Bioactive glass–polymer nanocomposites: a comprehensive review on unveiling their biomedical applications
Radhakrishnan
Sreena
ab,
Gurusamy
Raman
*c,
Geetha
Manivasagam
a and
A. Joseph
Nathanael
 *a
*a
aCentre for Biomaterials, Cellular and Molecular Theranostics (CBCMT), Vellore Institute of Technology (VIT), Vellore 632014, Tamil Nadu, India. E-mail: joseph.nathanael@vit.ac.in
bSchool of Biosciences & Technology (SBST), Vellore Institute of Technology (VIT), Vellore 632014, Tamil Nadu, India
cDepartment of Life Sciences, Yeungnam University, Gyeongsan, South Korea. E-mail: bioramg@gmail.com
First published on 1st October 2024
Abstract
Most natural and synthetic polymers are promising materials for biomedical applications because of their biocompatibility, abundant availability, and biodegradability. Their properties can be tailored according to the intended application by fabricating composites with other polymers or ceramics. The incorporation of ceramic nanoparticles such as bioactive glass (BG) and hydroxyapatite aids in the improvement of mechanical and biological characteristics and alters the degradation kinetics of polymers. BG can be used in the form of nanoparticles, nanofibers, scaffolds, pastes, hydrogels, or coatings and is significantly employed in different applications. This biomaterial is highly preferred because of its excellent biocompatibility, bone-stimulating activity, and favourable mechanical and degradation characteristics. Different compositions of nano BG are incorporated into the polymer system and studied for positive results such as enhanced bioactivity, better cell adherence, and proliferation rate. This review summarizes the fabrication and the progress of natural/synthetic polymer-nano BG systems for biomedical applications such as drug delivery, wound healing, and tissue engineering. The challenges and the future perspectives of the composite system are also addressed.
1. Introduction
Bioactive glass (BG) has become a major subject of interest in various biomedical applications since its discovery in 1970 by Hench et al.1 This inorganic silica-based material has gained a lot of attention in the field of biomaterials because of its innate nature to bond with the bone by the formation of a hydroxycarbonate apatite layer (HCA).2,3 The main composition includes 46.1 mol% SiO2, 24.4 mol% Na2O, 26.9 mol% CaO and 2.6 mol% P2O5 and was called 45S5 and Bioglass®.3 The application of BG is not limited to orthopedic and dental implants but is also used in the regeneration of tissues such as skin, cartilage,4 biosensors,5 and diagnostic systems.6 It has also been discovered that the nanoscale dimension of BG plays a key role in cell-to-cell communication in biological tissues.7 The change in topography and surface-to-volume ratio from bulk to nano dimension initiates improvement in various properties of biomaterials, especially promoting cell adhesion and proliferation.8 BG nanoparticles are typically silicates, phosphates, or borates combined with different proportions of sodium oxide or calcium oxide that are used as glass modifiers. Binary, ternary, and quaternary systems of BG can be synthesized by sol–gel, microemulsion, flame synthesis, and laser spinning techniques. The size and the morphology of the synthesized particles can be tuned by varying the composition and reaction conditions, thereby ultimately changing their properties.9–13Synthetic or natural biodegradable polymers are widely employed owing to their low immunogenicity, biocompatibility, and degradability. They can be natural (collagen, alginate, silk-fibroin) or synthetic (polycaprolactone (PCL), poly vinyl alcohol (PVA)).12 Various polymer matrices can be modified by adding BG nanoparticles to change their physiochemical, mechanical, and biological properties.10 Fabrication of nanocomposites is a promising area for biomedical applications since combining two or more materials leads to further enhancement of their properties.13 The mechanical properties such as elastic moduli, compressive strength, and bone-bonding abilities are improved.4,14 The addition of BG nanoparticles to the polymers alters the degradation rate. Also, it shows a positive impact on osteoblast proliferation due to the elution of ions from BG.15 Rapid improvements in nanocomposite engineering have opened the door to a wide range of biomedical uses, such as drug delivery, wound-healing, sensors, tissue engineering, and diagnostic systems. These composites combine the flexibility of the polymer with the bioactive properties of BG.16
This review summarizes the various techniques adopted for the fabrication of BG–polymer nanocomposites and their major biomedical applications. Primary emphasis is placed on the current research landscape of various BG compositions with ions such as zinc (Zn), strontium (Sr), and magnesium (Mg) and their composites with natural or synthetic polymers. The biomedical applications of BG-based nanocomposites, such as tissue engineering, drug delivery, and wound healing, are also highlighted.
2. Types of BG
BG can be of many types, namely the conventional silicate type commonly known as 45S5 BGs, phosphate glasses, and borate glasses.17 BG is also doped with elements, such as Zn,18 copper (Cu),19 Sr,20 Mg,21 selenium (Se), and tellurium (Te),22 and various other ions to form composites for improvement in properties. Borosilicate, boro phosphate glasses,23 and borate-based BGs are of recent interest because of their positive results in treating chronic wounds such as diabetic ulcers.17,24 Phosphate-based BGs are also highly preferred owing to their solubility and bioactivity.25 The structure of 45S5 BG and the tetrahedral sites of Si and PO43− are shown in Fig. 1.1,26 These three types of BGs can be used as a modifier in natural polymers such as silk fibroin,27,28 collagen,29 gelatin, and chitosan and synthetic polymers such as PCL,30–33 PVA34,35 and polylactic acid (PLA).36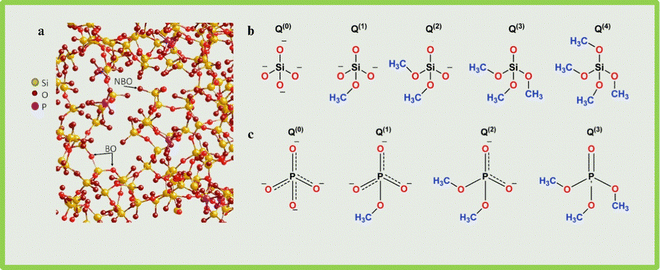 | ||
| Fig. 1 (a) Structure of 45S5 BG. NBO and BO stand for non-bridging oxygen and bridging oxygen (reproduced with permission from ref. 1. Copyright 2013, Elsevier); (b) schematic representation of tetrahedral units of silicate glass; and (c) schematic representation of tetrahedral units of phosphate glass. In figure (b) and (c), Q stands for quaternary which represents the number of bridging oxygens that are connected to each tetrahedron (reproduced with permission from ref. 26 under CC-BY license) -Created using Biorender. | ||
2.1. Silicate BG
The fundamental building block of silicate-based BGs is the SiO4 tetrahedron that can covalently bond to other tetrahedra.37 Silicate-based BGs are extensively used in research for biomedical applications as fillers with polymer matrices.38 Addition of silica particles facilitates higher bioactivity and improved biocompatibility. They also improve the resorption rate and porosity further inducing the formation of calcium phosphate that is found in natural bone.39,40 In addition, they can also directly bond to the bone tissue by forming HCA.41 The dissolution of ions such as silicon, calcium, and phosphorus improves the expression of genes in the bone cells and aids in stem cell differentiation. At the same time, they can also promote angiogenesis with specific ions, as mentioned above.38 The most important feature responsible for the bioactivity is the low SiO2 and high CaO to P2O5 ratio. The commercial glass 45S5 is a silicate glass, and its biocompatibility has been very well established over the years. One of the main limitations pertaining to 45S5 BG is that it is very challenging to form three-dimensional scaffolds owing to the difficulties faced in sintering the particles to form a network.172.2. Phosphate BG
Phosphate glasses comprise an inorganic phosphate network covalently connected with three tetrahedral phosphate units.42 The P2O5 network in the phosphate bio-glass has a wide range of solubility that can be tuned by varying the composition or using a modifier.43 They are widely used as inorganic fillers in bone tissue engineering because of their likeliness to natural bone.40 Incorporation of phosphate-based BG nanoparticles not only modulates the physiochemical properties but also has a great influence on the biological properties.38 Low-dissolution phosphate BG has been shown to increase osteoblastic cell proliferation and to participate with a number of bone-associated growth factors.44 There is a direct influence on the degradation of the polymer–phosphate BG in a way that the degradation is fast with the decrease in calcium oxide content.452.3. Borate BG
One of the most important glass-forming oxides is borate-based BG, which is highly preferred because of its high strength and easily tunable properties.46 Borate-based BG is widely used due to its bioactivity; however, the biological applications are uncertain because the borate ions released during dissolution can cause cytotoxicity.41,47 In addition to this, borate glasses find use in a variety of drug-release applications related to orthopaedic infections.48 The apatite layer formed post-implantation is induced by the borate ion layer formation in contrast to the silicate-rich layer formation in silicate BG.41 To overcome their limitation, aluminium oxide or strontium oxide is partially replaced with borate ions to decrease the rate of dissolution. These ions can facilitate better osteoblast cell differentiation.383. Fabrication techniques for polymer–BG nanocomposites
Owing to the individual advantages of the polymers and ceramics, combining them and fabricating them into composites has been a milestone for biomedical applications. The composites can be fabricated by using conventional as well as advanced 3D printing techniques. The composites can be fabricated in various forms such as foams, membranes, sheets, single layer or multi-layer scaffolds or fibres. Based on the technique employed, the form of the composites will vary. Some of the most important techniques used in composite fabrication are phase separation, freeze drying, solvent casting technique, foam replica method, electrospinning, salt leaching method and various additive manufacturing techniques such as fused deposition modelling, stereolithography, and selective laser sintering.49,50 Some of the major techniques adopted for the fabrication of polymer–BG composites will be discussed in this section.3.1. Freeze drying/lyophilization
This technique of composite fabrication has been conventionally employed and the terms freeze-drying and lyophilization are very much similar in meaning but the former is more accurate. It works on the principle of sublimation and the solvent used is usually water. This method of composite scaffold fabrication aids in producing scaffolds with different pore sizes that can be tuned according to the pre-freezing time. The advantages of the process are that there is no separate step for leaching as in salt leaching and highly porous structures that are random or oriented are formed. The disadvantages are that this method is very time consuming and is very expensive.49,51 Natural polymers such as alginate, chitosan,52,53 collagen,51 and silk fibroin54 and synthetic polymers such as PLGA55 can be made into composites using this technique.3.2. Solvent casting
This method is widely employed for fabricating composite films reinforced with nanoparticles such as bioactive glass or any other filler. It involves stirring the polymer solution along with the filler at high speed or under ultrasonication that will induce mixing and further the mixture will be cast into moulds. It is a very simple technique with good reproducibility. The solvent employed for dissolving the polymer will be removed by drying at appropriate temperature. The method can be employed to fabricate very thin sheets or membranes. The disadvantages of this method are non-uniform distribution of the nanoparticles, and brittleness and shrinkage that occurs after the drying process. Various synthetic and natural polymers can be used in this method.49,563.3. Electrospinning
Electrospinning is a fabrication technique in which the polymer will be made into a viscous solution and an electric field will be applied that will aid in the formation of nanofibers and further be collected on a metal plate. The polymer solution can be incorporated with any nanoparticle such as bioactive glass. A high voltage power supply will be required for the generation of nanofibers. The generated fibrous mat will show porosity and the fiber diameter can be easily controlled and varied. The fibers have a very high surface area and the method is comparatively inexpensive. The major disadvantage of this method is that the pore size decreases with increasing fibre thickness and this technique is not applicable for all the polymers. Synthetic polymers such as PLGA,57 PVA,58 PCL,59 and PLA60,61 and natural polymers such as collagen62 and chitosan63 have been employed to make composites along with bioactive glass particles.493.4. 3D printing
3D printing is an additive manufacturing technique that can aid in the formation of three dimensional composite structures. It is based on computer aided design and is employed to fabricate intricate structures. There are multiple types of 3D printing techniques such as stereolithography, selective laser melting, selective laser sintering, fused deposition modelling and robocasting method. Each method involves different ways of sample preparation and has its own set of advantages and disadvantages. The main aim of the method is that it can be employed to make patient specific composites according to the requirement. The precursor composite solution can be in the form of filaments, powders or printable inks. The cells can also be loaded along with the ink solution facilitating the 3D bioprinting approach. It will be more of a biomimetic kind of approach mimicking the natural tissues of the body.49,64,653.5. Gas foaming
Gas foaming is a composite fabrication technique where porogens will be used to create pores rather than the usage of any solvent. Highly porous structures will be formed and can be controlled. The processing time involved is very long and interconnectivity between the pores can be a challenge.494. Natural polymer–BG nanocomposites
Natural polymers have been widely employed for various biomedical applications, especially in the regeneration of tissues, since they mimic the extracellular matrix of the human body.66 Even though they have advantages such as biocompatibility, bioactivity, and biodegradability, they have disadvantages like poor mechanical strength, low availability, and cost. Owing to their poor mechanical strength, they are used as composites to alter the mechanical properties and the degradation rate.67,68 They can be classified as either protein-based or polysaccharide-based polymers. Protein-based polymers include silk fibroin, gelatin, fibrin, elastin, and keratin. Polysaccharide-based polymers include chitosan, alginate, starch, dextran, chitin, cellulose, and agarose.49 The following section discusses naturally derived polymer–BG nanocomposites.4.1. Polysaccharide-based polymer–BG nanocomposites
Generally, polysaccharides can be obtained from different sources such as animals (chitin, chitosan), plants (starch, cellulose), and microorganisms (dextran, xanthan gum). Variation in molecular weight and polysaccharide chains can alter their physio-chemical properties. The properties can be tuned according to the need for various biomedical applications.69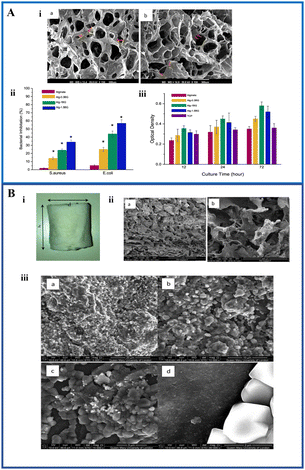 | ||
Fig. 2 (A) (i) SEM micrographs of the alginate–BG composite at different concentrations. The red arrows indicate the distribution of BG in the scaffold; (ii) antibacterial activity of the composite scaffold against Staphylococcus aureus and Escherichia coli; and (iii) cell viability assessment of the composite scaffolds using MTT assay (reproduced with permission from ref. 75. Copyright 2019, Elsevier); (B) (i) fabricated composite scaffold; (ii) SEM images of the (a) fabricated composite scaffold and (b) open pore microarchitecture of the fabricated composite scaffold; and (iii) SEM images of the ICIE silicate based BG-alginate scaffold immersed in SBF after 2 weeks: (a) 5000×, (b) 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000×, (c) 15 000×, (c) 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000×, and (d) 60 000×, and (d) 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000× (reproduced with permission from ref. 76 under CC-BY license). 000× (reproduced with permission from ref. 76 under CC-BY license). | ||
Another study utilized ICIE16 silicate-based BG modified with Sr and Zn ions and further combined it with alginate to fabricate a nanocomposite scaffold by the freeze-drying method as indicated in Fig. 2B(i). The ICIE16 was developed as an alternative of 45S5 BG with a combination of 48% SiO2, 6.6% Na2O, 32.9% CaO, 2.5% P2O5, and 10% K2O which has a comparatively large sintering window.77Fig. 2B(ii) shows the SEM micrograph of the scaffold with open pores and the pore size was very much appropriate for osteoinduction. The maximum pore size was found to be 308.9 μm. The amorphous nature of the BG facilitated an enhanced bioactivity evident by the formation of the apatite layer when incubated in SBF (Fig. 2B(iii)). Calcite formation was observed on the surface of the composite between 120 hours and 2 weeks.76 Erol et al. fabricated and characterized a new type of boron-based BG coated with alginate with Cu-releasing ability. The SEM images confirmed the presence of alginate. It was found that the mechanical ability and the bioactivity were increased because of the coating and further facilitated copper ion release in a controlled way.78
Bio-silica based BG–alginate composite putty was fabricated to act as a bone support material and was evaluated for in vitro capability, bioactivity, and cytotoxicity behaviour. The putties exhibited non-Newtonian behaviour that is suitable for a variety of surgical applications. This composite exhibited higher bioactivity in terms of apatite formation when compared to the commercial silica-based BG–alginate composite.79 Zn-substituted mesoporous BG was made into a composite along with alginate and methyl cellulose to fabricate bio-ink for bone regeneration applications. The addition of BG nanoparticles resulted in alteration of the rheological properties as well as an increase in the amount of ion release in the manufactured bio-ink.80
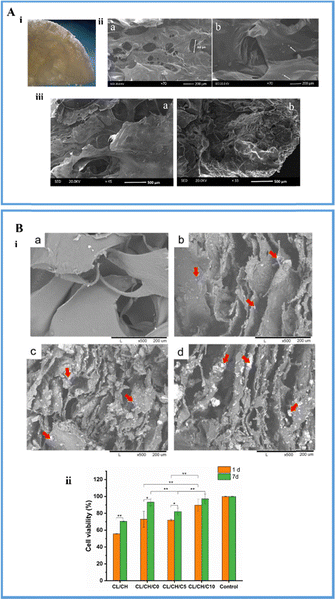 | ||
| Fig. 3 (A) (i) The image of the fabricated composite scaffold; (ii) SEM micrographs of the composite scaffold: (a) dispersion of the BG nanoparticles in the scaffold and (b) the pore size of the scaffold is 200 μm; (iii) SEM image of the composite scaffold (a) before and (b) after immersion in simulated body fluid for 7 days (reproduced with permission from ref. 84. Copyright 2020, John Wiley and Sons); (B) (i) SEM micrographs of the composite scaffolds: (a) chitosan–collagen, (b) chitosan–collagen with undoped BG, (c) chitosan–collagen with 5 moles% cerium doped BG and (d) chitosan–collagen with 10 moles% cerium doped BG where the red arrows show the dispersion of BG particles in the polymer matrix; and (ii) cell viability assays of the fabricated composite scaffolds (reproduced with permission from ref. 85 under CC.BY license). | ||
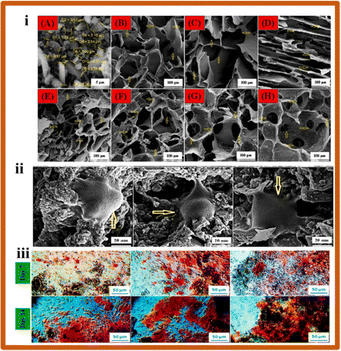 | ||
| Fig. 4 (i) SEM micrographs of the scaffolds fabricated with different compositions (10 wt% starch, 2, 3, and 4 wt% Aloe vera, 2, 3, and 4 wt% BG and 0.5, 1 and 1.5 wt% quail egg shell); (ii) SEM images of MG-63 cells adhered on S5 (10 wt% starch, 2 wt% Aloe vera, 2 wt% BG and 0.5 wt% quail egg shell), S6 (10 wt% starch, 3 wt% Aloe vera, 3 wt% BG and 1 wt% quail egg shell), and S7 (10 wt% starch, 4 wt% Aloe vera, 4 wt% BG and 1.5 wt% quail egg shell) scaffolds after 72 hours; (iii) optical images of Alizarin red staining assay on composite scaffolds (S5, S6, S7) after 7 and 14 days of cell culture (reproduced with permission from ref. 92 Copyright 2022, Elsevier). | ||
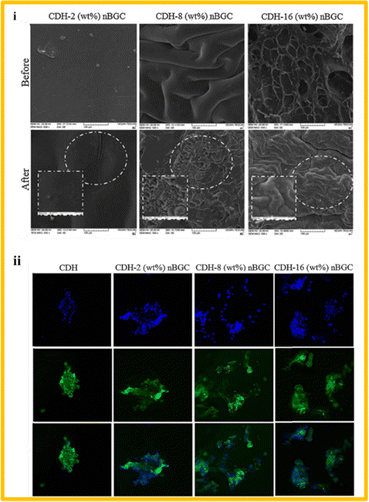 | ||
| Fig. 5 (i) SEM images of the fabricated composite scaffolds made of dextran and BG at different concentrations, before and after immersing in simulated body fluid for 28 days and (ii) immunofluorescence images of human osteoblast cells cultured on the composite scaffolds (reproduced with permission from ref. 97. Copyright 2018, Elsevier). | ||
4.2. Protein based polymer–BG nanocomposites
Proteins are made of amino acids, and if the side chain changes, it can change both physiochemical and biological properties of the protein. Protein based polymer–BG nanocomposites have disadvantages, like making people sick and having different purification results from one batch to the next. Some examples of biomedical applications of protein-based biopolymers are as scaffolds for tissue engineering and drug delivery systems, among other things. Proteins can be made to break down at different rates, so they can be made to fit the needs of a specific application. Some of the biopolymers made from proteins that are used to make nanocomposites are collagen, gelatin, and silk fibroin.69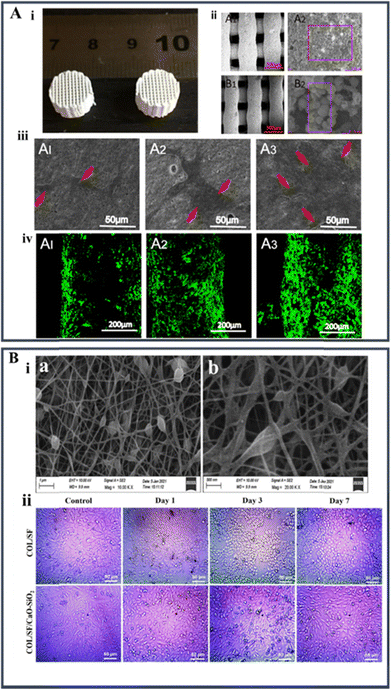 | ||
| Fig. 6 (A) (i) Optical image of 3D printed silk fibroin-mesoporous BG nanoparticle scaffolds; (ii) SEM micrograph images of the 3D printed scaffolds after immersion in simulated body fluid for 28 days (A1 and A2: mesoporous BG/silk fibroin scaffold; B1 and B2: mesoporous BG/polycaprolactone scaffold); (iii) SEM images of the human bone marrow stem cells attached on mesoporous BG/silk fibroin scaffolds after 7 days of cell seeding; and (iv) LCSM images of the cells seeded on mesoporous BG/silk fibroin scaffolds for one week. The cell actin and nuclei were labelled with Alexa Fluor 488 phalloidin (reproduced with permission from ref. 27 under CC-BY license); (B) (i) SEM images of the composite nanofiber and (ii) light microscopic images showing the in vitro biocompatibility of Saos-2 of the collagen–silk fibroin system and the composite system with BG at 1, 3, and 7 days (reproduced with permission from ref. 104 under CC-BY license). | ||
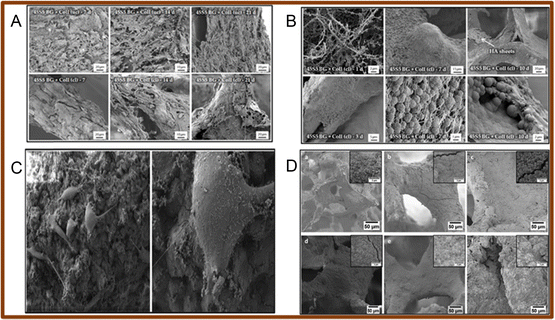 | ||
| Fig. 7 (A) SEM images of MG-63 cells seeded on collagen/BG scaffolds (uc – un-crosslinked; cl – crosslinked); (B) SEM images of the collagen/BG scaffolds after immersion for 1, 7 and 10 days (uc – un-crosslinked; cl – crosslinked) (reproduced with permission from ref. 91 under CC-BY license); (C) SEM images of the MG-63 cell line seeded on the surface of gelatin/Mn-mesoporous BG nanoparticles at low and high magnifications; (D) SEM images of the scaffold immersed in simulated body fluid: (a) and (d) 45S5 uncoated BG scaffolds, (b) and (e) Mn-mesoporous BG coated scaffold, (c) and (f) gelatin/Mn-mesoporous BG coated scaffold after 3 (a)–(c) and 21 (d)–(f) days of incubation (reproduced with permission from ref. 109. Copyright 2019, Elsevier). | ||
Table 1 discusses the different natural polymers made into nanocomposites with bioactive glass along with their key findings and application.
| Polymer | BG | Ion doping | Technique | Key findings | Applications | Ref. |
|---|---|---|---|---|---|---|
| Alginate | SiO2–P2O5–CaO–ZnO–MgO | Zn and Mg | Scaffold – freeze drying | Compressive strength: 1.7 MPa; improvement in degradation property; induced the formation of apatite on the surface after immersion in SBF | Bone tissue engineering | 75 |
| Alginate | ICIE 16M | Sr and Zn | Scaffold– freeze drying | Average pore size of 110 μm; average Young's modulus: 1.83 ± 0.66 MPa; crystal growth observed after immersion of the scaffold in SBF after 120 hours | Bone tissue engineering | 76 |
| Alginate | SiO2–CaO–Na2O–P2O5 (biosilica) | Nil | Composite putty – mechanical mixing | Exhibited pseudo plastic fluid behavior; bio silica-based composite putty showed higher bioactivity; not cytotoxic below 5 mg mL−1 concentration level | Bone tissue engineering | 79 |
| Alginate | 80SiO2–18B2O3–2SeO2 | Se | Composite – freeze drying | The presence of nano BG in the scaffold reduced the swelling and degradation time at 48 hours; positive antimicrobial effect against Staphylococcus aureus | Wound healing | 112 |
| Alginate | S53P4 (53% SiO2, 20% CaO, 23% Na2O and 4% P2O5) | Nil | Bioink fabrication – mechanical mixing, 3D printing | Increased compression strength in cross-linked (5.45 ± 0.01 MPa) scaffolds when compared to un-crosslinked (5.08 ± 0.37 MPa) scaffolds; effective anti-bacterial and antifungal activity; increase in cell viability in MC3T3-E1 cells to 100% in 14 days | Bone infections | 113 |
| Chitosan | 55% SiO2, 40% CaO, 5% P2O5 | Nil | Scaffolds – freeze gelation | Pore size: 150–300 μm; scaffold with 20% BG nanoparticles showed better surface area; 9% loss in weight observed after immersion in phosphate buffer saline (PBS); increased bioactivity due to the addition of chitosan to the scaffold | Bone tissue engineering | 84 |
| Chitosan | 60% SiO2, 36% CaO, 4% P2O5 | Nil | Composite scaffold – needle punching technique + dip coating | Porosity: 77.52%; pore size ∼50 μm, compression strength: 7.68 ± 0.38 MPa; water absorption capacity – 59%; elastic modulus: 0.46 ± 0.02 GPa | Bone tissue engineering | 114 |
| Chitosan | 64% SiO2, 26% CaO, 5% P2O5, 5% MgO | Mg | Films – solvent casting | Increased bioactivity after immersion in SBF | Orthopaedic and maxillofacial application | 115 |
| Increased cell adherence and proliferation in Saos-2 osteoblast-like cells | ||||||
| Chitosan | 6% Na2O, 8% K2O, 8% MgO, 22% CaO, 54% B2O3, 2% P2O5 | Nil | Injectable bone cement – mechanical mixing | Compressive strength was found to be 31 ± 2 MPa, enhanced cell proliferation and alkaline phosphatase activity in the MC3T3-E1 cell line; bone formation stimulated in rabbit femoral condyle 12 weeks post-implantation | Bone tissue engineering | 116 |
| Chitosan | 55% SiO2–40% CaO–5% P2O5 | Nil | Composite – solvent casting | Increased metabolic activity against human periodontal ligament cells | Periodontal regeneration | 117 |
| Starch | 64% SiO2–31% CaO–5% P2O5 | Nil | Composite scaffold– freeze drying | Cell viability greater than 95%. Increased compressive strength, expression of bone differentiation markers ranged between 30 and 75% | Bone regeneration | 92 |
| Starch | 45S5 BG | Nil | Scaffold – freeze drying | Calcium phosphate layer formed on the surface proving their bioactivity. Enhanced adhesion and proliferation on rat stromal cells and expression of markers such as osteocalcin and osteopontin | Bone tissue engineering | 118 |
| Starch | 50% SiO2, 42% CaO, 8% P2O5 | Nil | Composite –solvent casting and salt leaching | Increased tensile strength and Young's modulus. Improved formation of the apatite layer when immersed in SBF; the degradation rate decreased as the amount of BG increased | Bone tissue engineering | 119 |
| Starch | 45S5 BG | Nil | Twin screw extrusion–injection molding | The modulus was found to be 3.8 GPa and the ultimate tensile strength was found to be 38.6 MPa | Temporary bone replacement, fracture fixation | 120 |
| Dextran | 64% SiO2, 31% CaO, 5% P2O5 | Nil | Hydrogel – mechanical mixing, freeze drying | Average particle size: 77 nm, increased apatite layer formation after incubation in SBF; enhanced adherence and spreading of osteosarcoma cells (Saos-2) | Bone regeneration | 98 |
| Dextran | 64% SiO2, 31% CaO, 5% P2O5 | Nil | Scaffold – freeze drying | Average pore size: 240 μm, higher BG content leads to agglomeration and reduced compressive modulus. Increased human osteoblast cell proliferation and alkaline phosphatase activity | Bone tissue engineering | 97 |
| Silk fibroin | Mesoporous BG | Nil | Composite scaffolds – 3D printing | Enhanced compressive strength – 20 MPa. No cell toxicity observed against bone marrow stem cells. Increased expression of bone formation markers | Bone tissue engineering | 28 |
| Silk fibroin | 70S30C – 70% SiO2, 25% CaO, 5% P2O5 | Sr | Composite scaffolds – 3D printing | Enhanced bone formation in vivo. High expression levels of biomarkers. Increased proliferation of mesenchymal stem cells | 3D bone constructs | 102 |
| Silk fibroin/chitosan | 45S5 BG | Cu | Hydrogels – mechanical mixing | Promoted osteogenesis and vascularization. Improved cell adhesion in MC3T3-E1 cells | Bone tissue engineering | 103 |
| Silk fibroin/alginate | Mesoporous BG | Nil | Hydrogels-mechanical mixing | Large pores for the loading of insulin growth factor; elastic modulus higher than 5 kPa; high mechanical strength | Drug delivery + bone tissue engineering | 121 |
| Silk fibroin/collagen | CaO–SiO2 (25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75) 75) |
Nil | Nanofibers – electrospinning | Enhanced osteogenic ability; highest thermal stability; compatible with Saos-2 cells | Repair osteoporotic bone defects | 104 |
| Silk fibroin | 58% SiO2–23% CaO–9% P2O5 | Nil | Films – solvent casting method | Increased hydrophilicity; improved bioactivity; supports the growth of osteoblast cells | Bone tissue engineering | 122 |
| Collagen | S53P4 BG–53% SiO2–23% Na2O–20% CaO–4% P2O5 | Nil | Gel scaffolds – plastic compression | Quick mineralization | Delivery of stem cells for bone regeneration | 107 |
| Apatite formation found within 1 day; increased compressive modulus | ||||||
| Collagen | 60% SiO2–34% CaO–4% P2O5–2% CuO | Cu | Scaffolds – freeze drying | 1.9 fold increase in compression; 3.6 times increase in calcium deposition; 66% inhibition against S. aureus; enhanced vascularisation | Bone tissue engineering | 29 |
| Collagen | 45S5 BG | Nil | Scaffolds – foam replica method | Compressive modulus: 0.18 ± 0.03 MPa; biocompatible and showed increased adherence and proliferation towards the MG-63 cell line | Bone tissue engineering | 106 |
| Collagen | 45S5 BG | Nil | Composite gel – plastic compression | Immediate calcium phosphate formation; increase in compression modulus; increased cell viability towards the MC3T3-E1 cell line | Bone tissue engineering | 108 |
| Collagen/chitosan | 80% SiO2, 15% CaO, 5% P2O5 | Ce | Scaffolds – freeze drying | >50% of cell proliferation observed when the cells were seeded on the composite for 24 hours; no toxicity of cells observed (<25% of inhibition observed) | Bone tissue engineering | 85 |
| Collagen | 58% SiO2, 38% CaO, 4% P2O5 | Nil | Membrane/scaffold – electrospinning | Rapid bioactivity; excellent biocompatibility; higher alkaline phosphatase activity | Bone tissue engineering | 123 |
| Gelatin | 45S5 BG | Nil | Sponge like scaffolds – foam replica method | Increased compressive strength; enhanced bioactivity; increased drug delivery ability | Drug delivery | 110 |
| Gelatin | 49.2% SiO2, 23.4% Na2O, 25.5% CaO, 1.7% P2O5 | Nil | Scaffolds – freeze drying | Porosity ranged between 79 and 84%; compressive strength varied between 1.9 and 5.7 MPa; enhanced bioactivity and osteogenic ability | Bone tissue engineering | 30 |
| Gelatin | 45S5 BG | Mn | Scaffolds + coating – foam replica method, dip coating | Enhanced biological activity towards the MG-63 cell line; increased bioactivity and biocompatibility | Bone tissue engineering | 109 |
| Gelatin | 95% SiO2, 2.5% CaO, 2.5% CuO | Cu | Scaffolds + coating – freeze drying, dip coating | Improved bioactivity; enhanced osteogenic ability; improved mechanical properties | Bone regeneration | 111 |
| Gelatin | 45S5 BG | Nil | Scaffold–solvent casting + lamination technique | Total porosity – 85%; pore size ranged from 200 to 500 μm | Bone regeneration | 124 |
| Positive biological activity towards endothelial cells; increased bone regeneration and angiogenic ability |
5. Synthetic polymer–BG nanocomposites
Synthetic biopolymers are materials that can be easily tuned according to the required properties and can be made into different structures.49 Their main advantages, compared to natural polymers, are extended shelf life, economical nature, ability to form any shape, and enhanced biological properties. The problem of employing synthetic polymers is that they lack the sites to bond cells, and various chemical treatments are required for the bonding to occur. The major synthetic polymers used for biomedical applications such as tissue engineering and drug delivery include PLA, poly lactic co glycolic acid (PLGA), poly glycolic acid (PGA), PVA, and poly hydroxy butyrate (PHB). These polymers exhibit varying levels of mechanical properties, biodegradability, and biocompatibility. They can be tuned according to the requirement of the application. These polymers are made into composites to improve their mechanical and biological properties.5.1. PLA–BG composites
PLA is a biodegradable aliphatic poly ester and is one of the most important synthetic polymers. It is highly in use because of its excellent properties, such as biodegradability and biocompatibility.125 It is also environmentally friendly since it can be isolated from feedstock that can be renewed and are not toxic. It is utilized in many applications, but has some limitations, such as the degradation rate being very slow, and hydrophobicity, and, thus, blending with other polymers or ceramics ameliorates the disadvantages.126 PLA and BG composite scaffolds were fabricated by the gel pressing technique. The scaffolds were made in two different compositions, and physio-chemical characterization was performed. The SEM micrographs of the scaffolds are shown in Fig. 8A(i) in which BG70–PLA30 scaffolds are homogeneous and dense, whereas BG30–PLA70 scaffolds show cracks due to the higher sintering temperature because of the higher percentage of PLA. The bioactivity test confirmed apatite layer formation on the surface of the scaffold when immersed in SBF for 14 and 28 days. The scaffold with a higher quantity of BG showed increased bioactivity making its presence essential as shown in Fig. 8A(ii). The degradation analysis of the scaffolds were analysed using phosphate buffer saline and was found to degrade with respect to new bone formation.36 In another study, composite scaffolds made of BG and PLA were fabricated using an extrusion-based 3D printing method. The scaffold's bioactivity was assessed by immersion in SBF and characterized by SEM. Apatite formation was observed in 1 day itself. The scaffold was highly porous with an interconnected structure. Weight loss (degradation analysis) was studied using SBF.127 PLA was coated onto the surface of the BG scaffolds. The scaffolds were fabricated by robocasting. The presence of this polymer increased the mechanical properties in terms of compression, toughness, and bending.128 Mesoporous BG was made into a composite with PLA by 3D bioprinting methodology. The Auto CAD design and the optical images of the scaffolds are shown in Fig. 8B(i) and (ii). The pore size of the composite ranged between 500 and 700 μm. Enhanced mechanical strength was observed, especially the compressive modulus of the composite was found to be 91.5 MPa. The HCA layer was formed on the third day after immersion in SBF. Enhanced cell adherence and proliferation of MG-63 cells when seeded in the bioprinted scaffolds containing BG nanoparticles when compared to the control scaffold was observed as indicated in Fig. 8B(iii).129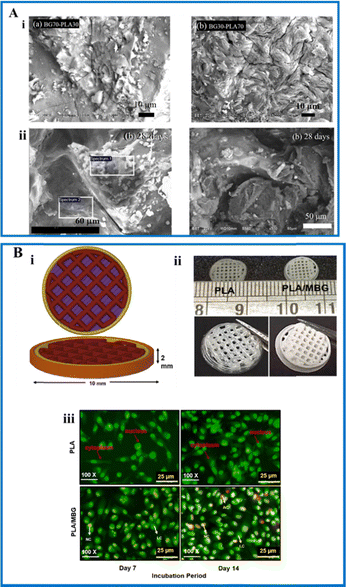 | ||
| Fig. 8 (A) (i) SEM images of BG70-PLA30 and BG30-PLA70 scaffolds and (ii) SEM images of the scaffolds (left side: BG70-PLA30 and right side: BG30-PLA70) immersed in simulated body fluid after 28 days (reproduced with permission from ref. 36 under CC-BY license); (B) (i) CAD design of the scaffold; (ii) optical images of the fabricated 3D printed construct; and (iii) live and dead staining of MG-63 cells seeded on 3D printed PLA and PLA with BG construct at 7 and 14 days (reproduced with permission from ref. 129. Copyright 2022, Elsevier). | ||
5.2. PCL–BG composites
PCL is an aliphatic polymer that is semi-crystalline and hydrophobic, preventing the adhesion and proliferation of cells. It shows adequate biocompatibility and mechanical strength.113 Enhancement of bioactivity is achieved by chemical treatments or surface functionalization. Degradation is very slow, and it takes about 2 to 4 years to get degraded by hydrolysis of the esters.130 The main advantages of using PCL are that it is non-toxic, has good mechanical properties and controls cell proliferation and adhesion. The drawbacks of PCL are its low bioactivity and the incapability to attach cells onto its surface.49,131 To improve the properties, PCL is made into a composite with other polymers or ceramics. In one study, PCL nanofibers were fabricated, and were embedded with BG nanoparticles and a drug called simvastatin. The presence of BG improved the rate of degradation and improved the rate of apatite formation when incubated in SBF. Drug release studies performed on phosphate buffer saline showed that the drug was released in a very controlled manner.132 PCL and mesoporous BG were made into a composite using 3D printing technology. Increasing the amount of BG resulted in enhancing the hydrophilicity. The composite showed apatite formation owing to the bioactivity of BG. In vitro analysis showed increased cell adhesion, proliferation, and up-regulation of various biomarkers such as Run-x2 and collagen I.133 Hybrid scaffolds using PCL and BG were fabricated by the porogen leaching method. The scaffold network was found to be highly porous and initiated apatite formation within 24 hours of immersion in SBF. Degradation analysis reported 13.2% weight loss at the end of 8 weeks. The scaffold supported the growth of osteoblast cells and was found to be non-toxic. Similarly, the in vivo analysis showed that the scaffold is biocompatible, bioactive, and biodegradable.1345.3. PVA–BG composites
PVA is a semi-crystalline, well-hydrated polymer with a hydroxyl group. It is formed from the hydrolysis of polyvinyl acetate. The major advantages of using PVA are that it is biocompatible, non-toxic, and has strength like that of cartilage. The drawbacks of using PVA are that there are no cell adhesion sites and very less ingrowth of cells.49 PVA is used for applications in the biomedical domain, such as tissue engineering and designing novel drug delivery systems because of its properties. Modification can be easily done according to the requirement of the application.135 In one study, the cobalt ion was incorporated into PVA–BG scaffolds fabricated by the foam replica method. The formed scaffolds were highly porous in nature, forming a network-like structure. The fabricated composite supposedly had physical, mechanical, and biological properties same as that of natural bone. In vitro assays performed on umbilical vein endothelial cells showed no cytotoxicity and good mitochondrial activity. Ion release was very fast, and the elastic modulus was 20.19 MPa.34 Injectable hydrogels composed of BG and PVA were fabricated by the physical cross-linking method. The bioactive glass particles were uniformly distributed into the hydrogel network. An increase in the compression strength by 325% and elastic modulus by 150% was observed. The in vivo analysis in adult male rats showed that the composite scaffolds were biocompatible, and no inflammation was seen.136 PVA was made into a nanofiber composite along with chitosan and BG by the electrospinning approach and loaded with a drug to treat osteoporosis.137 Strontium-doped mesoporous BG was made into a composite with PVA using 3D printing technology. The presence of strontium aids in quick bone regeneration. The amount of BG directly corresponded to the bioactive layer formed in the composite after immersion in SBF. In vitro results indicated that the composite was biocompatible when tested on MC3T3-E1 cells.1385.4. PHB–BG composites
PHB is a highly crystalline homo-polymer that is non-toxic and bio-compatible. It has a slow degradation rate, and it is more advantageous than PLA.49 It is a member of polyhydroxyalkanoates, and is highly preferred in biomedical applications owing to its properties like biocompatibility and non-toxicity to cells such as osteoblasts, fibroblasts, and endothelial and epithelial cells. The drawbacks include hydrophilicity and slow degradation. PHB is made into a composite with other polymers or ceramics to improve its properties. Ceramic nanoparticles are used as reinforcements for improving the mechanical strength and the rate of degradation. In one study, nano-fibrous scaffolds were fabricated using BG and PHB by an electrospinning technique. The particles were uniformly distributed in the network structure. The bioactivity results showed that the surface of the scaffolds had increased the apatite formation in 21 days due to the presence of BG.139 A composite of PHB, PCL, and BG was fabricated using an electrospinning technique. The composite formed a porous network, and the average fiber diameter increased with respect to the quantity of BG. The Young's modulus decreased from 101 MPa without the addition of BG and varied between 67 and 87 MPa in composites. The strain values increased 40 to 50 times compared to pure electro-spun scaffolds. In vitro analysis showed that the fabricated scaffold was biocompatible and supported the adhesion and proliferation of the MG-63 cell line.140 PHB/chitosan and multi-walled carbon nanotubes (MWCNTs) were coated on BG and titania scaffolds fabricated by the foam replication method. A high percentage of porosity was observed in the scaffolds, and the surface roughness was increased. The degradation rate was improved, and enhanced bioactivity was found when immersed in SBF.141Table 2 discusses the different synthetic polymers made into nanocomposites with bioactive glass along with their key findings and application.
| Polymer | BG | Ion doping | Technique | Key findings | Applications | Ref. |
|---|---|---|---|---|---|---|
| PLA | SiO2–CaO–Na2O–P2O5 | Nil | Scaffolds – gel pressing technique | Improved bioactivity; steady degradation rate | Bone tissue engineering | 36 |
| PLA | 45S5 BG | Nil | Composite – 3D printing | Apatite layer observed on the surface of the composite in 1 day | Bone regeneration | 127 |
| PLA | 45S5 BG | Nil | Scaffold – robocasting | Enhancement of mechanical properties | Bone tissue engineering | 128 |
| PLA | Mesoporous BG | Nil | Composite – 3D bioprinting | Increased compression modulus (6.18 GPa); increased apatite formation; yield strength was found to be 80.2 MPa | Bone tissue engineering | 129 |
| PLA/chitosan | SiO2, CaO, P2O5, CeO2, CuO, Ag2O | Ce/Cu/Ag | Nanofibers – electrospinning | Antibacterial activity against E. coli; improved bioactivity; enhanced mechanical strength | Bone trauma | 142 |
| PCL | BG | Nil | Nanofibers – electrospinning | Improved bioactivity; increased mechanical strength; improved degradation rate | Bone regeneration | 132 |
| PCL | 58S BG | Nil | Composite – 3D printing | Increase in hydrophilicity; increased mechanical strength; expression of markers such as Run-x2 and collagen I | Bone tissue engineering | 133 |
| PCL | 58S BG | Nil | Scaffold – porogen leaching method | Enhanced bioactivity; increased mechanical strength; supported cell adhesion and proliferation on primary osteoblasts | Bone tissue engineering | 134 |
| PCL | SiO2–CaO–P2O5 | Nil | Scaffolds – robocasting | Enhanced biocompatibility with the Saos-2 cell line; increased angiogenesis; evident new bone formation in osteoporotic sheep; enhanced bioactivity | Bone tissue engineering | 143 |
| PVA | 45S5 BG | Cu | Scaffolds – foam replica method | Increased angiogenic properties; good mitochondrial activity; increased bioactivity; good cell adhesion and proliferation | Bone tissue engineering | 34 |
| PVA | 45S5BG | Nil | Injectable hydrogels – physical crosslinking | Self-standing hydrogel obtained; compressive strength increased by 325%; elastic modulus increased by 150% | Bone tissue engineering | 136 |
| PVA | 46% SiO2, 24% CaO, 24% Na2O, 6% P2O5 | Nil | Nano-fibrous composite – electrospinning | Drug release efficiency: 93%; sustained drug release was observed; the nanofibers ranged between 20 and 125 nm in diameter | Drug delivery system | 137 |
| PVA | SiO2–CaO–P2O5 | Sr | Composite – 3D printing | Highly ordered mesoporous structure; increased bioactivity; cell cytocompatibility towards the MC3T3-E1 cell line | Bone tissue engineering | 138 |
| PHB | 58S BG | Nil | Nanofibrous scaffolds – electrospinning | Increased bioactivity; uniform distribution of BG particles in the porous network | Bone tissue engineering | 139 |
| PHB | 58S BG (60% SiO2, 36% CaO, 4% P2O5) | Nil | Nanofibrous scaffolds – electrospinning | Strain increased 40 to 50 times; no toxicity observed with the MG-63 cell line | Bone tissue engineering | 140 |
| PHB/chitosan/MWCNT | 45S5 BG | Nil | Scaffolds – foam replication method | Interconnected porous structure; increased degradation rate; enhanced bioactivity; increased surface roughness | Bone tissue engineering | 141 |
| PHB | 45S5 BG | Nil | Films – solvent casting | Young's modulus: 0.8 to 1.6 MPa; increased bioactivity; good biocompatibility; increased cell proliferation | Bone tissue engineering | 144 |
6. Biomedical applications of BG nanocomposite materials
BG is already present in a few of the commercially available products. A toothpaste sold under the brand name “Sensodyne” contains Novamin®, a proprietary BG composition that is highly effective in remineralizing teeth.145 This is one of the most popular examples of mass-scale products that utilize the numerous biological properties of BGs. In the following sections, a few interesting research works on the applications of BG biopolymer composites are discussed.6.1. Tissue engineering
Besides their major contribution to bone-based materials, BG-based polymer composites have also been used in tissue engineering due to their osteogenic, angiogenic, and antibacterial properties.146 Along with these capabilities, the immunomodulatory capabilities of the BG nanoparticles also greatly influence the tissue regeneration process of the body. Inorganic materials, such as silica and calcium phosphate, the major components in BGs, have already been found to have immunomodulatory capabilities.147–149 The dissolution products of BG have been found to stimulate a favourable response in target cells (e.g., stem cells, fibroblasts, etc.) that contributes to the tissue regeneration process. The BG-composites are also employed for a wide variety of soft tissue engineering applications as indicated in Fig. 9.150 | ||
| Fig. 9 Illustration of the use of BG-composites in various aspects of soft tissue engineering (reproduced with permission from ref. 150 under CC-BY 4.0 license). | ||
BG locally establishes a favourable microenvironment for stem cell differentiation by regulating immune cell activities and reducing the inflammatory response.147,148 Various strategies such as incorporating active inorganic ions, the programmed release of immunomodulatory agents, functionalization, and manipulation of surface features have been used for obtaining an immunomodulatory response from BG-containing biomaterials (Fig. 10).146 These immunomodulatory capabilities are found to have a great impact in engineering soft tissues such as wound healing and even cancer therapy.151,152
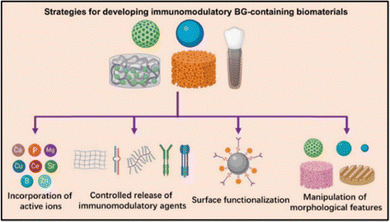 | ||
| Fig. 10 Strategies used for developing immunomodulatory BG containing biomaterials (reproduced with permission from ref. 146 (CC-BY 4.0)). | ||
6.2. Drug delivery
Mesoporous BGs have widely been employed for drug delivery applications because of their drug-loading capabilities. BG-based composite systems provide an interesting avenue for multifunctional drug loading and delivery due to the possibility of optimizing release kinetics to suit targeted drug delivery. BG nanoparticles have been loaded with numerous therapeutics ranging from therapeutic ions, macromolecules (e.g., DNA), to small molecules to target various tissue sites for the treatment of pathological conditions ranging from bone diseases, inflammation, to even cancer. One of the major advantages of BGs as drug carriers arises from their non-inert nature inside the body. The dissolution of BG inside the biological environment also leads to the promotion of tissue regeneration along with drug release. Another advantage of BG-based systems is the localized drug delivery mechanism that eliminates the challenges of the peak-plateau effect, frequent administration, a requirement of heavy dosage, patient compliance, and non-specific targeting faced in traditional drug delivery routes.152 Various routes have been used for drug delivery through BG–biopolymer composites which include scaffolds, microparticles, hydrogels, etc. Electrospinning of polymer-bioactive nano-fibers is an interesting route for achieving drug delivery in a sustained manner over a longer duration. In a study evaluating the drug release kinetics of RIS osteoporotic drug (Risedronate sodium) loaded chitosan–PVA nanofibers, BG nanoparticles were added to achieve sustained release. It was found that the CS/PVA nanofibers loaded with BG showed only 30% RIS release compared to 80% release in non-BG-containing fibers in 30 min duration. The release kinetics of BG-containing drug-loaded CS/PVA nanofibers were further studied over 96 hours. Only 72.21% of the drug was released in that duration, signifying significant improvement over non-BG-loaded fibers.137 BGs containing micro and nano-spheres are another avenue used for the controlled release of drugs. PHBV-BG microspheres have been explored to deliver the popular phototherapeutic drug curcumin. The microspheres showed good biological properties in terms of cytotoxicity and bioactivity. The microspheres showed a sustained release of the drug over 24 hours of immersion in phosphate-buffered saline.153 Similar to this, there are multiple researchers working on using the polymer–BG composite as a drug delivery vehicle.6.3. Wound healing
BG has the capability of releasing various ions when in contact with biological fluids that can stimulate various wound healing-related processes. BGs also have other capabilities, such as the antibacterial effect that largely increases the success rate of wound healing.24 BGs can play a role in the multiple stages of the wound healing process. Therapeutic ions can play an important role in the secretion of growth factors.154–156 Cu-containing mesoporous BG and nano-fibrillated cellulose composites were evaluated for their biological response through gene expression in 3T3 fibroblast cells. It was found that there was up-regulation of VEGF, PDGF, and FGF growth factors due to the release of copper ions from the nanoparticles.157 Another study examined the angiogenic capabilities of sol–gel based 58S (60SiO2·36CaO·4P2O5, mol%) and 80S (80SiO2·15CaO·5P2O5, mol%) nano BGs by dissolving in cell culture media. The gene expression studies showed 2- to 3-fold more upregulation of VEGF and FGF in glass extract media compared to control media in umbilical vein cells of humans, respectively. These results are attributed to the presence of silicon and calcium ions eluted from the BG nanoparticles.158 These capabilities of BGs to promote angiogenesis are being widely explored for the development of wound-healing dressings and ointments.159 BGs doped with ions such as Zn and Ag have also been found to vastly impact wound healing by inhibiting microbial growth.160 A gelatin/chitosan/BG composite was used to fabricate a nanofibrous membrane for wound healing. In the evaluation of antibacterial activity against Gram-negative Escherichia coli (E. coli) and Gram-positive Actinomyces viscosus (A. viscosus) using the disc diffusion method, the membrane showed significant inhibition of bacterial growth. The membrane also showed no inflammation and degraded within weeks of being implanted in a rat model.161 Different BG containing polymer composites have been used as ointments,159,162,163 hydrogels112,148,164,165 and dressings161,166 for the treatment of wounds, with all studies showing encouraging results.7. Challenges and future outlook
One of the major advantages of nano BGs is their market presence. BGs are not restricted to laboratories but are used in various medical and everyday products such as toothpaste. Various formulations have already received approval from authorities such as the US Food and Drug Administration (FDA). The specificity and the optimization of combining biopolymers and nano BG and their influence on the intended application must be well understood. This will make the development and marketing of BG–polymer-based medical products easier and less tedious. However, there are numerous challenges related to their scalability for producing BG nanoparticles on a larger scale. Most studies are limited to in vitro or preliminary in vivo studies, and the long-term impact of the developed composites has not been explored. Due to the higher number of materials involved, there are also concerns regarding the feasibility of the composites for clinical translation. In the future, advanced fabrication technologies such as 4D printing or 5D printing will be used to fabricate stimuli responsive composites or to make complex curved structures. Another major factor that affects the mass acceptance and application of composite materials is the cost. Currently, BG nanoparticles are very steeply priced, making them unaffordable for a significant global population. Thus, for broader acceptance and application of the BG–polymer composites, various concerns related to cost, scalability, and regulations must be addressed.8. Conclusion
BG polymer composites are an interesting avenue for future research due to their potential for application in multiple areas such as bone and tissue regeneration, wound healing, and drug delivery. The physiochemical and biological properties of BG make them a perfect material for these applications. Apart from the apatite layer forming capability, BG-based systems provide an interesting avenue owing to their unique characteristics like controllable drug release kinetics, biodegradability, and biocompatibility. The important properties of BG, such as bioactivity and solubility, are improved at the nanoscale. The properties of the biopolymers involved are tuned according to the application. Composites fabricated out of biopolymers and nano BG help to develop personalized scaffolds for repair and regeneration. The drug-releasing capability, mechanical strength, and degradation kinetics are also altered when made into a composite. Optimization in pre-clinical studies will also help to develop a proper understanding of this combination. Due to these features, we may expect numerous new advancements in the fabrication techniques as well with respect to the biomedical applications of polymer–BG nanocomposites in the coming years.Author contributions
Conceptualization: AJN, RS, and GM, writing – original draft preparation: RS and AJN; writing – review and editing: AJN, GM, RG and RS; supervision: AJN, GM, and RG. All authors have read and agreed to the published version of the manuscript.Data availability
This is a review article. But all the original contents are available on request.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
AJN would like to acknowledge the financial support from the Department of Biotechnology, Government of India, through the Ramalingaswami Re-entry fellowship (D.O. No. BT/HRD/35/02/2006) and Faculty Startup Venture (FSV2024) from Vellore Institute of Technology. RS acknowledges the UGC-Savitribai Jyotirao Phule Fellowship for Single Girl Child (202223UGCES-SJSGC-14584) which supported her PhD. The authors would also like to acknowledge the support of Biorender software for making figures.References
- J. R. Jones, Review of Bioactive Glass: From Hench to Hybrids, Acta Biomater., 2013, 9(1), 4457–4486, DOI:10.1016/j.actbio.2012.08.023.
- H. O. Simila and A. R. Boccaccini, Sol-Gel Bioactive Glass Containing Biomaterials for Restorative Dentistry: A Review, Dent. Mater., 2022, 38(5), 725–747, DOI:10.1016/j.dental.2022.02.011.
- L. L. Hench, R. J. Splinter, W. C. Allen and T. K. Greenlee, Bonding Mechanisms at the Interface of Ceramic Prosthetic Materials, J. Biomed. Mater. Res., 1971, 5(6), 117–141, DOI:10.1002/jbm.820050611.
- Á. J. Leite and J. F. Mano, Biomedical Applications of Natural-Based Polymers Combined with Bioactive Glass Nanoparticles, J. Mater. Chem. B, 2017, 5(24), 4555–4568, 10.1039/C7TB00404D.
- A. Baranowska, M. Kochanowicz, A. Wajda, M. Leśniak, J. M. Żmojda, P. Miluski, I. Zgłobicka, K. J. Kurzydłowski and D. Dorosz, Luminescence Sensing Method for Degradation Analysis of Bioactive Glass Fibers, Sensors, 2021, 21(6), 2054, DOI:10.3390/s21062054.
- Mesoporous Bioactive Glasses in Cancer Diagnosis and Therapy: Stimuli-Responsive, Toxicity, Immunogenicity, and Clinical Translation - Sharifi - 2022 - Advanced Science, Wiley Online Library. https://onlinelibrary-wiley-com.egateway.vit.ac.in/doi/10.1002/advs.202102678 (accessed 2024-08-30) Search PubMed.
- E. Engel, A. Michiardi, M. Navarro, D. Lacroix and J. A. Planell, Nanotechnology in Regenerative Medicine: The Materials Side, Trends Biotechnol., 2008, 26(1), 39–47, DOI:10.1016/j.tibtech.2007.10.005.
- L. Zhang and T. J. Webster, Nanotechnology and Nanomaterials: Promises for Improved Tissue Regeneration, Nano Today, 2009, 4(1), 66–80, DOI:10.1016/j.nantod.2008.10.014.
- P. Saravanapavan, J. R. Jones, R. S. Pryce and L. L. Hench, Bioactivity of Gel–Glass Powders in the CaO-SiO2 System: A Comparison with Ternary (CaO-P2P5-SiO2) and Quaternary Glasses (SiO2-CaO-P2O5-Na2O), J. Biomed. Mater. Res., Part A, 2003, 66A(1), 110–119, DOI:10.1002/jbm.a.10532.
- Bioactıve Glass-Polymer Nanocomposites for Bone Tıssue Regeneration Applicatıons: A Revıew – Erol-Taygun - 2019 - Advanced Engineering Materials, Wiley Online Library. https://onlinelibrary-wiley-com.egateway.vit.ac.in/doi/10.1002/adem.201900287 (accessed 2024-08-30) Search PubMed.
- F. Quintero, J. Pou, R. Comesaña, F. Lusquiños, A. Riveiro, A. B. Mann, R. G. Hill, Z. Y. Wu and J. R. Jones, Laser Spinning of Bioactive Glass Nanofibers, Adv. Funct. Mater., 2009, 19(19), 3084–3090, DOI:10.1002/adfm.200801922.
- IJMS | Free Full-Text | Biopolymer-Based Nanoparticles for Drug/Gene Delivery and Tissue Engineering. https://www.mdpi.com/1422-0067/14/1/1629 (accessed 2024-08-30).
- N. M. Alves, I. B. Leonor, H. S. Azevedo, R. L. Reis and J. F. Mano, Designing Biomaterials Based on Biomineralization of Bone, J. Mater. Chem., 2010, 20(15), 2911–2921, 10.1039/B910960A.
- M. Mattioli-Belmonte, C. De Maria, C. Vitale-Brovarone, F. Baino, M. Dicarlo and G. Vozzi, Pressure-Activated Microsyringe (PAM) Fabrication of Bioactive Glass–Poly(Lactic-Co-Glycolic Acid) Composite Scaffolds for Bone Tissue Regeneration, J. Tissue Eng. Regener. Med., 2017, 11(7), 1986–1997, DOI:10.1002/term.2095.
- J. Ródenas-Rochina, J. L. G. Ribelles and M. Lebourg, Comparative study of PCL-HAp and PCL-bioglass composite scaffolds for bone tissue engineering, J. Mater. Sci.: Mater. Med., 2013, 24(5), 1293–1308, DOI:10.1007/s10856-013-4878-5.
- P. Ducheyne, Bioglass Coatings and Bioglass Composites as Implant Materials, J. Biomed. Mater. Res., 1985, 19(3), 273–291, DOI:10.1002/jbm.820190309.
- M. N. Rahaman, D. E. Day, B. Sonny Bal, Q. Fu, S. B. Jung, L. F. Bonewald and A. P. Tomsia, Bioactive Glass in Tissue Engineering, Acta Biomater., 2011, 7(6), 2355–2373, DOI:10.1016/j.actbio.2011.03.016.
- D. Kim, Y.-S. Shim, S.-Y. An and M.-J. Lee, Role of Zinc-Doped Bioactive Glass Encapsulated with Microspherical Gelatin in Localized Supplementation for Tissue Regeneration: A Contemporary Review, Molecules, 2021, 26(7), 1823, DOI:10.3390/molecules26071823.
- Y.-E. Choe, Y.-J. Kim, S.-J. Jeon, J.-Y. Ahn, J.-H. Park, K. Dashnyam, N. Mandakhbayar, J. C. Knowles, H.-W. Kim, S.-K. Jun, J.-H. Lee and H.-H. Lee, Investigating the Mechanophysical and Biological Characteristics of Therapeutic Dental Cement Incorporating Copper Doped Bioglass Nanoparticles, Dent. Mater., 2022, 38(2), 363–375, DOI:10.1016/j.dental.2021.12.019.
- Á. J. Leite, A. I. Gonçalves, M. T. Rodrigues, M. E. Gomes and J. F. Mano, Strontium-Doped Bioactive Glass Nanoparticles in Osteogenic Commitment, ACS Appl. Mater. Interfaces, 2018, 10(27), 23311–23320, DOI:10.1021/acsami.8b06154.
- M. Shoaib, A. Bahadur, S. Iqbal, M. M. AL-Anazy, A. Laref, M. A. Tahir, P. A. Channar, S. Noreen, M. Yasir, A. Iqbal and K. W. Ali, Magnesium Doped Mesoporous Bioactive Glass Nanoparticles: A Promising Material for Apatite Formation and Mitomycin c Delivery to the MG-63 Cancer Cells, J. Alloys Compd., 2021, 866, 159013, DOI:10.1016/j.jallcom.2021.159013.
- Y. Zhang, M. Hu, W. Zhang and X. Zhang, Homology of Selenium (Se) and Tellurium (Te) Endow the Functional Similarity of Se-Doped and Te-Doped Mesoporous Bioactive Glass Nanoparticles in Bone Tissue Engineering, Ceram. Int., 2022, 48(3), 3729–3739, DOI:10.1016/j.ceramint.2021.10.155.
- Biocompatible Glasses Advanced Structured Materials, ed. J. Marchi, Springer International Publishing, Cham, 2016, vol. 53 DOI:10.1007/978-3-319-44249-5.
- S. Naseri, W. C. Lepry and S. N. Nazhat, Bioactive Glasses in Wound Healing: Hope or Hype?, J. Mater. Chem. B, 2017, 5(31), 6167–6174, 10.1039/C7TB01221G.
- E. A. A. Néel, D. M. Pickup, S. P. Valappil, R. J. Newport and J. C. Knowles, Bioactive Functional Materials: A Perspective on Phosphate-Based Glasses, J. Mater. Chem., 2009, 19(6), 690–701, 10.1039/B810675D.
- K. Deshmukh, T. Kovářík, T. Křenek, D. Docheva, T. Stich and J. Pola, Recent Advances and Future Perspectives of Sol–Gel Derived Porous Bioactive Glasses: A Review, RSC Adv., 2020, 10(56), 33782–33835, 10.1039/D0RA04287K.
- X. Du, D. Wei, L. Huang, M. Zhu, Y. Zhang and Y. Zhu, 3D Printing of Mesoporous Bioactive Glass/Silk Fibroin Composite Scaffolds for Bone Tissue Engineering, Mater. Sci. Eng., C, 2019, 103, 109731, DOI:10.1016/j.msec.2019.05.016.
- J. C. Moses, S. K. Nandi and B. B. Mandal, Multifunctional Cell Instructive Silk-Bioactive Glass Composite Reinforced Scaffolds Toward Osteoinductive, Proangiogenic, and Resorbable Bone Grafts, Adv. Healthcare Mater., 2018, 7(10), 1701418, DOI:10.1002/adhm.201701418.
- E. J. Ryan, A. J. Ryan, A. González-Vázquez, A. Philippart, F. E. Ciraldo, C. Hobbs, V. Nicolosi, A. R. Boccaccini, C. J. Kearney and F. J. O’Brien, Collagen Scaffolds Functionalised with Copper-Eluting Bioactive Glass Reduce Infection and Enhance Osteogenesis and Angiogenesis Both in Vitro and in Vivo, Biomaterials, 2019, 197, 405–416, DOI:10.1016/j.biomaterials.2019.01.031.
- A. Thomas and J. Bera, Preparation and Characterization of Gelatin-Bioactive Glass Ceramic Scaffolds for Bone Tissue Engineering, J. Biomater. Sci., Polym. Ed., 2019, 30(7), 561–579, DOI:10.1080/09205063.2019.1587697.
- G. S. Kozehkonan, M. Salehi, S. Farzamfar, H. Ghanbari, M. Adabi and A. Amani, Preparation and Characterization of PCL Polymeric Scaffolds Coated with Chitosan/Bioactive Glass/Gelatin Nanoparticles Using the Tips Methodology for Bone Tissue Engineering, Nanomed. J., 2019, 6(4), 311–320 CAS.
- S. Dasgupta, K. Maji and S. K. Nandi, Investigating the Mechanical, Physiochemical and Osteogenic Properties in Gelatin-Chitosan-Bioactive Nanoceramic Composite Scaffolds for Bone Tissue Regeneration: In Vitro and in Vivo, Mater. Sci. Eng., C, 2019, 94, 713–728, DOI:10.1016/j.msec.2018.10.022.
- R. Soni, N. V. Kumar, S. Chameettachal, F. Pati and S. Narayan Rath, Synthesis and Optimization of PCL-Bioactive Glass Composite Scaffold for Bone Tissue Engineering, Mater. Today: Proc., 2019, 15, 294–299, DOI:10.1016/j.matpr.2019.05.008.
- A. G. S. de Laia, B. R. Barrioni, T. M. Valverde, A. M. de Goes, M. A. de Sá and M. de Magalhães Pereira, Therapeutic cobalt ion incorporated in poly(vinyl alcohol)/bioactive glass scaffolds for tissue engineering, J. Mater. Sci., 2020, 55, 8710–8727, DOI:10.1007/s10853-020-04644-0.
- L. Pang, Y. Shen, H. Hu, X. Zeng, W. Huang, H. Gao, H. Wang and D. Wang, Chemically and Physically Cross-Linked Polyvinyl Alcohol-Borosilicate Gel Hybrid Scaffolds for Bone Regeneration, Mater. Sci. Eng., C, 2019, 105, 110076, DOI:10.1016/j.msec.2019.110076.
- G. Carbajal-De la Torre, N. N. Zurita-Méndez, M. Ballesteros-Almanza, L. de, J. Ortiz-Ortiz, M. Estévez and M. A. Espinosa-Medina, Characterization and Evaluation of Composite Biomaterial Bioactive Glass–Polylactic Acid for Bone Tissue Engineering Applications, Polymers, 2022, 14(15), 3034, DOI:10.3390/polym14153034.
- W. Vogel, Glass Chemistry, ed. W. Vogel, Springer, Berlin, Heidelberg, 1994 DOI:10.1007/978-3-642-78723-2.
- M. Dziadek, E. Stodolak-Zych and K. Cholewa-Kowalska, Biodegradable Ceramic-Polymer Composites for Biomedical Applications: A Review, Mater. Sci. Eng., C, 2017, 71, 1175–1191, DOI:10.1016/j.msec.2016.10.014.
- K.-H. Shin, Y.-H. Koh, W.-Y. Choi and H.-E. Kim, Production of Porous Poly(ε-Caprolactone)/Silica Hybrid Membranes with Patterned Surface Pores, Mater. Lett., 2011, 65(12), 1903–1906, DOI:10.1016/j.matlet.2011.03.096.
- A. Hoppe, N. S. Güldal and A. R. Boccaccini, A Review of the Biological Response to Ionic Dissolution Products from Bioactive Glasses and Glass-Ceramics, Biomaterials, 2011, 32(11), 2757–2774, DOI:10.1016/j.biomaterials.2011.01.004.
- G. Kaur, O. p Pandey, K. Singh, D. Homa, B. Scott and G. Pickrell, A Review of Bioactive Glasses: Their Structure, Properties, Fabrication and Apatite Formation, J. Biomed. Mater. Res., Part A, 2014, 102(1), 254–274, DOI:10.1002/jbm.a.34690.
- R. K. Brow, Review: The Structure of Simple Phosphate Glasses, J. Non-Cryst. Solids, 2000, 263–264, 1–28, DOI:10.1016/S0022-3093(99)00620-1.
- M. Shah Mohammadi, I. Ahmed, B. Marelli, C. Rudd, M. N. Bureau and S. N. Nazhat, Modulation of Polycaprolactone Composite Properties through Incorporation of Mixed Phosphate Glass Formulations, Acta Biomater., 2010, 6(8), 3157–3168, DOI:10.1016/j.actbio.2010.03.002.
- V. Salih, K. Franks, M. James, G. W. Hastings, J. C. Knowles and I. Olsen, Development of soluble glasses for biomedical use Part II: The biological response of human osteoblast cell lines to phosphate-based soluble glasses, J. Mater. Sci.: Mater. Med., 2000, 11(10), 615–620, DOI:10.1023/a:1008901612674.
- H.-W. Kim, E.-J. Lee, I.-K. Jun, H.-E. Kim and J. C. Knowles, Degradation and Drug Release of Phosphate Glass/Polycaprolactone Biological Composites for Hard-Tissue Regeneration, J. Biomed. Mater. Res., Part B, 2005, 75B(1), 34–41, DOI:10.1002/jbm.b.30223.
- S. G. Motke, S. P. Yawale and S. S. Yawale, Infrared spectra of zinc doped lead borate glasses, Bull. Mater. Sci., 2002, 25, 75–78, DOI:10.1007/BF02704599.
- A. Yao, D. Wang, W. Huang, Q. Fu, M. N. Rahaman and D. E. Day, In Vitro Bioactive Characteristics of Borate-Based Glasses with Controllable Degradation Behavior, J. Am. Ceram. Soc., 2007, 90(1), 303–306, DOI:10.1111/j.1551-2916.2006.01358.x.
- X. Liu, Z. Xie, C. Zhang, H. Pan, M. N. Rahaman, X. Zhang, Q. Fu and W. Huang, Bioactive borate glass scaffolds: in vitro and in vivo evaluation for use as a drug delivery system in the treatment of bone infection, J. Mater. Sci.: Mater. Med., 2010, 21(2), 575–582, DOI:10.1007/s10856-009-3897-8.
- M. S. B. Reddy, D. Ponnamma, R. Choudhary and K. K. Sadasivuni, A Comparative Review of Natural and Synthetic Biopolymer Composite Scaffolds, Polymers, 2021, 13(7), 1105, DOI:10.3390/polym13071105.
- D. Vukajlovic, J. Parker, O. Bretcanu and K. Novakovic, Chitosan Based Polymer/Bioglass Composites for Tissue Engineering Applications, Mater. Sci. Eng., C, 2019, 96, 955–967, DOI:10.1016/j.msec.2018.12.026.
- C. Katrilaka, N. Karipidou, N. Petrou, C. Manglaris, G. Katrilakas, A. N. Tzavellas, M. Pitou, E. E. Tsiridis, T. Choli-Papadopoulou and A. Aggeli, Freeze-Drying Process for the Fabrication of Collagen-Based Sponges as Medical Devices in Biomedical Engineering, Materials, 2023, 16(12), 4425, DOI:10.3390/ma16124425.
- W. Zhang, X. Hou, H. Wang, D. Kong and Y. Zhou, Preparation of Chitosan-Sodium Alginate/Bioactive Glass Composite Cartilage Scaffolds with High Cell Activity and Bioactivity, Ceram. Int., 2023, 49(2), 1987–1996, DOI:10.1016/j.ceramint.2022.09.164.
- C. Shi, X. Hou, D. Zhao, H. Wang, R. Guo and Y. Zhou, Preparation of the Bioglass/Chitosan-Alginate Composite Scaffolds with High Bioactivity and Mechanical Properties as Bone Graft Materials, J. Mech. Behav. Biomed. Mater., 2022, 126, 105062, DOI:10.1016/j.jmbbm.2021.105062.
- M. R. Bidgoli, I. Alemzadeh, E. Tamjid, M. Khafaji and M. Vossoughi, Fabrication of Hierarchically Porous Silk Fibroin-Bioactive Glass Composite Scaffold via Indirect 3D Printing: Effect of Particle Size on Physico-Mechanical Properties and in Vitro Cellular Behavior, Mater. Sci. Eng., C, 2019, 103, 109688, DOI:10.1016/j.msec.2019.04.067.
- A.-M. Haaparanta, P. Uppstu, M. Hannula, V. Ellä, A. Rosling and M. Kellomäki, Improved Dimensional Stability with Bioactive Glass Fibre Skeleton in Poly(Lactide-Co-Glycolide) Porous Scaffolds for Tissue Engineering, Mater. Sci. Eng., C, 2015, 56, 457–466, DOI:10.1016/j.msec.2015.07.013.
- F. Wahid; T. Khan; Z. Hussain and H. Ullah, 30 - Nanocomposite Scaffolds for Tissue Engineering; Properties, Preparation and Applications, in Applications of Nanocomposite Materials in Drug Delivery, ed. A. M. Inamuddin, A. Mohammad, Woodhead Publishing Series in Biomaterials; Woodhead Publishing, 2018, pp. 701–735 DOI:10.1016/B978-0-12-813741-3.00031-5.
- S. Chen, Z. Jian, L. Huang, W. Xu, S. Liu, D. Song, Z. Wan, A. Vaughn, R. Zhan, C. Zhang, S. Wu, M. Hu and J. Li, Mesoporous Bioactive Glass Surface Modified Poly(Lactic-Co-Glycolic Acid) Electrospun Fibrous Scaffold for Bone Regeneration, Int. J. Nanomed., 2015, 10, 3815–3827, DOI:10.2147/IJN.S82543.
- M. Mahmoudi, P. Alizadeh and M. Soltani, Wound Healing Performance of Electrospun PVA/70S30C Bioactive Glass/Ag Nanoparticles Mats Decorated with Curcumin: In Vitro and in Vivo Investigations, Biomater. Adv., 2023, 153, 213530, DOI:10.1016/j.bioadv.2023.213530.
- E. Piatti, M. Miola, L. Liverani, E. Verné and A. R. Boccaccini, Poly(ε-Caprolactone)/Bioactive Glass Composite Electrospun Fibers for Tissue Engineering Applications, J. Biomed. Mater. Res., Part A, 2023, 111(11), 1692–1709, DOI:10.1002/jbm.a.37578.
- D. A. Canales, F. Reyes, M. Saavedra, L. Peponi, A. Leonés, H. Palza, A. R. Boccaccini, A. Grünewald and P. A. Zapata, Electrospun Fibers of Poly (Lactic Acid) Containing Bioactive Glass and Magnesium Oxide Nanoparticles for Bone Tissue Regeneration, Int. J. Biol. Macromol., 2022, 210, 324–336, DOI:10.1016/j.ijbiomac.2022.05.047.
- F. Serio, M. Miola, E. Vernè, D. Pisignano, A. R. Boccaccini and L. Liverani, Electrospun Filaments Embedding Bioactive Glass Particles with Ion Release and Enhanced Mineralization, Nanomaterials, 2019, 9(2), 182, DOI:10.3390/nano9020182.
- D. Dhinasekaran, S. Vimalraj, A. R. Rajendran, S. Saravanan, B. Purushothaman and B. Subramaniam, Bio-Inspired Multifunctional Collagen/Electrospun Bioactive Glass Membranes for Bone Tissue Engineering Applications, Mater. Sci. Eng., C, 2021, 126, 111856, DOI:10.1016/j.msec.2020.111856.
- Q. Chen, J. Wu, Y. Liu, Y. Li, C. Zhang, W. Qi, K. W. K. Yeung, T. M. Wong, X. Zhao and H. Pan, Electrospun Chitosan/PVA/Bioglass Nanofibrous Membrane with Spatially Designed Structure for Accelerating Chronic Wound Healing, Mater. Sci. Eng., C, 2019, 105, 110083, DOI:10.1016/j.msec.2019.110083.
- L. He, J. Yin and X. Gao, Additive Manufacturing of Bioactive Glass and Its Polymer Composites as Bone Tissue Engineering Scaffolds: A Review, Bioengineering, 2023, 10(6), 672, DOI:10.3390/bioengineering10060672.
- A.-V. Do; R. Smith; T. M. Acri; S. M. Geary and A. K. Salem, 9 - 3D Printing Technologies for 3D Scaffold Engineering, in Functional 3D Tissue Engineering Scaffolds, Deng, Y., Kuiper, J., ed. Woodhead Publishing, 2018, pp. 203–234 DOI:10.1016/B978-0-08-100979-6.00009-4.
- J. F. Mano, G. A. Silva, H. S. Azevedo, P. B. Malafaya, R. A. Sousa, S. S. Silva, L. F. Boesel, J. M. Oliveira, T. C. Santos, A. P. Marques, N. M. Neves and R. L. Reis, Natural Origin Biodegradable Systems in Tissue Engineering and Regenerative Medicine: Present Status and Some Moving Trends, J. R.Soc., Interface, 2007, 4(17), 999–1030, DOI:10.1098/rsif.2007.0220.
- P. Palmero, 15 - Ceramic–Polymer Nanocomposites for Bone-Tissue Regeneration, in Nanocomposites for Musculoskeletal Tissue Regeneration, ed. H. Liu, Woodhead Publishing, Oxford, 2016, pp. 331–367 DOI:10.1016/B978-1-78242-452-9.00015-7.
- I. Armentano, M. Dottori, E. Fortunati, S. Mattioli and J. M. Kenny, Biodegradable Polymer Matrix Nanocomposites for Tissue Engineering: A Review, Polym. Degrad. Stab., 2010, 95(11), 2126–2146, DOI:10.1016/j.polymdegradstab.2010.06.007.
- R. Sergi, D. Bellucci and V. Cannillo, A Review of Bioactive Glass/Natural Polymer Composites: State of the Art, Materials, 2020, 13(23), 5560, DOI:10.3390/ma13235560.
- S. N. Pawar and K. J. Edgar, Alginate Derivatization: A Review of Chemistry, Properties and Applications, Biomaterials, 2012, 33(11), 3279–3305, DOI:10.1016/j.biomaterials.2012.01.007.
- K. Y. Lee and D. J. Mooney, Alginate: Properties and Biomedical Applications, Prog. Polym. Sci., 2012, 37(1), 106–126, DOI:10.1016/j.progpolymsci.2011.06.003.
- P. R. Gabbai-Armelin, D. A. Cardoso, E. D. Zanotto, O. Peitl, S. C. G. Leeuwenburgh, J. A. Jansen, A. C. M. Renno and J. J. J. P. van den Beucken, Injectable Composites Based on Biosilicate® and Alginate: Handling and in Vitro Characterization, RSC Adv., 2014, 4(86), 45778–45785, 10.1039/C4RA07522F.
- Q. Zeng, Y. Han, H. Li and J. Chang, Bioglass/Alginate Composite Hydrogel Beads as Cell Carriers for Bone Regeneration, J. Biomed. Mater. Res., Part B, 2014, 102(1), 42–51, DOI:10.1002/jbm.b.32978.
- Y. Luo, C. Wu, A. Lode and M. Gelinsky, Hierarchical Mesoporous Bioactive Glass/Alginate Composite Scaffolds Fabricated by Three-Dimensional Plotting for Bone Tissue Engineering, Biofabrication, 2012, 5(1), 015005, DOI:10.1088/1758-5082/5/1/015005.
- D. Zamani, F. Moztarzadeh and D. Bizari, Alginate-Bioactive Glass Containing Zn and Mg Composite Scaffolds for Bone Tissue Engineering, Int. J. Biol. Macromol., 2019, 137, 1256–1267, DOI:10.1016/j.ijbiomac.2019.06.182.
- J. Hatton, G. R. Davis, A.-H. I. Mourad, N. Cherupurakal, R. G. Hill and S. Mohsin, Fabrication of Porous Bone Scaffolds Using Alginate and Bioactive Glass, J. Funct. Biomater., 2019, 10(1), 15, DOI:10.3390/jfb10010015.
- F. Westhauser, F. Hohenbild, M. Arango-Ospina, S. I. Schmitz, S. Wilkesmann, L. Hupa, A. Moghaddam and A. R. Boccaccini, Bioactive Glass (BG) ICIE16 Shows Promising Osteogenic Properties Compared to Crystallized 45S5-BG, Int. J. Mol. Sci., 2020, 21(5), 1639, DOI:10.3390/ijms21051639.
- M. M. Erol, V. Mouriňo, P. Newby, X. Chatzistavrou, J. A. Roether, L. Hupa and A. R. Boccaccini, Copper-Releasing, Boron-Containing Bioactive Glass-Based Scaffolds Coated with Alginate for Bone Tissue Engineering, Acta Biomater., 2012, 8(2), 792–801, DOI:10.1016/j.actbio.2011.10.013.
- A. C. Özarslan, Y. B. Elalmis and S. Yücel, Production of Biosilica Based Bioactive Glass-Alginate Composite Putty as Bone Support Material, and Evaluation of in Vitro Properties; Bioactivity and Cytotoxicity Behavior, J. Non-Cryst. Solids, 2021, 561, 120755, DOI:10.1016/j.jnoncrysol.2021.120755.
- V. Guduric, N. Belton, R. F. Richter, A. Bernhardt, J. Spangenberg, C. Wu, A. Lode and M. Gelinsky, Tailorable Zinc-Substituted Mesoporous Bioactive Glass/Alginate-Methylcellulose Composite Bioinks, Materials, 2021, 14(5), 1225, DOI:10.3390/ma14051225.
- I. Bano, M. Arshad, T. Yasin, M. A. Ghauri and M. Younus, Chitosan: A Potential Biopolymer for Wound Management, Int. J. Biol. Macromol., 2017, 102, 380–383, DOI:10.1016/j.ijbiomac.2017.04.047.
- F. Croisier and C. Jérôme, Chitosan-Based Biomaterials for Tissue Engineering, Eur. Polym. J., 2013, 49(4), 780–792, DOI:10.1016/j.eurpolymj.2012.12.009.
- S. G. Caridade, E. G. Merino, N. M. Alves, V. Bermudez, Z. de, A. R. Boccaccini and J. F. Mano, Chitosan Membranes Containing Micro or Nano-Size Bioactive Glass Particles: Evolution of Biomineralization Followed by in Situ Dynamic Mechanical Analysis, J. Mech. Behav. Biomed. Mater., 2013, 20, 173–183, DOI:10.1016/j.jmbbm.2012.11.012.
- H. Oudadesse, S. Najem, S. Mosbahi, N. Rocton, J. Refifi, H. El Feki and B. Lefeuvre, Development of Hybrid Scaffold: Bioactive Glass Nanoparticles/Chitosan for Tissue Engineering Applications, J. Biomed. Mater. Res., Part A, 2021, 109(5), 590–599, DOI:10.1002/jbm.a.37043.
- H. F. Hammouda, M. M. Farag, M. M. F. El Deftar, M. Abdel-Gabbar and B. M. Mohamed, Effect of Ce-Doped Bioactive Glass/Collagen/Chitosan Nanocomposite Scaffolds on the Cell Morphology and Proliferation of Rabbit's Bone Marrow Mesenchymal Stem Cells-Derived Osteogenic Cells, J. Genet. Eng. Biotechnol., 2022, 20(1), 33, DOI:10.1186/s43141-022-00302-x.
- H. G. H. Hammad and M. N. F. Salama, Porosity Pattern of 3D Chitosan/Bioactive Glass Tissue Engineering Scaffolds Prepared for Bone Regeneration, Open Dent. J., 2021, 14, 15–41, DOI:10.2174/1874210602115010041.
- H. Nosrati, M. Banitalebi-Dehkordi, M. Khodaei, E. Sharifi, S. Asadpour, K. Mansouri and M. Soleimannejad, Preparation and in Vitro Characterization of Electrospun Scaffolds Composed of Chitosan, Gelatin and 58S Bioactive Glass Nanoparticles for Skin Tissue Engineering, J Shahrekord Univ Med Sci, 2022, 24(1), 1–6 CrossRef CAS.
- Structure and Physicochemical Properties of Starch, SpringerLink. https://link-springer-com.egateway.vit.ac.in/chapter/10.1007/978-981-13-0725-6_1 (accessed 2024-08-30) Search PubMed.
- G. Liu, Z. Gu, Y. Hong, L. Cheng and C. Li, Electrospun Starch Nanofibers: Recent Advances, Challenges, and Strategies for Potential Pharmaceutical Applications, J. Controlled Release, 2017, 252, 95–107, DOI:10.1016/j.jconrel.2017.03.016.
- A. Aidun, A. Zamanian and F. Ghorbani, Novel Bioactive Porous Starch–Siloxane Matrix for Bone Regeneration: Physicochemical, Mechanical, and in Vitro Properties, Biotechnol. Appl. Biochem., 2019, 66(1), 43–52, DOI:10.1002/bab.1694.
- J. Mastalska-Popławska, M. Sikora, P. Izak and Z. Góral, Applications of Starch and Its Derivatives in Bioceramics, J. Biomater. Appl., 2019, 34(1), 12–24, DOI:10.1177/0885328219844972.
- M. Soltani and P. Alizadeh, Aloe Vera Incorporated Starch-64S Bioactive Glass-Quail Egg Shell Scaffold for Promotion of Bone Regeneration, Int. J. Biol. Macromol., 2022, 217, 203–218, DOI:10.1016/j.ijbiomac.2022.07.054.
- G. A. Silva, O. P. Coutinho, P. Ducheyne, I. M. Shapiro and R. L. Reis, The Effect of Starch and Starch-Bioactive Glass Composite Microparticles on the Adhesion and Expression of the Osteoblastic Phenotype of a Bone Cell Line, Biomaterials, 2007, 28(2), 326–334, DOI:10.1016/j.biomaterials.2006.07.009.
- G. A. Silva, F. J. Costa, O. P. Coutinho, S. Radin, P. Ducheyne and R. L. Reis, Synthesis and Evaluation of Novel Bioactive Composite Starch/Bioactive Glass Microparticles, J. Biomed. Mater. Res., Part A, 2004, 70A(3), 442–449, DOI:10.1002/jbm.a.30099.
- E. Díaz-Montes, Dextran: Sources, Structures, and Properties, Polysaccharides, 2021, 2(3), 554–565, DOI:10.3390/polysaccharides2030033.
- L. Cai, J. Li, S. Quan, W. Feng, J. Yao, M. Yang and W. Li, Dextran-Based Hydrogel with Enhanced Mechanical Performance via Covalent and Non-Covalent Cross-Linking Units Carrying Adipose-Derived Stem Cells toward Vascularized Bone Tissue Engineering, J. Biomed. Mater. Res., Part A, 2019, 107(6), 1120–1131, DOI:10.1002/jbm.a.36580.
- P. Nikpour, H. Salimi-Kenari, F. Fahimipour, S. M. Rabiee, M. Imani, E. Dashtimoghadam and L. Tayebi, Dextran Hydrogels Incorporated with Bioactive Glass-Ceramic: Nanocomposite Scaffolds for Bone Tissue Engineering, Carbohydr. Polym., 2018, 190, 281–294, DOI:10.1016/j.carbpol.2018.02.083.
- P. Nikpour, H. Salimi-Kenari and S. M. Rabiee, Biological and bioactivity assessment of dextran nanocomposite hydrogel for bone regeneration, Prog. Biomater., 2021, 10, 271–280, DOI:10.1007/s40204-021-00171-6.
- B. Kundu, R. Rajkhowa, S. C. Kundu and X. Wang, Silk Fibroin Biomaterials for Tissue Regenerations, Adv. Drug Delivery Rev., 2013, 65(4), 457–470, DOI:10.1016/j.addr.2012.09.043.
- T. P. Nguyen, Q. V. Nguyen, V.-H. Nguyen, T.-H. Le, V. Q. N. Huynh, D.-V. N. Vo, Q. T. Trinh, S. Y. Kim and Q. V. Le, Silk Fibroin-Based Biomaterials for Biomedical Applications: A Review, Polymers, 2019, 11(12), 1933, DOI:10.3390/polym11121933.
- J. Zhao, Z. Zhang, S. Wang, X. Sun, X. Zhang, J. Chen, D. L. Kaplan and X. Jiang, Apatite-Coated Silk Fibroin Scaffolds to Healing Mandibular Border Defects in Canines, Bone, 2009, 45(3), 517–527, DOI:10.1016/j.bone.2009.05.026.
- S. Midha, S. Kumar, A. Sharma, K. Kaur, X. Shi, P. Naruphontjirakul, J. R. Jones and S. Ghosh, Silk Fibroin-Bioactive Glass Based Advanced Biomaterials: Towards Patient-Specific Bone Grafts, Biomed. Mater., 2018, 13(5), 055012, DOI:10.1088/1748-605X/aad2a9.
- J. Wu, K. Zheng, X. Huang, J. Liu, H. Liu, R. Aldo, Y. Wan, X. Guo and Z. Shao, Thermally Triggered Injectable Chitosan/Silk Fibroin/Bioactive Glass Nanoparticle Hydrogels for in situ Bone Formation in Rat Calvarial Bone Defects, Acta Biomater., 2019, 91, 60–71, DOI:10.1016/j.actbio.2019.04.023.
- J. Wu, S. Wang, Z. Zheng and J. Li, Fabrication of Biologically Inspired Electrospun Collagen/Silk Fibroin/Bioactive Glass Composited Nanofibrous Scaffold to Accelerate the Treatment Efficiency of Bone Repair, Regener. Ther., 2022, 21, 122–138, DOI:10.1016/j.reth.2022.05.006.
- E. J. Sheehy; G. M. Cunniffe and F. J. O’Brien, 5 - Collagen-Based Biomaterials for Tissue Regeneration and Repair, in Peptides and Proteins as Biomaterials for Tissue Regeneration and Repair, ed. M. A. Barbosa, M. C. L. Martins, Woodhead Publishing, 2018, pp. 127–150 DOI:10.1016/B978-0-08-100803-4.00005-X.
- J. Hum and A. R. Boccaccini, Collagen as Coating Material for 45S5 Bioactive Glass-Based Scaffolds for Bone Tissue Engineering, Int. J. Mol. Sci., 2018, 19(6), 1807, DOI:10.3390/ijms19061807.
- H. Park, A.-M. Collignon, W. C. Lepry, J. L. Ramirez-GarciaLuna, D. H. Rosenzweig, C. Chaussain and S. N. Nazhat, Acellular Dense Collagen-S53P4 Bioactive Glass Hybrid Gel Scaffolds Form More Bone than Stem Cell Delivered Constructs, Mater. Sci. Eng., C, 2021, 120, 111743, DOI:10.1016/j.msec.2020.111743.
- B. Marelli, C. E. Ghezzi, D. Mohn, W. J. Stark, J. E. Barralet, A. R. Boccaccini and S. N. Nazhat, Accelerated Mineralization of Dense Collagen-Nano Bioactive Glass Hybrid Gels Increases Scaffold Stiffness and Regulates Osteoblastic Function, Biomaterials, 2011, 32(34), 8915–8926, DOI:10.1016/j.biomaterials.2011.08.016.
- Q. Nawaz, M. A. Ur Rehman, J. A. Roether, L. Yufei, A. Grünewald, R. Detsch and A. R. Boccaccini, Bioactive Glass Based Scaffolds Incorporating Gelatin/Manganese Doped Mesoporous Bioactive Glass Nanoparticle Coating, Ceram. Int., 2019, 45(12), 14608–14613, DOI:10.1016/j.ceramint.2019.04.179.
- T. Reiter, T. Panick, K. Schuhladen, J. A. Roether, J. Hum and A. R. Boccaccini, Bioactive Glass Based Scaffolds Coated with Gelatin for the Sustained Release of Icariin, Bioact. Mater., 2019, 4, 1–7, DOI:10.1016/j.bioactmat.2018.10.001.
- K. Zheng, J. Wu, W. Li, D. Dippold, Y. Wan and A. R. Boccaccini, Incorporation of Cu-Containing Bioactive Glass Nanoparticles in Gelatin-Coated Scaffolds Enhances Bioactivity and Osteogenic Activity, ACS Biomater. Sci. Eng., 2018, 4(5), 1546–1557, DOI:10.1021/acsbiomaterials.8b00051.
- A. M. El-Kady, A. A. Ali and A. El-Fiqi, Controlled Delivery of Therapeutic Ions and Antibiotic Drug of Novel Alginate-Agarose Matrix Incorporating Selenium-Modified Borosilicate Glass Designed for Chronic Wound Healing, J. Non-Cryst. Solids, 2020, 534, 119889, DOI:10.1016/j.jnoncrysol.2020.119889.
- R. L. M. S. Oliveira, A. P. N. Alves, L. Barbosa, A. P. Silva, G. L. de Cena, K. Conceição, D. B. Tada, E. Trichês and S. de, 3D Printing of Bioactive Glass S53P4/Sodium Alginate Sintering-Free Scaffolds, Bioprinting, 2022, 27, e00226, DOI:10.1016/j.bprint.2022.e00226.
- J. Yang, T. Long, N.-F. He, Y.-P. Guo, Z.-A. Zhu and Q.-F. Ke, Fabrication of a chitosan/bioglass three-dimensional porous scaffold for bone tissue engineering applications, J. Mater. Chem. B, 2014, 2, 6611–6618, 10.1039/C4TB00940A.
- G. M. Luz and J. F. Mano, Chitosan/Bioactive Glass Nanoparticles Composites for Biomedical Applications, Biomed. Mater., 2012, 7(5), 054104, DOI:10.1088/1748-6041/7/5/054104.
- X. Cui, W. Huang, Y. Zhang, C. Huang, Z. Yu, L. Wang, W. Liu, T. Wang, J. Zhou, H. Wang, N. Zhou, D. Wang, H. Pan and M. N. Rahaman, Evaluation of an Injectable Bioactive Borate Glass Cement to Heal Bone Defects in a Rabbit Femoral Condyle Model, Mater. Sci. Eng., C, 2017, 73, 585–595, DOI:10.1016/j.msec.2016.12.101.
- J. Mota, N. Yu, S. G. Caridade, G. M. Luz, M. E. Gomes, R. L. Reis, J. A. Jansen, X. F. Walboomers and J. F. Mano, Chitosan/Bioactive Glass Nanoparticle Composite Membranes for Periodontal Regeneration, Acta Biomater., 2012, 8(11), 4173–4180, DOI:10.1016/j.actbio.2012.06.040.
- G. A. Silva, A. Pedro, F. J. Costa, N. M. Neves, O. P. Coutinho and R. L. Reis, Soluble Starch and Composite Starch Bioactive Glass 45S5 Particles: Synthesis, Bioactivity, and Interaction with Rat Bone Marrow Cells, Mater. Sci. Eng., C, 2005, 25(2), 237–246, DOI:10.1016/j.msec.2005.01.012.
- S. N. F. S. Adam, A. F. Osman and R. Shamsudin, Tensile Properties, Biodegradability and Bioactivity of Thermoplastic Starch (TPS)/Bioglass Composites for Bone Tissue Engineering, Sains Malays., 2018, 47(6), 1303–1310, DOI:10.17576/jsm-2018-4706-27.
- I. B. Leonor, R. A. Sousa, A. M. Cunha, R. L. Reis, Z. P. Zhong and D. Greenspan, Novel starch thermoplastic/Bioglass® composites: Mechanical properties, degradation behavior and in vitro bioactivity, J. Mater. Sci.: Mater. Med., 2002, 13(10), 939–945, DOI:10.1023/a:1019800411229.
- Q. Min, X. Yu, J. Liu, Y. Zhang, Y. Wan and J. Wu, Controlled Delivery of Insulin-like Growth Factor-1 from Bioactive Glass-Incorporated Alginate-Poloxamer/Silk Fibroin Hydrogels, Pharmaceutics, 2020, 12(6), 574, DOI:10.3390/pharmaceutics12060574.
- H. Zhu, N. Liu, X. Feng and J. Chen, Fabrication and Characterization of Silk Fibroin/Bioactive Glass Composite Films, Mater. Sci. Eng., C, 2012, 32(4), 822–829, DOI:10.1016/j.msec.2012.01.033.
- H.-W. Kim, J.-H. Song and H.-E. Kim, Bioactive glass nanofiber–collagen nanocomposite as a novel bone regeneration matrix, J. Biomed. Mater. Res., Part A, 2006, 79(3), 698–705, DOI:10.1002/jbm.a.30848.
- M. Kazemi, M. Azami, B. Johari, M. Ahmadzadehzarajabad, B. Nazari, S. Kargozar, S. Hajighasemlou, M. Mozafari, M. Soleimani, A. Samadikuchaksaraei and M. Farajollahi, Bone Regeneration in Rat Using a Gelatin/Bioactive Glass Nanocomposite Scaffold along with Endothelial Cells (HUVECs, Int. J. Appl. Ceram. Technol., 2018, 15(6), 1427–1438, DOI:10.1111/ijac.12907.
- M. A. Ghalia and Y. Dahman, Biodegradable Poly(Lactic Acid)-Based Scaffolds: Synthesis and Biomedical Applications, J. Polym. Res., 2017, 24(5), 74, DOI:10.1007/s10965-017-1227-2.
- M. S. Singhvi, S. S. Zinjarde and D. V. Gokhale, Polylactic Acid: Synthesis and Biomedical Applications, J. Appl. Microbiol., 2019, 127(6), 1612–1626, DOI:10.1111/jam.14290.
- S. A. M. Estrada, I. O. Armendáriz, A. T. García, J. F. H. Paz and C. A. R. González, Evaluation of in Vitro Bioactivity of 45S5 Bioactive Glass/Poly Lactic Acid Scaffolds Produced by 3D Printing, Int. J. Compos. Mater., 2017, 7(5), 144–149 CAS.
- S. Eqtesadi, A. Motealleh, F. H. Perera, A. Pajares and P. Miranda, Poly-(Lactic Acid) Infiltration of 45S5 Bioglass® Robocast Scaffolds: Chemical Interaction and Its Deleterious Effect in Mechanical Enhancement, Mater. Lett., 2016, 163, 196–200, DOI:10.1016/j.matlet.2015.10.073.
- S. Pant, S. Thomas, S. Loganathan and R. B. Valapa, 3D Bioprinted Poly(Lactic Acid)/Mesoporous Bioactive Glass Based Biomimetic Scaffold with Rapid Apatite Crystallization and in vitro Cytocompatability for Bone Tissue Engineering, Int. J. Biol. Macromol., 2022, 217, 979–997, DOI:10.1016/j.ijbiomac.2022.07.202.
- D. R. Chen, J. Z. Bei and S. G. Wang, Polycaprolactone Microparticles and Their Biodegradation, Polym. Degrad. Stab., 2000, 67(3), 455–459, DOI:10.1016/S0141-3910(99)00145-7.
- B. Joseph, R. Augustine, N. Kalarikkal, S. Thomas, B. Seantier and Y. Grohens, Recent Advances in Electrospun Polycaprolactone Based Scaffolds for Wound Healing and Skin Bioengineering Applications, Mater. Today Commun., 2019, 19, 319–335, DOI:10.1016/j.mtcomm.2019.02.009.
- M. Kouhi, M. Morshed, J. Varshosaz and M. H. Fathi, Poly (ε-Caprolactone) Incorporated Bioactive Glass Nanoparticles and Simvastatin Nanocomposite Nanofibers: Preparation, Characterization and in Vitro Drug Release for Bone Regeneration Applications, Chem. Eng. J., 2013, 228, 1057–1065, DOI:10.1016/j.cej.2013.05.091.
- C. Wang, C. Meng, Z. Zhang and Q. Zhu, 3D Printing of Polycaprolactone/Bioactive Glass Composite Scaffolds for in Situ Bone Repair, Ceram. Int., 2022, 48(6), 7491–7499, DOI:10.1016/j.ceramint.2021.11.293.
- Polycaprolactone/bioactive glass hybrid scaffolds for bone regeneration on Portico. https://access.portico.org/Portico/auView?auId=ark:%2F27927%2Fphz61nhbt0w (accessed 2024-08-30) Search PubMed.
- A. Kumar and S. S. Han, PVA-Based Hydrogels for Tissue Engineering: A Review, Int. J. Polym. Mater. Polym. Biomater., 2017, 66(4), 159–182, DOI:10.1080/00914037.2016.1190930.
- A. Abd El-Fattah, M. Nageeb Hassan, A. Rashad, M. Marei and S. Kandil, Viscoelasticity, Mechanical Properties, and in Vivo Biocompatibility of Injectable Polyvinyl Alcohol/Bioactive Glass Composite Hydrogels as Potential Bone Tissue Scaffolds, Int. J. Polym. Anal. Charact., 2020, 25(5), 362–373, DOI:10.1080/1023666X.2020.1790253.
- M. S. El-Okaily, A. M. EL-Rafei, M. Basha, N. T. Abdel Ghani, M. M. H. El-Sayed, A. Bhaumik and A. A. Mostafa, Efficient Drug Delivery Vehicles of Environmentally Benign Nano-Fibers Comprising Bioactive Glass/Chitosan/Polyvinyl Alcohol Composites, Int. J. Biol. Macromol., 2021, 182, 1582–1589, DOI:10.1016/j.ijbiomac.2021.05.079.
- J. Jiménez-Holguín, A. López-Hidalgo, S. Sánchez-Salcedo, J. Peña, M. Vallet-Regí and A. J. Salinas, Strontium-Modified Scaffolds Based on Mesoporous Bioactive Glasses/Polyvinyl Alcohol Composites for Bone Regeneration, Materials, 2020, 13(23), 5526, DOI:10.3390/ma13235526.
- R. Iron, M. Mehdikhani, E. Naghashzargar, S. Karbasi and D. Semnani, Effects of Nano-Bioactive Glass on Structural, Mechanical and Bioactivity Properties of Poly (3-Hydroxybutyrate) Electrospun Scaffold for Bone Tissue Engineering Applications, Mater. Technol., 2019, 34(9), 540–548, DOI:10.1080/10667857.2019.1591728.
- Y. Ding, W. Li, T. Müller, D. W. Schubert, A. R. Boccaccini, Q. Yao and J. A. Roether, Electrospun Polyhydroxybutyrate/Poly(ε-Caprolactone)/58S Sol–Gel Bioactive Glass Hybrid Scaffolds with Highly Improved Osteogenic Potential for Bone Tissue Engineering, ACS Appl. Mater. Interfaces, 2016, 8(27), 17098–17108, DOI:10.1021/acsami.6b03997.
- M. Parvizifard and S. Karbasi, Physical, Mechanical and Biological Performance of PHB-Chitosan/MWCNTs Nanocomposite Coating Deposited on Bioglass Based Scaffold: Potential Application in Bone Tissue Engineering, Int. J. Biol. Macromol., 2020, 152, 645–662, DOI:10.1016/j.ijbiomac.2020.02.266.
- Y. Goh, M. Akram, A. Alshemary and R. Hussain, Antibacterial Polylactic Acid/Chitosan Nanofibers Decorated with Bioactive Glass, Appl. Surf. Sci., 2016, 387, 1–7, DOI:10.1016/j.apsusc.2016.06.054.
- N. Gómez-Cerezo, L. Casarrubios, M. Saiz-Pardo, L. Ortega, D. de Pablo, I. Díaz-Güemes, B. Fernández-Tomé, S. Enciso, F. M. Sánchez-Margallo, M. T. Portolés, D. Arcos and M. Vallet-Regí, Mesoporous Bioactive Glass/ε-Polycaprolactone Scaffolds Promote Bone Regeneration in Osteoporotic Sheep, Acta Biomater., 2019, 90, 393–402, DOI:10.1016/j.actbio.2019.04.019.
- S. K. Misra, D. Mohn, T. J. Brunner, W. J. Stark, S. E. Philip, I. Roy, V. Salih, J. C. Knowles and A. R. Boccaccini, Comparison of Nanoscale and Microscale Bioactive Glass on the Properties of P(3HB)/Bioglass® Composites, Biomaterials, 2008, 29(12), 1750–1761, DOI:10.1016/j.biomaterials.2007.12.040.
- S.-M. Hsu, M. Alsafadi, C. Vasconez, C. Fares, V. Craciun, E. O’Neill, F. Ren, A. Clark and J. Esquivel-Upshaw, Qualitative Analysis of Remineralization Capabilities of Bioactive Glass (NovaMin) and Fluoride on Hydroxyapatite (HA) Discs: An In Vitro Study, Materials, 2021, 14(14), 3813, DOI:10.3390/ma14143813.
- K. Zheng, W. Niu, B. Lei and A. R. Boccaccini, Immunomodulatory Bioactive Glasses for Tissue Regeneration, Acta Biomater., 2021, 133, 168–186, DOI:10.1016/j.actbio.2021.08.023.
- F. Zhao, W. Xie, W. Zhang, X. Fu, W. Gao, B. Lei and X. Chen, 3D Printing Nanoscale Bioactive Glass Scaffolds Enhance Osteoblast Migration and Extramembranous Osteogenesis through Stimulating Immunomodulation, Adv. Healthcare Mater., 2018, 7(16), 1800361, DOI:10.1002/adhm.201800361.
- Y. Zhu, Z. Ma, L. Kong, Y. He, H. F. Chan and H. Li, Modulation of Macrophages by Bioactive Glass/Sodium Alginate Hydrogel Is Crucial in Skin Regeneration Enhancement, Biomaterials, 2020, 256, 120216, DOI:10.1016/j.biomaterials.2020.120216.
- J. M. Sadowska and M.-P. Ginebra, Inflammation and Biomaterials: Role of the Immune Response in Bone Regeneration by Inorganic Scaffolds, J. Mater. Chem. B, 2020, 8(41), 9404–9427, 10.1039/D0TB01379J.
- M. H. Kaou, M. Furkó, K. Balázsi and C. Balázsi, Advanced Bioactive Glasses: The Newest Achievements and Breakthroughs in the Area, Nanomaterials, 2023, 13(16), 2287, DOI:10.3390/nano13162287.
- V. Miguez-Pacheco, L. L. Hench and A. R. Boccaccini, Bioactive Glasses beyond Bone and Teeth: Emerging Applications in Contact with Soft Tissues, Acta Biomater., 2015, 13, 1–15, DOI:10.1016/j.actbio.2014.11.004.
- K. Zheng, B. Sui, K. Ilyas and A. R. Boccaccini, Porous Bioactive Glass Micro- and Nanospheres with Controlled Morphology: Developments, Properties and Emerging Biomedical Applications, Mater. Horiz., 2021, 8(2), 300–335, 10.1039/D0MH01498B.
- A. E. Aguilar-Rabiela, A. Leal-Egaña, Q. Nawaz and A. R. Boccaccini, Integration of Mesoporous Bioactive Glass Nanoparticles and Curcumin into PHBV Microspheres as Biocompatible Composite for Drug Delivery Applications, Molecules, 2021, 26(11), 3177, DOI:10.3390/molecules26113177.
- X. Wang, J. Chang and C. Wu, Bioactive Inorganic/Organic Nanocomposites for Wound Healing, Appl. Mater. Today, 2018, 11, 308–319, DOI:10.1016/j.apmt.2018.03.001.
- I. Cacciotti, Bivalent cationic ions doped bioactive glasses: the influence of magnesium, zinc, strontium and copper on the physical and biological properties, J. Mater. Sci., 2017, 52, 8812–8831, DOI:10.1007/s10853-017-1010-0.
- E. O’Neill, G. Awale, L. Daneshmandi, O. Umerah and K. W.-H. Lo, The Roles of Ions on Bone Regeneration, Drug Discovery Today, 2018, 23(4), 879–890, DOI:10.1016/j.drudis.2018.01.049.
- X. Wang, F. Cheng, J. Liu, J.-H. Smått, D. Gepperth, M. Lastusaari, C. Xu and L. Hupa, Biocomposites of Copper-Containing Mesoporous Bioactive Glass and Nanofibrillated Cellulose: Biocompatibility and Angiogenic Promotion in Chronic Wound Healing Application, Acta Biomater., 2016, 46, 286–298, DOI:10.1016/j.actbio.2016.09.021.
- C. Mao, X. Chen, G. Miao and C. Lin, Angiogenesis Stimulated by Novel Nanoscale Bioactive Glasses, Biomed. Mater., 2015, 10(2), 025005, DOI:10.1088/1748-6041/10/2/025005.
- C. Lin, C. Mao, J. Zhang, Y. Li and X. Chen, Healing Effect of Bioactive Glass Ointment on Full-Thickness Skin Wounds, Biomed. Mater., 2012, 7(4), 045017, DOI:10.1088/1748-6041/7/4/045017.
- S. Pourshahrestani, E. Zeimaran, N. A. Kadri, N. Gargiulo, S. Samuel, S. V. Naveen, T. Kamarul and M. R. Towler, Gallium-Containing Mesoporous Bioactive Glass with Potent Hemostatic Activity and Antibacterial Efficacy, J. Mater. Chem. B, 2015, 4(1), 71–86, 10.1039/C5TB02062J.
- W. Ma, X. Yang, L. Ma, X. Wang, L. Zhang, G. Yang, C. Han and Z. Gou, Fabrication of Bioactive Glass-Introduced Nanofibrous Membranes with Multifunctions for Potential Wound Dressing, RSC Adv., 2014, 4(104), 60114–60122, 10.1039/C4RA10232K.
- C. Mao, C. Lin and Xiaofeng, Enhanced healing of full-thickness diabetic wounds using bioactive glass and Yunnan baiyao ointments, J. Wuhan Univ. Technol., Mater. Sci. Ed., 2014, 29, 1063–1070, DOI:10.1007/s11595-014-1044-y.
- S. M. Mârza, K. Magyari, S. Bogdan, M. Moldovan, C. Peştean, A. Nagy, F. Tăbăran, E. Licarete, S. Suarasan, A. Dreanca, L. Baia and I. Papuc, Skin Wound Regeneration with Bioactive Glass-Gold Nanoparticles Ointment, Biomed. Mater., 2019, 14(2), 025011, DOI:10.1088/1748-605X/aafd7d.
- Y. Zhou, L. Gao, J. Peng, M. Xing, Y. Han, X. Wang, Y. Xu and J. Chang, Bioglass Activated Albumin Hydrogels for Wound Healing, Adv. Healthcare Mater., 2018, 7(16), 1800144, DOI:10.1002/adhm.201800144.
- L. Kong, Z. Wu, H. Zhao, H. Cui, J. Shen, J. Chang, H. Li and Y. He, Bioactive Injectable Hydrogels Containing Desferrioxamine and Bioglass for Diabetic Wound Healing, ACS Appl. Mater. Interfaces, 2018, 10(36), 30103–30114, DOI:10.1021/acsami.8b09191.
- Z. Lin, W. Gao, L. Ma, H. Xia, W. Xie, Y. Zhang and X. Chen, Preparation and Properties of Poly(ε-Caprolactone)/Bioactive Glass Nanofibre Membranes for Skin Tissue Engineering, J. Bioact. Compat. Polym., 2018, 33(2), 195–209, DOI:10.1177/0883911517715659.
| This journal is © The Royal Society of Chemistry 2024 |




