immunity cycle, Acta Pharm. Sin. B, 2022, 12(1), 107–134 CrossRef CAS PubMed.
- C. Lin, H. Hao, L. Mei and M. Wu, Metal-free two-dimensional nanomaterial-mediated photothermal tumor therapy, Smart Mater. Med., 2020, 1, 150–167 CrossRef.
- M. J. Kalmutzki, N. Hanikel and O. M. Yaghi, Secondary building units as the turning point in the development of the reticular chemistry of MOFs, Sci. Adv., 2018, 4(10), eaat9180 CrossRef CAS PubMed.
- M. Pourmadadi, et al., UiO-66 nanoparticles as a drug delivery system: A comprehensive review, J. Drug Delivery Sci. Technol., 2023, 104690 CrossRef CAS.
- J. Winarta, et al., A decade of UiO-66 research: a historic review of dynamic structure, synthesis mechanisms, and characterization techniques of an archetypal metal–organic framework, Cryst. Growth Des., 2019, 20(2), 1347–1362 CrossRef.
- B. M. Jarai, et al., Evaluating UiO-66 metal–organic framework nanoparticles as acid-sensitive carriers for pulmonary drug delivery applications, ACS Appl. Mater. Interfaces, 2020, 12(35), 38989–39004 CrossRef CAS PubMed.
- S. Bazzazan, et al., Engineered UIO-66 metal-organic framework for delivery of curcumin against breast cancer cells: An in vitro evaluation, J. Drug Delivery Sci. Technol., 2023, 79, 104009 CrossRef CAS.
- S. Yao, Z. Wang and L. Li, Application of organic frame materials in cancer therapy through regulation of tumor microenvironment, Smart Mater. Med., 2022, 3, 230–242 CrossRef.
- L. Alili, et al., Combined cytotoxic and anti-invasive properties of redox-active nanoparticles in tumor–stroma interactions, Biomaterials, 2011, 32(11), 2918–2929 CrossRef CAS PubMed.
- Y. Li, et al., Acquired Superoxide-Scavenging Ability of Ceria Nanoparticles, Angew. Chem., Int. Ed., 2015, 54(6), 1832–1835 CrossRef CAS PubMed.
- C. Xu and X. Qu, Cerium oxide nanoparticle: a remarkably versatile rare earth nanomaterial for biological applications, NPG Asia Mater., 2014, 6(3), e90–e90 CrossRef CAS.
- A. Karakoti, et al., Redox-active radical scavenging nanomaterials, Chem. Soc. Rev., 2010, 39(11), 4422–4432 RSC.
- S. S. Lee, et al., Antioxidant properties of cerium oxide nanocrystals as a function of nanocrystal diameter and surface coating, ACS Nano, 2013, 7(11), 9693–9703 CrossRef CAS PubMed.
- S. Gao, et al., Construction of cerium-based oxide catalysts with abundant defects/vacancies and their application to catalytic elimination of air pollutants, J. Mater. Chem. A, 2023, 11, 19210–19243 RSC.
- M. Lammert, et al., Cerium-based metal organic frameworks with UiO-66 architecture: synthesis, properties and redox catalytic activity, Chem. Commun., 2015, 51(63), 12578–12581 RSC.
- R. Re, et al., Antioxidant activity applying an improved ABTS radical cation decolorization assay, Free Radical Biol. Med., 1999, 26(9–10), 1231–1237 CrossRef CAS PubMed.
- İ. Gülçin, R. Elias, A. Gepdiremen and L. Boyer, Antioxidant activity of lignans from fringe tree (Chionanthus virginicus L.), Eur. Food Res. Technol., 2006, 223(6), 759–767 CrossRef.
- I. Gülçin, Comparison of in vitro antioxidant and antiradical activities of L-tyrosine and L-Dopa, Amino Acids, 2007, 32(3), 431–438 CrossRef PubMed.
- E. S. Contreras-Guzmán and F. C. Strong, III, Determination of Tocopherols (Vitamin E) by Reduction of Cupric Ion, J. AOAC Int., 2020, 65(5), 1215–1221 CrossRef.
- A. M. Ebrahim and T. J. Bandosz, Ce(III) Doped Zr-Based MOFs as Excellent NO2 Adsorbents at Ambient Conditions, ACS Appl. Mater. Interfaces, 2013, 5(21), 10565–10573 CrossRef CAS.
- M. Farrag, Comparative study of size-selected gold clusters (Au38) and gold nanoparticles over porous cerium-based metal–organic frameworks with UiO-66 architecture for aerobic oxidation of cinnamyl alcohol, Res. Chem. Intermed., 2021, 47(6), 2589–2604 CrossRef CAS.
- H. Liu, et al., Modified UiO-66 as photocatalysts for boosting the carbon-neutral energy cycle and solving environmental remediation issues, Coord. Chem. Rev., 2022, 458, 214428 CrossRef CAS.
- F. Zsila, Z. Bikádi and M. Simonyi, Circular dichroism spectroscopic studies reveal pH dependent binding of curcumin in the minor groove of natural and synthetic nucleic acids, Org. Biomol. Chem., 2004, 2(20), 2902–2910 RSC.
- P. Somu and S. Paul, A biomolecule-assisted one-pot synthesis of zinc oxide nanoparticles and its bioconjugate with curcumin for potential multifaceted therapeutic applications, New J. Chem., 2019, 43(30), 11934–11948 RSC.
- G. Huang, et al., Curcumin-loaded nanoMOFs@ CMFP: A biological preserving paste with antibacterial properties and long-acting, controllable release, Food Chem., 2021, 337, 127987 CrossRef CAS PubMed.
- S.-N. Kim, et al., Metal-organic frameworks, NH2-MIL-88 (Fe), as carriers for ophthalmic delivery of brimonidine, Acta Biomater., 2018, 79, 344–353 CrossRef CAS.
- H. R. Abid, et al., Adsorption of CH4 and CO2 on Zr-metal organic frameworks, J. Colloid Interface Sci., 2012, 366(1), 120–124 CrossRef CAS PubMed.
- M. R. Azhar, et al., One-pot synthesis of binary metal organic frameworks (HKUST-1 and UiO-66) for enhanced adsorptive removal of water contaminants, J. Colloid Interface Sci., 2017, 490, 685–694 CrossRef CAS PubMed.
- X. Qiu, et al., Cerium-Based Metal–Organic Frameworks with UiO Architecture for Visible Light-Induced Aerobic Oxidation of Benzyl Alcohol, Sol. RRL, 2020, 4(8), 1900449 CrossRef CAS.
- H. Liu, H. Zhu and S. Zhu, Reversibly Dispersible/Collectable Metal–Organic Frameworks Prepared by Grafting Thermally Responsive and Switchable Polymers, Macromol. Mater. Eng., 2015, 300(2), 191–197 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4tb01206b |
| This journal is © The Royal Society of Chemistry 2024 |