Surface engineering of orthopedic implants for better clinical adoption
Shivi
Tripathi
ab,
Ansheed
Raheem
c,
Madhusmita
Dash
d,
Prasoon
Kumar
e,
Ahmad
Elsebahy
f,
Harpreet
Singh
g,
Geetha
Manivasagam
*c and
Himansu Sekhar
Nanda
 *ab
*ab
aBiomaterials and Biomanufacturing Laboratory, Discipline of Mechanical Engineering, PDPM Indian Institute of Information Technology Design and Manufacturing, Jabalpur 482005, MP, India. E-mail: himansu@iiitdmj.ac.in
bInternational Centre for Sustainable and Net Zero Technologies, PDPM-Indian Institute of Information Technology Design and Manufacturing Jabalpur, Madhya Pradesh 482005, India
cCentre for Biomaterials, Cellular and Molecular Theranostics & School of Mechanical Engineering, Vellore Institute of Technology (VIT), Vellore, 632014, Tamil Nadu, India. E-mail: geethamanivasagam@vit.ac.in
dSchool of Minerals, Metallurgical and Materials Engineering, Indian Institute of Technology Bhubaneswar, Argul, Khordha, Odisha 752050, India
eBiodesign and Medical device laboratory, Department of Biotechnology and Medical Engineering, National Institute of Technology, Rourkela, 769008, Odisha, India
fCenter for Translational Oral Research (TOR), Department of Clinical Dentistry, University of Bergen, Årstadveien 19, Bergen 5009, Norway
gDr B R Ambedkar National Institute of Technology Jalandhar, Grand Trunk Road, Barnala Amritsar Bypass Rd, Jalandhar, Punjab 14401111, India
First published on 9th October 2024
Abstract
Musculoskeletal disorders are on the rise, and despite advances in alternative materials, treatment for orthopedic conditions still heavily relies on biometal-based implants and scaffolds due to their strength, durability, and biocompatibility in load-bearing applications. Bare metallic implants have been under scrutiny since their introduction, primarily due to their bioinert nature, which results in poor cell–material interaction. This challenge is further intensified by mechanical mismatches that accelerate failure, tribocorrosion-induced material degradation, and bacterial colonization, all contributing to long-term implant failure and posing a significant burden on patient populations. Recent efforts to improve orthopedic medical devices focus on surface engineering strategies that enhance the interaction between cells and materials, creating a biomimetic microenvironment and extending the service life of these implants. This review compiles various physical, chemical, and biological surface engineering approaches currently under research, providing insights into their potential and the challenges associated with their adoption from bench to bedside. Significant emphasis is placed on exploring the future of bioactive coatings, particularly the development of smart coatings like self-healing and drug-eluting coatings, the immunomodulatory effects of functional coatings and biomimetic surfaces to tackle secondary infections, representing the forefront of biomedical surface engineering. The article provides the reader with an overview of the engineering approaches to surface modification of metallic implants, covering both clinical and research perspectives and discussing limitations and future scope.
1. Introduction
Musculoskeletal disorders are caused by aging, road accidents, war and sports injuries, poor posture, and certain medical conditions that affect our bones, joints, muscles, and connective tissues.1 According to the World Health Organization (WHO), these disorders impact around 1.71 billion people globally (about 22% of the world population).2 These disorders are a significant global health concern and can impact the quality of life. Orthopedic implants are essential for addressing these disorders, particularly in the case of severe bone fractures, as they need realignment and stabilization for proper healing or regeneration of bones.3,4 Materials used for orthopedic implants must be biocompatible, which is the property of an implant material to perform its intended function within a living organism without eliciting unfavorable local or systemic responses.4,5 Apart from biocompatibility, materials used in implants must have strong mechanical properties to maintain their shape when subjected to repetitive loading and unloading cycles during movement.6 They endure the various forces experienced by the human body, including bending, twisting, and shearing stress. Hence, metallic materials are usually chosen for load-bearing orthopedic applications based on their mechanical strength to withstand physical loads and the degree of elasticity required to resist failure under repeated biomechanical pressures.7 Biometals used in orthopedic implants include American Society for Testing Materials (ASTM) A276 stainless steel (SS),8 ASTM F75 cobalt–chromium (Co–Cr) alloys,9 ASTM F136 Ti alloys,10 and ASTM graded (AZ31A, AZ31B, AZ61A, AZ80A, ZK60, WE54A, WE43A, ZMX100 etc.) Mg alloys.11SS is widely used in various medical procedures due to its availability, affordability, and corrosion resistance.12 It is a common choice for implants in cardiovascular, orthopedic, dental, craniofacial, and otorhinolaryngology procedures.13 However, SS lacks biofunctional features,14 antithrombotic,15 osteoconductivity,16 antibacterial properties,17 and bioactivity,18 which can lead to implant-associated infections.19 Co–Cr alloys, on the other hand, offer strong corrosion resistance in chloride-rich environments. This property makes them suitable for orthopedic implants because chloride ions are present in all body fluids, including blood plasma, interstitial fluid, cell fluid, and extracellular fluid.20 Despite their superior anti-corrosion performance, the higher cost of Co–Cr alloys limits their use in the medical market.21 Cobalt-based alloys are also associated with stress-shielding effects due to significantly higher Young's modulus (220–230 GPa) than cortical bone (20–30 GPa).22 There are also concerns about their long-term biocompatibility due to stress-shielding effects and toxic elements like nickel, chromium, and cobalt release.23–25 Titanium (Ti) and its alloys are popular for medical applications due to their mechanical properties, like human bone. They are used in artificial joints, dental implants, and stabilizing healing bones. However, Ti implants can fail due to stress shielding, lack of biological activity, and poor antimicrobial properties, leading to wear, discomfort, and the risk of bacterial colonization.26,27 Other issues associated with Ti-implants (Ti-6Al-4V ELI (Grade 23), ATI Ti-6Al-4V (Grade 5)) include the release of harmful elements like vanadium (V) and aluminum (Al) from its alloys. The release of such undesirable elements could lead to the risk of cytotoxicity and neurotoxicity.28 Additionally, these implants often require a second surgery for removal. However, as third-generation materials, Magnesium (Mg) alloys can biodegrade within the human body, eliminating the need for this additional surgery while also promoting bone regeneration.29 Their mechanical properties closely resemble cortical bone, with Young's moduli (40–45 GPa) and densities (1.7–1.9 g cm−3) similar to cortical bone (Young's modulus 20–30 GPa, density 1.8–2.0 g cm−3).30,31 The compressive yield strength of Mg alloy (ranging from 21–170 MPa) is more similar to that of cortical bone (105–115 MPa) than other metal implants.32 However, Mg's low standard potential hinders the formation of a protective layer, leading to ongoing corrosion in the blood environment.33 The presence of hydrogen as a corrosion byproduct can delay wound healing and elevate the risk of blood flow obstruction.34 The rapid degradation of Mg in the body can also affect local body fluid pH, potentially causing protein deposition or inflammation.35,36 It was observed that when an implant is placed in vivo, there is a reduction in the pH of the body fluids in the vicinity of the implants. Laing et al.37 reported that in the vicinity of a newly inserted metallic implant, the pH decreases from 7.4 to 4 due to the accumulation of hematomas, which last for several weeks. This reduction of local pH can accelerate implant corrosion, weaken cell–material interaction.
Consequently, despite progress in surgical methodologies, sterile environments, and postoperative management, a significant number of metallic implants continue to exhibit inadequate osseointegration with the adjacent biological tissues, leading to eventual failure.38 This is due to the formation of fibrous capsules (part of the foreign body reaction),39 biofilm formation,40 changes in surface roughness,41 applied load,42 stress conditions,43 accelerated corrosion, and the patient's immune response.44 Often, surgical intervention is needed to address these issues.45
The need for revision surgeries poses life-threatening risks to patients and can lead to non-compliance with medical treatments with high costs. The expenses, potential dangers, and discomfort associated with revision surgery create a strong demand for engineering-based modifications of implant surfaces that promote quick osseointegration. It is increasingly vital for bone implants to interact effectively with surrounding tissues. This interaction is crucial to prevent failure and ensure long-lasting implant performance.46
Surface modification through functional coatings offers a promising approach to overcoming challenges related to the poor interaction between implants and tissue, which can be caused by material corrosion, wear, biofilm formation, and fibrous encapsulation.47 Consequently, there has been a continuous increase in research and development focused on surface modification, primarily through functional coatings, to enhance the effectiveness and reliability of metallic orthopedic implants for clinical use. This is evident from a significant rise in published articles (1336) across 25 disciplines from 2003 to 2023 (Web of Science).48 For example, numerous attempts to alter the surface of implants have been made to address the shortcomings of bare implants for biomedical applications.49 Incorporating thin layers of hydroxyapatite (HAp) using a wide range of surface modification techniques has been considered a gold standard for surface modification of bone implants. Applying HAp coatings through air plasma spraying (APS) is a widely adopted industrial technique in medical industries.50 Its popularity stems from its reliability, efficiency, and cost-effectiveness. For instance, Kowalski et al.51 studied the bioactive characteristics of plasma-sprayed HAp coatings on Ti alloys at three varying distances of axial injection (at distances of 100 mm, 120 mm, and 140 mm, respectively). The study investigated the impact of plasma-spray distance on in vitro biocompatibility, cytotoxicity, and the adhesion of Staphylococcus aureus (S. aureus) bacterial cells to three HAp-coated Ti samples. The viability of L929 mouse fibroblasts and human Hs68 fibroblasts was evaluated using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) reduction assay. The results showed that murine fibroblasts exposed to HAp-coated Ti had viabilities of 83 ± 3% (at 100 mm), 87 ± 5% (at 120 mm), and 84 ± 13% (at 140 mm) for different HAp-coated Ti samples. Similarly, the metabolic activities of human fibroblasts co-cultured with HAp-coated Ti were 89 ± 7% (at 100 mm), 90 ± 4% (at 120 mm), and 89 ± 5% (at 140 mm). The THP1-Blue™ NF-κB cells were utilized in the monocyte activation assay to assess the immunocompatibility of the bare and HAp-coated Ti samples. The results demonstrated that the HAp-coated sample did not contribute to monocyte activation, which confirmed the material's immunosafety and negligible endotoxin contamination. The modified vortex method assessed bacterial attachment to HAp and Ti surfaces. The results indicated that the quantity of S. aureus bacterial cells retained on HAp coatings was approximately 1.4 × 106, while it was around 6 × 104 for the bare Ti substrate. In addition to its antibacterial properties, it is crucial to consider the corrosion resistance of implants to extend their lifespan. Zeolitic imidazolate framework-8 (ZIF-8) nanoparticles play a vital role in this regard.52 These nanoparticles enhance the impermeability of coatings and create longer diffusion paths for corrosive substances, effectively reducing the chances of corrosion. Polyvinylidene fluoride (PVDF) coatings on the implants offer the advantages of biocompatibility, chemical resistance, mechanical strength, low friction, non-toxicity, and ease of application. Layered double hydroxides (LDHs) are inorganic materials with a layered structure that offer a cost-effective and environmentally friendly approach to protect metal substrates from corrosion.53 Yin et al.54 produced superhydrophobic ZIF-8/PVDF/LDH (SZPL) coatings on Mg alloy to enhance the corrosion resistance properties. The SEM photomicrographs elucidated distinctive attributes of both the bare Mg substrate and SZPL-coated Mg substrate. The bare Mg substrate is smooth but has visible scratches. On the other hand, the SZPL-coated Mg surface has a rough texture and robust interfacial cohesion between the coating and substrate, indicating a substantial bond with a thickness of approximately 47 μm. The Tafel polarization curves and electrochemical impedance spectroscopy were used to evaluate the corrosion rate of the Mg alloy substrate. The SZPL coating displayed excellent corrosion protection, with an efficiency of over 99.9%, providing long-lasting and efficient protection for Mg alloys. Although these passive coatings are commonly used, but only provide a static, protective layer and cannot actively respond to changes in the surrounding microenvironment.55 This limitation hinders their capacity to provide dynamic, adaptive functionalities such as responsive antibacterial properties, self-healing capabilities, and multifunctional characteristics. These features are essential for preventing infections, enhancing implant performance, and increasing durability, ultimately reducing the need for revision surgeries in coated implants.
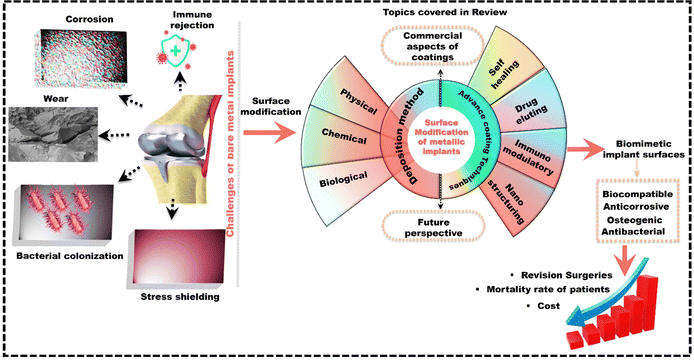
This review focuses on bridging the gap in the literature by exploring smart coatings for metallic implants to overcome the limitations of passive coatings. It covers the commercial aspects and various coating deposition methods while also exploring smart self-healing and drug-eluting coatings. Furthermore, as a strong correlation exists between soft tissue integration and the immune response, the research community is increasingly focusing on an immunomodulation-based strategy. This approach aims to enhance implant integration by regulating the immune response, improving bone–implant fixation, stimulating new bone growth, reducing infection risk, and minimizing the need for revision surgeries. Additionally, the review highlights surface texturing for biomimetic interfaces in implants to tackle secondary infections, especially antibiotic-resistant bacterial infections, which significantly contribute to mortality rates. Fig. 1 offers a detailed overview of the challenges associated with bare metal implants and how the topics covered in this review aim to address and alleviate these challenges. Overall, the advanced surface engineering techniques for metallic implants discussed in this review are expected to enhance their clinical adoption by reducing the need for revision surgeries, which in turn lowers the associated costs and patient mortality rates.
2. Commercial implant coatings: current market insights
The global market for functional implant coatings is expected to reach $2832.17 million, growing at a compound annual growth rate (CARG) of 9.3% from 2023 to 2030. Specifically, in 2023, North America dominated this market, accounting for around 40.3% of the total revenue in the medical coatings for implants sector.56 Several factors contribute to the growth in the functional coatings sector. Among these are the superior technical qualities of coated implants compared to conventional implants, driven by the desire for an enhanced quality of life. This growth is also fueled by increasing healthcare spending, which accounts for about 19.7% of the US GDP.57 This spending is expected to rise 5.4% annually (from 2019 to 2028), reaching $6.2 trillion by 2028.58 Factors such as the prevalence of chronic disorders, orthopedic diseases, technological advancements in imaging technologies, surgery equipment, and an aging population contribute to this surge. According to the United Nations, in 2019, there were 703 million older people worldwide. By 2050, this number is projected to double to reach 1.5 billion.59 This significant increase in the elderly population is expected to drive market growth. This is because some senior individuals who require implants may need revision surgery.60 As a result, the importance of implementing functional coatings on the implant becomes essential in addressing the necessity for revision surgeries. This necessity is reflected in the projected growth of the global market for drug-eluting implants, expected to reach approximately $8.5 billion by the end of 2026, which marks a significant increase from $6.05 billion in 2019, with an average annual growth rate of around 6.28. The market for antibacterial coatings, a type of drug-eluting coatings for medical implants, is projected to expand at a CAGR of 16.4% from 2021 to 2030, reaching $1072.27 million by 2030.61The growth of the antibacterial coatings market for medical implants is primarily driven by a significant increase in implant infections, advancements in antibacterial coating technologies, and the widespread use of cardiovascular and orthopedic implants.62 Additionally, the growing elderly population and the increasing prevalence of bone-related disorders and cardiovascular diseases worldwide are further driving market growth. Key players in the global antibacterial coatings market include Aap Implantate AG,63 AST Products,64 Inc., BioCote Ltd,65 Covalon Technologies Ltd,66 and Specialty Coating Systems, Inc.67 Other manufacturers like Himed,68 DOT GmbH,69 Hydromer,70 and Medicoat AG71 also invest in technological advancements and new product development to provide top-notch solutions in medical, functional coatings.72 For example, Himed MATRIX HA® utilizes high-purity raw HAp powder to coat Ti dental implants and medical devices.68 Likewise, DOT GmbH is one of the top global providers of medical coating technologies for orthopedic and dental implants and instruments. Hydromer company also provides hydrophilic medical device coatings that offer lubricity and other functionalities like enhanced adhesion, etc.70 Medicoat is a top company in plasma spray technology. It coats medical devices with porous osteoconductive coatings and produces and distributes Ti and HAp spray powders.71 These technological advancements provided by various companies are essential for improving and preserving the long-term functional integration between implants and soft tissue and advancing healthcare. The United States has one of the best healthcare systems in the world. There has been an increase in healthcare investment in the country due to the COVID-19 outbreak.73 The demand for antibacterial-coated medical equipment in the U.S. is growing due to government and hospital efforts to prevent infections acquired in healthcare settings.61 These factors mentioned above contribute to the market's growth. This highlights the importance of functional coatings in orthopedic implantation, not only for improved functionality but also to prevent the need for revision surgeries and reduce patient readmission after implantation. Among the various coating techniques, plasma spray technology is the dominant player, commanding a significant 55.3% market revenue share.74 The United States Food and Drug Administration (FDA) has exclusively approved plasma spraying as one of the popular techniques among thermal spray methods for depositing HAp coatings on medical implants and devices.75 This method is crucial for applying thick coatings on ceramic or metal implants, especially in orthopedic, trauma, dental, and other applications. Commonly used materials like Ti, HAp, and dual Ti/HAp play a vital role in biological fixation, surface roughness, and maintaining a chemical composition similar to bone minerals on the implant's surface in contact with the bone. The existing coated implants in the market, such as ZrO2-coated Zr from Smith and Nephew,76 TiN-coated CoCr or Ti6Al4V from Implantcast (Link Orthopaedic),77 TiNbN-coated CoCr from OHST Medical Technology,78 TiN-coated Ti6Al4V from Endotec79 and BONIT® from DOT GmbH,80 aim to enhance implant performance and longevity while reducing the risk of adverse reactions in patients. For example, George et al.81 reviewed the record of 120 patients who underwent total knee arthroplasty. The study compared the outcomes of using oxidized zirconium (surface-modified Zr) femoral implants with bare Co–Cr alloy. The oxidized zirconium knee implants exhibit lower wear and damage than bare CoCr implants.82 This suggests a possible decrease in the production of metal debris, a major reason for revisions, which could result in implants lasting longer.83 Apart from orthopedics, there are various commercially available drug-eluting coating and stents like Taxus®,84 Lutonix®,85 Cypher®,86 and Biomatrix® for coronary restenosis treatment.87 This advancement may result in improved long-term outcomes for those with coronary artery disease.88 However, the cost of drug-eluting stents is a concern, being 3 to 4 times more expensive than bare metal stents from both hospital and societal perspectives. This cost disparity raises concerns about affordability. Additionally, drug-eluting coatings have drawbacks, such as the inability to reload the drug and its short-term release.89 Although there are a lot of coatings in the market (Table 1), continuous improvements in active functional coatings are essential to transitioning from the research and development phase to the commercialization stage.90 The commercialization of coatings is a complex process that needs optimal equilibrium between quality, cost, and efficiency. Maintaining high quality is a non-negotiable aspect of medical device development.91 Cutting corners in this process could result in market failures. Being quick to market can be advantageous in the fast-paced healthcare field.92 However, rushing development can degrade quality and raise costs later on.93 Hence, cost-benefit analysis, outsourcing, and risk management need to be considered for successfully bringing functional coatings for metal implants to market.94,95
| Coating | Type | Ref. |
|---|---|---|
| jHemo PC® phosphorylcholine hemocompatible coatings | Chemical coating (covalent bond to substrate) | 96 |
| Hygea® heparin antithrombogenic coating | Chemical coating (chemical bond to substrate) | 96 |
| Lipocoat biocompatible coatings | Biological coating (noncovalent attachment of molecule to substrate) | 97 |
| Zolidd® coating | Biological coating | 98 |
3. Surface modification techniques
Surface modification techniques are commonly employed to coat or change a outer layer of material and create an interface with unique functionalities that differ from those in the bulk material.99 When choosing an appropriate surface modification method for implants, it is essential to consider fundamental properties such as corrosion resistance, wear resistance, fatigue strength, biocompatibility, and degradation of the implant material.100 Surface modification of implants is essential as it increases surface area, enhances protein absorption,101 strengthens cell adhesion and growth, and improves integration with surrounding bone.96 Therefore, choosing the suitable method to apply coating onto the surface of metal implants is a crucial consideration in orthopedic procedures. Various surface engineering approaches (physical, chemical, and biological) are employed to alter the surface properties of metallic implants and scaffolds, addressing concerns related to uncoated metal implants.1023.1 Physical techniques
Physical coating techniques refer to the various methods and processes used to apply a thin layer of functional material onto a surface of the substrate. These methods include physical vapour deposition (PVD), condensation, sputtering, plasma spray, dip coating, or mechanical techniques like grit blasting. The method deposits the coating material onto the surface of the substrate without altering the implant bulk material's chemical state.103 Deering et al.104 utilized the dip coating technique to apply a coating of polymethyl methacrylate–alumina (PMMA–Al2O3) composite films onto the porous stainless steel (SS) scaffolds (with approximately diameter of 275 μm). SS scaffolds were immersed in a solution of PMMA–Al2O3 and subsequently allowed to dry (Fig. 2(a)). The SEM images could confirm the material deposition over the surface of SS scaffolds (Fig. 2(b)). The cell viability assay demonstrated that PMMA–Al2O3 coated SS exhibited increased cellular metabolic activity from the first to the seventh day (Fig. 2(c)). These findings suggest that incorporating PMMA–Al2O3 composite coatings onto porous scaffolds enhances the osseointegration potential compared to uncoated scaffolds. Coatings on porous SS scaffolds modify surface topography, chemistry, and wettability, which enhances cell interactions and boosts cell viability. Hence, the dip coating technique shows significant potential in forming composite coatings on porous implants, enhancing the potential osteoconductivity within their internal pores.105 However, the high-temperature sintering process (usually over 1000 °C) can weaken the metal implants and reduce their ability to bond effectively, resulting poor adhesion. Drying films can lead to cracks from material shrinkage due to environmental factors. Using a cleanroom or laminar flow hood and heat treatment for drying can increase costs and limit dip coating scalability.106 Additionally, the main drawbacks of dip coating are poor adhesion and inconsistent coating thickness. PVD techniques provide more durable and uniform coatings compared to dip coating. Unlike dip coating, PVD is a vacuum-based process where the material is heated until it evaporates and condenses onto the substrate. This results in more uniform, durable coatings, and less prone to defects and inhomogeneities. Uddin et al.107 used the PVD technique to apply Titanium nitride (TiN) coatings onto titanium substrates (Fig. 3(a)). The tribomechanical properties of these coatings with two different roughness levels, including a roughness of Ra 0.4 ± 0.05 μm (rough) and a roughness of Ra 0.1 ± 0.01 μm (fine), were compared. The coating thicknesses for deposition times of 7 minutes, 15 minutes, 30 minutes, and 60 minutes were 1.0 μm, 2.9 μm, 3.3 μm, and 4.0 μm, respectively (Fig. 3(b) shows the coating thickness of 2.9 μm). TiN-coated samples showed a significant decrease in corrosion current density from 1.110 μA cm−2 to 0.145 μA cm−2 (Fig. 3(c)) and lower wear rates. The rough samples had even lower wear rates due to improved hardness and adhesion (Fig. 3(d)).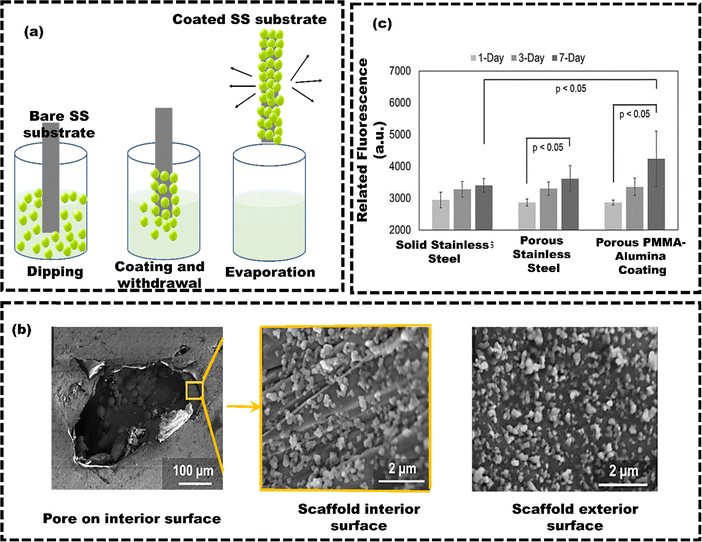 | ||
| Fig. 2 (a) Working principle of dip coating, (b) surface morphology of the PMMA–Al2O3 composite coating at the interior and exterior of a scaffold, (c) viability of cells on solid, porous, and coated porous additive manufacturing samples, reproduced from Deering et al.104 with permission from Elsevier, copyright (2020). | ||
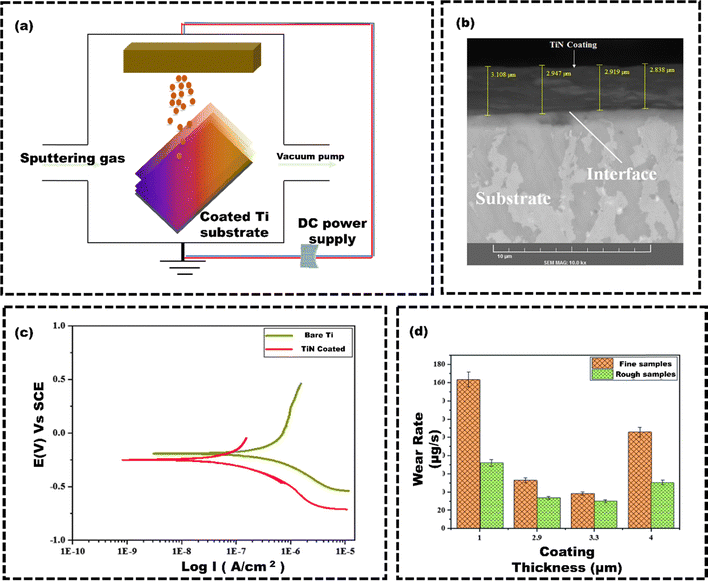 | ||
| Fig. 3 (a) Working principle of PVD technique, (b) SEM image of TiN coating on Ti substrate, (c) potentiodynamic polarization curves for the bare Ti and TiNcoated samples in SBF, (d) effect of coating thickness and roughness on wear rate, reproduced from Uddin et al.107 with permission from Springer US, copyright (2019). | ||
Apart from these advantages, PVD has several limitations. For example, the PVD technique can degrade polymers, lowering the molecular weight of the film.108 Large-area deposition with solid metal or oxide sources is laborious and time-consuming. Controlling film composition is difficult except with laser ablation. PVD is also expensive due to the need for advanced equipment, and coating complex shapes is challenging because the process requires direct vapor contact, making it hard to reach all areas.109 However, complex-shaped components can be effectively coated using the plasma spray method, which operates at atmospheric pressure, making it a more cost-effective alternative. Plasma spray deposition of HAp on metal implants is a widely adopted method due to its affordability. Ashely et al.110 used a plasma spray technique to develop zinc/silicon/silver/hydroxyapatite (ZnSiAg–HAp) composite coatings on Ti substrates for orthopedic and dental applications (Fig. 4(a)). Composite coatings of 75 μm were applied using a 25-kW plate power and a 110 mm spray distance from the supersonic plasma nozzle. The supersonic nozzle introduced powder at 510 m s−1, much faster than conventional nozzles (5 m s−1), resulting in less powder heating. Minimizing powder heating during plasma spray coating has several advantages. It conserves energy, lowers operating costs, and preserves coating integrity, which, in turn, leads to the production of higher-quality coatings. The surface morphology of ZnSiAg–HAp exhibited both roughness and porosity (Fig. 4(b)). The rate of bone mineralization was 68% for the ZnSiAg–HAp coatings, compared to 55% for HAp alone. Even though the HAp coating led to increased osteoid formation, the ZnSiAg–HAp coating demonstrated superior overall bone formation (Fig. 4(c) and (d)). Unlike HAp coatings that only attach bone tissue to the surface, ZnSiAg–HAp coatings enable more significant bone tissue infiltration, indicating improved bone ingrowth and demonstrating that the plasma spray technique enhances osteointegration in metallic implants. Still, plasma spray coating of implants can have drawbacks, such as high thermal stresses from the decomposition of bioceramics at high temperatures. Additionally, defects like unmelted particles, voids, and cracks can occur if spraying parameters are not correctly optimized.111 This can be overcome by post-coating heat treatment processes. Singh et al.112 investigated the effects of post-coating heat treatment on the phase composition, microstructure, and mechanical and electrochemical corrosion properties of HAp-coated 316 L stainless steel substrates. The vacuum plasma spray technique was used to deposit reinforced HAp coatings, specifically HAp with 10 wt% Al2O3 (SHAA) and HAp with 10 wt% ZrO2 (SHAZ). The study showed that plasma-sprayed coatings had superior wear resistance than heat-treated ones. However, post-coating heat treatment enhanced corrosion resistance by densifying the microstructure, restoring HAp integrity, reducing porosity, and improving nano hardness and shear strength (Fig. 4(e)). Despite reducing the hardness of the top layer due to partially melted particles (Fig. 4(f)), the heat treatment made the coatings more suitable for durable orthopedic implants.
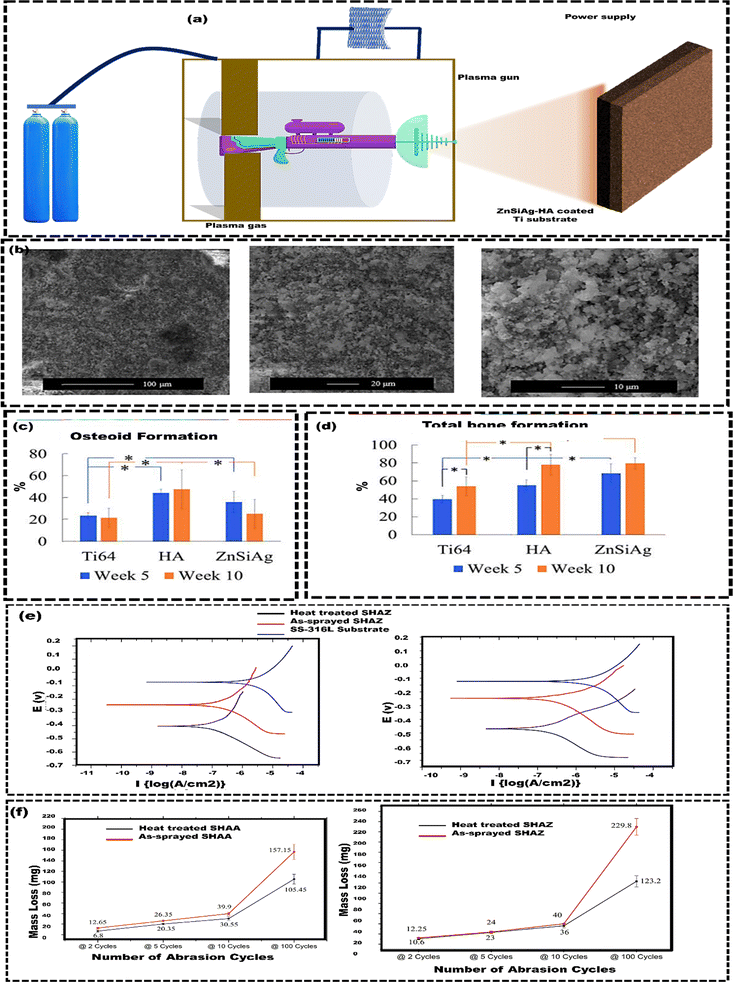 | ||
| Fig. 4 (a) Working principle of plasma spray coating technique, (b) surface characteristics of the ZnSiAg–HA coating, including the surface roughness and porosity, (c) the percentage of total osteoid formation around the implant (250 μm radius), (d) total bone formation in % around the implant (250 μm radius), reproduced from Ashely et al.110 with permission from Elsevier, copyright (2019) (e) Tafel polarization curves of un-coated SS-316L substrate, as-sprayed SHAZ, and heat-treated SHAZ coating in Hank's balanced salt solution, (f) abrasion wear behavior (using taber abrasion tester) of as-sprayed and heat treated SHAA and SHAZ coating, reproduced from Singh et al.112 with permission from Elsevier, copyright (2018). | ||
Direct metal fabrication (DMF) is a three-dimensional (3D) printing method that creates a stable coating–substrate interface, even when the coating and substrate have different physical and chemical properties.113 Kim et al.114 investigated the osseointegration ability of Ti powder-coated machined Co–Cr alloy using the DMF technique. At 24 hours, the DMF-coated samples showed lower cell proliferation than the machined samples. However, by 96 hours, cell proliferation in the DMF group increased fivefold from 24 hours (Fig. 5(a) and (b)). This increase was attributed to the highly porous surface with an average pore size of 200 μm to 500 μm and 65% porosity, enhancing nutrient and oxygen diffusion. Subsequently, after three months, the distal femurs were extracted for analysis (Fig. 5(c)). In vivo, DMF-coated rods had a significantly higher bone-to-implant contact of 72.3 ± 6.2% compared to 47.6 ± 6.9% for machined rods after three months (Fig. 5(d)). This Ti coating is effective for cementless knee and hip implants. Likewise, interest in nanoscale metallic materials is driven by their high specificity and large surface area, which increase reactivity.115In vitro studies also indicate that nanosized ceramic coatings provide superior mechanical strength and wear resistance compared to conventional powder coatings.116 Hazim et al.117 deposited a nanostructured coating of TiO2 infused with Ag over Co–Cr alloys using the APS technique and high-velocity oxy-fuel (HVOF) techniques. The interface between the coating and substrate displays minimal oxide formation and no visible delamination or cracks. The HVOF coating demonstrated a slightly thicker layer per spray compared to the APS coating. This difference was due to the higher feed rate used in the HVOF coating process (Fig. 5(e)). HVOF spray coating showed the lowest wear loss, followed by APS sprayed coating and bare Co–Cr alloy (Fig. 5(f)). The HVOF sprayed coating was harder, less porous, and had slower particle detachment, resulting in lower wear than the APS sprayed coating. Bare Co–Cr alloy samples had deeper scars and more debris than the coated samples. These differences are due to the nanoscale TiO2–Ag particles in the coatings.
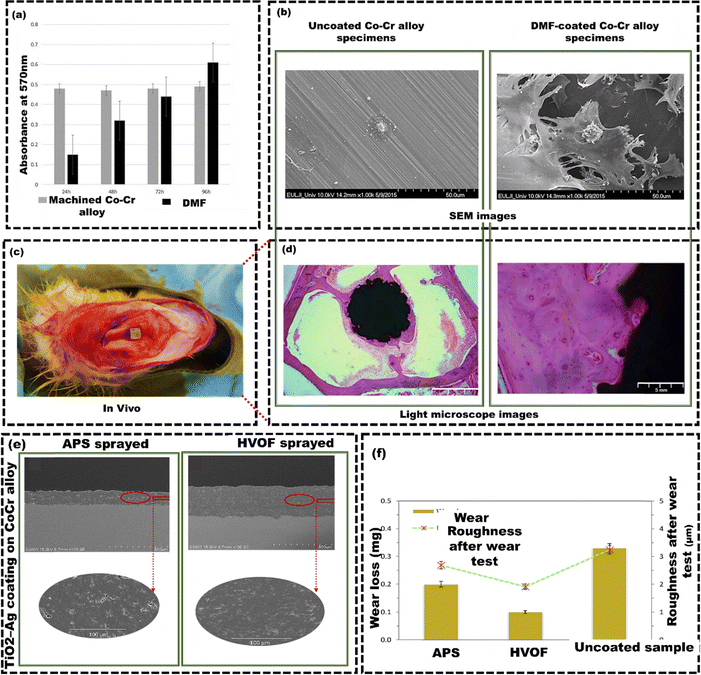 | ||
| Fig. 5 (a) SEM images showing osteoblast cells on machined and DMF-coated Co–Cr alloy specimens after 6 hours of incubation, (b) light microscope images showing the bone-to-implant contact on machined and DMF-coated samples, (c) a DMF-coated rod placed inside the medullary canal of the right femur, (d) osteoblast cell proliferation for DMF-coated and machined specimens, reproduced from Kim et al.114 with permission from Springer Nature, copyright (2019) (e) The cross-section of a nanostructured TiO2–Ag coating on a Co–Cr alloy by APS spraying technique and HVOF spraying technique, (f) average wear loss and surface roughness of APS TiO2–Ag coated, HVOF TiO2–Ag coated, and uncoated Co–Cr alloys, reproduced from Hazim et al.117 with permission from Elsevier, copyright (2019). | ||
Silane treatment effectively protects Mg alloy from corrosion by forming a silanol group that bonds with the alloy surface, enhancing adhesion.118 Additionally, incorporating graphene oxide (GO) extends the diffusion path for corrosion media. It provides favorable tribological properties, biostability, and biocompatibility for biological applications.119 Liu et al.120 utilized the layer-by-layer (LBL) method to fabricate S/GO composite coatings on AZ91 Mg alloy (AZ91SG) to enhance the corrosion resistance of AZ91 Mg alloy (Fig. 6(a)). AZ91SG showed a much slower release of Mg ions, ranging from 0.12 mM cm−2 after 24 hours to 0.34 mM cm−2 after 72 hours. In contrast, AZ91 had a higher release of Mg ions, starting from 1.44 mM cm−2 after 24 hours and reaching 4.32 mM cm−2 after 72 hours (Fig. 6(b)). Furthermore, the fluctuations of pH in simulated body fluid (SBF) containing AZ91 and AZ91SG indicated that the SBF containing AZ91SG was less acidic than SBF containing AZ91 due to the shielding effect of silane/GO composite coatings (Fig. 6(c)). Moreover, AZ91SG exhibited superior resistance to corrosion compared to the uncoated AZ91 alloy. This is evident from the lower rate of hydrogen evolution, indicating a reduction in corrosion for the underlying Mg in AZ91SG (Fig. 6(d)). Eben et al.121 employed a 3D inkjet printing technique to enhance the corrosion resistance of bioresorbable AZ31 Mg alloy. The AZ31 Mg alloy was coated with poly(ester urethane) urea virgin (PEUU-V), poly(ester urethane) urea with phosphorylcholine (PEUU-PC), and poly(ester urethane) urea with sulfobetaine (PEUU-SB). PEUU-V with 20 layers had the best corrosion resistance of 109.3 kΩ, while PEUU-SB with 5 layers had the lowest of 33.93 kΩ. However, all coated AZ31 Mg alloys performed better than the bare AZ31 Mg alloy, which had a resistance below 20 kΩ.
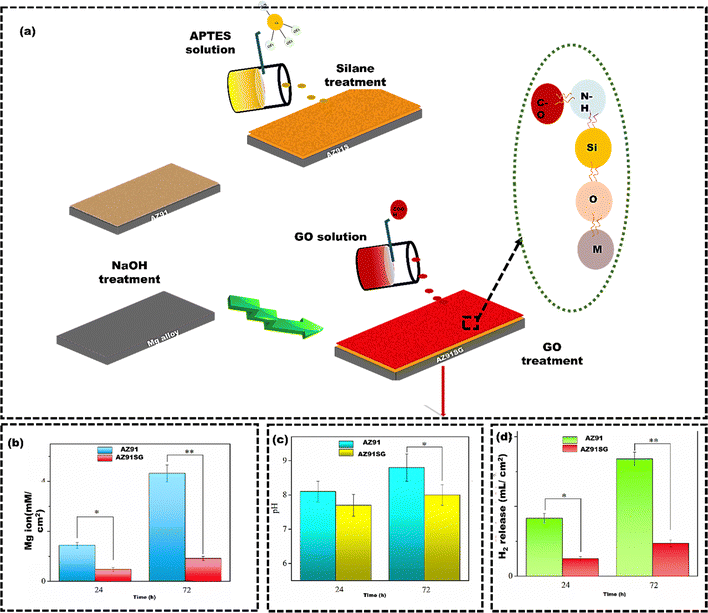 | ||
| Fig. 6 (a) Schematic representation of the layer-by-layer assembly of silane and GO sheets over AZ91 Mg alloy substrate, (b) the measurement of released Mg ions using inductively coupled plasma-optical emission spectroscopy (ICP-OES), (c) pH change (d) the hydrogen (H2) release rates from AZ91SG compared to AZ91 as the control group. Reproduced from Liu et al.120 with permission from MDPI, copyright (2021). | ||
3.2 Chemical techniques
Chemical modification techniques are employed to modify the surface of large metallic samples, which can be challenging to achieve using physical techniques.122,123 Chemical techniques are utilized to modify the surface of implants through various chemical reactions, such as carbonization and oxidation. The coating material, often in liquid, gas, or solution, is applied onto the bare substrate. During this application, chemical reactions occur between the substrate's reactive groups and those on the coating material.124 These chemical reactions create strong covalent bonds, firmly attaching the coating to the substrate. The coating method, material properties, and substrate characteristics influence the bonding mechanism.125 Some of the widely used chemical surface modification techniques for metallic implants include chemical vapour deposition (CVD), sol–gel deposition, anodic oxidation, layer-by-layer spin (LBL) technique, etc.126 In recent years, polymeric multilayer coatings with multiple functions have displayed significant potential. Hyaluronic acid (HA) is known for its viscoelasticity, biocompatibility, biodegradability, and bacterial anti-adhesive properties. It is widely used in joint fluid supplementation for arthritis.127 However, its ability to prevent bacterial adhesion varies with bacteria type, load, and conditions. HA coatings are often combined with amoxicillin (AX), which is effective against Gram-positive and Gram-negative bacteria. Fucoidan (FU) is recognized for its potent antioxidant properties. In a notable study by Matej et al.,128 a composite coating of AX-infused FU/HA was engineered and deployed on medical-grade stainless steel (MSS) samples referred to as 4HA3AX, 2HA3AX2FU, and 3HA3AX1FU. The coatings were applied using LBL spin coating to enhance antioxidant effectiveness and inhibit biofilm formation (Fig. 7(a)). The inhibition zone test demonstrated the antibacterial effectiveness of the released AX against S. aureus and Escherichia coli (E. coli), as indicated by the formation of inhibition zones (Fig. 7(b)). The live/dead assay confirmed the biocompatibility and viability of the cells, indicating that a significantly higher percentage of cells were alive compared to the number of dead cells on the coated MSS (Fig. 7(c)). However, the 2HA3AX2FU substrate had reduced cell viability due to rapid FU layer degradation. Overall, the spin coating of HA, FU, and AX improved biofilm inhibition and antioxidant activity compared to the bare MSS substrate. However, the spin coating is sensitive to temperature and humidity, which can affect the thickness and quality of the film. Additionally, spin coating may not be suitable for creating complex film structures or for films that require a very thick coating.129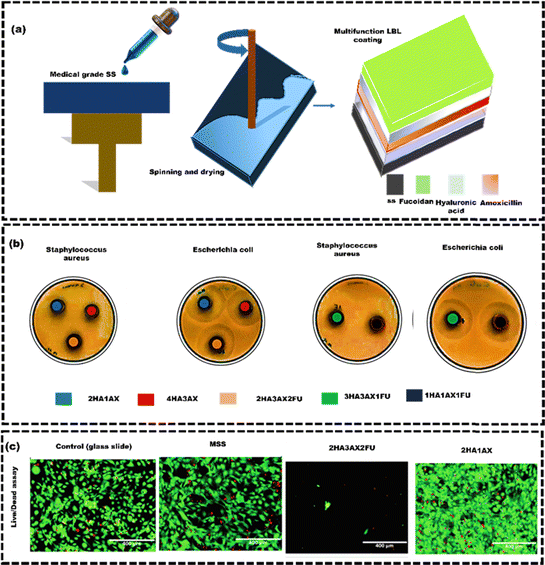 | ||
| Fig. 7 (a) A schematic representation of multifunctional coatings consisting of HA/AX/FU on MSS disks, (b) photographs showing the inhibition zones in the samples (1HA1AX1FU: black colour, 2HA1AX: blue colour, 2HA3AX2FU: orange colour, 3HA3AX1FU: green colour and 4HA3AX: red colour), and (c) microscopic images obtained from live/dead staining showcasing all the tested samples, reproduced from Matej et al.128 with permission from Elsevier, copyright (2023). | ||
The sol–gel technique addresses thickness concerns by converting a liquid precursor into a gel-like silica network through hydrolysis and condensation reactions. This improves adhesion, reduces porosity, and increases coating thickness.130,131 Forsterite, a crystalline magnesium silicate ceramic, can be coated onto Mg alloy, where it interacts with calcium and phosphate ions to create a protective layer that produces hydroxyapatite (HAp), a natural bone mineral.132
Sharmid et al.133 employed the sol–gel technique (Fig. 8(a)) to apply a nano forsterite coating on AZ91 Mg alloy to enhance biocompatibility and control their corrosion in a physiological environment (Fig. 8(b)). The potentiodynamic polarization electrochemical tests demonstrated a reduction in corrosion current density from 11 μA cm−2 (for uncoated samples) to 4.3 μA cm−2 (for coated samples) (left side of Fig. 8(c)). The reduced corrosion current density was due to the formation of the stable and protective oxide layer, effectively shielding against corrosive substances. The forsterite coating significantly improves Mg alloy corrosion resistance, reduces Mg ion release, enhances biocompatibility, ultimately lowering the risk of tissue damage and inflammation. In addition, the weight loss of the coated AZ91 Mg alloy was also reduced (right side of Fig. 8(c)). While sol–gel technology offers benefits, still it has limitations, such as slow kinetics, poor wear resistance, and weak bonding. The process is laborious and prone to cracking due to contraction, and it is not suitable for large-scale coatings due to expensive, moisture-sensitive precursors. Additionally, uneven particle distribution can lead to non-uniform coating thickness, potentially causing cytotoxicity and implant failure.134,135 Hence, a simple, solvent-free method is essential for creating an osteoinductive metal surface for successful implantation. CVD is commonly used for its versatile, solvent-free approach that improves biocompatibility. Youn et al.136 utilized the CVD technique to coat the Ti substrate with poly(glycidyl methacrylate) (pGMA) to enhance the osteogenic properties of the Ti (Fig. 9(a)). The bare Ti substrates attached 40.2 ± 32.7 ng of rhBMP2, while Ti-pGMA substrates showed 243.9 ± 25.7 ng rhBMP2 attachment. SEM and AFM images initially showed smooth surfaces for both, but after rhBMP2 immobilization, Ti-pGMA-BMP2 surfaces appeared textured, indicating suitable rhBMP2 attachment. This confirms that pGMA enhances consistent and efficient rhBMP2 immobilization on Ti surfaces (Fig. 9(b) and (c)). The osteogenic gene expression results also supported the finding that a significant quantity of rhBMP2 adhered to the modified Ti surfaces. This adhesion had a noticeable positive impact on osteogenic differentiation and calcium deposition (Fig. 9(d) and (e)). The Ti-pGMA-BMP2 group showed slightly more cell viability than the bare Ti and Ti-pGMA groups, indicating minimal toxicity to hASCs. However, the efficiency can be affected at times due to the specific challenges. For example, in the CVD process, substrates are heated up to 900 °C, making them unsuitable for heat-sensitive materials.137 Key concerns include the chemical and safety risks from toxic, corrosive, and flammable precursor gases.129 Additionally, advanced CVD variants come with higher costs due to the need for complex reactors and vacuum systems.138
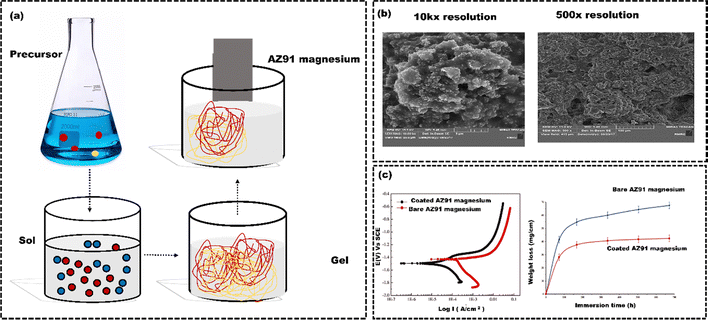 | ||
| Fig. 8 (a) Working principle of sol–gel technique, (b) SEM images of the forsterite coating on the AZ91 magnesium alloy substrate at different resolutions, (c) potentiodynamic polarization behavior of uncoated and forsterite-coated samples in an SBF and weight loss of both forsterite-coated and uncoated samples after immersion in an SBF, reproduced from Sharmid et al.133 with permission from Elsevier, copyright (2023). | ||
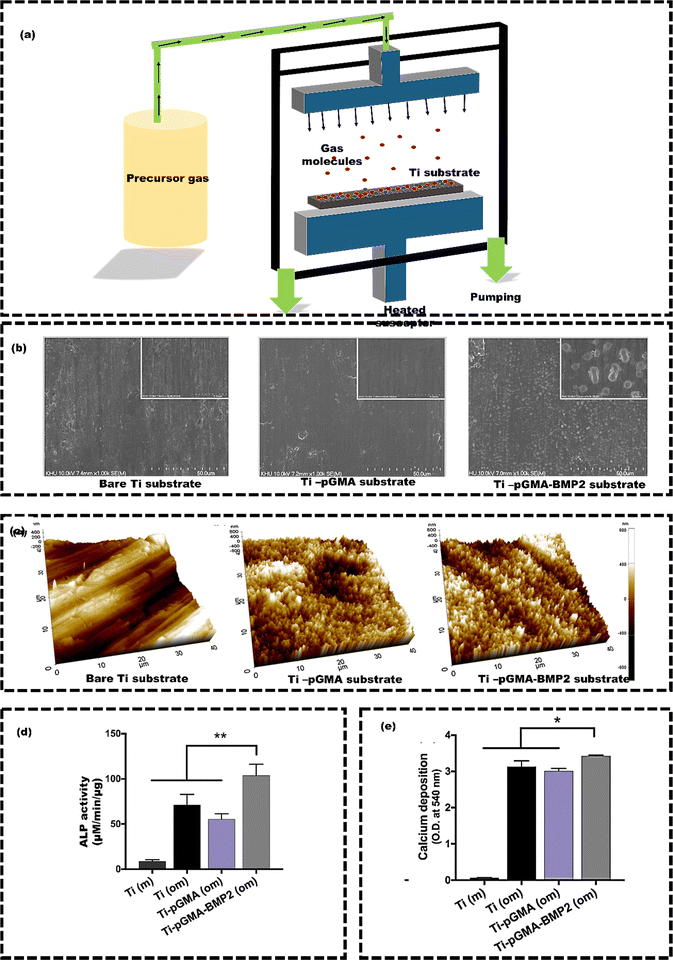 | ||
| Fig. 9 (a) Working principle of CVD, (b) SEM images of coated and uncoated Ti substrate, (c) AFM images of coated and uncoated Ti substrates, (d) the osteogenic activity of hASCs on Ti in non-osteogenic medium (m), Ti-pGMA in osteogenic medium (om), and Ti-pGMA-BMP2 (om) (e) Calcium deposition on Ti in non-osteogenic medium (m), Ti-pGMA in osteogenic medium (om), and Ti-pGMA-BMP2 (om), reproduced from Youn et al.136 with permission from Elsevier, copyright (2019). | ||
Hence, researchers are more interested in other chemical methods for coatings like the anodization technique.139 Sahar et al.140 employed the anodization method to coat pure Ti and compared bone healing around the pure Ti implants with anodized Ti implants after inserting them into the tibiae of rats (n = 15). Five rats received pure Ti implants, five received coated implants (TiO2/Ti), and the remaining five were the control groups (Fig. 10(a)). The SEM images revealed the anodic oxidation resulted in the formation of a nano-porous oxide layer on the Ti implant (Fig. 10(b)), which significantly improved the cell attachment and proliferation compared to the uncoated Ti implant. The surface roughness of the anodized Ti implants at the interface between the implant and bone significantly promoted the formation of physico-chemical bondage with the adjacent hard tissues (Fig. 10(c)). The TiO2 surface increased the contact area, enabling bone growth into the interconnected 3D pores. Apart from anodization method, electrodeposition can also be used to coat Ti alloys. Like, Katic et al.141 used electrodeposition to apply calcium phosphate (CaP) coatings on Ti6Al7Nb (Ti, 6% aluminum (Al), and 7% niobium (Nb)), enhancing its corrosion resistance. Electrochemical impedance spectroscopy showed that CaP-coated Ti6Al7Nb had significantly higher corrosion resistance (131.6 ± 24.7 kΩ cm2) than uncoated Ti6Al7Nb (58.6 ± 16.0 kΩ cm2). The CaP coating also improved cell adhesion, bone growth, and implant stability. However, coatings using the electrodeposition method have poor adhesion between the coated layer and the implant surface, resulting in delamination of the coated layer. The delamination of coated layers can lead to inflammation and bone loss, which may require additional surgeries.142 Electrochemical methods for coating nanoparticles can help prevent this problem. Inorganic nanoparticles like Ag, zinc (Zn), and copper (Cu) are known for their antibacterial properties.143 Polypyrrole (PPy) regulates and enhances the behaviour of HAp nanoparticles for improved functionality.144 Luo et al.145 used the pulse electrochemical method to develop PPy/HAp/ZnO–Cu, PPy/HAp/ZnO–Ag, PPy/HAp/Ag–Cu, and PPy/HAp/ZnO–Ag–Cu multi-ion antibacterial composite coatings over the Ti surface to enhance the antibacterial properties against S. aureus and E. coli bacteria. After seven days, BMSCs shed on PPy/HAp/Ag–Cu, PPy/HAp/ZnO–Cu, and PPy/HAp/ZnO–Ag–Cu coatings but adhered and extended well on the PPy/HAp/ZnO–Ag coating (Fig. 11(a)). Immunofluorescence staining (FITC) results suggested that the composite coating effectively stimulated the differentiation of bone forming cells (BMSCs) into osteoblasts (Fig. 11(b)). The observed effects are due to HAp in the coating, which activates osteoblast-specific genes in BMSCs, leading to growth factor secretion and BMSC differentiation into osteoblasts. Ag, ZnO, and Cu nanoparticle composite coatings exhibit bactericidal effects by releasing Ag+, Cu2+, and Zn2+ ions. These ions disrupt bacterial membranes and elevate reactive oxygen species (ROS), leading to protein oxidation, DNA damage, and lipid peroxidation in bacteria (Fig. 11(c)). The coatings can also acquire antibacterial properties by combining Ag ions with zeolite. Zeolite-A, a synthetic zeolite with a three-dimensional framework structure, is effectively utilized as a carrier for controlling the release of antibacterial agents. Quing et al.146 used selective laser melting to create porous 316 L SS scaffolds and applied zeolite-A coatings with or without Ag ions via hydrothermal crystallization (Fig. 12(a)). In agar diffusion tests, the Ag-ZCs coating significantly reduced microbial activity, leaving almost no colonies on the agar plate (Fig. 12(b)). The bacterial inhibition was due to burst release of Ag+ ion for up to 7 days (Fig. 12(c)). The CCK-8 assay confirmed good cell adhesion and distribution on the scaffolds (Fig. 12(d) and (e)). Ag-incorporated zeolite coatings on 3D-printed SS scaffolds showed promise for orthopedic implants.
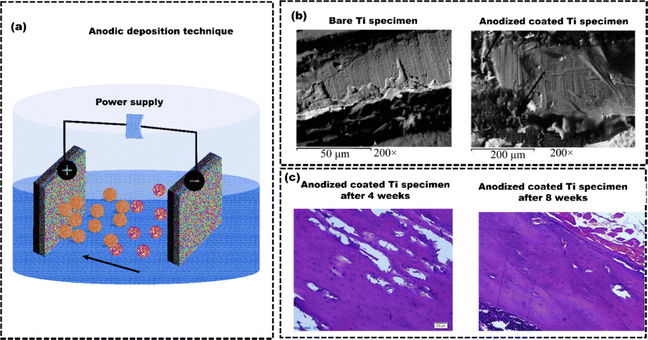 | ||
| Fig. 10 (a) Schematic illustration for anodic oxidation technique, (b) SEM images of uncoated Ti specimen and anodized coated Ti specimen after implantation, (c) Photomicrographs of histological sections stained with Hematoxylin and eosin dyes from bone samples after implantation, reproduced from Sahar et al.140 | ||
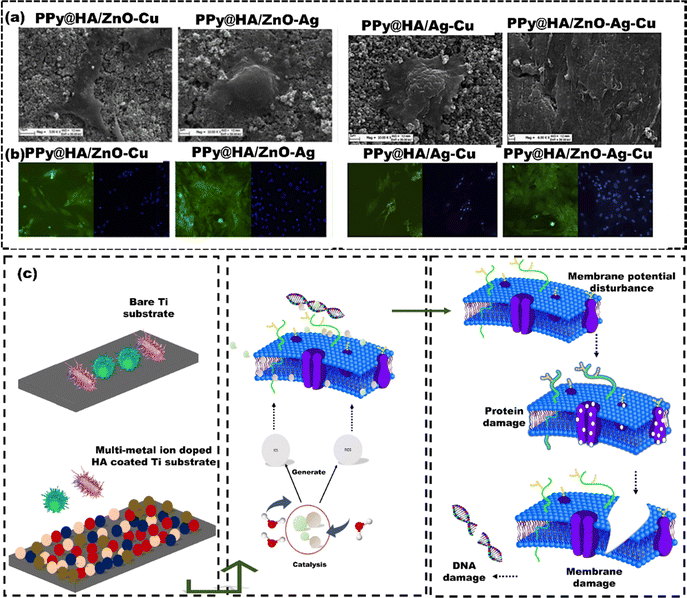 | ||
| Fig. 11 (a) SEM images of various coatings over Ti-substrate (b) FITC images showing growth of BMSCs on the surface of each composite coating after seven days of cell culture (c) shematics illustrating the mechanism of action of composite coatings against E. coli and S. aureus bacteria, reproduced from Luo et al.145 with permission from Elsevier, copyright (2021). | ||
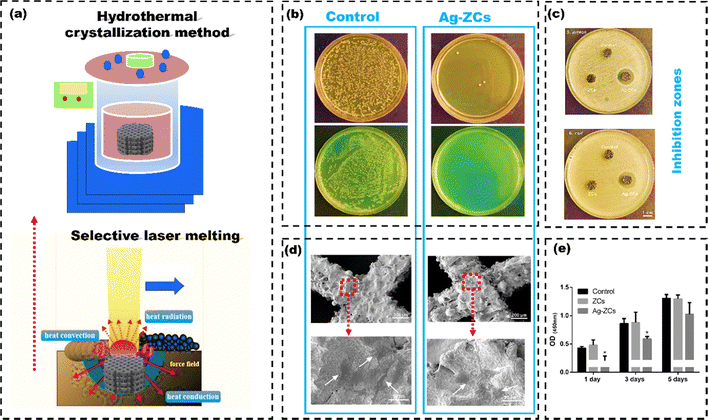 | ||
| Fig. 12 (a) 3D printing of 316 L stainless steel scaffold using Dimetal-280 printer (selective laser melting), and functional coating using hydrothermal crystallization technique, (b) E. coli and S. aureus growth on Luria-Bertani agar plates after 24-hour incubation of the samples (c) inhibition zones (d) SEM images of BMSCs grown over the scaffolds and (e) cell proliferation of BMSCs with different scaffold's, reproduced from Quing et al.146 with permission Elsevier, copyright (2020). | ||
Overall, chemical techniques enable precise molecular or nanoscale surface modifications, offering enhanced control and versatility, but require careful consideration of material sensitivities and process details.
3.3 Biological techniques
Physical and chemical surface modification techniques indirectly improve the implant cell material interaction by altering the surface properties (wettability, roughness, corrosion, wear resistance, and functional groups) of implant.147 On the other hand, biological surface modification techniques directly impact and regulate cellular functions, such as reducing inflammation, promoting stem cell differentiation, and stimulating blood vessel formation.148 Biological coating techniques involve immobilizing proteins, enzymes, growth factors, antibodies, genes, and cells onto surfaces through physical adsorption, chemical conjugation, self-crosslinking, and electrospinning.149Applying biological coating through the physical adsorption method usually entails immersing the implant in a solution containing the desired bioactive molecules, facilitating spontaneous adsorption.150 The adsorption process primarily relies on factors such as surface chemistry, topography, hydrophilicity, electrostatic interactions among bioactive molecules, and their interactions with the surface and solution properties. A surface treated with biological substances significantly improves osteoconduction, osteodifferentiation, and osteointegration.151 Osteoprogenitor cells are the mesenchymal stem cells that can differentiate into osteoblasts. Osteoblasts release various growth factors throughout the process of osteogenesis, including bone morphogenetic proteins (BMPs), which attract other mesenchymal stem cells and encourage them to transform into additional osteoblasts. As a result, osteogenic growth factors such as Fibroblast Growth Factor 2 (FGF2), Transforming Growth Factor Beta (TGF-β2), platelet-derived growth factor (PDGF), and BMP2 can be incorporated as biological coatings on metallic implants to augment the osteoconductivity.152 Keleci et al.153 evaluated the effects of PDGF and BMP6 on TiO2 surfaces in early osseointegration and bone formation using osteoblastic cells. Ti surfaces were anodized, followed by BMP6 adsorption, and coated with PDGF-containing silk fibroin (SF) using electrospinning, creating Ti-BMP6-PDGF-SF plates. Mouse calvaria osteoblastic cells (MC3T3-E1) were used to study cell–material interactions, including cell proliferation, mineralization, and gene expression. The PDGF and BMP6 release was slower on SF-coated surfaces, leading to improved MC3T3-E1 cell growth and mineralization but decreased RUNX2 and ALPL gene expression by day 28, promoting early osseointegration (Fig. 13(a) and (b)). Yin et al.154 applied a mussel adhesive protein (MAP) coating onto alkali-treated Ti with nanonetwork structures (TNS) using a physical adsorption method to create TNS-MAP implants. The study assessed the osteogenic potential of MAP coatings by examining their effects on cell adhesion, cell proliferation, gene expression, and subsequent implantation in an animal model. The TNS-MAP coating promoted rapid cell adhesion and proliferation (15 minutes and 30 minutes after incubation), which was attributed to the surface nanostructure and improved hydrophilicity of the coated surface (Fig. 13(c)). The in vivo experiments demonstrated new bone formation around the implants and at the bone–implant interface. The TNS-MAP implant exhibited a higher level of new bone integration on its surface compared to the TNS implant (Fig. 13(d)). Despite its advantages, the physical adsorption method has drawbacks for immobilizing biomolecules on implant surfaces. Due to weak bonds like hydrogen bonds and electrostatic attraction, it offers limited control over biomolecule density and retention.155 This makes detachment through desorption or displacement, causing the surface's bioactive properties to diminish quickly. Like the Ti surfaces coated with bone morphogenic protein via the physical adsorption method lost 96% of its bioactivity within the first few hours of interaction with biological fluids.156 Furthermore, this approach is unable to properly manage the molecule's surface density and orientation, which is essential for controlling how the implant interacts with biological agents such as proteins, cells, and tissues.157 Additionally, physical adsorption can induce conformational changes or denaturation in proteins or peptides, potentially altering their bioactivity. To overcome these constraints, specific covalent bonding techniques like chemical conjugation offer a chance to immobilize bioactive molecules on substrates.158 This method offers the distinct advantage of immobilizing precise amounts of bioactive molecules and allowing control over their orientation on the substrate. The scientific community widely accepts polydopamine coatings for orthopedic implants. This is because they are non-toxic, biocompatible, mimic natural properties, easy to obtain, and can prevent adverse reactions like inflammation and platelet adhesion on metal surfaces.159 For example, Yu et al.160 utilized a novel polydopamine coating to attach collagen to the surface of a Ti implant covalently. The results showed that the collagen coating on the Ti surface, supported by polydopamine and substrate, showed more even distribution and coverage than collagen adsorbed using physical adsorption. The covalently immobilized collagen coating is biologically active. It enhances the adhesion of MC3T3-E1 cells, supports cell proliferation, and promotes the early-stage osteogenic differentiation of pre-osteoblasts. Still, one major drawback of covalent bonding is the significant loss of biomolecule activity that can occur after immobilization. For example, depending on the method, enzymes can lose up to 98% of their enzymatic activity after immobilization.161 Thus, assessing the functional activity of biomimetic covalent immobilizations is essential, mainly when using protein crosslinking methods. However, bioinspired coatings chemically bonded to metal surfaces could enhance the long-term therapeutic effects of metal implants in biomedical applications. Utilizing biological molecules to coat the implants enhances biocompatibility, tissue integration, and regeneration, thereby improving osseointegration and accelerated healing.45 However, DAC® is the only commercially available biological coating material made of biopolymers (hyaluronan) used to effectively prevent bacterial colonization over the implant surface to avoid biofilm formation. There is an increased risk of infection in vertebral surgery when more vertebral levels are involved in instrumented arthrodesis. The utilization of DAC® in vertebral surgery, along with the localized administration of antibiotics within the first 72 hours (the period during which the hydrogel gets absorbed), prevents bacterial attachment and the proliferation of harmful microorganisms, consequently averting the formation of biofilms.162 Ensuring sterility in the early stages of surgery is crucial. It helps in preventing early and late infections linked to implants or surgery, primarily within the first year after the procedure. This significantly improves bone integration. Every prosthetic implant placed and shielded with the DAC® antibiotic-loaded system displayed complete integration with the host bone, resulting in no implant movement. The shelf life of the coatings is approximately 6 months to 24 months. Despite its numerous advantages, applying biological coatings of biomolecules on implant surfaces presents several challenges. These challenges include ensuring stability, achieving uniformity, maintaining molecule adhesion, preserving bioactivity, sterilizing without causing damage, avoiding adverse immune responses, and effectively managing costs and scalability.151,163 Hence, it is of utmost essential to address these issues through dedicated research and the development of more efficient techniques for biomolecule application.
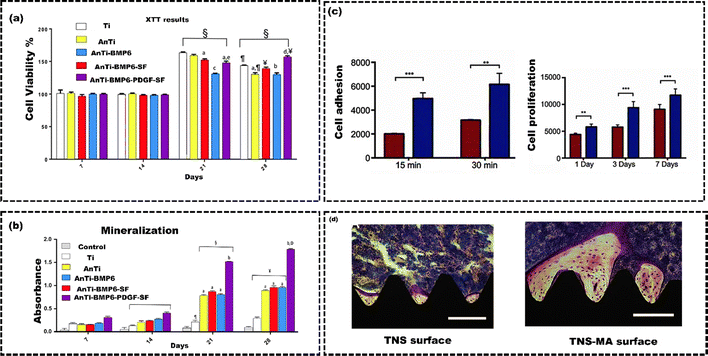 | ||
| Fig. 13 (a) MC3T3-E1 cell viability, (b) mineralization analysis of MC3T3-E1 cells on different surfaces (Ti, anodized Ti, BMP6-loaded Ti, silk fibroin electrospun BMP6-loaded anodized Ti, and PDGF-loaded silk fibroin electrospun BMP6-loaded anodized Ti), reproduced from Keleci et al.153 with permission from John Wiley & Sons, copyright (2020) (c) Cell adhesion and proliferation on TNS and TNS-MAP, (d) Villanueva staining depict the morphology of bone tissue surrounding the implant on the TNS and TNS-MAP surfaces, Reproduced from Yin et al.154 with permission from Taylor & Fransis, copyright (2019). | ||
4. Smart coatings
Surface functionalization has consistently aimed to enhance specific material responses when interacting with its surroundings. The idea of a functional coating has evolved extensively, and numerous surface functionalization methods are available for implants. However, the operational temperature fluctuation may cause thermal deterioration and chemical ageing of the existing functional coatings, which may cause the coatings to separate and shatter after some period of time.164 For example, most polymer-based coatings lose their toughness and chemical stability at high temperatures due to the cross-linking of polymers and changes in chemical composition, resulting in inadequate substrate adhesion.165 Similarly, after implant placement in vivo, there is a competition between bacterial colonization and tissue integration to gain control over the implant's surface. This competition is called the “race for the surface”. This bacterial colonization of the implant induces infection. This infection hampers the body's immune response and results in bone resorption, ultimately leading to the septic loosening of the implant. Hence, it is common to coat implants with bioactive substances or antibiotics to overcome implant failure. This approach aims to promote integration with bone tissue, prevent infection, and inhibit the formation of biofilms.166In vitro studies have shown promising results for several bioactive coating techniques. Nevertheless, their practical efficacy is impeded by antibiotic resistance stemming from the sustained release of antibiotics, even in the absence of an active infection.62 Hence, there is a critical need to employ smart coating techniques to overcome such drawbacks, such as bacterial resistance and rapid drug release observed in existing coating methods. By doing so, osteoconduction and osteoinduction can be enhanced for better outcomes.55The notion of a “smart” coating is relatively new. It pertains to functional coatings capable of reacting to specific stimuli from internal or external factors. In contrast to conventional existing coatings that display passive characteristics, smart coatings represent the next evolutionary advancement in creating orthopedic implant coatings with significantly improved capabilities. The smart coatings actively respond to intrinsic or extrinsic stimuli, resulting in significantly more advanced and dynamic functionality. These stimuli include changes in pH caused by UV exposure, aggressive ions, and various chemical reactions.167 For example, BMP-2 is frequently utilized to stimulate bone growth.168 Nevertheless, its efficacy is concentration-dependent. Lower concentrations facilitate bone remodelling, while higher concentrations may trigger bone resorption. It is essential to control and limit the release of BMP-2 from the implant to enhance the desired effect of promoting bone growth (osteoinduction).169 Existing coating methods have significant drawbacks when coating BMP onto an implant surface. These methods involve superficial adsorption of BMP using functional molecules, resulting in a quick release of BMP upon exposure to the external environment.170 This rapid release diminishes the osteogenic effect of BMP, leading to decreased effectiveness in promoting bone growth. The solution to this issue is harnessing the benefits of smart coating technology. Smart coatings allow for the active regulation of BMP-2 release, ensuring its effectiveness in promoting bone growth.171 Thus, smart coatings like self-healing and drug-eluting are the next-generation coating technologies that redefine the functional coatings for metallic implants.
4.1 Self-healing coatings
Coatings can prevent the unwanted corrosion of metal implants. However, it is challenging to avoid surface scratches on the coatings of implants during surgical procedures.172 When there is partial damage to the coating, it creates openings for electrolytes to enter. As a result, significant local corrosion may occur, potentially leading to an early coating failure and a shortened lifespan for an implant.173 Thus, repairing microcracks is crucial to prevent potential issues and extend the implant's life. Self-healing coatings protect the implant surfaces from damage caused by harsh environments by automatically triggering the repair mechanisms against corrosion. Self-healing coatings can repair microcracks using extrinsic or intrinsic self-healing mechanisms.174 Intrinsic self-healing coating materials possess the inherent capability to repair micro-scale damage through the reversible bonding of the polymer matrix.175 The damaged surface's reversible bonding and intrinsic self-healing ability are primarily due to physical and chemical interactions at the damaged site. When polymeric coating materials are heated, the polymer chains move around and create new connections, closing the crack, known as physical interaction (Fig. 14(a)). The chemical interactions can be covalent (Diels–Alder and retro Diels–Alder processes), supramolecular (host–guest interactions and supramolecular interactions) or non-covalent (ionic, hydrogen bonds, π–π interactions) (Fig. 14(b)). Chen et al.176 fabricated propylene polycarbonate (PPC)/polyurethane (urea) (PU) elastomer that utilizes its intrinsic self-healing ability through non-covalent interactions. The PPC-PU elastomer showed that the flexible structure of the PPC main chain made the PU macromolecular chain less rigid, allowing it to move more freely. This motion led to the formation of multiple flexible hydrogen bonds. It intensified the connections between the molecular chains through van der Waals forces. Consequently, there was a constant and dynamic exchange of hydrogen bonding pairs. These interacting hydrogen bonds and van der Waals forces created multiple networks of dynamic cross-linking based on non-covalent bonds. This enabled the self-healing ability of the elastomer.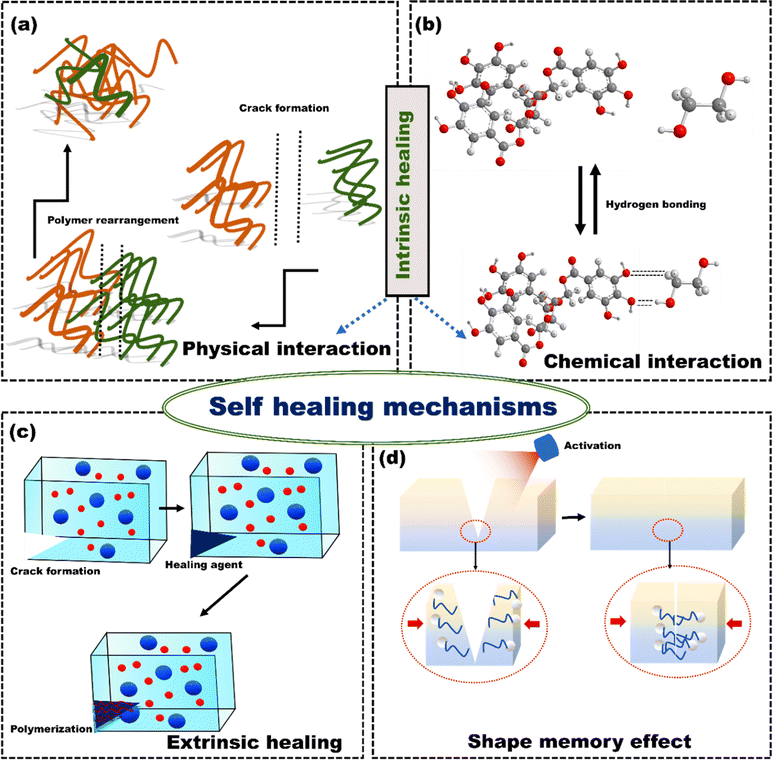 | ||
| Fig. 14 (a) Schematic of physical self-healing process, (b) self-healing via hydrogen bond formation in a functional unit of tannic acid and polyethylene glycol, (c) microcapsules based self-healing process, and (d) schematic illustration of shape memory assisted self-healing process, reproduced from Awaja et al.175 with permission from Elsevier, copyright (2016). | ||
The extrinsic self-healing mechanism differs entirely from intrinsic self-healing processes.177 The coating material does not possess inherent reversible bonding or healing properties. A separate healing agent is incorporated into the coating during manufacturing to enable healing. These corrosion inhibitors (healing agents) are stored in containers such as capsules, vascular networks, or nanoparticles.178 When cracks appear in the coating, the capsules break, and the corrosion inhibitor is released into the cracks, filling them up due to a capillary effect (Fig. 14(c)). As a result, the damaged surface is repaired, and its integrity is restored through a curing process. Shape memory materials can be used as self-healing coatings because they regain their original shape from substantial and permanent deformations when exposed to specific stimuli such as temperature and electrical or magnetic fields (Fig. 14(d)). Ma et al.179 developed a nanocomposite coating that offers two-fold self-healing for corrosion protection. The core of the nanoparticles was made of titanium nitride (TiN), while the shell was composed of mesoporous silica (SiO2) containing benzotriazoles (BTA) as a corrosion inhibitor (TiN-BTA SiO2 NP). These NPs were embedded in epoxy (shape memory polymer) coatings to impart self-healing characteristics against corrosion. When these coatings were subjected to near-infrared (NIR) light, the nanoparticle played a dual role in self-healing. The corrosion inhibitors (BTA) were released from TiN-BTA SiO2 NPs when the NIR light heated the TiN core. The heat generated by the TiN cores activated the epoxy coating's shape memory effect, which could repair the scratch. Both electrochemical impedance spectroscopy and scanning electrochemical microscopy (SECM) measurements showed that the combination of inhibitor adsorption and scratch closure effectively prevented the corrosion.
Plasma electrolytic oxidation (PEO) uses high-voltage anodization to produce a dual-layer oxide structure, with a dense layer at the base and a porous layer on top.180 This leads to biocompatible coatings featuring controlled corrosion, superior wear resistance, and strong adhesion. However, these coatings exhibit limited bioactivity and are susceptible to pitting corrosion.181
Polydopamine (PDA), derived from dopamine, presents strong reactivity. It is an ideal candidate for bioactive coatings and scaffolds that encourage apatite formation resembling bone.182 Therefore, Farshid et al.183 applied a duplex PEO/PDA coating on AZ91 alloy to enhance its self-healing properties, corrosion resistance, and bioactivity for bone implant applications. The potentiodynamic polarization tests conducted in a phosphate buffer solution revealed that the PDA/PEO coating demonstrated enhanced corrosion resistance (36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 318.267 ± 151 kΩ) compared to both the PEO coating (1116.537 ± 120 kΩ) and the bare AZ91 substrate (3.016 ± 1 kΩ), attributed to its ability to fill porosities and reduce the infiltration of corrosive ions. The self-healing potential of the coatings was analyzed by generating surface scratches and then evaluating the corrosion response of the samples using electrochemical impedance spectroscopy tests. These evaluations were carried out at specific time intervals of 15 minutes, 30 minutes, 60 minutes, and 90 minutes following immersion of the scratched samples in a phosphate buffer solution. The Nyquist curves from electrochemical impedance spectroscopy test results for the PEO coating indicated reduced capacitive loop diameters with extended immersion time. In contrast, the duplex PDA/PEO coatings exhibited an enlargement in capacitive loop diameters, signifying varying degrees of self-healing capability. Additionally, using culturing of MG63 cell lines, PDA/PEO samples demonstrated significantly higher cell viability (64.9%) compared to bare AZ91 (35.1%) and PEO-coated samples (52.3%). Several investigations have been conducted to enhance the healing capabilities of self-healing coatings for metallic implants. However, the healing performance depends on the surrounding environment and the size of the damage.184 In academia, there remains a need for long-term studies examining the anticorrosion performance and fatigue resistance of both extrinsic and intrinsic self-healing materials. The evaluation methods for gauging research outcomes should better align with the extended timeframes needed to assess long-term anticorrosion performance. Some research has examined how extrinsic coatings perform over a more extended period. However, intrinsic coatings seem especially promising because they can heal repeatedly. Therefore, enhancing active materials and coating designs is crucial for further improving self-healing coatings.
318.267 ± 151 kΩ) compared to both the PEO coating (1116.537 ± 120 kΩ) and the bare AZ91 substrate (3.016 ± 1 kΩ), attributed to its ability to fill porosities and reduce the infiltration of corrosive ions. The self-healing potential of the coatings was analyzed by generating surface scratches and then evaluating the corrosion response of the samples using electrochemical impedance spectroscopy tests. These evaluations were carried out at specific time intervals of 15 minutes, 30 minutes, 60 minutes, and 90 minutes following immersion of the scratched samples in a phosphate buffer solution. The Nyquist curves from electrochemical impedance spectroscopy test results for the PEO coating indicated reduced capacitive loop diameters with extended immersion time. In contrast, the duplex PDA/PEO coatings exhibited an enlargement in capacitive loop diameters, signifying varying degrees of self-healing capability. Additionally, using culturing of MG63 cell lines, PDA/PEO samples demonstrated significantly higher cell viability (64.9%) compared to bare AZ91 (35.1%) and PEO-coated samples (52.3%). Several investigations have been conducted to enhance the healing capabilities of self-healing coatings for metallic implants. However, the healing performance depends on the surrounding environment and the size of the damage.184 In academia, there remains a need for long-term studies examining the anticorrosion performance and fatigue resistance of both extrinsic and intrinsic self-healing materials. The evaluation methods for gauging research outcomes should better align with the extended timeframes needed to assess long-term anticorrosion performance. Some research has examined how extrinsic coatings perform over a more extended period. However, intrinsic coatings seem especially promising because they can heal repeatedly. Therefore, enhancing active materials and coating designs is crucial for further improving self-healing coatings.
4.2 Drug-eluting coatings
Infection remains a prominent and formidable complication in orthopedic surgery.185 These infections cause implant failure, necessitating revision surgeries and prolonged hospitalization, which significantly amplifies the financial burden and escalates the mortality risk.186 The drug delivery system ensures that medications reach the intended location. The administered dose should release the drug gradually over a defined timeframe, ensuring it achieves the maximum positive impact while causing minimal harm to nearby or distant healthy tissue.187 Conventional systemic drug delivery through intermittent oral or intravenous methods leads to rapid elevation of drug levels in the bloodstream shortly after the dose is administered.188 When the concentration of the administered drug in the bloodstream is too high, it may lead to unacceptable toxic side effects in patients. Conversely, if the drug falls below the therapeutic level, it becomes ineffective.189 The distribution of therapeutic drugs throughout the body can reduce effectiveness because the liver metabolizes them, known as first-pass metabolism. Furthermore, these drugs may attach to plasma proteins, which hinders their therapeutic impact. The conventional method of drug delivery can also be inefficient because drugs must act within a specific therapeutic window on the target, and systemic circulation can slow down this critical process. Therefore, local drug therapy has become widely accepted for infections in implantation sites.190 This approach delivers a concentrated drug dose to a specific area without harming the rest of the body, effectively targeting the implantation site. Drug-eluting coatings are the most promising method for localized drug therapy at the implantation site.191The development of the first subcutaneous drug-eluting implant in the 1930s, led by R. Deanesly and A. S. Parkes for sustaining therapeutic hormone levels like testosterone propionate and oestrone, marked the beginning of extensive research in implantable drug delivery.192 Furthermore, this approach has been extended to integrate multifaceted features into metal implants. These features are essential for combating infections related to implants and improving osseointegration, particularly in orthopedic and dental applications.193 To build on this legacy, Moes et al.194 developed a mesoporous nanocomposite using bioactive glass containing 70% of SiO2(70S) and silk fibroin to mimic the natural organo-apatite structure of bones (Fig. 15(a)). These nanocomposites could effectively load and deliver antibiotics like gentamicin and doxycycline. The mesoporous nanocomposites were modified to specifically interact with the silanol network of the bioactive glass by tailoring 3-(aminopropyl)-triethoxysilane. This modification improved the nanocomposite's capacity to load and retain a hydrophobic drug (dexamethasone). These drugs loaded mesoporous nanocomposites were assembled into conformal coatings on SS substrates for their versatility as antibacterial, osteoinductive, and immunomodulatory surfaces. The study confirmed that the coatings with antibiotics showed antibacterial effects against S. aureus and E. coli and remained biologically active for 28 days. The coatings loaded with dexamethasone were beneficial for attaching MSCs and promoting bone formation. Moreover, these coatings were found to have an immunomodulatory effect, encouraging the activation of M2 macrophages for bone healing. Rahmati et al.195 utilized doxycycline coating over titanium–zirconium (TiZr) implants to facilitate the controlled release of doxycycline and improve osseointegration of TiZr implants. Coin-shaped TiZr substrates, both with and without doxycycline, were implanted into the tibia of eight rabbits for the evaluation of its osseointegration performance. Furthermore, bone ingrowth and attachment were examined by inserting TiZr dental implants into the jawbone of six dogs (Fig. 15(b)). The in vitro results confirmed that doxycycline was physically present on the surface of the TiZr implant and released in a controlled manner. Both in vivo studies, conducted over different periods, found no significant differences between the groups with and without the doxycycline coating. This suggests that coating the TiZr implant with doxycycline did not adversely affect the implant's integration with the surrounding bone or the maintenance of the required doxycycline level. However, in an acidic pH of 3, more doxycycline was released, likely due to the faster breakdown of the coating. Therefore, Soares et al.196 developed and evaluated a dual polymeric film coating designed for controlled localized antibiotic delivery to combat implant-related infections on SS implants. This coating consisted of layers of chitosan and polycaprolactone (PCL). The coating was impregnated with polymethyl methacrylate (PMMA) microspheres carrying either vancomycin or daptomycin (antibiotics). This was then compared to a coating containing microspheres without antibiotics (acting as a negative control) and coatings with antibiotics present in their free, unencapsulated form, which served as the positive control. The agar diffusion test evaluated the antimicrobial activity of the samples against S. aureus ATCC 25923 and clinically isolated Methicillin-resistant Staphylococcus aureus (MRSA). For the S. aureus ATCC 25923 strains that were susceptible to daptomycin, the inhibition zone diameters were 13.9 ± 1.3 mm compared to 20.6 ± 0.2 mm (positive control). In contrast to the positive control group, with a mean inhibition zone diameter of 21.7 ± 0.4 mm, daptomycin-resistant MRSA strains displayed smaller zones of approximately 16.6 ± 2.2 mm. The inclusion of PMMA microspheres in the coatings demonstrated a controlled daptomycin release, exhibiting an ideal pattern of initial rapid release followed by a prolonged, sustained release compared to the free drug within the coatings. The findings indicated that coatings containing daptomycin suppressed bacterial growth. However, there was no observable inhibition zone for the sample lacking antibiotics (negative control). The coating loaded with vancomycin exhibited no antibacterial activity. This could be due to either a limited diffusion of the drug in the agar medium or the diffusion levels falling below the reported minimum inhibitory concentration (MIC) of vancomycin for S. aureus. Specifically, the MIC for the ATCC 25923 strain is 2 μg mL−1, while it varies from 4 to 8 μg mL−1 for MRSA. Daptomycin-loaded films effectively combat susceptible S. aureus strains, highlighting their promise as drug-eluting coatings for implant-related infection prevention.
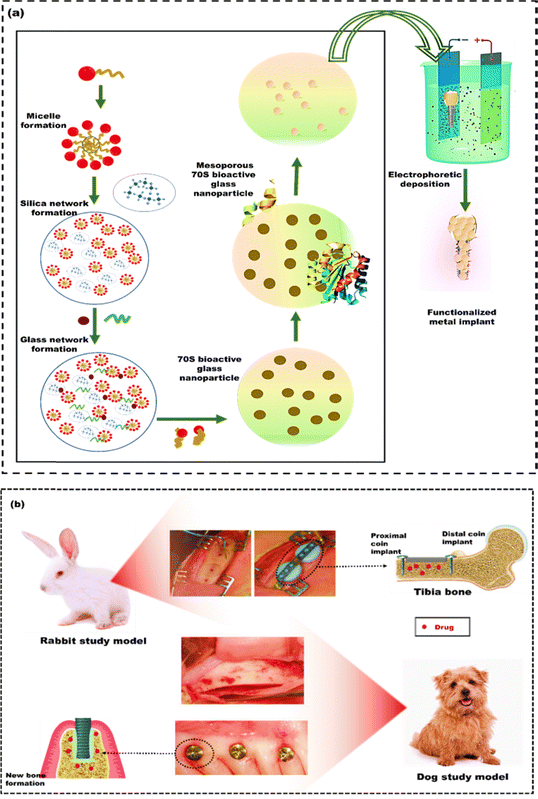 | ||
| Fig. 15 (a) Synthesis of silk bioactive glass nanoparticle for coating the SS implant using electrophoretic deposition, reproduced from Moes et al.194 with permission from American Chemistry Society, copyright (2022) (b) Preclinical images of rabbit and dog implantation procedures, reproduced from Rahmati et al.195 with permission from Elsevier, copyright (2020). | ||
While numerous studies have explored the use of drug-eluting coatings for metallic implants to treat implant-related infections, most of them have conducted in vitro analysis, with only a few reports on its application in animal models.190 Hence, the pharmaceutical field is constantly evolving to meet the challenges of implants and their physiological environments. This progress stems from innovations in pharmacology and pharmacokinetics. Concurrently, with the development of metallic materials, there have been significant improvements in implant processing technology.197 This has led to the creation of devices that align with human biomechanics and release drugs for extended periods. For example, devices like XIENCE Sierra™ Stentcan efficiently store and release drugs at a controlled pace, resulting in extended drug delivery periods, sometimes several months, while enhancing overall stability.198 However, despite these advancements in localized drug delivery approaches, it's worth noting that burst release still remains the predominant challenge in this field.199 This issue continues to impede the long-term sustained release of drugs from implanted devices.200 Additional efforts are required to optimize the drug release rates in coatings on metallic implants for clinical trials.
Recent surveys conducted in the US, Europe, and Japan reveal that only a small number of drug-eluting coated implant products, such as Expert Tibial Nail PROTect™, have successfully made their way into the market.201 A key reason drug-eluting coatings have not been widely adopted in clinical practice despite significant research is the gap in communication and collaboration between researchers and regulatory agencies.202 Developing antimicrobial orthopedic medical devices is a multifaceted endeavor that involves overcoming regulatory challenges to secure approval from the U.S. FDA.202 The FDA categorizes these devices according to their primary mode of action (PMOA), influencing whether they are evaluated solely by the Center for Devices and Radiological Health (CDRH) or as combination products requiring input from multiple regulatory centers.203 Devices with antimicrobial properties derived from physical modifications, like engineered surfaces or magnetic fields, might be classified as standard devices. Conversely, those utilizing chemical interactions, such as antibiotics, are treated as combination products, complicating the approval process.204 The FDA has established three classes for medical devices: class I, II, and III. Class I devices are deemed low-risk and typically do not require premarket approval. Class II devices generally undergo the 510(k)-submission process, where they must demonstrate substantial equivalence to existing products. Class III devices, considered a higher risk, require a more stringent Premarket Approval (PMA) process, often involving extensive clinical data.205 The recent implementation of the European Medical Device Regulation (MDR) has further complicated the landscape, requiring consultation with medicinal authorities for devices that release substances absorbed into the body.206 This has heightened regulatory demands, making it increasingly more complex for manufacturers to introduce new antimicrobial implants. Regulatory bodies like the FDA and their European counterparts face significant challenges in setting clear criteria for demonstrating safety and efficacy. Manufacturers often need help to conduct clinical trials that satisfy these criteria, mainly when device success depends on a small number of patients with severe infections. Additionally, the effectiveness criteria for antimicrobial devices can be ambiguous, often focusing on laboratory results that may not align with real-world outcomes. This uncertainty can discourage investment in new technologies, as companies may need clarification on the financial viability of their products.207
Despite these obstacles, some antimicrobial orthopedic devices have successfully entered the market, though often at considerable cost due to stringent regulatory requirements. For example, few devices, such as gentamicin-releasing coatings, have received clearance.208 In contrast, many others need significant approval barriers. Overall, the complex regulatory environment creates significant obstacles to developing and commercializing antimicrobial orthopedic devices, slowing innovation and limiting access to life-saving healthcare technologies. Further research is needed to understand how drugs bind to coated and uncoated metallic implant surfaces. This will help achieve more precise therapeutic effects with consistent release patterns. Additionally, better communication and collaboration between researchers and regulatory agencies is necessary to address current gaps.209
5. Immuno modulation in functional coatings
One of the major caveats of orthopedic implantation is the inflammatory response caused by the interaction of the foreign object with human immune cells, hindering their integration with surrounding tissues, thus gradually leading to implant failure.210 To address this issue, immunomodulation strategies have been developed to modify the immune microenvironment, thereby improving the biocompatibility and functionality of the implants. These strategies use physical, chemical, or biological cues on an implant surface or within the implanted scaffold to influence the behavior of immune cells and their interactions with other cells involved in bone regeneration, such as stem cells and osteoblasts.211,212 By leveraging immunomodulation, orthopedic implants can enhance tissue repair and regeneration while reducing the risk of infection and inflammation. Immunomodulating functional coatings have emerged as a promising approach for strengthening the integration of orthopedic implants with host tissues. By modulating the immune response to the implant, these coatings can promote tissue regeneration and reduce the risk of implant rejection or infection.211 These coatings have shown great potential in preclinical studies, and continued research holds promise for improving the long-term outcomes of orthopedic implant procedures. Osteoimmunology is a necessary science that needs to be understood before beginning with implantation. It elucidates how metallic implants affect bone remodelling, which involves a balance between osteoblasts (bone-forming cells) and osteoclasts (bone-resorbing cells). Osteoblasts and osteoclasts are regulated by various cytokines and growth factors produced by immune cells, such as macrophages, T cells, B cells, and dendritic cells.213 These immune cells can recognize metallic implants as foreign bodies and initiate an inflammatory response that promotes or inhibits bone healing.214 For example, some pro-inflammatory cytokines, such as TNF-alpha and IL-1 beta, can stimulate osteoclast activity and bone resorption. In contrast, anti-inflammatory cytokines, such as IL-4 and IL-10, can inhibit osteoclast activity and enhance bone formation. Moreover, some immune cells can directly interact with osteoblasts and osteoclasts through cell surface receptors, modulating their differentiation and function.215Understanding the interaction of immune cells with metallic implants helps to develop new strategies to improve the biocompatibility and bioactivity of metallic implants by modifying their surface properties. To influence their immunomodulatory effects, surface modification techniques using functional coatings can alter metallic implant's chemical composition, topography, wettability, charge, and bioactivity.216 For instance, coating metallic implants with CaP or other bioactive materials can enhance their osteogenic properties and facilitate bone apposition.217 Similarly, creating porous or rough surfaces on metallic implants can increase their surface area and promote cell adhesion and tissue ingrowth. Furthermore, incorporating bioactive molecules, such as growth factors or anti-inflammatory agents, into metallic implants can modulate the immune response and favour bone healing.45,159
Immunological tools can be utilized to manipulate diverse biological pathways to attain a desirable cell–material interaction. Specifically, the four fundamental mechanisms that can be targeted for immune modulation include inflammation, tissue fibrosis, angiogenesis, and osteogenesis.218–220 Alkhoury et al.221 used surface coatings made of HAp or heparin (Hep) combined with chitosan prepared as multilayers through the layer-by-layer (LbL) technique. The finding suggested that multilayers with HAp or Hep can reduce inflammation by limiting the adhesion of macrophages, multinucleated giant cells (MNGC) formation, and IL-1β release. This study elucidated the potential of the anti-inflammatory activity of glycosaminoglycans. It showed how the NF-κB signaling pathway influences inflammation. Barone et al.222 utilized a small molecule MCC950 as a potent and selective inhibitor of the NLRP3 inflammasome, incorporated into the silicone coating of implantable medical devices, to prevent FBR in mice. Unlike dexamethasone treatment, which blocks the FBR and hinders axon regeneration, MCC950 did not affect tissue regeneration. This study suggested that the local inhibition of NLRP3 is a promising approach for preventing FBR and promoting long-term usage of implantable electronic medical devices, particularly in neural applications. Friedrich et al.223 used HAp-coated superparamagnetic iron oxide nanoparticles (SPION HAp) to investigate the immunogenicity of these particles under in vitro conditions. The HAp-coated nanoparticles were synthesized using a co-precipitation method. Their impact on the pro-and anti-inflammatory cytokines was assessed. The study revealed that SPION HAp could modulate the inflammatory property based on its interaction. Elevated levels of cytokines can be advantageous for stimulating the immune system in specific therapeutic applications like cancer treatment.
Conversely, controlling the release of cytokines can be more advantageous in preventing the aggravation of inflammatory conditions. This knowledge provides critical information on the inflammatory processes that follow in vivo applications of SPION.223 Li et al.224 immobilized the anti-inflammatory cytokine interleukin 4 (IL-4) on a Ti substrate using a Graphene oxide (GO) coating (Fig. 16(a)). This study aimed to regulate the macrophage-related inflammatory response and improve implant performance. The GO/IL-4 coating showed good biocompatibility and promoted macrophage polarization to the M2 phenotype in vitro. Conditioned media from macrophages grown with coating promoted proliferation, migration, and osteogenic differentiation of bone stem cells. In vivo experiments also indicated that GO/IL-4 coatings enhanced the regulation of the inflammatory response at the implant–bone interface. It effectively facilitated the transition from the inflammatory phase to the reparative phase by promoting the presence of M2 macrophages. Implants coated with GO/IL-4 demonstrated superior contact and integration with the bone. These findings suggested that the macrophage-related inflammatory response is critical for implant osseointegration. The GO/IL-4 coating could be a promising approach for promoting implant osseointegration by regulating immune function.224 Macrophage polarization is essential during implantation because it affects the host's response to biomaterials, tissue repair, and remodeling.225 Macrophages are immune cells that can adopt different phenotypes depending on the local microenvironment. M1 macrophages are pro-inflammatory and can cause tissue damage and FBR. In contrast, M2 macrophages are anti-inflammatory and can promote tissue regeneration and angiogenesis.225,226 Macrophage polarization is critical in metallic implantation, as it can influence the immune response and the integration of the implant with the surrounding bone tissue. Some of the factors related to implants that affect this polarization are the physical and chemical properties of the biomaterials, such as surface topography, composition, degradation rate, and stiffness.227–231 These factors can modulate the interactions between macrophages and biomaterials and the secretion of cytokines and growth factors that regulate macrophage polarization. Therefore, designing biomaterials that induce a favourable macrophage response is a promising strategy to enhance implant-to-bone osteointegration and tissue engineering outcomes.
 | ||
| Fig. 16 (a) Immunomodulation induced by a functional coating of IL-4 with Graphene Oxide leads to M2 polarization and subsequent BMSC differentiation, reproduced from Li et al.224 with the permission from American Chemistry Society, copyright (2020). (b) Laser surface texturing of metal substrate, Reproduced from Sirdeshmukh et al.232 with permission Elsevier, copyright (2021). | ||
Surface modification using laser texturing has garnered a lot of attention recently as a promising surface engineering technique to modulate cell–material interaction.233 Laser texturing can create specific micro- and nano-scale patterns on implant surfaces. These patterns can influence the behaviour of cells and proteins that interact with the implant. By altering the surface topography, it's possible to affect how immune cells, like macrophages, respond to the implant (Fig. 16(b)). This response can be tuned to promote a more favourable immune reaction, reducing inflammation and enhancing healing.232 Another highlight is that the textured implant surface can influence the adsorption of proteins from body fluids. This protein layer can mediate the interaction between the implant and cells, including immune cells.234 The right texture can promote the adsorption of proteins that favour a more regenerative, rather than inflammatory, immune response. In a study demonstrated by Yang Liu et al.235 in 2021, a femtosecond laser, in combination with sandblasting, was used to modify the surface of a Ti alloy. While the sandblasting created beneficial roughness with an average roughness of 3.77 ± 0.29 μm, the femtosecond laser texturing created a mastoid columnar structure with irregular holes, which altered properties like morphology and wettability. The modified Ti surfaces promoted anti-inflammatory factors and decreased the production of pro-inflammatory factors through the modulation of macrophage polarization caused by the engineered surface. The study used interleukin-1 beta (IL-1β) and interleukin-6 (IL-6) as key pro-inflammatory factors, while IL-4 and IL-10 were identified as anti-inflammatory factors. The study revealed that the sandblasted surface with femtolaser texturing enhanced early osseointegration and improved anti-inflammatory properties. Using laser texturing on metal surfaces can enhance the performance of implants. This process enhances surface characteristics critical for improved cell responses and osseointegration, thereby reducing the need for subsequent revision procedures.
6. Biomimetic interfaces and nanosurface engineering for reducing bacterial resistance
Antibiotic-resistant bacterial infections are estimated to cause 700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 deaths per year.236 This number could grow to 10 million by 2050 if drug-resistant bacteria keep evolving at the same rate.237 A biofilm forms when bacteria attach to a surface, multiply, and establish a colony. When bacterial cells come into contact with the surface of the material, their appendages enhance the attachment between the bacteria and the material.238 It is essential to prevent biofilm formation, which can cause infections and lead to the failure of medical implants, to ensure the proper functioning of the implant.239,240 At the initial stage, bacterial attachment is considered to be reversible. Therefore, we can inhibit biofilm formation at this point. However, after this stage, the bacteria undergo growth and multiplication, forming extracellular polymeric substances (EPS).241 The EPS functions as a protective barrier for the bacteria, enhancing their resistance to external environmental influences. Hence, the attachment of bacterial cells is irreversible at this stage. Therefore, it is possible to prevent bacteria from adhering initially by introducing micro- or nano-structured topography on the surface.242 This strategy has gained substantial interest in the past decade. Materials with distinctive micro- and nano-scale surface textures and features have proven effective in preventing bacterial attachment and biofilm formation.243 Micro- and nano-scale surface textures in implants can significantly alter bacterial responses by affecting cell morphology, membrane elasticity, and the expression of genes linked to bacterial adhesion.244 It was proposed that when the dimensions of the topographical features are smaller than the bacterial cells, the available contact area for bacterial attachment is minimized. Therefore, when topographical features are at sub-micron and nanometer scales, the surface prevents bacterial attachment. Therefore, by controlling the surface topography of metallic implants, bacterial attachment can be prevented without using traditional antimicrobial agents.245 Nanostructures can influence bacterial behavior by regulating the expression of structural genes associated with adhesion. For instance, exposure to nanostructures can inhibit the expression of fimbriae that facilitate bacterial adhesion to surfaces.246 Fimbriae are the long hairlike structures found on the surface of bacteria. Rizello et al.247 demonstrated that nanostructured substrates cause significant changes in the morphological, genetic, and proteomic responses of adherent E. coli bacteria. Atomic force microscopy and electron microscopy results showed that type 1 fimbriae in E. coli decrease when interacting with nanostructures, and real-time polymerase chain reaction (PCR) analyses confirmed a reduction in the expression of genes related to fimbriae in their presence. Additionally, nanostructures can alter protein secretion pathways and the composition of bacterial cell wall proteins. The size and composition of coating materials also significantly impact bacterial behavior. For instance, a 20 μm pore size coating enhances osteogenic cell proliferation and adhesion. In contrast, a 100 nm thick poly(ε-caprolactone)-gelatin-forsterite nanocomposite coating on titanium improves MG63 cell proliferation and viability. Surfaces with a roughness of 1.7 nm promote higher cell proliferation than smoother surfaces with a roughness of 0.6 nm.248
000 deaths per year.236 This number could grow to 10 million by 2050 if drug-resistant bacteria keep evolving at the same rate.237 A biofilm forms when bacteria attach to a surface, multiply, and establish a colony. When bacterial cells come into contact with the surface of the material, their appendages enhance the attachment between the bacteria and the material.238 It is essential to prevent biofilm formation, which can cause infections and lead to the failure of medical implants, to ensure the proper functioning of the implant.239,240 At the initial stage, bacterial attachment is considered to be reversible. Therefore, we can inhibit biofilm formation at this point. However, after this stage, the bacteria undergo growth and multiplication, forming extracellular polymeric substances (EPS).241 The EPS functions as a protective barrier for the bacteria, enhancing their resistance to external environmental influences. Hence, the attachment of bacterial cells is irreversible at this stage. Therefore, it is possible to prevent bacteria from adhering initially by introducing micro- or nano-structured topography on the surface.242 This strategy has gained substantial interest in the past decade. Materials with distinctive micro- and nano-scale surface textures and features have proven effective in preventing bacterial attachment and biofilm formation.243 Micro- and nano-scale surface textures in implants can significantly alter bacterial responses by affecting cell morphology, membrane elasticity, and the expression of genes linked to bacterial adhesion.244 It was proposed that when the dimensions of the topographical features are smaller than the bacterial cells, the available contact area for bacterial attachment is minimized. Therefore, when topographical features are at sub-micron and nanometer scales, the surface prevents bacterial attachment. Therefore, by controlling the surface topography of metallic implants, bacterial attachment can be prevented without using traditional antimicrobial agents.245 Nanostructures can influence bacterial behavior by regulating the expression of structural genes associated with adhesion. For instance, exposure to nanostructures can inhibit the expression of fimbriae that facilitate bacterial adhesion to surfaces.246 Fimbriae are the long hairlike structures found on the surface of bacteria. Rizello et al.247 demonstrated that nanostructured substrates cause significant changes in the morphological, genetic, and proteomic responses of adherent E. coli bacteria. Atomic force microscopy and electron microscopy results showed that type 1 fimbriae in E. coli decrease when interacting with nanostructures, and real-time polymerase chain reaction (PCR) analyses confirmed a reduction in the expression of genes related to fimbriae in their presence. Additionally, nanostructures can alter protein secretion pathways and the composition of bacterial cell wall proteins. The size and composition of coating materials also significantly impact bacterial behavior. For instance, a 20 μm pore size coating enhances osteogenic cell proliferation and adhesion. In contrast, a 100 nm thick poly(ε-caprolactone)-gelatin-forsterite nanocomposite coating on titanium improves MG63 cell proliferation and viability. Surfaces with a roughness of 1.7 nm promote higher cell proliferation than smoother surfaces with a roughness of 0.6 nm.248
Materials with similar antibacterial properties have been developed by drawing inspiration from natural surfaces. Many natural materials resist fouling and bacteria because of their unique surface features.249 The nanopillars and nano spikes on these natural materials physically damage bacterial cells that come into contact with them, leading to cell rupture and death.250 This has led to the development of analogous materials and structures designed to prevent biofilm formation and actively eliminate bacteria upon contact. These bio-inspired materials often replicate the surface topography found in natural materials like lotus leaves, wings of cicadas, dragonflies, etc.251 For instance, lotus leaves have surfaces covered with micropapillae that range from 3 to 11 μm in diameter. Each micro papillae is covered with nanotubules that are 100 nm in diameter. This hierarchical structure is crucial for giving lotus leaves their self-cleaning and antifouling properties.252 Various methods have been developed to create such types of nanostructures on orthopedic implants, including electron beam lithography,253 colloidal lithography,254 nano-imprinting,255 and soft lithography,256 each with strengths and limitations regarding precision, cost, and potential pattern defects. Jiang et al.252 created hierarchical structures inspired by lotus leaves using UV-lithography and showed that these bio-inspired designs exhibit antifouling properties similar to those of the natural material. The hierarchical structures made bacterial cells burst when they came into contact with the surface. Similarly, numerous other studies have demonstrated the fabrication of sharp nanoneedles and nanopillars that mimic the structures on dragonfly and cicada wings, effectively killing bacteria through mechanobactericidal mechanisms.257 Brennan et al.258 used a micro-embossing technique to replicate the structures found on shark skin. The rectangular micrometer ribs created on synthetic material effectively disrupt S. aureus colonization. The results showed that the rectangular micrometer ribs provided the surface with antifouling properties. This indicates that synthetic materials with similar properties can be created by mimicking the molecular structures of natural materials. While biomimetic designs represent an innovative approach, the exact antibacterial mechanisms of surface topographies are still under investigation. Different bacteria respond uniquely to nanostructures; for example, Gram-negative bacteria may be distorted and killed by specific nanostructures and Gram-positive bacteria may react differently due to variations in their cell wall structures.259 The optimal adjustment of physical parameters such as aspect ratio, diameter, spacing, and stiffness for these nanostructures remains to be determined.260 This adjustment is needed to ensure that bio-inspired surfaces can effectively eliminate Gram-negative and Gram-positive bacteria and prevent biofilm formation. Hence, gaining a thorough understanding of design optimization will help address the current challenges with mechanobactericidal surfaces and facilitate their adoption in clinical settings.
7. Conclusion and future perspective
Multifunctional coatings have attracted worldwide attention in orthopedic implantation because of their capabilities to enhance the cell–material interaction and corrosion resistance properties of metallic implants. This article thoroughly reviewed up-to-date progress on different surface modification techniques for implants made from various biometals, highlighting biocompatibility and implant life. Despite their benefits, metallic implants with current passive coatings have several limitations. These include aseptic loosening, poor cell–material interactions, and bacterial colonization, which can lead to post-operative complications. Additionally, their limited ability to adapt to environmental changes or external stimuli restricts their effectiveness in specific applications. To improve the performance and reliability of metallic implants, the review emphasizes the development of smart coatings. These coatings enable the controlled release of therapeutic agents, such as drugs or corrosion inhibitors, from nanocontainers or capsules in response to various triggers. They also provide the ability to repair localized damage autonomously, ensuring effective prevention. Additionally, it outlines the importance of functional coatings in immunomodulation, which is useful in creating a favorable immune environment, as failure of implants inserted in osteomyelitis patients and cancer patients is the greatest challenge owing to their poor immune responses. Immunomodulation involves regulating the behavior of immune cells such as neutrophils, macrophages, and T cells, promoting their differentiation to an anti-inflammatory phenotype, and facilitating the regeneration process. Overall, these smart multifunctional coatings discussed in this review mitigate the issues of traditional passive coatings of metallic implants. However, a significant issue with smart self-healing coatings is the potential increase in free volume within self-healing coatings. When embedded capsules release corrosion inhibitors, the empty spaces create voids that could compromise the integrity of coatings. This increased free volume may allow corrosive substances to penetrate, potentially leading to coating failure. Also, these coatings are only effective for minor damage, and their healing capability in a specific area diminishes after a single repair cycle. Similarly, drug-eluting coatings show promise in combating infections. However, it elevates the chances of antimicrobial resistance (AMR). AMR is a significant concern for the scientific community, as the potential failure of implants caused by bacterial colonization presents a major challenge for the implant industry.Therefore, optimizing drug release kinetics and incorporating alternative antimicrobial strategies to lessen dependence on conventional antibiotics is essential. While surface texturing for creating biomimetic interfaces on metallic implants has been discussed, there remains significant scope for further exploration. In addition, machine learning (ML) and data science can be used in the future to address the challenges arising from the multi-parametric optimization of surface features on implants. ML, a branch of artificial intelligence, has gained popularity for resolving challenging modeling and optimization issues across a wide range of engineering specialties.261 Emerging ML techniques have the power to improve and speed up the creation of novel coatings, potentially transforming this sector. ML algorithms such as linear regression, Support Vector Regressor, Random Forest Regressor, and Extra Tree Regressor may be used to optimize a methodology for predicting coating properties (Fig. 17(a)).262 For instance, Hrishita S et al.261 utilized linear regression, decision tree, and random forest regressor on the EIS data obtained from the fouling microneedle surface to predict the degree of fouling on the microneedle. Also, strain-sensing systems can detect multiple implant strains (Fig. 17(b)).263 These systems continuously monitor the biomechanics of the implants to identify any potential implant failures in advance. Sensors-integrated coatings may pave a path to detect any inappropriate situations and prevent more failures. Additional clinical studies are needed to assess the effectiveness of multifunctional smart coatings on implants that monitor and respond to implant health through a closed-loop system (Fig. 17(c)). These studies should also explore the potential of these coatings to provide real-time feedback on the underlying metal. Further, incorporating regulatory considerations into research practices is essential for the successful clinical translation of these smart coatings and for ensuring the safety, effectiveness, and accessibility of metallic implants.
 | ||
| Fig. 17 (a) ML for prediction surface and biological properties settings, reproduced from Sharma et al.261 with permission from IEEE, copyright (2023). (b) Smart coating containing nanocontainers, reproduced from Wang et al.263 with permission from American Chemistry Society, copyright (2019), (c) strategy for the commercialization of coated implants: step (1) assessing the surface and biological characteristics of coatings, step (2) investigating various methods of functional coatings, step (3) enhancing the clinical effectiveness of coated implants by conducting thorough in vivo testing and comprehensive in vitro assessments, step (4) approval from different regulatory agencies, step (5) ready to use in clinical settings. Funding and Acknowledgements. | ||
Author contributions
All the authors listed have made a substantial, direct, and intellectual contribution to the work and approved for its publication.Data availability
The authors declare that no primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
The authors declare no conflict of interest in the publication of this article.Acknowledgements
This work was supported by a start-up research grant (SRG/2019/001504) from the Science and Engineering Research Board (SERB), Department of Science and Technology (DST), Government of India, the funding from Faculty Initiation Grant (FIG), PDPM-IIITDM Jabalpur, International Centre for Sustainable and Net Zero Technologies, PDPM-IIITDM Jabalpur and the Ministry of Education (MOE), Government of India.References
- D. Zhao, F. Witte, F. Lu, J. Wang, J. Li and L. Qin, Biomaterials, 2017, 112, 287–302 CrossRef CAS PubMed.
- Musculoskeletal health. https://www.who.int/news-room/fact-sheets/detail/musculoskeletal-conditions (accessed 2023-03-01)., 2022.
- Q. Chen and G. A. Thouas, Mater. Sci. Eng., R, 2015, 87, 1–57 CrossRef.
- C. Li, C. Guo, V. Fitzpatrick, A. Ibrahim, M. J. Zwierstra, P. Hanna, A. Lechtig, A. Nazarian, S. J. Lin and D. L. Kaplan, Nat. Rev. Mater., 2019, 5, 61–81 Search PubMed.
- H. Ahirwar, Y. Zhou, C. Mahapatra, S. Ramakrishna, P. Kumar and H. S. Nanda, Coatings, 2020, 10, 264 CrossRef.
- H. Ahirwar, V. K. Gupta and H. S. Nanda, Comput. Methods Biomech. Biomed. Eng., 2021, 24, 1742–1751 CrossRef PubMed.
- A. Nouri and C. Wen, Structural Biomaterials, Elsevier, 2021, pp. 127–156 Search PubMed.
- S. Mohandesnezhad, M. Etminanfar, S. Mahdavi and M. S. Safavi, Surf. Interfaces, 2022, 28, 101604 CrossRef CAS.
- T. J. Fleming, A. Kavanagh, G. Duggan, B. O’Mahony and M. Higgens, J. Mater. Res. Technol., 2019, 8, 4417–4424 CrossRef CAS.
- E. dos S. Monteiro, F. Moura de Souza Soares, L. F. Nunes, A. I. Carvalho Santana, R. Sérgio de Biasi and C. N. Elias, J. Mater. Res. Technol., 2020, 9, 16329–16338 CrossRef.
- I. Antoniac, M. Miculescu, V. Mănescu (Păltânea), A. Stere, P. H. Quan, G. Păltânea, A. Robu and K. Earar, Materials, 2022, 15, 1148 CrossRef CAS PubMed.
- M. H. Fathi, E. M. Zahrani and A. Zomorodian, Mater. Lett., 2009, 63, 1195–1198 CrossRef CAS.
- S. Virtanen, I. Milošev, E. Gomez-Barrena, R. Trebše, J. Salo and Y. T. Konttinen, Acta Biomater., 2008, 4, 468–476 CrossRef CAS PubMed.
- M. Ribeiro, F. J. Monteiro and M. P. Ferraz, Biomatter, 2012, 2, 176–194 CrossRef PubMed.
- S. Yuan, J. Yin, W. Jiang, B. Liang, S. O. Pehkonen and C. Choong, Colloids Surf., B, 2013, 106, 11–21 CrossRef CAS PubMed.
- A. Bekmurzayeva, W. J. Duncanson, H. S. Azevedo and D. Kanayeva, Mater. Sci. Eng., C, 2018, 93, 1073–1089 CrossRef CAS PubMed.
- F. Batool, H. Özçelik, C. Stutz, P.-Y. Gegout, N. Benkirane-Jessel, C. Petit and O. Huck, J. Tissue Eng., 2021, 12, 204173142110414 Search PubMed.
- J. R. Dunkelberger and W.-C. Song, Cell Res., 2010, 20, 34–50 CrossRef CAS PubMed.
- J. Spałek, P. Ociepa, P. Deptuła, E. Piktel, T. Daniluk, G. Król, S. Góźdź, R. Bucki and S. Okła, Int. J. Mol. Sci., 2022, 23, 2575 CrossRef PubMed.
- C. Qian, X. Wu, F. Zhang and W. Yu, J. Prosthet. Dent., 2016, 116, 112–118 CrossRef CAS PubMed.
- S. Omarov, N. Nauryz, D. Talamona and A. Perveen, Metals, 2022, 13, 82 CrossRef.
- S. Teoh, Int. J. Fatigue, 2000, 22, 825–837 CrossRef CAS.
- P. Sahoo, S. K. Das and J. Paulo Davim, Mechanical Behaviour of Biomaterials, Elsevier, 2019, pp. 1–45 Search PubMed.
- A. Vaicelyte, C. Janssen, M. Le Borgne and B. Grosgogeat, Crystals, 2020, 10, 1151 CrossRef CAS.
- R. Kumar and A. Agrawal, J. Mech. Behav. Biomed. Mater., 2023, 142, 105852 CrossRef CAS PubMed.
- J. Quinn, R. McFadden, C.-W. Chan and L. Carson, iScience, 2020, 23, 101745 CrossRef CAS PubMed.
- C. Liu, Z. Ren, Y. Xu, S. Pang, X. Zhao and Y. Zhao, Scanning, 2018, 2018, 1–15 Search PubMed.
- B. C. Costa, C. K. Tokuhara, L. A. Rocha, R. C. Oliveira, P. N. Lisboa-Filho and J. Costa Pessoa, Mater. Sci. Eng., C, 2019, 96, 730–739 CrossRef CAS PubMed.
- J. Draxler, E. Martinelli, A. M. Weinberg, A. Zitek, J. Irrgeher, M. Meischel, S. E. Stanzl-Tschegg, B. Mingler and T. Prohaska, Acta Biomater., 2017, 51, 526–536 CrossRef CAS PubMed.
- L. Wei and Z. Gao, RSC Adv., 2023, 13, 8427–8463 RSC.
- V. S. Telang, R. Pemmada, V. Thomas, S. Ramakrishna, P. Tandon and H. S. Nanda, Curr. Opin. Biomed. Eng., 2021, 17, 100264 CrossRef CAS.
- M. Haghshenas, J. Magnesium Alloys, 2017, 5, 189–201 CrossRef CAS.
- V. Tsakiris, C. Tardei and F. M. Clicinschi, J. Magnesium Alloys, 2021, 9, 1884–1905 CrossRef CAS.
- Y. F. Zheng, X. N. Gu and F. Witte, Mater. Sci. Eng., R, 2014, 77, 1–34 CrossRef.
- A. Atrens, Surface Modification of Magnesium and its Alloys for Biomedical Applications, Elsevier, 2015, pp. 3–28 Search PubMed.
- W. Jin, G. Wang, Z. Lin, H. Feng, W. Li, X. Peng, A. M. Qasim and P. K. Chu, Corros. Sci., 2017, 114, 45–56 CrossRef CAS.
- N. Eliaz, Materials, 2019, 12, 407 CrossRef CAS PubMed.
- R. M. Grzeskowiak, J. Schumacher, M. S. Dhar, D. P. Harper, P.-Y. Mulon and D. E. Anderson, Front. Surg., 2020, 7, 601244 CrossRef PubMed.
- J. L. Hernandez, J. Park, S. Yao, A. K. Blakney, H. V. Nguyen, B. H. Katz, J. T. Jensen and K. A. Woodrow, Biomaterials, 2021, 273, 120806 CrossRef CAS PubMed.
- A. Wiessner, T. Wassmann, J. M. Wiessner, A. Schubert, B. Wiechens, T. Hampe and R. Bürgers, Int. J. Mol. Sci., 2023, 24, 1779 CrossRef CAS PubMed.
- Y. Lu, L. Wang, A. M. O. Dal Piva, J. P. M. Tribst, I. Nedeljkovic, C. J. Kleverlaan and A. J. Feilzer, J. Mech. Behav. Biomed. Mater., 2023, 143, 105944 CrossRef CAS PubMed.
- A. Pandey and S. Sahoo, J. Bionic Eng., 2023, 20, 470–494 CrossRef.
- A. Chmielewska and D. Dean, Acta Biomater., 2024, 173, 51–65 CrossRef CAS PubMed.
- I. Sudoma, Y. Goncharova, B. Dons’koy and D. Mykytenko, J. Reprod. Immunol., 2023, 157, 103943 CrossRef CAS PubMed.
- S. Kligman, Z. Ren, C.-H. Chung, M. A. Perillo, Y.-C. Chang, H. Koo, Z. Zheng and C. Li, J. Clin. Med., 2021, 10, 1641 CrossRef CAS PubMed.
- C. Stewart, B. Akhavan, S. G. Wise and M. M. M. Bilek, Prog. Mater. Sci., 2019, 106, 100588 CrossRef CAS.
- Z. Zhang, Q. Song, Y. Jin, Y. Feng, J. Li and K. Zhang, Metals, 2023, 13, 386 CrossRef CAS.
- Web of Science Core Collection.
- H. Ahirwar, A. Sahu, V. K. Gupta, P. Kumar and H. S. Nanda, Comput. Methods Biomech. Biomed. Eng., 2022, 25, 1262–1275 CrossRef PubMed.
- E. S. Bogya, Z. Károly and R. Barabás, Ceram. Int., 2015, 41, 6005–6012 CrossRef CAS.
- S. Kowalski, W. Gonciarz, R. Belka, A. Góral, M. Chmiela, W. Lechowicz, W. Kaca and W. Żórawski, Coatings, 2022, 12, 1317 CrossRef CAS.
- M. Jian, B. Liu, G. Zhang, R. Liu and X. Zhang, Colloids Surf., A, 2015, 465, 67–76 CrossRef CAS.
- A. C. Bouali, M. Serdechnova, C. Blawert, J. Tedim, M. G. S. Ferreira and M. L. Zheludkevich, Appl. Mater. Today, 2020, 21, 100857 CrossRef.
- X. Yin, P. Mu, Q. Wang and J. Li, ACS Appl. Mater. Interfaces, 2020, 12, 35453–35463 CrossRef CAS PubMed.
- M. U. Joshi, S. P. Kulkarni, M. Choppadandi, M. Keerthana and G. Kapusetti, Smart Mater. Med., 2023, 4, 661–679 CrossRef.
- Credence Research, Medical coatings for Implant market by product, by technology, growth, future perspective, competetion, 2023.
- A. B. Martin, M. Hartman, J. Benson, A. Catlin and The National Health Expenditure Accounts Team, Health Aff., 2023, 42, 6–17 CrossRef PubMed.
- North American Healthcare IT Market Trends & Growth Drivers.
- J. Wong, H. R. Doherty, M. Singh, S. Choi, N. Siddiqui, D. Lam, N. Liyanage, G. Tomlinson and F. Chung, BMC Anesthesiol., 2022, 22, 290 CrossRef PubMed.
- L. E. Bayliss, D. Culliford, A. P. Monk, S. Glyn-Jones, D. Prieto-Alhambra, A. Judge, C. Cooper, A. J. Carr, N. K. Arden, D. J. Beard and A. J. Price, Lancet, 2017, 389, 1424–1430 CrossRef PubMed.
- Dublin, Global Antibacterial Coatings for Medical Implants Market Report to 2030 – Featuring Covalon Technologies, Dot, Harland Medical Systems and Hydromer Among Others, 2022.
- X. Chen, J. Zhou, Y. Qian and L. Zhao, Mater. Today Bio, 2023, 19, 100586 CrossRef CAS PubMed.
- R. E. Hendea, D. Raducanu, A. Claver, J. A. García, V. D. Cojocaru, A. Nocivin, D. Stanciu, N. Serban, S. Ivanescu, C. Trisca-Rusu and R. S. Campian, J. Funct. Biomater., 2023, 14, 400 CrossRef CAS PubMed.
- L. Bonilla-Gameros, P. Chevallier, X. Delvaux, L. A. Yáñez-Hernández, L. Houssiau, X. Minne, V. P. Houde, A. Sarkissian and D. Mantovani, Nanomaterials, 2024, 14, 609 CrossRef CAS PubMed.
- Khaoula Sebbar and Amal El Aabedy.
- S. Negi, Next-Generation Antimicrobial Nanocoatings for Medical Devices and Implants, Elsevier, 2024, pp. 181–204 Search PubMed.
- D. Suzuki, Y. Nonoguchi, K. Shimamoto and N. Terasaki, ACS Appl. Mater. Interfaces, 2023, 15, 9873–9882 CrossRef CAS PubMed.
- N. Su, C. Villicana, C. Zhang, J. Lee, S. Sinha, A. Yang and F. Yang, Theranostics, 2023, 13, 4512–4525 CrossRef CAS PubMed.
- T. A. Kämmerer, V. Palarie, E. Schiegnitz, V. Topalo, A. Schröter, B. Al-Nawas and P. W. Kämmerer, J. Oral Pathol. Med., 2017, 46, 61–66 CrossRef PubMed.
- K. O. Böker, L. Gätjen, C. Dölle, K. Vasic, S. Taheri, W. Lehmann and A. F. Wolfgang, Int. J. Mol. Sci., 2023, 24, 11608 CrossRef PubMed.
- L. Major, D. F. Kopp, R. Major and J. M. Lackner, J. Microsc., 2024, 295, 177–190 CrossRef CAS PubMed.
- Medical Device Coating Market by Coating Type (Hydrophilic and Hydrophobic), Material (Metals, Ceramics and Polymers), Application (Medical Devices and Medical Implants) and Device Type (Gynecology, General surgery, Cardiovascular, Dentistry, Neurology, Orthopedics and others): Opportunity Analysis and Industry Forecast, 2020–2030.
- A. D. Kaye, C. N. Okeagu, A. D. Pham, R. A. Silva, J. J. Hurley, B. L. Arron, N. Sarfraz, H. N. Lee, G. E. Ghali, J. W. Gamble, H. Liu, R. D. Urman and E. M. Cornett, Best Pract. Res. Clin. Anaesthesiol., 2021, 35, 293–306 CrossRef PubMed.
- Medical Coatings For Implants Market Size, Share & Trends Analysis Report By Product (Hydroxyapatite), By Technology (Plasma Spray), By Application, By Region, And Segment Forecasts, 2023–2030.
- L. Sun, J. Therm. Spray Technol., 2018, 27, 1280–1290 CrossRef CAS.
- B. M. Frye, K. R. Laughery and A. E. Klein, Arthroplast. Today, 2021, 8, 103–109 CrossRef PubMed.
- M. Herbster, J. Döring, J. Nohava, C. H. Lohmann, T. Halle and J. Bertrand, J. Mech. Behav. Biomed. Mater., 2020, 112, 104034 CrossRef CAS PubMed.
- C. Skjöldebrand, J. L. Tipper, P. Hatto, M. Bryant, R. M. Hall and C. Persson, Mater. Today Bio, 2022, 15, 100270 CrossRef PubMed.
- S. Calderon, C. F. A. Alves, N. K. Manninen, A. Cavaleiro and S. Carvalho, Coatings, 2019, 9, 682 CrossRef CAS.
- A. Eggert, B. A. Buhren, H. Schrumpf, M. Haversath, M. Ruppert, M. Jäger, R. Krauspe and C. Zilkens, J. Funct. Biomater., 2022, 13, 176 CrossRef CAS PubMed.
- G. George, N. Durig, S. Lee, S. Tanner, R. Snider and T. Pace, ISRN Biomater., 2013, 2013, 1–7 CrossRef.
- R. W. McCalden, K. D. Charron, R. D. Davidson, M. G. Teeter and D. W. Holdsworth, J. Bone Jt. Surg., Br. Vol., 2011, 93B, 409–413 CrossRef PubMed.
- C. Y. Hu and T.-R. Yoon, Biomater. Res., 2018, 22, 33 CrossRef CAS PubMed.
- D. Carrié, J. Berland, S. Verheye, K. E. Hauptmann, M. Vrolix, C. Musto, S. Berti, A. Dibié, E. Maupas, D. Antoniucci and J. Schofer, Int. J. Cardiol., 2020, 301, 50–55 CrossRef PubMed.
- S. O. Trerotola, T. F. Saad and P. Roy-Chaudhury, J. Vasc. Interv. Radiol., 2020, 31, 1–14 CrossRef PubMed.
- K. Kurihara, S. Kawamoto, A. Kimura, A. Tanaka, K. Yabe, H. Nomoto, Y. Osaka, T. Miyazaki, A. Suzuki, Y. Ono, K. Otomo and T. Sasano, J. Cardiol. Ther., 2022, 11, 433–444 CrossRef PubMed.
- Marcelo Azevedo, Magdalena Baczynska, Greg Callaway and Oliver Ramsbottom.
- ThermoFisher Sci.
- Elham Nemati and ahmad gholami.
- A. Thakur, A. Kumar, S. Kaya, R. Marzouki, F. Zhang and L. Guo, Coatings, 2022, 12, 1459 CrossRef CAS.
- Technical experts of ISO/TC 210, ISO 13485:2016 – Medical devices – A practical guide, 2016.
- O. M. Neve, J. A. Boerman, W. B. van den Hout, J. J. Briaire, P. P. G. van Benthem and J. H. M. Frijns, Ear Hear., 2021, 42, 1338–1350 CrossRef PubMed.
- G. Asensio, B. Vázquez-Lasa and L. Rojo, J. Clin. Med., 2019, 8, 1982 CrossRef CAS PubMed.
- H. Dong, H. Liu, N. Zhou, Q. Li, G. Yang, L. Chen and Y. Mou, Coatings, 2020, 10, 1012 CrossRef CAS.
- Chris Nocchi, The Balance Between Quality, Cost, and Speed in Medical Device Development.
- jMedtech Group Pioneering Medical Polymer Solutions.
- LipoCoat.
- Zolidd® (0) Medicine Prosthesis and Orthopedy.
- S. Thanigaivel, A. K. Priya, D. Balakrishnan, K. Dutta, S. Rajendran and M. Soto-Moscoso, Biochem. Eng. J., 2022, 187, 108522 CrossRef CAS.
- K. Moghadasi, M. S. Mohd Isa, M. A. Ariffin, M. Z. Mohd Jamil, S. Raja, B. Wu, M. Yamani, M. R. Bin Muhamad, F. Yusof, M. F. Jamaludin, M. S. bin Ab Karim, B. Binti Abdul Razak and N. bin Yusoff, J. Mater. Res. Technol., 2022, 17, 1054–1121 CrossRef CAS.
- P. Roach, D. Eglin, K. Rohde and C. C. Perry, J. Mater. Sci.: Mater. Med., 2007, 18, 1263–1277 CrossRef CAS PubMed.
- Z. Liu, X. Liu and S. Ramakrishna, Biotechnol. J., 2021, 16, 2000116 CrossRef CAS PubMed.
- M. O. Mavukkandy, S. A. McBride, D. M. Warsinger, N. Dizge, S. W. Hasan and H. A. Arafat, J. Membr. Sci., 2020, 610, 118258 CrossRef CAS.
- J. Deering, A. Clifford, A. D’Elia, I. Zhitomirsky and K. Grandfield, Mater. Lett., 2020, 274, 128057 CrossRef CAS.
- W. Qing, F. Liu, H. Yao, S. Sun, C. Chen and W. Zhang, Adv. Colloid Interface Sci., 2020, 282, 102207 CrossRef CAS PubMed.
- K. A. Kravanja and M. Finšgar, Mater. Des., 2022, 217, 110653 CrossRef CAS.
- G. M. Uddin, M. Jawad, M. Ghufran, M. W. Saleem, M. A. Raza, Z. U. Rehman, S. M. Arafat, M. Irfan and B. Waseem, Int. J. Adv. Manuf. Technol., 2019, 102, 1391–1404 CrossRef.
- A. Baptista, F. Silva, J. Porteiro, J. Míguez and G. Pinto, Coatings, 2018, 8, 402 CrossRef.
- M. Qadir, Y. Li and C. Wen, Acta Biomater., 2019, 89, 14–32 CrossRef CAS PubMed.
- A. A. Vu, S. F. Robertson, D. Ke, A. Bandyopadhyay and S. Bose, Acta Biomater., 2019, 92, 325–335 CrossRef CAS PubMed.
- H. Singh, R. Kumar, C. Prakash and S. Singh, Mater. Today: Proc., 2022, 50, 612–628 CAS.
- A. Singh, G. Singh and V. Chawla, J. Mech. Behav. Biomed. Mater., 2018, 85, 20–36 CrossRef CAS PubMed.
- F. A. Shah, O. Omar, F. Suska, A. Snis, A. Matic, L. Emanuelsson, B. Norlindh, J. Lausmaa, P. Thomsen and A. Palmquist, Acta Biomater., 2016, 36, 296–309 CrossRef CAS PubMed.
- S. C. Kim, W. L. Jo, Y. S. Kim, S. Y. Kwon, Y. S. Cho and Y. W. Lim, Tissue Eng. Regener. Med., 2019, 16, 11–18 CrossRef CAS PubMed.
- G. Das, R. D. Kalita, P. Gogoi, A. K. Buragohain and N. Karak, Appl. Clay Sci., 2014, 90, 18–26 CrossRef CAS.
- O. Yilmaz and A. Yorgancioglu, Polymeric Nanomaterials in Nanotherapeutics, Elsevier, 2019, pp. 299–331 Search PubMed.
- M. H. Hassim, M. H. Idris, M. A. M. Yajid and S. Samion, J. Mater. Res. Technol., 2019, 8, 2290–2299 CrossRef CAS.
- M. F. Montemor, R. Pinto and M. G. S. Ferreira, Electrochim. Acta, 2009, 54, 5179–5189 CrossRef CAS.
- A. N. Ghulam, O. A. L. dos Santos, L. Hazeem, B. Pizzorno Backx, M. Bououdina and S. Bellucci, J. Funct. Biomater., 2022, 13, 77 CrossRef CAS PubMed.
- T. Liu, S. Rui and S. Li, Coatings, 2021, 11, 515 CrossRef CAS.
- E. Adarkwa, R. Kotoka and S. Desai, Med. Devices Sens., 2021, 4, e10167 CrossRef CAS.
- T. Govindarajan and R. Shandas, Polymers, 2014, 6, 2309–2331 CrossRef.
- S. Kang, A. Haider, K. C. Gupta, H. Kim and I. Kang, Coatings, 2022, 12, 999 CrossRef CAS.
- J.-O. Carlsson and P. M. Martin, Handbook of Deposition Technologies for Films and Coatings, Elsevier, 2010, pp. 314–363 Search PubMed.
- B. Fotovvati, N. Namdari and A. Dehghanghadikolaei, J. Manuf. Mater. Process., 2019, 3, 28 CAS.
- W. Sun, W. Liu, Z. Wu and H. Chen, Macromol. Rapid Commun., 2020, 41, 1900430 CrossRef CAS PubMed.
- M. Dovedytis, Z. J. Liu and S. Bartlett, Eng. Regen., 2020, 1, 102–113 Search PubMed.
- M. Bračič, S. Potrč, M. Finšgar, L. Gradišnik, U. Maver, H. Budasheva, D. Korte, M. Franko and L. Fras Zemljič, Appl. Surf. Sci., 2023, 611, 155621 CrossRef.
- M. A. Butt, Coatings, 2022, 12, 1115 CrossRef CAS.
- D. Navas, S. Fuentes, A. Castro-Alvarez and E. Chavez-Angel, Gels, 2021, 7, 275 CrossRef CAS PubMed.
- G. J. Owens, R. K. Singh, F. Foroutan, M. Alqaysi, C.-M. Han, C. Mahapatra, H.-W. Kim and J. C. Knowles, Prog. Mater. Sci., 2016, 77, 1–79 CrossRef CAS.
- R. Choudhary, S. K. Venkatraman, I. Bulygina, A. Chatterjee, J. Abraham, F. Senatov, S. Kaloshkin, A. Ilyasov, M. Abakumov, M. Knyazeva, D. Kukui, F. Walther and S. Swamiappan, J. Australas. Ceram. Soc., 2020, 8, 1051–1065 CrossRef.
- P. Siahmard, R. Amini Najafabadi, A. Meysami, M. Meysami and T. Isfahani, Results Eng., 2023, 18, 101138 CrossRef CAS.
- J. P. Fernández-Hernán, B. Torres, A. J. López and J. Rams, Gels, 2022, 8, 426 CrossRef PubMed.
- A. Dehghanghadikolaei and B. Fotovvati, Materials, 2019, 12, 1795 CrossRef CAS PubMed.
- Y. H. Youn, S. J. Lee, G. R. Choi, H. R. Lee, D. Lee, D. N. Heo, B.-S. Kim, J. B. Bang, Y.-S. Hwang, V. M. Correlo, R. L. Reis, S. G. Im and I. K. Kwon, Mater. Sci. Eng., C, 2019, 100, 949–958 CrossRef CAS PubMed.
- N. Schalk, M. Tkadletz and C. Mitterer, Surf. Coat. Technol., 2022, 429, 127949 CrossRef CAS.
- M. Sabzi, S. H. Mousavi Anijdan, M. Shamsodin, M. Farzam, A. Hojjati-Najafabadi, P. Feng, N. Park and U. Lee, Coatings, 2023, 13, 188 CrossRef CAS.
- L. Sun, G. Yuan, L. Gao, J. Yang, M. Chhowalla, M. H. Gharahcheshmeh, K. K. Gleason, Y. S. Choi, B. H. Hong and Z. Liu, Nat. Rev. Methods Primers, 2021, 1, 5 CrossRef CAS.
- S. A. Fadl-allah, M. Quahtany and N. S. El-Shenawy, J. Biomater. Nanobiotechnol., 2013, 04, 74–83 CrossRef.
- J. Katić, S. Krivačić, Ž. Petrović, D. Mikić and M. Marciuš, Coatings, 2023, 13, 640 CrossRef.
- R. Drevet and H. Benhayoune, Coatings, 2022, 12, 539 CrossRef CAS.
- V. Tsikourkitoudi, B. Henriques-Normark and G. A. Sotiriou, Curr. Opin. Chem. Eng., 2022, 38, 100872 CrossRef.
- D. Samanta, J. L. Meiser and R. N. Zare, Nanoscale, 2015, 7, 9497–9504 RSC.
- J. Luo, B. Mamat, Z. Yue, N. Zhang, X. Xu, Y. Li, Z. Su, C. Ma, F. Zang and Y. Wang, Colloid Interface Sci. Commun., 2021, 43, 100435 CrossRef CAS.
- Y. Qing, K. Li, D. Li and Y. Qin, Mater. Sci. Eng., C, 2020, 108, 110430 CrossRef CAS PubMed.
- E. Buck, H. Li and M. Cerruti, Macromol. Biosci., 2020, 20, 1900271 CrossRef CAS PubMed.
- R. Li, K. Liu, X. Huang, D. Li, J. Ding, B. Liu and X. Chen, Adv. Sci., 2022, 9, 2105152 CrossRef CAS PubMed.
- A. Balaji, S. K. Jaganathan, M. V. Vellayappan, A. A. John, A. P. Subramanian, M. SelvaKumar, H. Mohandas, S. R. M and E. Supriyanto, RSC Adv., 2015, 5, 69660–69679 RSC.
- C. Chen, S.-M. Zhang and I.-S. Lee, Surf. Coat. Technol., 2013, 228, S312–S317 CrossRef CAS.
- M. P. Nikolova and M. D. Apostolova, Materials, 2022, 16, 183 CrossRef PubMed.
- K.-H. Frosch, I. Sondergeld, K. Dresing, T. Rudy, C. H. Lohmann, J. Rabba, D. Schild, J. Breme and K. M. Stuermer, J. Orthop. Res., 2003, 21, 213–223 CrossRef CAS PubMed.
- H. G. Keceli, C. Bayram, E. Celik, N. Ercan, M. Demirbilek and R. M. Nohutcu, J. Periodontal Res., 2020, 55, 694–704 CrossRef CAS PubMed.
- D. Yin, S. Komasa, S. Yoshimine, T. Sekino and J. Okazaki, Int. J. Nanomed., 2019, 14, 3831–3843 CrossRef CAS PubMed.
- S. Liu, M. Bilal, K. Rizwan, I. Gul, T. Rasheed and H. M. N. Iqbal, Int. J. Biol. Macromol., 2021, 190, 396–408 CrossRef CAS PubMed.
- S. Smith, K. Goodge, M. Delaney, A. Struzyk, N. Tansey and M. Frey, Nanomaterials, 2020, 10, 2142 CrossRef CAS PubMed.
- X. Sun, X. Xu, R. Xue, L. Zhang and L. Liu, Mater. Chem. Phys., 2023, 293, 126910 CrossRef CAS.
- K. Pugdee, Y. Shibata, N. Yamamichi, H. Tsutsumi, M. Yoshinari, Y. Abiko and T. Hayakawa, Dent. Mater. J., 2007, 26, 647–655 CrossRef CAS PubMed.
- A. Bandyopadhyay, I. Mitra, S. B. Goodman, M. Kumar and S. Bose, Prog. Mater. Sci., 2023, 133, 101053 CrossRef CAS PubMed.
- X. Yu, J. Walsh and M. Wei, RSC Adv., 2014, 4, 7185 RSC.
- L. Sánchez-López, Biomed. Eng. Adv., 2024, 7, 100111 CrossRef.
- E. C. Rodríguez-Merchán, D. J. Davidson and A. D. Liddle, Int. J. Mol. Sci., 2021, 22, 10243 CrossRef PubMed.
- M. Pagel and A. G. Beck-Sickinger, Biol. Chem., 2017, 398, 3–22 CAS.
- Z.-C. Hu, B. Liu, L. Wang, Y.-H. Cui, Y.-W. Wang, Y.-D. Ma, W.-W. Sun and Y. Yang, Coatings, 2020, 10, 732 CrossRef CAS.
- C. R. Matos-Pérez, J. D. White and J. J. Wilker, J. Am. Chem. Soc., 2012, 134, 9498–9505 CrossRef PubMed.
- L. Zhao, P. K. Chu, Y. Zhang and Z. Wu, J. Biomed. Mater. Res., Part B, 2009, 91B, 470–480 CrossRef CAS PubMed.
- D. Tejero-Martin, M. Rezvani Rad, A. McDonald and T. Hussain, J. Therm. Spray Technol., 2019, 28, 598–644 CrossRef CAS.
- D. Halloran, H. W. Durbano and A. Nohe, J. Dev. Biol., 2020, 8, 19 CrossRef CAS PubMed.
- J. N. Zara, R. K. Siu, X. Zhang, J. Shen, R. Ngo, M. Lee, W. Li, M. Chiang, J. Chung, J. Kwak, B. M. Wu, K. Ting and C. Soo, Tissue Eng., Part A, 2011, 17, 1389–1399 CrossRef CAS PubMed.
- H. Haimov, N. Yosupov, G. Pinchasov and G. Juodzbalys, J. Oral Maxillofac. Res., 2017, 8 DOI:10.5037/jomr.2017.8201.
- A. Szwed-Georgiou, P. Płociński, B. Kupikowska-Stobba, M. M. Urbaniak, P. Rusek-Wala, K. Szustakiewicz, P. Piszko, A. Krupa, M. Biernat, M. Gazińska, M. Kasprzak, K. Nawrotek, N. P. Mira and K. Rudnicka, ACS Biomater. Sci. Eng., 2023, 9, 5222–5254 CrossRef CAS PubMed.
- G. Wang and H. Zreiqat, Materials, 2010, 3, 3994–4050 CrossRef CAS PubMed.
- D. R. Unune, G. R. Brown and G. C. Reilly, Vacuum, 2022, 203, 111298 CrossRef CAS.
- N. N. F. Nik Md Noordin Kahar, A. F. Osman, E. Alosime, N. Arsat, N. A. Mohammad Azman, A. Syamsir, Z. Itam and Z. A. Abdul Hamid, Polymers, 2021, 13, 1194 CrossRef PubMed.
- F. Awaja, S. Zhang, M. Tripathi, A. Nikiforov and N. Pugno, Prog. Mater. Sci., 2016, 83, 536–573 CrossRef CAS.
- J. Chen, F. Li, Y. Luo, Y. Shi, X. Ma, M. Zhang, D. W. Boukhvalov and Z. Luo, J. Mater. Chem. A, 2019, 7, 15207–15214 RSC.
- S. J. Garcia, Eur. Polym. J., 2014, 53, 118–125 CrossRef CAS.
- M. Ramesh, L. R. Kumar, A. Khan and A. M. Asiri, Self-Healing Composite Materials, Elsevier, 2020, pp. 415–427 Search PubMed.
- L. Ma, J. Wang, D. Zhang, Y. Huang, L. Huang, P. Wang, H. Qian, X. Li, H. A. Terryn and J. M. C. Mol, Chem. Eng. J., 2021, 404, 127118 CrossRef CAS.
- N. Van Phuong, B. R. Fazal and S. Moon, Surf. Coat. Technol., 2017, 309, 86–95 CrossRef.
- J. Yang, X. Lu, C. Blawert, S. Di and M. L. Zheludkevich, Surf. Coat. Technol., 2017, 319, 359–369 CrossRef CAS.
- R. Batul, T. Tamanna, A. Khaliq and A. Yu, Biomater. Sci., 2017, 5, 1204–1229 RSC.
- S. Farshid, M. Kharaziha and M. Atapour, J. Magnesium Alloys, 2023, 11, 592–606 CrossRef CAS.
- Y. Zhao, J. Bai, F. Xue, R. Zeng, G. Wang, P. K. Chu and C. Chu, Smart Mater. Manuf., 2023, 1, 100022 Search PubMed.
- A. I. Ribau, J. E. Collins, A. F. Chen and R. J. Sousa, J. Arthroplasty, 2021, 36, 752–766 CrossRef PubMed.
- K. N. Malizos and L. A. Poultsides, Infection and Local Treatment in Orthopedic Surgery, Springer Berlin Heidelberg, Berlin, Heidelberg, 2007, pp. 1–10 Search PubMed.
- S. Adepu and S. Ramakrishna, Molecules, 2021, 26, 5905 CrossRef CAS PubMed.
- J. C. Quarterman, S. M. Geary and A. K. Salem, Eur. J. Pharm. Biopharm., 2021, 159, 21–35 CrossRef CAS PubMed.
- H. Wen, H. Jung and X. Li, AAPS J., 2015, 17, 1327–1340 CrossRef CAS PubMed.
- M. Livingston and A. Tan, J. Med. Device, 2016, 10, MED-15-1164, DOI:10.1115/1.4031718.
- G. A. Lakalayeh, M. Rahvar, N. Nazeri and H. Ghanbari, Med. Eng. Phys., 2022, 108, 103878 CrossRef PubMed.
- L. W. Kleiner, J. C. Wright and Y. Wang, J. Controlled Release, 2014, 181, 1–10 CrossRef CAS PubMed.
- A. R. Johnson, S. P. Forster, D. White, G. Terife, M. Lowinger, R. S. Teller and S. E. Barrett, Expert Opin. Drug Delivery, 2021, 18, 577–593 CrossRef CAS PubMed.
- J. C. Moses and B. B. Mandal, ACS Appl. Mater. Interfaces, 2022, 14, 14961–14980 CrossRef CAS PubMed.
- M. Rahmati, S. P. Lyngstadaas, J. E. Reseland, I. Andersbakken, H. S. Haugland, M. López-Peña, A. G. Cantalapiedra, F. M. Guzon Muñoz and H. J. Haugen, Bioact. Mater., 2020, 5, 787–797 Search PubMed.
- Í. Soares, J. Faria, A. Marques, I. A. C. Ribeiro, C. Baleizão, A. Bettencourt, I. M. M. Ferreira and A. C. Baptista, ACS Omega, 2022, 7, 23096–23106 CrossRef PubMed.
- X. Ma, Y. Gao, D. Zhao, W. Zhang, W. Zhao, M. Wu, Y. Cui, Q. Li, Z. Zhang and C. Ma, Nanomaterials, 2021, 12, 47 CrossRef PubMed.
- XIENCE SierraTM Stent IFU. XIENCE AlpineTM Stent IFU.
- J. Lou, H. Duan, Q. Qin, Z. Teng, F. Gan, X. Zhou and X. Zhou, Pharmaceutics, 2023, 15, 484 CrossRef CAS PubMed.
- E. Adarkwa, A. Roy, J. Ohodnicki, B. Lee, P. N. Kumta and S. Desai, Int. J. Bioprint., 2023, 9, 661 CrossRef CAS PubMed.
- DePuy Synthes Addresses Clinical Demand to Reduce the Incidence of Implant Related Infections with Expert Tibial Nail PROtect.
- P. Ghelich, M. Kazemzadeh-Narbat, A. Hassani Najafabadi, M. Samandari, A. Memić and A. Tamayol, Adv. NanoBiomed Res., 2022, 2, 2100073 CrossRef CAS PubMed.
- A. Hasan, A. Memic, N. Annabi, M. Hossain, A. Paul, M. R. Dokmeci, F. Dehghani and A. Khademhosseini, Acta Biomater., 2014, 10, 11–25 CrossRef CAS PubMed.
- M. Polívková, T. Hubáček, M. Staszek, V. Švorčík and J. Siegel, Int. J. Mol. Sci., 2017, 18, 419 CrossRef PubMed.
- C. of C. Joe Byrne, Navigating FDA Approval: Understanding 510 K vs. PMA.
- J. Huusko, U.-M. Kinnunen and K. Saranto, BMC Health Serv. Res., 2023, 23, 310 CrossRef PubMed.
- M. Paul, Y. Dishon-Benattar, Y. Dickstein and D. Yahav, JAC-Antimicrob. Resist., 2023, 5, dlad005 CrossRef PubMed.
- I. Negut, B. Bita and A. Groza, Polymers, 2022, 14, 1611 CrossRef CAS PubMed.
- Y. Duan, Y. Wu, R. Yan, M. Lin, S. Sun and H. Ma, Prog. Org. Coat., 2021, 155, 106232 CrossRef CAS.
- L. Amengual-Peñafiel, L. A. Córdova, M. Constanza Jara-Sepúlveda, M. Brañes-Aroca, F. Marchesani-Carrasco and R. Cartes-Velásquez, Jpn. Dent. Sci. Rev., 2021, 57, 12–19 CrossRef PubMed.
- A. B. Gardner, S. K. C. Lee, E. C. Woods and A. P. Acharya, Biomed. Res. Int., 2013, 2013, 1–7 CrossRef PubMed.
- B. Zhang, Y. Su, J. Zhou, Y. Zheng and D. Zhu, Adv. Sci., 2021, 8, 2100446 CrossRef CAS PubMed.
- J. Jann, S. Gascon, S. Roux and N. Faucheux, Int. J. Mol. Sci., 2020, 21, 7597 CrossRef CAS PubMed.
- C. Guder, S. Gravius, C. Burger, D. C. Wirtz and F. A. Schildberg, Front. Immunol., 2020, 11 DOI:10.3389/fimmu.2020.00058.
- J. Zupan, M. Jeras and J. Marc, Biochem. Med., 2013, 43–63 CrossRef CAS PubMed.
- P. Jiang, Y. Zhang, R. Hu, B. Shi, L. Zhang, Q. Huang, Y. Yang, P. Tang and C. Lin, Bioact. Mater., 2023, 27, 15–57 CAS.
- X. Hou, L. Zhang, Z. Zhou, X. Luo, T. Wang, X. Zhao, B. Lu, F. Chen and L. Zheng, J. Funct. Biomater., 2022, 13, 187 CrossRef CAS PubMed.
- C. N. Serhan, N. Chiang and T. E. Van Dyke, Nat. Rev. Immunol., 2008, 8, 349–361 CrossRef CAS PubMed.
- D. Zhang, Q. Chen, C. Shi, M. Chen, K. Ma, J. Wan and R. Liu, Adv. Funct. Mater., 2021, 31, 2007226 CrossRef CAS.
- S. Bose, S. Tarafder and A. Bandyopadhyay, Ann. Biomed. Eng., 2017, 45, 261–272 CrossRef PubMed.
- H. Alkhoury, A. Hautmann, B. Fuhrmann, F. Syrowatka, F. Erdmann, G. Zhou, S. Stojanović, S. Najman and T. Groth, Int. J. Mol. Sci., 2020, 21, 3724 CrossRef CAS PubMed.
- D. G. Barone, A. Carnicer-Lombarte, P. Tourlomousis, R. S. Hamilton, M. Prater, A. L. Rutz, I. B. Dimov, G. G. Malliaras, S. P. Lacour, A. A. B. Robertson, K. Franze, J. W. Fawcett and C. E. Bryant, Proc. Natl. Acad. Sci. U. S. A., 2022, 119, e2115857119 CrossRef CAS PubMed.
- B. Friedrich, J.-P. Auger, S. Dutz, I. Cicha, E. Schreiber, J. Band, A. R. Boccacccini, G. Krönke, C. Alexiou and R. Tietze, Int. J. Mol. Sci., 2021, 22, 4143 CrossRef CAS PubMed.
- Q. Li, B. Liang, F. Wang and Z. Wang, ACS Biomater. Sci. Eng., 2020, 6, 5215–5229 CrossRef CAS PubMed.
- B. N. Brown, B. D. Ratner, S. B. Goodman, S. Amar and S. F. Badylak, Biomaterials, 2012, 33, 3792–3802 CrossRef CAS PubMed.
- A. Mamilos, L. Winter, V. H. Schmitt, F. Barsch, D. Grevenstein, W. Wagner, M. Babel, K. Keller, C. Schmitt, F. Gürtler, S. Schreml, T. Niedermair, M. Rupp, V. Alt and C. Brochhausen, Cells, 2023, 12, 276 CrossRef CAS PubMed.
- S. Derakhshanfar, R. Mbeleck, K. Xu, X. Zhang, W. Zhong and M. Xing, Bioact. Mater., 2018, 3, 144–156 Search PubMed.
- P. L. Graney, S. Ben-Shaul, S. Landau, A. Bajpai, B. Singh, J. Eager, A. Cohen, S. Levenberg and K. L. Spiller, Sci. Adv., 2020, 6, eaay6391 CrossRef CAS PubMed.
- Y. Yu, Z. Yue, M. Xu, M. Zhang, X. Shen, Z. Ma, J. Li and X. Xie, PeerJ, 2022, 10, e14053 CrossRef PubMed.
- U. Saqib, S. Sarkar, K. Suk, O. Mohammad, M. S. Baig and R. Savai, Oncotarget, 2018, 9, 17937–17950 CrossRef PubMed.
- J. Kzhyshkowska, A. Gudima, V. Riabov, C. Dollinger, P. Lavalle and N. E. Vrana, J. Leukoc. Biol., 2015, 98, 953–962 CrossRef CAS PubMed.
- N. Sirdeshmukh and G. Dongre, Mater. Today: Proc., 2021, 44, 2348–2355 CAS.
- S. Capuani, G. Malgir, C. Y. X. Chua and A. Grattoni, Bioeng. Transl. Med., 2022, 7, e10300 CrossRef PubMed.
- R. Saran, K. Ginjupalli, S. D. George, S. Chidangil and V. K. Unnikrishnan, Heliyon, 2023, 9, e17457 CrossRef CAS PubMed.
- Y. Liu, Z. Rui, W. Cheng, L. Song, Y. Xu, R. Li and X. Zhang, Regen. Biomater., 2021, 8, rbab006 CrossRef PubMed.
- M. E. A. de Kraker, A. J. Stewardson and S. Harbarth, PLoS Med., 2016, 13, e1002184 CrossRef PubMed.
- S. K. Ahmed, S. Hussein, K. Qurbani, R. H. Ibrahim, A. Fareeq, K. A. Mahmood and M. G. Mohamed, J. Med. Surg. Public Heal., 2024, 2, 100081 CrossRef.
- A. K. Epstein, A. I. Hochbaum, P. Kim and J. Aizenberg, Nanotechnology, 2011, 22, 494007 CrossRef CAS PubMed.
- A. Elbourne, R. J. Crawford and E. P. Ivanova, J. Colloid Interface Sci., 2017, 508, 603–616 CrossRef CAS PubMed.
- S. Achinas, N. Charalampogiannis and G. J. W. Euverink, Appl. Sci., 2019, 9, 2801 CrossRef CAS.
- S. Sharma, J. Mohler, S. D. Mahajan, S. A. Schwartz, L. Bruggemann and R. Aalinkeel, Microorganisms, 2023, 11, 1614 CrossRef CAS PubMed.
- A. Francone, S. Merino, A. Retolaza, J. Ramiro, S. A. Alves, J. V. de Castro, N. M. Neves, A. Arana, J. M. Marimon, C. M. S. Torres and N. Kehagias, Surf. Interfaces, 2021, 27, 101494 CrossRef CAS PubMed.
- S. V. Oopath, A. Baji, M. Abtahi, T. Q. Luu, K. Vasilev and V. K. Truong, Adv. Mater. Interfaces, 2022, 10, 2201425 CrossRef.
- L. C. Hsu, J. Fang, D. A. Borca-Tasciuc, R. W. Worobo and C. I. Moraru, Appl. Environ. Microbiol., 2013, 79, 2703–2712 CrossRef CAS PubMed.
- S. K. Paikra, S. Bauri and M. Mishra, J. Cluster Sci., 2024, 35, 1687–1707 CrossRef CAS.
- S. Kreve and A. C. Dos Reis, Jpn. Dent. Sci. Rev., 2021, 57, 85–96 CrossRef PubMed.
- L. Rizzello, A. Galeone, G. Vecchio, V. Brunetti, S. Sabella and P. P. Pompa, Nanoscale Res. Lett., 2012, 7, 575 CrossRef PubMed.
- M. Karimi Kichi, R. Torkaman, H. Mohammadi, A. Toutounchi, M. Kharaziha and F. Alihosseini, Mater. Today Commun., 2020, 24, 101326 CrossRef CAS.
- L. Luo, Y. Zhou, X. Xu, W. Shi, J. Hu, G. Li, X. Qu, Y. Guo, X. Tian, A. Zaman, D. Hui and Z. Zhou, Nanotechnol. Rev., 2020, 9, 1562–1575 CrossRef CAS.
- N. Lin, P. Berton, C. Moraes, R. D. Rogers and N. Tufenkji, Adv. Colloid Interface Sci., 2018, 252, 55–68 CrossRef CAS PubMed.
- M. Muneer, H. P. Kalappurackal, A. Balachandran and S. Lone, RSC Appl. Interfaces, 2024, 1, 648–666 RSC.
- R. Jiang, L. Hao, L. Song, L. Tian, Y. Fan, J. Zhao, C. Liu, W. Ming and L. Ren, Chem. Eng. J., 2020, 398, 125609 CrossRef CAS.
- S. Kuschlan, V. Gianotti, M. Laus, F. Pérez-Murano, J. Llobet, M. Fernandez-Regulez, C. Bonafos, M. Perego, G. Seguini, M. De Michielis and G. Tallarida, ECS Meet. Abstr., 2023, MA2023-02, 1536–1536.
- R. Olloghe Mandoukou, V. Vallejo Otero, A. Valour, M. Traynar, M. Royon, I. Verrier, O. Lebaigue, O. Dellea, N. N. Crespo-Monteiro and Y. Jourlin, in Advances in Optical Thin Films VIII, ed. M. Lequime and D. Ristau, SPIE, 2024, p. 51 Search PubMed.
- B. T. Sayed, M. M. Al-Sakhnini, A. A. Alzubaidi, A. H. R. Alawadi, A. J. Ibrahim and S. Askar, J. Electron. Mater., 2023, 52, 6943–6958 CrossRef CAS.
- J. Litowczenko, J. K. Wychowaniec, K. Załęski, Ł. Marczak, C. J. C. Edwards-Gayle, K. Tadyszak and B. M. Maciejewska, Biomater. Adv., 2023, 154, 213653 CrossRef CAS PubMed.
- G. Reid, J. C. McCormack, O. Habimana, F. Bayer, C. Goromonzi, E. Casey, A. Cowley and S. M. Kelleher, Materials, 2021, 14, 1910 CrossRef CAS PubMed.
- K. K. Chung, J. F. Schumacher, E. M. Sampson, R. A. Burne, P. J. Antonelli and A. B. Brennan, Biointerphases, 2007, 2, 89–94 CrossRef CAS PubMed.
- S. Wu, F. Zuber, K. Maniura-Weber, J. Brugger and Q. Ren, J. Nanobiotechnol., 2018, 16, 20 CrossRef PubMed.
- D. Li, L. Yang, H. Deng, T. Li and Z. Zhang, J. Mech. Behav. Biomed. Mater., 2023, 144, 105988 CrossRef CAS PubMed.
- H. Sharma, D. Kalita, U. Naskar, B. K. Mishra, P. Kumar and K. B. Mirza, IEEE Sens. J., 2023, 23, 18785–18797 CAS.
- A. Suwardi, F. Wang, K. Xue, M. Han, P. Teo, P. Wang, S. Wang, Y. Liu, E. Ye, Z. Li and X. J. Loh, Adv. Mater., 2022, 34, 2102703 CrossRef CAS PubMed.
- T. Wang, J. Du, S. Ye, L. Tan and J. Fu, ACS Appl. Mater. Interfaces, 2019, 11, 4425–4438 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |