Production process defects of MiXi beverage detected by neutral desorption extractive electrospray ionization mass spectrometry†
Lingling Pan‡
a,
Manman Qin‡ b,
Leting Wang
b,
Leting Wang c,
Lu Huangc,
Huiyu Xingc,
Ning Wang
c,
Lu Huangc,
Huiyu Xingc,
Ning Wang c,
Tongtong Yangc,
Liyun Hud,
Rui Su*d and
Huanwen Chenc
c,
Tongtong Yangc,
Liyun Hud,
Rui Su*d and
Huanwen Chenc
aScientific Research Department, Jiangxi University of Chinese Medicine, Nanchang 330004, China
bCenter for Translational Medicine, College of Traditional Chinese Medicine, Jiangxi University of Chinese Medicine, Nanchang, 330004, China
cThe Jiangxi Province Key Laboratory for Diagnosis, Treatment, and Rehabilitation of Cancer in Chinese Medicine, Jiangxi University of Chinese Medicine, Nanchang, 330004, China
dState Key Laboratory of Inorganic Synthesis and Preparative Chemistry, College of Chemistry, Jilin University, Changchun, 130012, China. E-mail: rsu@jlu.edu.cn; Fax: +86-431-85168986; Tel: +86-431-85168986
First published on 12th June 2025
Abstract
Process defects in the model production process of MiXi beverage, typical examples of extremely viscous food products, have been successfully detected using neutral desorption-extractive electrospray ionization mass spectrometry (ND-EESI-MS) without sample pretreatment. We employed statistical analysis to explore the correlation between process defects in production and metabolite ions in the fingerprints of MiXi samples with diverse qualities, which were acquired using ND-EESI-MS. Abnormal signals in the fingerprint profiles, such as the unexpected presence of heavy metal ions or pesticides, as well as changes in beneficial compounds like D-glucose, can indicate defects or issues encountered in the production process. Meanwhile, we quantitatively analysed the concentrations of heavy metals and pesticide residues in MiXi beverage, and the results showed a strong linear relationship (R2 > 0.99) between heavy metals and pesticide residues in the concentration range 0.50–200.00 μg L−1, with a limit of detection (LOD) of 0.04–1.44 μg L−1 and limit of quantification (LOQ) of 0.12–4.36 μg L−1, with an RSD of 0.39–3.66%. Furthermore, in contrast to previous methods, concealed defects occurring in the manufacturing process, including raw material screening, soaking, and extraction, can be directly detected by terminal product analysis, showing that ND-EESI-MS was a promising tool for quality control in viscous beverages and tracing defects in the production process.
1. Introduction
In the chemical and food industries, in general, a defect means any malfunction in the manufacturing line and/or abnormal conditions leading to unexpected properties for a product. It is important to address all defects before delivering the product to the client, to maintain the client relationship and avoid huge economic losses.1 Usually, the physical conditions of a production line are monitored online by instrumental methods, including infrared thermal imaging,2,3 equivalent circuit analysis,4 thin film characteristic analysis,5 as well as optical, electrical, and other technologies. Advances in the field have been systematically reviewed elsewhere.6,7To mitigate the impact of process defects, inspection procedures are implemented at multiple stages throughout food production. Additionally, sampling inspections are conducted before the products leave the factory. However, if substandard quality is identified during this final sampling, it becomes difficult to trace the exact stage at which the issue occurred. Consequently, not only must the entire batch be discarded, but the staff must also re-evaluate each stage of the subsequent batch's production. This leads to substantial economic losses and imposes significant time and labor demands on the production process. To date, the quality of the product has normally been evaluated by chemical analytical methods, including gas chromatography (GC),8,9 high performance liquid chromatography (HPLC),10–12 liquid chromatography-mass spectrometry (LC-MS),13,14 gas chromatography-mass spectrometry (GC-MS)15–17 and stable carbon isotope ratio analysis (SCIRA).18,19 Most of these methods require extensive preprocessing of the product, which may result in the loss of some sample information. Process defects alter the chemical composition of the product, giving rise to inconsistent chemical fingerprints of the final products. However, efforts have rarely been made to diagnose process defects by analyzing the chemical composition of the final product. Therefore, it is highly desirable to develop rapid and sensitive analytical methods for directly detecting potential process defects by analyzing the chemical composition of the final product.To demonstrate, a typically viscous and atherosclerotic rice food named a MiXi beverage was selected as the sample, which normally contains Chinese herbal medicines, such as Euryale ferox, yam, lotus seed, and Poria cocos. As formulated, it is rich in nutrients resulting from the complex production processes (Fig. S1†).20 The components of MiXi beverage are complex and diverse. They include proteins, carbohydrates, crude fibers, vitamins, and minerals that are essential to the human body. In addition, the beverage contains numerous bioactive substances, such as polysaccharides, triterpenoids, volatile oils, alkaloids, flavonoids, glycosides, steroids, and fatty acids, which are beneficial to the human body.21–23 The sample processing protocols are complicated, and it takes hours for a single run, which is time-consuming and laborious. This causes a lot of inconvenience for quality testing of products. Therefore, there is an urgent need to develop a system that is easy to implement, simple to operate, time-efficient, and capable of preserving sample integrity for the rapid and accurate detection of defective production steps or corresponding factors in the MiXi beverage production process.
Extractive electrospray ionization mass spectrometry (EESI-MS) allows for the direct analysis of diverse and intricate sample matrices without the need for any prior sample preparation. In EESI-MS, the initial step involves charging the chosen reagent (such as methanol or water) using electrospray ionization (ESI) to effectively generate an ionic reagent plume. This plume subsequently interacts with the sample plume in the open air, encompassing a substantial volume of approximately 20 cm3. During this interaction, the energetic charge is transferred to the analyte molecules derived from the sample mixture. Afterward, the analytes undergo ionization through extraction and collision processes that take place during interactions between the two plume sprays. As a result, the ionized analytes were introduced into the mass spectrometer for subsequent analysis.24 ND-EESI-MS is an advanced technique derived from EESI, achieved by introducing suitable neutral gas plumes like air or nitrogen to efficiently extract analytes from highly viscous samples (e.g. honey, cheese, or toothpaste).25–29 However, the use of ND-EESI-MS technology in process tracking for commercialized products has not yet been reported. Therefore, this study will establish an ND-EESI-MS analysis technology for analyzing the chemical composition of the MiXi beverage. Changes in the chemical composition of the products suggest that there may be loopholes in the selection of raw materials and process production and may help explain the origin of these changes in the chemical composition of the products. The chemical association with the raw material quality and production processes provides a powerful scientific tool for quality control and accurate diagnosis of defects in the MiXi beverage production process (Fig. 1).
 | ||
| Fig. 1 Workflow diagram of the study design: (a) schematic diagram of ND-EESI-MS platform; (b) flowchart of ND-EESI-MS analysis. | ||
2. Results and discussion
2.1. ND-EESI-MS fingerprint of MiXi beverage
The metabolomics approach is of great importance for identifying and associating chemical changes in food processing with the desired quality assessment of the final product.30 Unlike targeted metabolomics for the main purpose of quantitative analysis, the untargeted approach is a more common choice for exploratory research, as it covers a wider range of molecules and is more suitable for identifying new metabolites.31 To gain insight into the underlying relationships between molecules and process defects, we conducted molecular profiling of normal MiXi beverage samples and samples with manufactured defects. The total ion current (TIC) for MiXi beverage was visually analyzed by ND-EESI-MS in both positive ion and negative ion detection modes (Fig. 2). In the positive ion mode, a total of 1365 ions were detected, and 62 components were successfully identified by comparing them to the NIST database, MassBank database, and relevant literature. Likewise, in the negative ion mode, 584 ions were detected, and 17 components were identified.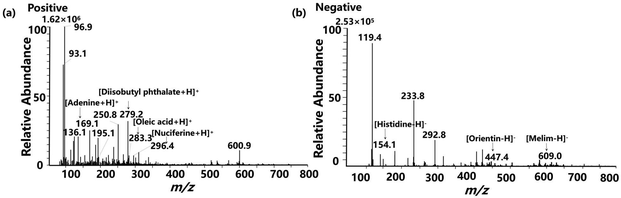 | ||
| Fig. 2 Mass spectra of MiXi beverage acquired by ND-EESI-MS in the (a) positive and (b) negative ion modes. | ||
Based on Table S1,† the identified nutrients include various triterpenoids, such as tumulosic acid ([M + H]+, m/z 487.4), 25-hydroxypachimic acid ([M + H]+, m/z 545.4), dehydrotumulosic acid ([M + H]+, m/z 485.4), and pachymic acid ([M + H]+, m/z 529.4). Additionally, nucleosides were detected, including adenosine ([M + H]+, m/z 268.1), and adenine ([M + H]+, m/z 136.1). The presence of alkaloids, such as neferine ([M + H]+, m/z 625.0), tuduranine ([M + H]+, m/z 298.1), and leonticine ([M + H]+, m/z 328.2), was also observed. Moreover, flavonoids, such as orientin ([M − H]−, m/z 447.0), isovitexin ([M − H]−, m/z 431.0), and melim ([M − H]−, m/z 609.0) were identified. Fatty acids, such as oleic acid ([M + H]+, m/z 283.3), acetic acid ([M + K]+, m/z 99.0), and tridecanoic acid ([M + Na]+, m/z 235.2), were also observed. Finally, amino acids, including histidine ([M − H]−, m/z 154.1), arginine ([M + H]+, m/z 175.1), asparagic acid ([M + H]+, m/z 134.1), and glutamic acid ([M + H]+, m/z 148.1), were detected.
2.2. Evaluation of quality control of MiXi beverage production process
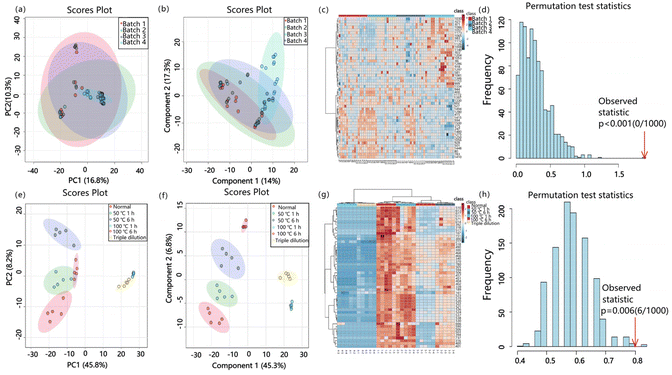
Extended or excessive heating can have detrimental effects on MiXi beverage, resulting in a decrease in volatile components and an increase in by-products, adversely affecting the overall quality. In addition, the duration of the soaking process plays a crucial role in the dissolution of water-soluble active ingredients. Coarse crushing of medicinal materials leads to a decrease in the content of active ingredients. Our findings indicate that relying solely on sensory methods to detect subpar MiXi beverage after subjecting them to varying treatments (heating at 50 °C for 1 h, heating at 100 °C for 1 h, heating at 100 °C for 6 h, and diluting with water thrice) may be insufficient. Accurately identifying defective products and localizing the specific production process responsible for the defect by detecting unique markers in the terminal product, represent valuable research.ND-EESI-MS was utilized to perform untargeted metabolomics analysis on both qualified normal MiXi beverage and five kinds of artificially prepared samples. Four batches of normal MiXi beverage cannot be distinguished in either the principal components analysis (PCA) model (an unsupervised statistical method, as depicted in Fig. 3a) or the PLS-DA model (Fig. 3b). The heatmap also showed that the signal intensities of the top 50 ions among the four groups of samples had no significant differences (Fig. 3c). In contrast, the molecular differences among the normal MiXi and five kinds of artificially prepared samples were readily visible in the PCA score plot (Fig. 3e), demonstrating clear separation between the groups. After conducting a multivariate analysis, 50 critical metabolites (p < 0.05) that significantly differentiated the six groups were observed (Fig. 3f and g).
We thoroughly evaluated the classification ability of the PLS-DA model by analyzing three crucial model parameters: R2X (explained variation in the X matrix), R2Y (explained variation in the Y matrix), and Q2Y (the predictive ability of the model). As depicted in Fig. S2a,† the Q2 value was 0.18182, indicating that the model lacked classification ability and was invalid. However, Fig. S2b† showed that the PLS-DA model was efficient in classifying the normal and defective groups with a Q2 value greater than 0.5. Moreover, no signs of overfitting were observed in Fig. 3d or h (p < 0.05), indicating that the model is robust and capable of accurate classification.
2.3. The effect of extraction temperature and time
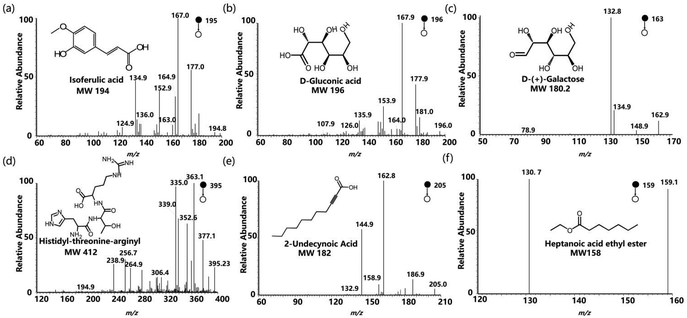
In the course of producing MiXi beverage, both the extraction and concentration process are carried out at temperatures between 40 and 60 °C.20,32 Employing a low-temperature extraction and concentration process guarantees the stability of the active components, as well as facilitating their full dissolution without the risk of decomposition. We investigated the effect of extraction temperature and time on the quality of MiXi beverage. To simulate and validate the effect of extraction temperature in the production process of MiXi beverage, we heated a normal commercial MiXi beverage soup at 50 °C for 1 h. However, we found no significant difference compared to normal MiXi beverage, as shown in Fig. S3a.† Despite conducting statistical analysis on samples subjected to heating at 50 °C for 1 h and 6 h, no obvious difference was found (Fig. S3b†). This observation reflects the suitability of the extraction temperature and time (30–40 min) used in the production of commercial MiXi beverage, ensuring the stability and consistency of the quality of the final product. Likewise, we conducted an experiment where the normal MiXi beverage were heated at 100 °C for 1 h, and we then compared the mass spectra with those of the normal MiXi beverage. As a result, we observed a significant distinction between the two groups, which was further supported by the heatmap analysis, revealing 46 ions with significant changes in their intensity induced by the high extraction temperature (Fig. 4a and b). Among them, m/z values, include 213, 282, 310, 137, 336, 525, 195, 231, 1395, 629, 196, 217, 124, 205, 127, 61, 681, 357, 161, 578, 160, 72, 203, 201, 148, 152, 519, 339, and 159, representing a set of unique marker ions that exhibit significant differences in intensity, highlighting the impact of the high extraction temperature on the quality of the final product. Some significant ions, such as isoferulic acid (m/z 195), heptanoic acid ethyl ester (m/z 159), and D-glucose acid (m/z 196), which can be used as marker ions for referring to defects induced by high-temperature extraction, were identified using MS/MS experiments (Fig. 5).A comparison between MiXi beverage heated at 100 °C for 1 h and those heated for 6 h at the same temperature revealed the presence of 26 different ions, of which 15 included m/z values of 137, 205, 163, 161, 169, 293, 555, 525, 556, 159, 201, 127, 145, 310 and 107 were selected as defect markers. The presence of these ions in the final product of MiXi beverage would indicate that the extraction or concentration process involved extended exposure to high-temperature, as depicted in Fig. 4c and d. Of particular note, m/z 163 ([D-(+)-galactose-H2O + H]+) and m/z 205 ([2-undecynoic acid + Na]+), generated by the de-CH3 of methyl 2-undecynoate after long-term high-temperature heating, were identified (Fig. 5).
2.4. The effect of soaking time
Furthermore, notable variations were observed between the normal group and the triple dilution group, in which m/z values of 230, 229, 173, 151, 184, 100, 201, 142, and 232 exhibited significant changes following the triple dilution (Fig. S3c†), indicating a significant decrease in the concentration of key components. This suggests that the soaking time was likely too short, resulting in a low rate of dissolution of active ingredients and that inadequate homogenization may also have contributed to a reduced rate of dissolution. Another potential cause of this defect may have been excessive water usage during the mixing process.2.5. Exogenous pesticide residue pollution
Pesticides are synthetic chemicals that exert significant biological effects and pose a substantial risk to both human health and the environment. Long-term exposure to and excess intake of these chemicals have been linked to various health hazards, including cancer, teratogenesis, and mutagenesis, as well as the potential transmission of viruses affecting the liver, kidneys, reproductive system, and nervous system.33,34 Therefore, countries worldwide have implemented stringent regulations regarding pesticide residue levels in both food and the environment. China's recently updated national food safety standard, GB 2763-2021, outlines specific residue limits for 34 pesticides and their metabolites in rice and Chinese medicines.35,36 ND-EESI-MS is advantageous for detecting trace compounds due to its ability to achieve low limits of detection (LODs).27,37,38 Thus, we employed the ND-EESI-MS method to detect four pesticides and their metabolites in terminal samples of MiXi beverage to trace the safety of the raw materials. The LODs of MiXi samples spiked with diazinon, β-endosulfan, carbendazim, and methyl parathion, calculated using signal-to-noise ratios (S/N) of 3.3, were found to be 0.04–0.43 μg L−1. The LOQs estimated as the analyte concentration producing S/N of ten were 0.12–1.30 μg L−1 (Table S2†). Moreover, the standard working curves for the four pesticide samples were constructed, and the linearities of the present method were evaluated. The correlation coefficients (r) for these curves ranged from 0.9933 to 0.9982, indicating a strong linear relationship between signal intensity and concentration. The correlation coefficient of diazinon is 0.9982, the highest among all analytes. Simultaneously, we compared our method with the conventional detection method LC-MS, as shown in Table S3.† The relative standard deviations (RSDs) of LC-MS ranged from 1.64% to 6.43%. The results indicate that the method developed in this study exhibits better and more stable repeatability than LC-MS. This improvement may be attributed to the multiple sample pretreatment steps in LC-MS, which introduce additional errors. Beyond the advantage of repeatability, LC-MS requires at least 10 h from sample processing to obtaining final results, whereas the entire ND-EESI-MS process takes only 1 min, significantly reducing analysis time and improving experimental efficiency.We analyzed the differential ions associated with changes in rice quality resulting from pesticide contamination. Compared to normal MiXi samples, we observed characteristic ions indicative of pesticide residues, in addition to quasi-molecular ions. Distinctive ion signatures were identified for each pesticide via differential ion analysis (Fig. S4†). Diazinon exhibited characteristic ions at m/z 93, 94, 149, and 311, with 311 serving as its diagnostic marker. α-Endosulfan displayed specific ions at m/z 121, 125, 135, 143, 153, 157, and 251, while β-endosulfan was distinguished by m/z 108, 134, 227, 117, and 228. Carbendazim showed ions at m/z 107, 122, 150, 177, and 199, and bifenthrin was uniquely marked by m/z 133. Methyl parathion demonstrated specificity through its characteristic ions at m/z 87, 147, and 310. Notably, shared ions (m/z 93, 94, 105, 107, 119, and 149) were observed across multiple analytes. The identification of these differential ions was accomplished by using secondary mass spectra, as depicted in Fig. S5.†
2.6. Heavy metal pollution
Medicinal plants are susceptible to the presence of heavy metals, including lead (Pb), cadmium (Cd), and mercury (Hg), and detecting their presence is of the utmost importance. Notably, the levels of heavy metal content can differ significantly across various plant species, geographical regions, growth stages, and specific parts used for medicinal purposes.39 Simultaneously, the proximity of food processing plants to regions experiencing significant environmental pollution, combined with inadequate water quality standards, can result in the accumulation of excessive levels of heavy metals. Therefore, screening out the characteristic ions of heavy metals through ND-EESI-MS analysis in terminal samples of MiXi beverage will be helpful for tracing heavy metal pollution in raw materials in MiXi processing, and guaranteeing safety, quality, and efficacy in food production.However, heavy metal ions are commonly found in the form of inorganic salts, and these salts do not readily evaporate under the typical conditions employed in organic mass spectrometry analysis. Consequently, they are unable to enter the mass spectrometer for ionization and subsequent analysis, presenting a notable obstacle in the detection of heavy metal ions using organic mass spectrometry. According to our previous work,40 we utilize EDTA as an extraction reagent, because its exceptional chelating capability facilitates the formation of complexes with more than 90% of metal ions. These complex ions facilitated the rapid detection of heavy metal ions using organic mass spectrometry. We performed quantitative analysis experiments in the negative ion mode. Table S4† summarizes the metals, limit of detection (LOD), and limit of quantification (LOQ) and national standard for drinking water (GB 5749-2022). Complexes of heavy metal ions with EDTA, such as [PbY]2− (m/z 248), [CdY]2− (m/z 201), [CrY]− (m/z 340), [HgHY]− (m/z 491), and [CuY]2− (m/z 176) were detected. The results unequivocally demonstrate that the LOD values for Pb2+, Cd2+, Cr3+, Hg2+, and Cu2+ in MiXi were consistently below the detection limits prescribed by the national standard for drinking water (GB 5749-2022). Among these metals, Cd2+ exhibited remarkable sensitivity, while Cu2+ showed relatively weaker sensitivity in detection compared to their respective national standard limits (Table S4†). When the proposed heavy metals in the terminal MiXi products were detected to exceed the concentration allowed in the national standard, this suggested existing defects in the processing steps, such as the introduction of substandard raw materials, exceeding the limits of heavy metals in raw material screening or during soaking, grinding, and extraction processes, where contaminated water was utilized.
The analytical performance of inductively coupled plasma mass spectrometry (ICP-MS) and the proposed ND-EESI-MS method for the determination of heavy metals in MiXi beverages was systematically evaluated, as summarized in Table S5.† The ICP-MS method exhibited relative standard deviations (RSDs) ranging from 0.85% to 38.49% and recoveries of 68.0–123.1%. In contrast, the ND-EESI-MS protocol demonstrates superior repeatability and accuracy compared to ICP-MS. The observed limitations of ICP-MS may arise from two critical factors. Digestion-mediated sample preparation introduces inherent variability due to incomplete recovery of analytes and potential adsorption of analytes onto the inner walls of the sample introduction system, thereby introducing systematic errors. The protracted workflow of ICP-MS, requiring a minimum of 13 h from sample pretreatment to data acquisition, exacerbates the risks of contamination and analyte degradation. Conversely, ND-EESI-MS eliminates digestion requirements and completes the entire analytical process within 1 min, significantly minimizing operational uncertainties.
Further, through a comparative analysis of the mass spectra between the normal MiXi samples and those supplemented with heavy metal ions, rigorous statistical analysis demonstrated the discernible influence of heavy metal ions on the quality of the MiXi beverage (Fig. S6†). When Pb2+ appears in rice samples, the differential ions observed are m/z 146, 165, 313, 150, 164, 145, 149, 684, 182, 158, 438, and 268. The introduction of Cd2+ generates differential ions at m/z 93, 155, 61, 97, 165, 63, 123, 91, 125, 206, 145, 261, 125, and 154. The presence of Cr3+ leads to the observation of differential ions at m/z 292, 344, and 62. Hg2+ results in the detection of differential ions at m/z 313 and 314. Finally, the addition of Cu2+ gives rise to differential ions observed at m/z 291, 292, 314, 313, 344, 164, and 62 in the MiXi samples.
The detection of specific product ions, such as D-glucuronic acid and 2-undecynoic acid, in MiXi beverage suggests the existence of process defects that lead to non-compliant product quality. These defects can be attributed to the use of inappropriate heating conditions during the extraction and concentration stages of the manufacturing process (Fig. 6a and b). The detected signals of pesticide residues, along with their associated metabolite ions and complex ions of heavy metals in the MiXi product, indicate the manifestation of process defects during the stage of raw material selection (Fig. 6c and d).
3. Materials and methods
3.1. Samples and chemicals
Different batches of MiXi beverage (batches 20220506; 20220507; 20220608; 20220816) produced by Jiangzhong Food Therapy Technology Co Ltd (in Nanchang, Jiangxi Province, China) were purchased from local markets and official flagship shops. Organophosphorus pesticides (HPLC grade purity, 96.8–99.5%), including diazinon, α-endosulfan, β-endosulfan, bifenthrin, methyl parathion and carbendazim were purchased from Aladdin (Shanghai, China). Heavy reagents (AR grade), including lead acetate, cadmium chloride, chromium nitrate, mercury nitrate, and copper nitrate, were purchased from Aladdin (Shanghai, China). Methanol, acetone and acetic acid (all LC-MS grade) were provided by ROE Scientific Inc. (Newark, U.S.A). Disodium ethylene diamine tetraacetate (EDTA) was purchased from Aladdin (Shanghai, China). The deionized water used for the experiments was provided by a Hetai Smart-DUVF water purifier (Shanghai, China).3.2. Study design
An untargeted metabolomics profiling method for discovering food production process defects was designed by detecting biomarkers of different quality samples of MiXi beverage based on ND-EESI-MS. The standard MiXi production process contains several steps: material selection, batch feeding, soaking, homogenization, extraction and concentration, and additive addition. Initial research mainly analyzed the influence of exogenous (heavy metals and organophosphorus pesticide pollution) and endogenous substances on the quality of MiXi beverages from the end product. This paper focused mainly on two exogenous and three other factors of improper soaking time, control of temperature and time.In brief, we prepared MiXi samples with defects resulting from insufficient soaking time by diluting normal batches of MiXi with ultrapure water three times. Additionally, MiXi samples with defects resulting from improper control of temperature and time were prepared by individually adjusting the heating temperature and time of normal batches of MiXi. Heavy metal and organophosphorus pesticide pollution samples (1 ng L−1 to 5 mg L−1) were produced by spiking normal batches of MiXi samples with a series of pollutant standard solutions. A summary of a representative artificially prepared sample is provided in Table S6.† A total of 67 samples, including four batches of normal MiXi samples and seven kinds of prepared MiXi samples, were analyzed using ND-EESI-MS analysis.
3.3. Analysis of pesticide residue and heavy metals in MiXi beverages by ND-EESI-MS
The voltages used for spray ionization were 4 kV and 3.5 kV under positive ion and negative ion detection modes, respectively. The capillary temperature, capillary voltage and tube lens voltage of the LTQ-MS were maintained at 200 °C, 1 V and 10 V, respectively. A gentle nitrogen sheath gas stream (0.1 MPa) was blown into the methanol solution to form a mixture gas of nitrogen/methanol, which continuously brings the ingredients in the MiXi beverage out into the ionization region for primary ion production. Subsequently, the spray solvent, a mixture of methanol–water–glacial acetic acid (47.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 47.5
47.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v) was passed through the quartz capillary at a flow rate of 5 μL min−1. Under the action of the electric field (spray solvent) and with the help of the nitrogen sheath gas stream (0.8 MPa), the spray solvent first obtains energy and forms charged reagent droplets. Conditional optimization results are shown in Fig. S7.† They are carriers of charge and energy and converge with analytes in three-dimensional space for ion exchange and ionization. The analyte in the sample is then extracted into the charged reagent droplets, followed by desolvation to generate ions of the analyte for subsequent mass spectrometry analysis. The width of the parent ion isolation was 1.0–1.5 Da, and the collision energy was 15% to 35% in collision induced dissociation (CID) experiments. Other conditions were optimized by the system itself. The mass spectrometry scan range was m/z 50–1000. The principle schematic diagram of ND-EESI-MS is shown in Fig. 1a.
5, v/v) was passed through the quartz capillary at a flow rate of 5 μL min−1. Under the action of the electric field (spray solvent) and with the help of the nitrogen sheath gas stream (0.8 MPa), the spray solvent first obtains energy and forms charged reagent droplets. Conditional optimization results are shown in Fig. S7.† They are carriers of charge and energy and converge with analytes in three-dimensional space for ion exchange and ionization. The analyte in the sample is then extracted into the charged reagent droplets, followed by desolvation to generate ions of the analyte for subsequent mass spectrometry analysis. The width of the parent ion isolation was 1.0–1.5 Da, and the collision energy was 15% to 35% in collision induced dissociation (CID) experiments. Other conditions were optimized by the system itself. The mass spectrometry scan range was m/z 50–1000. The principle schematic diagram of ND-EESI-MS is shown in Fig. 1a.
3.3.2.1. Linearity range and sensitivity. Linearity refers to the proportional relationship between analyte concentration and experimental response within the designated range. Mixed standard working solutions were prepared by serially diluting stock solutions of each target analyte to six concentration levels (Tables S12 and S13†). Each concentration was analyzed in triplicate using ND-EESI-MS (Section 3.3.1), with secondary characteristic fragment ions selected as quantitative ions. Calibration curves were plotted by correlating the mean signal intensity (y-axis) with analyte concentration (x-axis), and linearity was evaluated via the correlation coefficient (R2) (Tables S2 and S4†).
Method sensitivity was evaluated by determining the LOD and LOQ using the following relationships:
| LOD = 3.3σ/b, LOQ = 10σ/b |
3.3.2.2. Repeatability and accuracy. Repeatability, reflecting the consistency of results under identical conditions, was quantified via RSD. For pesticide residues and heavy metals, three concentration levels (low, medium, high) of spiked MiXi beverage blank samples were independently prepared in triplicate (Tables S14 and S15†). Each level was analyzed following Section 3.3.1, and RSD values were calculated from triplicate measurements to assess method repeatability.
Accuracy, expressed as spike recovery (%), was evaluated by spiking blank MiXi beverage samples with target analytes at three concentration levels. Triplicate spiked samples and three unspiked blanks were processed and analyzed as per Section 3.3.1. Recovery rates were calculated by comparing measured concentrations with theoretical spiked values (Tables S7 and S8†).
3.4. Analysis of pesticide residue in MiXi beverage by LC-MS
3.5. Analysis of heavy metals in MiXi beverage by ICP-MS
4. Conclusion
In this study, the ND-EESI-MS technique was employed to analyze the mass spectra of various substances in MiXi beverage, demonstrating its potential for rapid, online determination of complex matrix samples. ND-EESI-MS has high sensitivity, rapid analysis and excellent specificity, which are common characteristics of detection methods (such as LC-MS or GC-MS). Unlike the other methods, ND-EESI-MS enables direct online analysis of differential ions in MiXi beverage without the need for sample pretreatment. Through the identification of these distinctive ions, the technological defects within the production process of MiXi beverage can be deduced and traced back, providing valuable insights for raw material selection and production process monitoring to ensure beverage quality. The establishment and appropriate application of the ND-EESI-MS method can effectively monitor the quality of a MiXi beverage, and the proposed method showcases its remarkable efficacy and promising potential for wider implementation in food quality control and defect monitoring throughout the entire production process, solely by analyzing the terminal MiXi samples.Author contributions
Lingling Pan, Manman Qin, Lu Huang, Huiyu Xing and Ning Wang: performing data acquisition by ND-EESI-MS. Manman Qin, Lu Huang, Huiyu Xing, Tongtong Yang and Liyun Hu: conducting data preprocessing and statistical analysis. Rui Su: drafting the manuscript. Huanwen Chen: supervising the data interpretation. Rui Su had primary responsibility for the content of the manuscript. All authors provided critical input, revised, and approved the final version.Data availability
The authors declare that the data supporting the findings of this study are available within the paper. Should any data files be needed in any other format, they are available from the corresponding author upon reasonable request.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 82004005; 91959201), Jiangxi University of Chinese Medicine School-level Science and Technology Innovation Team Development Program (No. CXTD22005), High-level Talents of Chinese Medicine (No. 13030599) and Virtual Office for teaching and research (Xiao Zi [2022] No.41)References
- S. Felix, S. Ray Majumder, H. K. Mathews, M. Lexa, G. Lipsa, X. Ping, S. Roychowdhury and T. Spears, Sci. Rep., 2022, 12, 8503 CrossRef CAS PubMed.
- K. Liu, F. Wang, Y. He, Y. Liu, J. Yang and Y. Yao, Polymers, 2022, 15, 173 CrossRef PubMed.
- S. H. Go, A. Tugirumubano and H. G. Kim, Polymers, 2021, 13, 203 CrossRef CAS PubMed.
- I. G. Lee, Y. J. Yoon, K. S. Choi and I. P. Hong, Sensors, 2021, 21, 3076 CrossRef PubMed.
- W. T. Fan, P. T. Liu, P. Y. Kuo, C. M. Chang, I. H. Liu and Y. Kuo, Nanomaterials, 2021, 11, 3070 CrossRef CAS PubMed.
- Y. H. Liu, C. K. Wang, Y. Ting, W. Z. Lin, Z. H. Kang, C. S. Chen and J. S. Hwang, Int. J. Mol. Sci., 2009, 10, 4498–4514 CrossRef PubMed.
- Y. Wu, B. Cui and Y. Xiao, Materials, 2020, 13, 5643 CrossRef CAS PubMed.
- S. Belarbi, M. Vivier, W. Zaghouani, A. Sloovere, V. Agasse-Peulon and P. Cardinael, Food Chem., 2021, 359, 129932 CrossRef CAS PubMed.
- D. H. Wang, Z. Wang and J. T. Brenna, J. Agric. Food Chem., 2020, 68, 4973–4980 CrossRef CAS PubMed.
- K. Kosalková, I. C. Sánchez-Orejas, L. Cueto and C. García-Estrada, Methods Mol. Biol., 2021, 2296, 195–207 CrossRef PubMed.
- N. Kumar, D. Sangeetha and L. Kalyanraman, J. Chromatogr. Sci., 2021, 59, 154–164 CAS.
- Z. Liu, K. Ren, Y. Feng, T. Uong, S. Krepich and H. You, J. Agric. Food Chem., 2020, 68, 10142–10148 CrossRef CAS PubMed.
- V. Martinez-Rios, M. Pedersen, M. Pedrazzi, E. Gkogka, J. Smedsgaard and P. Dalgaard, Int. J. Food Microbiol., 2021, 338, 108952 CrossRef CAS PubMed.
- P. Zhong, X. Wei, X. Li, X. Wei, S. Wu, W. Huang, A. Koidis, Z. Xu and H. Lei, Compr. Rev. Food Sci. Food Saf., 2022, 21, 2455–2488 CrossRef PubMed.
- X. Chen, H. Chen, J. Xiao, J. Liu, N. Tang and A. Zhou, Food Res. Int., 2020, 138, 109717 CrossRef CAS PubMed.
- Z. Alsafra, G. Scholl and G. Eppe, J. AOAC Int., 2021, 104, 253–259 CrossRef PubMed.
- J. Yin, R. Lin, M. Wu, H. Ding, L. Han, W. Yang, X. Song, W. Li, H. Qu, H. Yu and Z. Li, Rapid. Commun. Mass Spectrom., 2021, 35, e9174 CrossRef CAS PubMed.
- M. Tosun, Food Chem., 2014, 165, 555–559 CrossRef CAS PubMed.
- J. Meng, Z. Liu, C. L. Gou, K. M. Rogers, W. J. Yu, S. S. Zhang, Y. W. Yuan and L. Zhang, J. Chromatogr. B: Analyt. Technol. Biomed. Life Sci., 2019, 1105, 104–112 CrossRef CAS PubMed.
- H. Zhong, M. Yao, H. Huang, M. Zhou and X. Zhu, A nutrient Mixi Beverage and its preparation process, China, CN110754593A, 2017.
- H. M. Wu, D. J. Xie, P. F. Jia, Z. S. Tang, D. Q. Shi, G. H. Shui, G. D. Wang and W. C. Yang, Plant Biotechnol. J., 2023, 21, 1757–1772 CrossRef CAS PubMed.
- J. H. Kim, H. A. Sim, D. Y. Jung, E. Y. Lim, Y. T. Kim, B. J. Kim and M. H. Jung, Int. J. Mol. Sci., 2019, 20, 4801 CrossRef CAS PubMed.
- N. Zhang, T. Liang, Q. Jin, C. Shen, Y. Zhang and P. Jing, Food Res. Int., 2019, 122, 191–198 CrossRef CAS PubMed.
- H. Chen, A. Venter and R. G. Cooks, Chem. Commun., 2006, 2042–2044, 10.1039/b602614a.
- Z. Wu, K. Chingin, H. Chen, L. Zhu, B. Jia and R. Zenobi, Anal. Bioanal. Chem., 2010, 397, 1549–1556 CrossRef CAS PubMed.
- Y. Liu, X. Zhang, Y. Ouyang, Z. Hu, L. Ma, J. Zhang, J. Lin and H. Chen, J. Mass Spectrom., 2011, 46, 794–803 CrossRef CAS PubMed.
- X. Y. Huang, X. W. Fang, X. Zhang, X. M. Dai, X. L. Guo, H. W. Chen and L. P. Luo, Anal. Bioanal. Chem., 2014, 406, 7705–7714 CrossRef CAS PubMed.
- J. Ding, H. Gu, S. Yang, M. Li, J. Li and H. Chen, Anal. Chem., 2009, 81, 8632–8638 CrossRef CAS PubMed.
- D. Wu, M. Cui, Y. Hao, L. Liu, Y. Zhou, W. Wang, A. Xue, K. Chingin and L. Luo, J. Agric. Food Chem., 2019, 67, 12945–12952 CrossRef CAS PubMed.
- G. Oms-Oliu, I. Odriozola-Serrano and O. Martín-Belloso, Food Res Int., 2013, 54, 1172–1183 CrossRef CAS.
- J. M. Cevallos-Cevallos, J. I. Reyes-De-Corcuera, E. Etxeberria, M. D. Danyluk and G. E. Rodrick, Trends Food Sci Technol., 2009, 20, 557–566 CrossRef CAS.
- S. Lin, Y. Bao, G. Zhu, Z. Guo, J. Chen, Y. He, R. Li and H. Li, A Pleurotus tuber-regium (Fr.) Sing Rice Dilute with Cough Relieving and Lung Moisturizing Effects and Its Preparation Method, China, CN113142552A, 2021.
- G. Chen, L. Shi, J. Wang, S. Zhu, J. Sheng, X. Yang and H. Xu, Food Addit. Contam.:Part B, 2023, 16, 176–184 CrossRef CAS PubMed.
- F. O. Sefiloglu, U. Tezel and I. A. Balcıoğlu, J. Agric. Food Chem., 2021, 69, 3298–3306 CrossRef CAS PubMed.
- J. Fan, D. Li, H. S. Chen, J. G. Huang, J. F. Xu, W. W. Zhu, J. G. Chen and F. Wang, Br. J. Pharmacol., 2019, 176, 297–316 CrossRef CAS PubMed.
- Z. Luo, L. Zhang, Y. Mou, S. Cui, Z. Gu, J. Yu and X. Ma, Anal. Bioanal. Chem., 2019, 411, 2447–2460 CrossRef CAS PubMed.
- M. Qin, Y. Qian, L. Huang, C. Zhong, M. Li, J. Yu and H. Chen, Front. Pharmacol., 2023, 14, 1110900 CrossRef CAS PubMed.
- M. Deng, T. Yu, H. Luo, T. Zhu, X. Huang and L. Luo, Front Pharmacol., 2017, 422, 111–118 CAS.
- E. S. Harris, S. Cao, B. A. Littlefield, J. A. Craycroft, R. Scholten, T. Kaptchuk, Y. Fu, W. Wang, Y. Liu, H. Chen, Z. Zhao, J. Clardy, A. D. Woolf and D. M. Eisenberg, Sci. Total Environ., 2011, 409, 4297–4305 CrossRef CAS PubMed.
- S. Shen, J. Xu, H. Chen and X. Fang, The Third National Symposium on Mass Spectrometry Analysis, Xiamen, China, 2017 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Additional data analysis results, including process flow chart, PLS-DA model, information of differential metabolites, tables of compounds identification, regression equations, LODs and LOQs of pesticide analytes. See DOI: https://doi.org/10.1039/d5an00299k |
| ‡ These authors contributed equally to this article. |
| This journal is © The Royal Society of Chemistry 2025 |