 Open Access Article
Open Access ArticleMagnetic-based tissue engineering: principles, applications, and future prospects in biofabrication
Hwanyong Choi a and
Jinah Jang
a and
Jinah Jang *abcde
*abcde
aDepartment of Mechanical Engineering, Pohang University of Science and Technology (POSTECH), Pohang, 37666, Republic of Korea. E-mail: jinahjang@postech.ac.kr
bCenter for 3D Organ Printing and Stem cells (COPS), Pohang University of Science and Technology (POSTECH), Pohang, 37666, Republic of Korea
cSchool of Interdisciplinary Bioscience and Bioengineering, Pohang University of Science and Technology (POSTECH), Pohang, 37666, Republic of Korea
dDepartment of Convergence IT Engineering, Pohang University of Science and Technology (POSTECH), Pohang, 37666, Republic of Korea
eInstitute for Convergence Research and Education in Advanced Technology, Yonsei University, Seoul, 03722, Republic of Korea
First published on 9th June 2025
Abstract
Magnetic-based tissue engineering (MagTE) is a rapidly advancing interdisciplinary field that integrates magnetic materials and external magnetic fields with tissue engineering principles to manipulate cells, biomaterials, and biological environments for developing functional tissue substitutes. This review provides a comprehensive overview of MagTE, covering its fundamentals, applications, and future directions within the biofabrication domain. The magnetic properties of paramagnetic, ferromagnetic, ferrimagnetic, and superparamagnetic materials are discussed, along with mechanisms of magnetic actuation through forces and torques. MagTE applications are categorized into cell manipulation and stimulation. Direct and indirect manipulation techniques also enable precise control of cell alignment, patterning, and assembly into complex three-dimensional structures, such as cell sheets, spheroids, and organoids. Stimulation approaches—mechanical, thermal, electrical, and biochemical—exploit interactions between magnetic particles and external fields to elicit specific physiological responses and support tissue regeneration. We then conclude by addressing the current limitations of MagTE and proposing strategies to overcome these challenges.
1. Introduction
Tissue engineering is an interdisciplinary field that integrates biomaterials, cells, and biochemical and physicochemical factors to develop biological substitutes capable of restoring, maintaining, or enhancing damaged tissues or organs.1 This field has demonstrated significant potential in addressing key challenges associated with existing medical technologies, such as organ donor shortages and immune rejection while accelerating tissue regeneration.2 Recent efforts in tissue engineering have focused on replicating the intricate three-dimensional (3D) structures and functions of the in vivo microenvironment by systematically delivering complex physical and chemical signals.3,4A notable direction within this evolving field is biofabrication, a subfield encompassing processes for artificially producing living tissues using cells and biomaterials as building blocks. Leveraging cutting-edge technologies, biofabrication achieves precision in tissue organization, cellular alignment, and functional integration.5–8 For example, the production of cellular aggregates (e.g., spheroids and organoids) utilizes self-assembling multicellular aggregates to mimic the 3D architecture and functionality of native tissues.9,10 At the same time, cell sheet methods create layered cell structures without scaffolds to facilitate integration with host tissues.11,12 In addition, bioprinting technology employs layer-by-layer deposition of bioinks—comprising cells, biomaterials, and growth factors—to construct complex tissue architectures with spatial control.2,13–19 Innovations such as cell-laden hydrogel systems20–22 and microfluidics23–26 enable dynamic simulations of tissue environments, facilitate vascularization, and address critical challenges like replicating the extracellular matrix (ECM), improving mechanical stability, and achieving functional complexity.
These advancements support customized tissue design, complex organ simulation, and automated mass production, expanding applications in regenerative medicine and the pharmaceutical industry.19,27 In regenerative medicine, biofabrication enables the design of custom implants tailored to individual patients, while in the pharmaceutical sector, biofabricated tissue models facilitate high-throughput drug screening, reduce reliance on animal testing, and enhance drug development and toxicology assessments.28,29 Moreover, biofabrication allows for disease modeling by recreating pathological conditions in vitro to study disease progression and therapeutic responses.30 Ultimately, the field aspires to create fully functional, transplantable organs, addressing organ shortages and revolutionizing organ replacement therapies.31 By integrating biological, mechanical, and chemical cues to guide cell behavior, promote tissue maturation, and mimic in vivo conditions—including gradients of oxygen, nutrients, and mechanical forces—biofabrication enables more realistic and effective tissue models.
Despite its progress, biofabrication faces challenges in replicating the functional complexity of in vivo tissues and organs.27,32,33 Current research focuses on refining fine structural details, optimizing intercellular signal transmission, and incorporating diverse cell types into tissue-specific microenvironments. Advanced vascularization strategies are being explored to ensure oxygen and nutrient supply, minimizing cell necrosis during scale-up.34–37 Additionally, advancements in equipment and materials aim to enable dynamic modulation of in vivo-like conditions after fabrication, including applying mechanical and chemical stimuli and inducing structural deformations.38,39 These capabilities are critical for simulating dynamic changes in clinical settings, such as post-implant remodeling and patient-specific stimulation protocols.
One emerging approach to address these limitations is magnetic-based tissue engineering (MagTE), which integrates magnetic materials and external magnetic fields to manipulate cells, biomaterials, and tissue environments. MagTE employs magnetic particles incorporated into cells or scaffolds, enabling remote interactions under an external magnetic field.40 For instance, magnetic-labeled cells, created by encapsulating magnetic particles within cells, can be precisely positioned and aligned using external fields. This simplifies the formation of 3D structures like spheroids and organoids while increasing efficiency.41–43 Magnetic particles also respond to external magnetic fields by generating forces, torques, vibrations, or oscillations, enabling non-invasive stimulation—including mechanical, thermal, and electrical stimuli—within tissues.44 These features allow for precise control over cell movement, alignment, drug delivery, and tissue formation, offering possibilities for patient-specific stimulation and post-implant remodeling. As such, MagTE is emerging as a transformative technology in next-generation tissue engineering.
This paper systematically examines the potential of MagTE in biofabrication. The fundamental principles of magnetic particles and magnetic fields are primarily summarized, followed by a review of current cell- and tissue-level applications. Beyond summarizing the fundamental mechanisms of magnetic actuation, this paper emphasizes the integration of MagTE into broader biofabrication workflows, exploring its roles not only in cellular and tissue assembly but also in functional maturation and clinical translation. In addition, the paper explores potential expansions of MagTE into areas such as magnetic field-based robotics, which have yet to be fully integrated into biofabrication. Finally, future directions and strategies for clinical applications of MagTE in the biofabrication field are discussed (Fig. 1).
 | ||
| Fig. 1 Illustration of an overview of this review: the fundamental principles and applications of magnetic-based tissue engineering. | ||
2. Fundamentals of magnetic-based tissue engineering
MagTE employs external magnetic fields to perform various functions essential for tissue engineering. To fully utilize MagTE, it is crucial to understand the selection and characteristics of magnetic particles, their manufacturing methods, and the properties of magnetic fields. The physical and chemical properties of magnetic particles, such as biocompatibility, remanence, and saturation magnetization, are influenced by factors such as particle size and material composition. Additionally, the applied magnetic field—whether static or dynamic—generates different operational mechanisms, including force and torque. This section will first introduce the types of magnetic particle, the methods used to implement magnetic fields and the effects these fields have on the actuation of magnetic particles.2.1. Magnetic properties of magnetic materials
To classify and understand magnetic materials, it is essential to examine several key parameters related to a material's magnetization, including magnetic susceptibility (χm), remanence (Mr), and coercivity (Hc). These parameters determine how a material responds to an external magnetic field and the magnetization state it retains once the field is removed.45Magnetic susceptibility (χm) quantifies how easily a material becomes magnetized when exposed to an external magnetic field. It is defined as the rate of change in magnetization with respect to the magnetic field, expressed as χm = ∂M/∂H. A higher susceptibility means the material exhibits significant magnetization even under a weak magnetic field. Magnetic susceptibility can be either positive (+) or negative (−), and its magnitude indicates the material's magnetization characteristics.
Generally, its magnetization increases until it reaches a point of saturation as a material is exposed to an external magnetic field. Beyond this point, further increases in the magnetic field do not result in greater magnetization. When the magnetic field is removed, the remaining magnetization is termed remanence (Mr). Materials with high remanence maintain strong magnetization even without an external magnetic field. Coercivity (Hc) is the strength of the reverse magnetic field required to remove the remanence. Materials with high coercivity are difficult to demagnetize, exhibiting permanent magnet-like properties, whereas those with low coercivity easily return to their initial state after the field is removed. These parameters provide crucial information about the magnetic behavior of materials and are essential for selecting the appropriate materials for specific applications.
Paramagnetic materials have magnetic moments that are randomly aligned in the absence of an external magnetic field, resulting in a net magnetization close to zero. When an external magnetic field is applied, the magnetic moments slightly align, producing a weak net magnetization. However, once the magnetic field is removed, the magnetization disappears due to thermal fluctuations. Although these materials exhibit positive magnetic susceptibility, their susceptibility is very small (χm ≪ 1), limiting their functionality as strong magnets. Examples include aluminum (Al), titanium (Ti), and tungsten (W) (Fig. 2A).46 Ferromagnetic materials contain magnetic domains, within which all atomic or molecular magnetic moments are aligned in the same direction, exhibiting spontaneous magnetization even without an external magnetic field. Applying an external magnetic field aligns all domains, achieving a saturated state. When the external magnetic field is removed, some domains remain aligned, resulting in significant remanence. These materials typically exhibit high magnetic susceptibility (χm ≫ 1). Examples include iron (Fe), nickel (Ni), cobalt (Co), and their alloys, which are commonly used as magnets due to their high remanence.46
Ferrimagnetic materials are similar to ferromagnetic materials in that they exhibit spontaneous magnetization. However, in these materials, some magnetic moments within the domains are aligned in opposite directions (antiparallel), although their magnitudes do not completely cancel out. This results in a non-zero net magnetization. Ferrimagnetic materials exhibit a more complex structure than ferromagnetic materials. Specifically, the spins of two groups (A and B) are aligned in opposite directions. However, the magnetic moments of these groups differ in magnitude, resulting in one side dominating and retaining strong magnetization even after the external magnetic field is removed. Ferrimagnetic materials exhibit high magnetic susceptibility and remanence, similar to ferromagnetic materials, but are structurally more complex. Representative examples include magnetite (Fe3O4) and maghemite (γ-Fe2O3) (Fig. 2C).46
Soft magnetic materials are a subset of ferromagnetic and ferrimagnetic materials with low coercivity and low remanence. These materials are easily magnetized and demagnetized, responding quickly to magnetic fields. However, they are not suitable as permanent magnets, as their remanence diminishes after the magnetic field is removed. Soft magnetic materials are primarily used in electromagnetic devices, such as transformer cores and motors, due to their low energy loss in alternating magnetic fields (AMF). Examples include carbonyl iron and silicon steel (Fig. 2C).47
Hard magnetic materials, on the other hand, have high coercivity and high remanence, allowing them to maintain strong magnetization even after the external magnetic field is removed. These materials can act as miniature permanent magnets, making them suitable for applications requiring torque-based deformations, such as rotation or bending in uniform magnetic fields. Examples include NdFeB, SmCo, and ferrites (e.g., BaFe12O19) (Fig. 2D).47
Superparamagnetic materials are typically ferromagnetic or ferrimagnetic materials that exhibit superparamagnetic behavior when the size of individual particles becomes much smaller than the magnetic domain size, on the nanometer scale. In these materials, thermal fluctuations can eliminate remanence when the external magnetic field is removed, causing their magnetization to rapidly randomize. Despite exhibiting high magnetization in the presence of a magnetic field, superparamagnetic materials lose their remanence once the field is removed. This behavior minimizes the risk of aggregation and potential side effects on surrounding tissues, making superparamagnetic materials ideal for biological and medical applications. Common examples include iron oxide nanoparticles (such as magnetite Fe3O4 and maghemite γ-Fe2O3) and gadolinium nanoparticles (Gd2O3) (Fig. 2B).48
Although magnetic classification explains their physical behavior, the decisive factor for clinical adoption is how each material interacts with living tissues. From a biomedical standpoint, superparamagnetic materials are regarded as comparatively safe because their magnetization disappears immediately after the external field is withdrawn, eliminating any residual magnetic force; clinically approved superparamagnetic iron-oxide nanoparticles (SPIONs) exemplify this benefit. Paramagnetic materials likewise lose magnetization once the field is removed, but their biocompatibility depends largely on chemical composition—gadolinium-based compounds, for example, can accumulate in tissues and raise toxicity concerns. Ferrimagnetic materials retain partial remanent magnetization, so long-term tissue residence and chronic cellular stimulation are possible; however, when these particles are downsized to the nanoscale, the associated risks are greatly reduced. Ferromagnetic materials present the greatest safety challenge: their strong remanence can impose mechanical stress on nearby cells, and corrosion or oxidation may release metal ions such as Fe3+, Co2+, or Ni2+ into the biological milieu.49
To suppress the potential cytotoxicity of magnetic particles, engineers routinely employ surface-coating strategies that physically isolate the metallic core from its environment. Biocompatible, inert layers of polyethylene glycol (PEG), poly(lactic-co-glycolic acid) (PLGA), dextran, or silica form an effective barrier, mitigating ion leaching, oxidative stress, and inflammatory responses.50 Further surface functionalization with targeting ligands or stealth coatings enhances colloidal stability, prolongs systemic circulation, and reduces nonspecific cellular uptake.51 Through these approaches, the safety of magnetic particles can be secured, thereby expanding their applicability in biomedical fields.
2.2. Principles of magnetic actuation
The actuation of magnetic particles is determined by the types of particle used and the form and characteristics of the external magnetic field applied to them. Magnetic actuation fundamentally relies on the interaction between the magnetic particles and the external magnetic field, inducing physical changes primarily through magnetic force and magnetic torque. The intensity of these effects is influenced by both the physical properties of the magnetic particles and the specific form of the external magnetic field, which collectively govern the macroscopic behavior of the materials and the resulting mode of operation.45| F = ∇(m·B), | (1) |
Magnetic torque (τ), in contrast, occurs under a uniform magnetic field and refers to the rotational force that causes the particle's magnetic moment to align with the field. This torque is expressed as:
| τ = m × B | (2) |
Magnetic torque induces rotational motion in particles, playing a crucial role in determining their orientation (eqn (2)). By adjusting the magnetic field accordingly, particles can be made to perform rotational or vibrational movements, which can then provide mechanical stimulation to cells or scaffolds, or generate heat for thermal effects. Together, the combination of magnetic forces and torques allows for the execution of a variety of tissue engineering functions depending on the specific characteristics of the magnetic particles and the external magnetic field (Fig. 3B).45
 | (3) |
Here, E denotes the induced electric field and B represents the magnetic flux density. This relationship, also known as Faraday's law, explains that when a magnetic field changes over time, an electric field is induced along a closed loop, thereby generating an electric current. Conversely, by controlling the magnitude and direction of the electric current, the strength and orientation of the magnetic field generated in a given space can be precisely modulated (eqn (3)).52 Magnetic fields are classified based on their characteristics into static and dynamic fields, as well as uniform and gradient fields. This classification is essential for selecting the appropriate magnetic field for specific applications.45 Static magnetic fields are generated using permanent magnets or direct current (DC) coils and maintain a constant strength and direction. Dynamic magnetic fields, in contrast, are generated using alternating current (AC) or electromagnetic induction, and their strength and direction vary over time. Dynamic magnetic fields can take forms such as alternating magnetic fields (AMF), rotating magnetic fields, and pulsed magnetic fields. These fields can induce repetitive movements or vibrations in magnetic particles, generating heat (hyperthermia) or applying mechanical stimulation to cells. Gradient magnetic fields are characterized by spatial variations in the magnetic field's strength and direction. These fields cause magnetic particles to experience forces that move or focus on them in specific directions. Gradient magnetic fields can easily be generated using permanent magnets or electromagnets arranged with a single pole, and are useful for adjusting the positioning or arrangement of particles. Uniform magnetic fields maintain consistent strength and direction across a specific area, typically generated using devices like Helmholtz coils. These fields are primarily used to induce rotational or bending movements in magnetic particles.
2.3. Magnetic particle sizes and their properties
Magnetic particles in MagTE enhance tissue engineering by leveraging magnetic fields at both cellular and tissue levels. Their size influences their properties and integration into cells or biomaterials, making it essential to summarize how particles of different sizes interact with cells and biomaterials and affect their functions.Magnetic nanoparticles (MNPs, 1–100 nm) possess a high surface area-to-volume ratio that facilitates efficient cellular uptake via endocytosis, enabling magnetic cellular labeling. Surface functionalization—with coatings such as carboxyl (–COOH), amino (–NH2), silanes, or polymers (e.g., PEG)—allows these nanoparticles to bind to proteins, peptides, antibodies, DNA, RNA, or signaling ligands. Thanks to these versatile properties, magnetically labeled cells are widely used for applications like cell tracking, magnetic field-guided cell manipulation, and drug delivery. Additionally, MNPs typically exhibit superparamagnetic behavior, meaning their magnetization vanishes when the external magnetic field is removed, which minimizes aggregation and enhances biocompatibility and safety. Their high magnetic moments and stability make them invaluable for tissue engineering and cell-based therapies.
Magnetic microparticles (MMPs, 1–100 μm) are significantly larger than MNPs and typically exhibit ferromagnetic or ferrimagnetic properties with stronger magnetization. Easily surface-modified for cell attachment through adsorption or covalent bonding, these particles enable magnetic cellular labeling53,54 and generate substantial forces and torques under external magnetic fields, allowing for the precise manipulation of cells into patterned structures. Moreover, MMPs can be incorporated into hydrogels or polymer scaffolds to enhance mechanical properties and promote cell attachment, with magnetic fields used to dynamically reshape scaffolds or control cell positioning. These features make MMPs indispensable for forming complex 3D tissue structures and creating dynamic environments conducive to biofabrication.
Magnetic particles—from nanoparticles to microparticles—integrate with cells and biomaterials in diverse ways, facilitating cell labeling, tissue structuring, scaffold enhancement, and precise cell manipulation. Moreover, the size of magnetic particles profoundly affects their in vivo behavior, including biodistribution, clearance, and long-term safety. MNPs typically exhibit enhanced permeability and retention effects, allowing them to accumulate in certain tissues, particularly tumors and inflamed regions. They are primarily cleared through renal excretion if their hydrodynamic diameter is below 5 nm, or via hepatobiliary routes for larger nanoparticles.55 However, as particle size increases, the management of in vivo degradation and clearance becomes increasingly challenging. Larger particles tend to exhibit slower or unpredictable degradation rates and prolonged tissue retention, complicating biocompatibility management.56 Consequently, for microscale or larger magnetic particles, direct in vivo injection is generally avoided. Instead, current applications primarily focus on their incorporation into biomaterial scaffolds, ex vivo cell manipulation prior to implantation, or external magnetic field-assisted assembly processes, thereby minimizing the need for systemic distribution and enhancing localized control. To successfully leverage magnetic particles in MagTE, it is crucial to carefully consider size-dependent properties—including magnetic behavior, biodegradation, clearance, and interaction with biological systems—during material selection and application design.
3. Magnetic-based tissue engineering
MagTE leverages the unique properties of magnetic particles, which respond to external magnetic fields, for diverse applications in tissue engineering. By incorporating magnetic particles into cells or biomaterials, these components can interact remotely with external magnetic fields. MagTE technologies, particularly in the field of biofabrication, can be broadly classified into two categories based on their primary functions: cell manipulation and cell stimulation.This section explores the development of MagTE technologies for specific purposes and methods and their subsequent applications.
3.1. Magnetic cell manipulation
A central application of MagTE is the precise control and assembly of magnetic cells. By fusing magnetic particles with cells or mixing them into cell culture media, magnetically labeled cells are created that can be non-invasively and remotely directed using magnetic fields. These fields, operating without physical contact, enable both direct and indirect cell manipulation—methods that allow cells to be aligned and patterned into desired configurations. As a result, tissue assembly processes are significantly advanced, facilitating the efficient fabrication of complex structures such as cell sheets, 3D spheroids, and organoids. These cell manipulation techniques are broadly categorized into two approaches: direct manipulation and indirect manipulation. | ||
| Fig. 4 Magnetic direct cell manipulation. (A-a) Schematic image of magnetically induced 3D cell alignment. (b) Cell chains of magnetic labeled cells were formatted by magnetic field. (c) Comparison of non-aligned and aligned cell chains. (d) The aligned myocardial tissue demonstrated more stable beating intervals. Reproduced from ref. 51 with permission from the American Chemical Society, copyright 2020. (B-a) Bright-field microscopy images of 3D spheroid formation on a magnetic platform, compared with control cultures (MNP-free), which exhibit spheroid disintegration beginning on day 4. (b) Fluorescent images of differentiated spheroids immunostained with various SG epithelial markers, including ductal and myoepithelial markers (KRT5, KRT14) and an acinar secretory epithelial marker (AQP5). (c) Representative images of mouse SG immunostained for nerves (TUBB3) and proliferative epithelial cells (Ki67), with nuclei counterstained using a nuclear dye, showing enhanced epithelial growth promoted by the transplanted organoids. Reproduced from ref. 62 with permission from Elsevier, copyright 2018. | ||
Cell sheets are single-layered structures of closely adhered cells, used extensively in biomedical applications due to their stability in tissue transplantation. Unlike traditional methods, such as injecting single-cell suspensions or attaching cultured cells to scaffolds, cell sheets provide a more reliable approach for stable tissue engineering.12,58 Furthermore, stacking multiple cell sheets creates multilayered structures used in vascularized tissues and complex biological assemblies.59 By utilizing magnetic forces in cell sheet fabrication, higher-density cell sheets can be produced more rapidly. Additionally, cell sheets can be detached from the substrate simply by toggling the magnetic field, eliminating the need for enzyme treatments. Ishii et al. effectively employed magnetic cellular labeling technology to create cell sheets for therapeutic angiogenesis via cell transplantation.60 Mesenchymal stem cells (MSCs), which are multipotent cells capable of differentiating into osteoblasts, chondrocytes, adipocytes, and smooth muscle cells, can secrete factors such as vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), and stromal cell-derived factor-1α upon transplantation, promoting vascular formation within tissues. To facilitate this, the MSCs were labeled through endocytosis of magnetite (Fe3O4) nanoparticles. Using magnetic fields, the researchers patterned the magnetized MSCs into densely packed cell sheet forms. Compared with unmagnetized MSCs, magnetized MSCs exhibited higher cell density and produced uniform, compact cell sheets. The magnetic field-induced alignment enhanced cell–cell interactions, accelerating cell sheet formation. The increased cell density further led to significantly elevated VEGF expression levels and enhanced angiogenic capability.
Spheroids, as 3D cell culture models, emulate natural cell–cell interactions by allowing cells to adhere to one another and maintain a three-dimensional structure during cultivation. Traditional spheroid fabrication methods rely on the natural aggregation of cells, which can be time-consuming and inconsistent.61,62 In contrast, the application of concentrated magnetic fields enables magnetic-labeled cells to assemble rapidly in three dimensions, resulting in more efficient spheroid formation. Magnetic spheroids, in particular, can be generated much faster than non-magnetic ones, as the magnetic particles within the cells are immediately attracted to specific locations. This process yields spheroids with a uniform size and shape, less affected by variations in cell density or culture conditions. Furthermore, magnetic spheroid fabrication platforms, designed for compatibility with well plates, minimize experimenter variability and improve reproducibility. A unique advantage of magnetic spheroids lies in their ability to remain manipulable using magnetic fields even after fabrication.63–66 Magnetic fields can be used to move or fix spheroids in specific locations with ease. Bowser et al. leveraged magnetic cellular labeling technology to mimic the hierarchical structure of the nervous system.67 Spinal cord cells were labeled with MNPs attached to their membranes and aggregated into spheroids using magnetic fields. During the bioprinting process, these spheroids were precisely positioned within hydrogels using external magnetic fields, enabling accurate spheroid arrangement. Adjustments to the spacing and orientation of the magnetic spheroids further enhanced neurite outgrowth and the electrical activity of the neural network compared with conventional spheroids. This study demonstrated that magnetic spheroids significantly improved cell alignment and network formation, offering superior precision and efficiency.
Magnetic organoids can similarly be manipulated through precise assembly and patterning. Unlike spheroids, organoids feature more complex structures and functions, making their fabrication challenging. MagTE enables the production of magnetic organoids with high precision. Adine et al. utilized magnetic cellular labeling technology to rapidly fabricate innervated salivary gland (SG) organoids.68 Human dental pulp stem cells (hDPSCs) were magnetized using a nanoparticle solution containing gold and iron oxide nanoparticles. The magnetized cells were efficiently and stably placed in ultra-low attachment 96-well plates and assembled into magnetic spheroids using neodymium magnets (Fig. 4B-a). Subsequently, fibroblast growth factor 10 (FGF10) signaling factor was introduced to differentiate the spheroid cells into ductal and myoepithelial salivary gland cell types while promoting the expression of secretory epithelial markers (Fig. 4B-b). This process successfully generated magnetic organoids containing diverse and functionally complex cell types. When implanted into damaged salivary glands in mouse models, these organoids stimulated epithelial growth and neural network regeneration, underscoring the potential of magnetic cell manipulation for therapeutic applications (Fig. 4B-c).
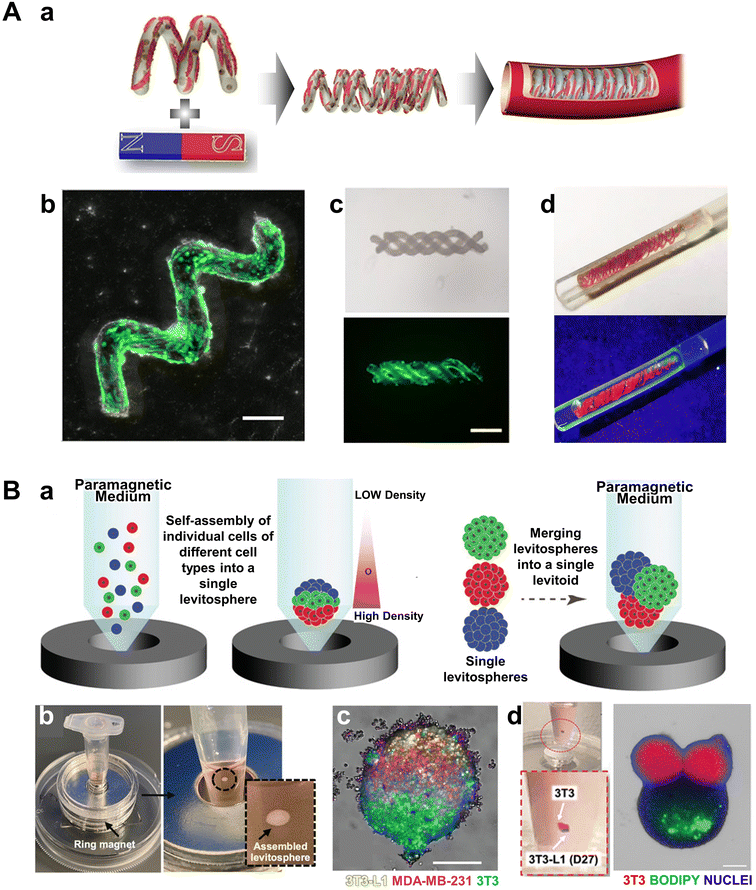 | ||
| Fig. 5 Magnetic indirect cell manipulation. (A-a) Schematic image of helical micromotors as dynamic cell microcarriers. (b) Confocal laser scanning microscopy images of the helical micromotor seeded with cells. (c) Bright-field and fluorescence microscopy images of the formed triple-helical microcarriers. (d) Images of micromotor-assembled tube packed in a hydrogel and kept in a microchannel. Reproduced from ref. 64 with permission from the American Chemical Society, copyright 2020. (B-a) Schematic image of heterogeneous multicellular levitosphere and density-based spatial organization of levitospheres into levitoids under levitation. (b) Assembled levitosphere within the ring magnet-based levitation platform. (c) Fluorescent image of a heterogeneous levitosphere composed of 3T3, 3T3-L1, and MDA-MB-231 cells, showing cell positioning based on their inherent density, resulting in the formation of distinct layers. (d) Confocal image of a levitoid formed by the assembly of prefabricated 3T3-L1 and 3T3 levitospheres. Reproduced from ref. 68 with permission from John Wiley and Sons, copyright 2022. | ||
Beyond magnetic spheroid formation, advanced techniques have been developed for spheroid fabrication and 3D multispheroid assembly without the need for magnetic cell labeling. These methods aim to harness the benefits of efficient 3D tissue formation through magnetic self-assembly while minimizing any potential effects of magnetic labeling on cell behavior. One such approach involves attaching magnetic particles to the surfaces of hydrogel microcapsules rather than directly labeling the cells themselves. Takeuchi et al. introduced a magnetic self-assembly procedure to create microstructured assemblies between toroid-shaped hydrogel microcapsules.72 In this method, magnetic particles were affixed to the surfaces of the toroids, which were then placed in a culture dish. By applying vibrations at 80–90 rpm, the researchers successfully induced the formation of 3D multispheroid tubular structures using magnetic forces. Hepatocytes were encapsulated within the hydrogel capsules to preserve their viability and function. The assembly process was optimized through the application of magnetic fields and vibration, allowing the structures to demonstrate functional hepatocyte characteristics, including albumin secretion and urea synthesis. This approach confirmed the feasibility of creating high-density cell aggregates while avoiding direct contact between magnetic particles and the cells.
Another technique, magnetic levitation, involves adding paramagnetic salts to the culture medium, enabling cells to float based on density differences in the presence of a magnetic field. In this floating state, cells align at the point where the forces of the magnetic field and gravity balance, naturally forming uniform 3D spheroids through cell–cell interactions. Unlike magnetic spheroids, which typically require single cells and uniform structures, this method allows for the hierarchical assembly of spheroids composed of multiple cell types, leading to the spontaneous formation of multilayered structures.73 Moncal et al. advanced this concept by developing a levitoid—a levitated spheroid assembly with a hierarchical arrangement (Fig. 5B-a and b).74 They first assembled various cell types (3T3, MDA-MB-231, 3T3-L1) into a multicellular structure called a levitosphere using magnetic levitation (Fig. 5B-c). Within the levitosphere, cells naturally layered according to density differences, based on cell type, resulting in a precise layer-by-layer arrangement (Fig. 5B-d). Additionally, the team then combined the levitospheres, each with a different cell composition, to form more complex 3D structures referred to as levitoids. By using magnetic forces to design the 3D tissue assemblies and spatial organization of cells, they presented an innovative approach for fabricating multicellular structures with complex architectures. A summary of these studies is provided in Table 1.
| Manipulation type | Magnetic particle type | Constructed structure | Primary function | Author | Ref. |
|---|---|---|---|---|---|
| Direct manipulation | Maghemite nanoparticles | 3D cardiomyocyte chains | Enhance contraction strength and functional heart tissue replication | Richard et al. | 57 |
| Magnetite nanoparticles | Mesenchymal stem cell sheets | Therapeutic angiogenesis through VEGF expression enhancement | Ishii et al. | 60 | |
| Magnetic nanoparticles | Spinal cord spheroids | Mimic neural hierarchy for improved neurite outgrowth and neural network activity | Bowser et al. | 67 | |
| Gold and iron oxide nanoparticles | Innervated salivary gland organoids | Stimulate epithelial growth and neural network regeneration in damaged salivary glands | Adine et al. | 68 | |
| Indirect manipulation | Magnetic nanoparticles | Helical hydrogel-based micro/nanomotors | Enable cell attachment, proliferation, and composite structure formation for tissue regeneration | Yu et al. | 70 |
| Magnetic nanoparticles | Helical micro/nanomotors | Improve cell-loading capacity, motility efficiency, and precise cell positioning during transport | Jeon et al. | 71 | |
| Ferrite microparticles | 3D multispheroid tubular structures | Create high-density cell aggregates while preserving hepatocyte viability and function | Takeuchi et al. | 72 | |
| Gadolinium salts | Layered multicellular 3D levitoids | Achieve precise layer-by-layer assembly of multicellular structures for complex 3D tissue engineering | Moncal et al. | 74 |
3.2. Magnetic stimulation
When an external magnetic field interacts with magnetic particles, it can induce various physical effects, such as magnetic force, torque, vibration, and oscillation. These effects depend on factors such as the intensity and form of the magnetic field (e.g., static or dynamic), as well as the type, size, and shape of the magnetic particles. Through these interactions, magnetic particles can apply mechanical, thermal, or biochemical stimulation to cells or tissues, triggering specific physiological responses. In this way, magnetic particles facilitate cell stimulation by delivering precise, non-invasive stimuli; when applied to magnetically labeled cells or magneto-responsive scaffolds, the resulting forces and motions can regulate cell growth and differentiation, enhance drug delivery, and support tissue formation.Cells sensitive to mechanical stimulation, such as endothelial cells (ECs) and mesenchymal stem cells (MSCs), respond rapidly to these forces by maturing or secreting factors necessary for angiogenesis and tissue regeneration. Mechanical stimulation delivery methods can be broadly classified into two categories: (1) directly magnetizing the cells (magnetically labeled cells) to pull or rotate the cells with magnetic fields, and (2) magnetizing the scaffold (magneto-responsive scaffold) to deform the scaffold with magnetic fields, indirectly transmitting forces to the cells.
In the first approach, cells respond directly to the magnetic field, which makes it easier to construct microtissues, such as cell aggregates and organized structures. In the second approach, the deformation of the scaffold generates compressive, tensile, and shear forces on the cells, promoting tissue regeneration in a 3D environment. Yang et al. magnetically labeled cells and assembled them into 3D microtissues using external magnetic fields.78 By applying additional magnetic fields, they provided mechanical stimulation to these microtissues. The shapes of the microtissues were deformed, and during deformation, cell proliferation was maintained and angiogenesis was promoted. Similarly, Abdel Fattah et al. explored a method for guiding tissue development through localized mechanical stimulation using MNPs.79 Traditional in vitro models are limited in their ability to accurately replicate the native biological environment because they only permit uniform external stimulation. To address this limitation, the research team developed magnetoids, which are magnetically responsive organoids created by incorporating magnetically labeled cells that generate mechanical forces in specific regions (Fig. 6A-a). Within the magnetoid, cells containing magnetic nanoparticles responded to an external magnetic field, causing morphological changes in designated directions (Fig. 6A-b). This interaction resulted in F-actin rearrangement and cytoskeleton remodeling. Applying a magnetic field facilitated the asymmetric growth of the tissue, promoting the formation of structured patterns essential for shaping tissue morphology and function (Fig. 6A-c). This study demonstrates that magnetic nanoparticle-mediated mechanical stimulation is a powerful tool for selectively controlling specific regions within cells, offering a novel approach for tissue engineering and regenerative medicine.
 | ||
| Fig. 6 Magnetic mechanical stimulation. (A-a) Schematic image of magnetoid generation. (b) Schematic representation of tissue displacement, along with tissue tracing measurement images and the corresponding heatmap. (c) Integrated fate expressions in scaled organoids, color-coded by expression frequency. Pattern formation of FOXA2 (ventral floor plate marker), NKX6.1 (intermediate domain marker), and PAX6 (dorsal marker) is induced in neural tissues. Reproduced from ref. 73 with permission from Springer Nature, copyright 2023. (B-a) Schematic image of the engineered hammock-shaped platform, featuring polyethylene terephthalate (PET) membranes secured onto a sliding hammock holder, specifically designed to fit into individual media-filled wells of a well plate. (b) Representative images of F-actin and cytokeratin in SAECs cultured under static and dynamic actuation conditions, showing enhanced expression of F-actin and cytokeratin under dynamic conditions. (c) Quantification of F-actin and cytokeratin expression in SAECs cultured under static and dynamic actuation conditions, demonstrating increased expression under dynamic conditions. Reproduced from ref. 76 with permission from Springer Nature, copyright 2024. | ||
An example of the second approach is described by Manjua et al., who applied physical deformation to magnetically actuated scaffolds using external magnetic fields.80 This mechanical stress on MSCs led to an increased secretion of physiologically active molecules, such as VEGF, and promoted the creation of vascularized 3D tissues. Spangenberg et al. aimed to support cartilage and bone tissue regeneration by developing magnetic hydrogel scaffolds capable of providing mechanical stimulation through magnetic deformation.81 They created magnetic inks by mixing magnetic microparticles (Fe3O4, 25% w/w) with alginate (alg) and methylcellulose (MC) to fabricate magnetically actuable scaffolds using 3D bioprinting techniques. They confirmed that the scaffolds deformed under external magnetic fields and found that adjusting the strand distance and scaffold design allowed for fine-tuning of the degree of deformation during stimulation. This demonstrated the potential to develop systems that could provide appropriate mechanical stimulation when cells are incorporated into the scaffolds. Wei et al. used magnetic fields to simulate the effects of mechanical forces and curvature on alveolar epithelial cells.82 They created a platform that applied the desired curvature and mechanical forces to a single-layered lung epithelium (Fig. 6B-a). By fabricating magnetically active PDMS (MagPDMS) films containing NdFeB particles, they created a hammock-shaped culture platform. When magnetic fields were applied using electromagnets, the scaffold deformed, inducing mechanical stimulation (Fig. 6B-b). The curvature of the hammock scaffold promoted the formation of a single-layered small airway epithelial cell (SAEC) epithelium and mechanical responses, confirming that dynamic stimulation through magnetic activation enhanced cell proliferation and cytoskeletal reinforcement (increased expression of F-actin and cytokeratin) (Fig. 6B-c). Thus, mechanical stimulation using magnetic particles offers several advantages over traditional physical stimulation devices. It is less invasive and allows for precise spatiotemporal control. As a result, magnetic stimulation is expected to play an increasingly important role in tissue engineering and regenerative medicine in the future.
The use of magnetic particles offers a non-invasive and precise method for applying thermal stimulation. This approach is based on the principle that magnetic particles when exposed to dynamic magnetic fields, specifically alternating magnetic fields (AMF), generate heat as their magnetic moments realign. By adjusting the intensity and frequency of the magnetic field, localized heat can be generated at specific locations, reducing the risk of damage to surrounding healthy tissues while precisely targeting the desired area. This technique, known as magnetic hyperthermia, has emerged as a prominent method for providing thermal stimulation in tissue engineering and regenerative medicine. In tissue regeneration, mild heat stimulation (41–42 °C) activates hypoxia-inducible factor (HIF-1α) in cells such as osteoblasts and chondrocytes, leading to increased expression of angiogenic factors like VEGF.83 This process promotes cell proliferation and differentiation, facilitating tissue repair and regeneration. Additionally, magnetic hyperthermia is employed in cancer treatment, bacterial infection management, and neural tissue regeneration, where it aids in tissue repair and maturation by activating key intracellular signaling pathways. Meikle et al. developed a magnetically controlled VEGF delivery system that uses superparamagnetic Fe3O4 nanoparticles functionalized with thermoresponsive poly(ε-lysine) dendrons and carboxybetaine (Fig. 7A-a).84 This configuration allows a strong binding of VEGF and its controlled release upon exposure to an alternating magnetic field (AMF). By applying a precisely tuned magnetic field, localized heating at 42 °C triggered the collapse of the dendron structures, enabling targeted VEGF release while minimizing premature diffusion at physiological temperatures (Fig. 7A-b). Unlike conventional VEGF delivery methods, which often struggle with sustained and gradient-based release, this approach provides a non-invasive and responsive platform for growth factor administration (Fig. 7A-c). Rosenfeld et al. demonstrated the potential of magnetothermal stimulation as a non-invasive method to enhance nerve regeneration by utilizing the heat dissipation properties of MNPs under alternating magnetic fields (AMF).85 Their study targeted the activation of heat-sensitive TRPV1 ion channels in dorsal root ganglion (DRG) explants, which triggered calcium influx and promoted axonal growth (Fig. 7B-a). They found that magnetothermal stimulation at physiologically relevant temperatures (around 42 °C) significantly increased neurofilament elongation and Schwann cell migration, both essential for effective nerve repair (Fig. 7B-b). Importantly, these effects were negated when a TRPV1 antagonist was introduced, as shown in Fig. 7B-c, which displays markedly reduced neurofilament outgrowth in DRG explants. This result confirms that the observed axonal elongation was specifically mediated by TRPV1 activation rather than nonspecific thermal stimulation. Unlike traditional thermal stimulation methods that rely on direct heating elements, this approach enables precise, remote, and localized heat application, thereby minimizing damage to surrounding healthy tissues. These findings highlight magnetothermal modulation as a promising, minimally invasive strategy for promoting tissue regeneration, particularly in neural repair applications. Yamaguchi et al. developed a magnetically controlled gene expression system using magnetite nanoparticles that generate localized heat up to 43–45 °C under an alternating magnetic field (AMF), thereby activating the heat shock protein (HSP70B′) promoter.86 This localized heating enabled a precise and remote induction of therapeutic gene expression, specifically tumor necrosis factor-α (TNF-α), in both in vitro and in vivo cancer models. By incorporating a transcriptional positive feedback loop with a tetracycline-responsive transactivator system, they significantly amplified gene expression upon AMF exposure, resulting in enhanced cytotoxic effects in cancer cells. This system provides a non-invasive approach for spatially and temporally regulating gene expression, offering potential applications in gene therapy and regenerative medicine. Unlike traditional thermal stimulation methods that rely on direct heating elements, this technique leverages magnetic nanoparticles to generate heat locally, minimizing collateral damage to the surrounding tissues while maximizing therapeutic efficacy.
 | ||
| Fig. 7 Magnetic thermal stimulation. (A-a) Schematic image of surface-functionalized superparamagnetic nanoparticles conjugated with thermoresponsive poly(epsilon-lysine) dendrons, tethered with carboxybetaine, enabling mild hyperthermia-controlled delivery of VEGF. (b) A graph presenting the results of the magnetic hyperthermia experiment. The magnetic hyperthermia analysis of Fe3O4@PAA and Fe3O4@PAA-C-ELP-G3K-Bet16 demonstrated that the latter exhibited reversible structural changes (ELP oscillation and collapse) at specific temperatures, indicating its potential for temperature-controlled drug delivery and hyperthermia therapy. (c) Release of hVEGF from Fe3O4@PAA-C-ELP-G3K-Bet16-VEGF after hyperthermia treatment. Reproduced from ref. 78 with permission from Elsevier, copyright 2016. (B-a) Schematic image of AMF-based DRG stimulation utilizing the magnetothermal effect of MNPs to activate TRPV1 and induce calcium influx. (b) Temperature curve of MNPs in response to AMF. (c) Immunostaining and image analysis of DRGs post-stimulation, quantifying neurofilament (NF) fluorescence intensity in 50 radial locations using a confocal microscopy-based algorithm. Reproduced from ref. 79 with permission from John Wiley and Sons, copyright 2022. | ||
To address these challenges, wireless electrical stimulation technologies that leverage magnetic particles have been developed. These technologies significantly reduce invasiveness by generating local electric fields in response to external magnetic fields. The underlying principle behind this technique is magnetoelectric coupling, which combines magnetostriction and piezoelectricity. Magnetostriction refers to the property of certain magnetic materials to physically deform when exposed to a magnetic field, while piezoelectricity is the ability of certain materials to generate an electric field when mechanically deformed. For example, magnetostrictive materials like CoFe2O4 undergo magnetic domain rearrangement and physical deformation when exposed to an external magnetic field. This deformation is transferred to piezoelectric materials such as BaTiO3, which then generates electric fields. These composite materials, typically consisting of a magnetostrictive core and a piezoelectric shell, are commonly known as magnetoelectric particles (MEPs).87
By adjusting the intensity and frequency of the external magnetic field, the magnitude and frequency of the electric signals generated by MEPs can be remotely controlled. This MEP-based wireless electrical stimulation technology allows electric fields to be delivered to cells or tissues solely through magnetic fields, eliminating the need for invasive electrode implantation and overcoming the limitations of traditional electrical stimulation systems. Qi et al. advanced the application of MEP-based wireless electrical stimulation by creating a scaffold embedded with CoFe2O4 (CFO) and BaTiO3 (BT) nanoparticles (Fig. 8A-a).88 Leveraging the magnetoelectric coupling effect, the scaffold generated localized electric fields in response to external magnetic fields. This wireless system effectively modulated cellular behavior, promoting cell proliferation and differentiation, and upregulating osteogenic markers such as Col-I, OCN, and Runx2 (Fig. 8A-c). Importantly, their findings confirmed that these biological effects resulted from the electrical stimulation within the scaffold rather than the magnetic field alone (Fig. 8A-b). These results highlight the potential of MEP-based wireless electrical stimulation as a non-invasive and efficient approach for tissue engineering applications, particularly in bone regeneration. Similarly, Zhang et al. explored the potential of MENPs for wireless neural stimulation, demonstrating that MENPs can synchronize neural activity with externally applied magnetic fields.89 They utilized CoFe2O4@BaTiO3 core–shell nanostructures to generate localized electric fields, effectively activating calcium transients and action potentials in hippocampal neurons. Furthermore, by comparing MENP-induced activation with traditional electrode-based stimulation, they confirmed that the observed neural responses were mediated by electrical signals generated by the MENPs rather than by the magnetic field alone. These findings highlight the potential of MENP-based wireless neural stimulation as a non-invasive and efficient approach for neural therapies.
 | ||
| Fig. 8 Magnetic electrical stimulation. (A-a) Schematic image of magnetically driven wireless electrical stimulation within a scaffold and the proposed mechanism of remote electrical stimulation influencing cell behavior. (b) Alizarin red staining image. It shows mineralized nodules, where alizarin red binds to calcium ions through chelation, forming a fuchsia-colored complex. (c) Expression levels of osteogenic markers Runx2, Col-I, and OCN were analyzed, showing enhanced expression when both CFO and BT were present within the scaffold compared with other conditions. Reproduced from ref. 82 with permission from Elsevier, copyright 2022. | ||
To address these challenges, non-invasive biochemical stimulation using external magnetic fields and magnetic particles has emerged as a promising alternative. These methods generally rely on magnetic fields and particles to concentrate bioactive substances at specific target sites, such as damaged tissues. This is achieved by inducing local environmental changes—such as pH alterations, enzymatic activity, or mechanical and thermal stimuli—that trigger the release of bioactive substances precisely where needed. Magnetic field-based stimulation facilitates the accurate delivery of therapeutic agents like drugs, proteins, and genes, which are loaded onto MNPs. This method offers the advantage of precise targeting, higher efficacy, and the use of lower concentrations over shorter durations, making it especially useful in tissue engineering. First, these bioactive substances can be directly delivered to target sites using an external magnetic field. MNP surface modifications by physically or chemically conjugating growth factors or therapeutic proteins to the surface of MNPs, such as coating with polymers (PEG, PVA, PLGA), functionalization with silica (SiO2), or conjugation with liposomes, enable the binding of various biomolecules to the MNP surface. Through these surface modifications, MNPs can effectively load chemical anticancer drugs (e.g., doxorubicin, paclitaxel), proteins, and genes (plasmid DNA, siRNA).93,94 This selective delivery method ensures the efficient accumulation of bioactive substances in damaged tissues or within deep areas of 3D scaffolds, where traditional delivery routes like blood flow are ineffective. However, simple surface modification methods have limitations in increasing drug-loading capacity or delivering multiple biomolecules simultaneously. Utilizing microcarriers made using MNPs offers several advantages over mere surface modifications. Microcarriers can increase the drug-loading capacity and allow for the simultaneous delivery of multiple bioactive molecules. Additionally, as structural supports, microcarriers maintain stable distribution within 3D scaffolds, enabling localized and sustained drug release. These advantages suggest that microcarrier-based systems can serve as more effective drug delivery vehicles in tissue regeneration and therapeutic applications. Yang et al. further validated this approach by developing MNP-incorporated microcarriers designed for the localized and controlled release of bioactive factors in tissue regeneration.95 Utilizing an external magnetic field, they directed these functionalized microcarriers to specific areas within 3D scaffolds, thereby enhancing the retention and bioavailability of growth factors such as BMP2 and VEGF. This targeted delivery resulted in significant improvements in osteogenic differentiation and vascularization, demonstrating the potential of magnetically responsive biomaterials in regenerative medicine. Their findings support the effectiveness of magnetic targeting in overcoming the limitations of conventional drug delivery methods, providing a more efficient strategy for directing therapeutic agents to hard-to-reach tissues.
Another notable technique is magnetofection, in which nucleic acid-loaded magnetic particles are rapidly attracted to the cell surface by external magnetic fields. This process is followed by endocytosis or other internalization mechanisms, resulting in higher gene delivery efficiency compared with conventional transfection methods. Magnetofection has proved particularly effective in cell cultures and 3D tissues, and it is especially beneficial for introducing stable genetic material into hard-to-transfect cells, such as stem or primary cells. This method shows promise for gene editing applications, including the introduction of growth factors, cytokines, and neural recovery proteins within tissues to stimulate regeneration and angiogenesis. Prosen et al. demonstrated the effectiveness of this approach by utilizing SPIONs functionalized with poly(acrylic) acid (PAA) and polyethyleneimine (PEI) for gene delivery into melanoma cells.96 Their study highlighted the robustness and reproducibility of the method, as the SPION–PAA–PEI complexes efficiently bound plasmid DNA and facilitated transfection under an external magnetic field. Compared with conventional non-viral gene delivery techniques, this strategy significantly enhanced transfection efficiency while maintaining high cell viability. Heun et al. further advanced this concept by integrating lentiviral vectors with MNPs to achieve targeted gene delivery using an external magnetic field (Fig. 9A-a).97 Their study demonstrated that this magnetically guided lentiviral system enabled precise, site-specific transduction, particularly in wound healing applications where HIF-1α-dependent angiogenesis was modulated (Fig. 9A-b). By leveraging magnetic targeting, they enhanced gene transfer efficiency while minimizing off-target effects (Fig. 9A-c). These findings highlight the potential of magnetically enhanced viral and non-viral gene delivery methods in regenerative medicine, offering improved precision and efficacy for therapeutic applications.
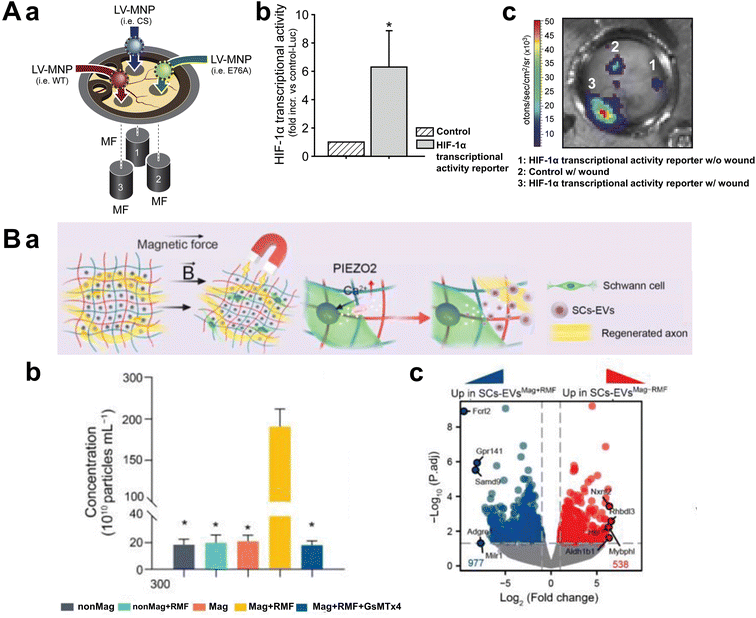 | ||
| Fig. 9 Magnetic biochemical stimulation. (A-a) Schematic image of lentiviral magnetic targeting of SHP-2. Lentiviral vectors carrying expression constructs for SHP-2 mutants (WT, CS, and E76A) and HIF1-TRE-Luc were conjugated with magnetic nanoparticles and individually applied to wounds under magnetic field exposure. (b) HIF-1α transcriptional activity. HIF-1α expression and activity in wounds were assessed using a lentiviral HIF-1 transcriptional activity reporter (HIF1-TRE-Luc) and compared with control transduction. (c) The representative bioluminescence image of luciferase activity in wounds within the dorsal skinfold chamber after magnetically assisted transduction with the HIF-1α transcriptional activity reporter and control, confirming increased HIF-1α activity only in the magnetically targeted lentiviral system. Reproduced from ref. 91 with permission from Elsevier, copyright 2017. (B-a) Schematic image of magneto-mechanical actuation of encapsulated Schwann cells in Mag-gel. (b) Quantification of EVs derived from SCs with different treatments by NTA. EV secretion increased by 18.5-fold upon applying RMF to Mag-gel. (c) Volcano plot of differentially expressed genes between SCs-EVs(Mag + RMF) and SCs-EVs(Mag-RMF). Red dots indicate significantly upregulated genes in SCs-EVs(Mag + RMF), blue dots represent significantly upregulated genes in SCs-EVs(Mag-RMF), and gray dots denote non-significant changes. The results demonstrate increased expression of genes related to axon growth, angiogenesis, and inflammation regulation in EVs following magnetic field application. Reproduced from ref. 94 with permission from John Wiley and Sons, copyright 2023. | ||
Therapeutic agents donors can also be loaded into magnetically responsive hydrogels or micro/nano-scaffolds. By applying alternating magnetic fields (AMF) or static magnetic fields, these materials can undergo shape or volume changes or increase in temperature, enabling controlled drug release at specific times. Such systems are advantageous in tissue engineering as they can simultaneously provide mechanical stimulation (through scaffold deformation) and biochemical signals (via drug release), broadening their applications in areas like angiogenesis and neural regeneration. For instance, when nitric oxide (NO) is bound to MNPs, exposure to an external magnetic field can trigger heating or vibration, releasing NO in a spatiotemporal manner at desired locations. Chiang et al. demonstrated the potential of magnetically controlled NO release for neural regeneration by developing a wirelessly chargeable gold yarn-dynamo (GY) system integrated into a silk microneedle platform.98 Their study utilized alternating magnetic fields (AMF) to induce localized heating and electrical stimulation, thereby triggering the on-demand release of NO from poly(S-nitrosoglutathione) (pGSNO) donors. This method effectively promoted neurogenesis and angiogenesis while reducing inflammatory responses in a traumatic brain injury (TBI) model. By integrating mechanical, electrical, and biochemical cues within a single system, they provided a non-invasive strategy for enhancing neural repair, highlighting the versatility of magnetically responsive biomaterials in regenerative medicine.
Additionally, extracellular vesicles (EVs) represent another promising application for magnetically controlled drug release platforms. EVs play pivotal roles in intercellular communication and are rich in beneficial proteins and miRNAs derived from stem cells or immune cells which aid in tissue repair and regeneration. Several studies have explored the use of magnetic particles to encapsulate EVs or load magnetic particles into cells, allowing for the concentration of EVs under magnetic fields at high densities. This approach offers a safer and more convenient alternative to cell therapies. Kim et al. utilized MSCs encapsulating MNPs to generate magnetic EVs, enhancing targeting precision and therapeutic effectiveness for ischemic lesions.99 Xia et al. reported that applying mechanical stimulation to Schwann cells within a magnetically responsive hydrogel scaffold induced EV secretion, thereby promoting the recovery of peripheral nerve injury sites (Fig. 9B-a).100 The magnetic scaffold, composed of polyacrylamide/hyaluronic acid hydrogel embedded with MNPs coated with PEG/polyethyleneimine (PEI), was highly sensitive to external rotating magnetic fields. Transcriptomic analysis revealed that magnetic stimulation enriched transcripts associated with axonal growth, angiogenesis, and inflammation regulation within the hydrogel, ultimately optimizing nerve regeneration in vivo (Fig. 9B-b and c). A summary of these studies is provided in Table 2.
| Stimulation type | Magnetic particle type | Stimulation strategy | Target cells | Constructed structure | Primary function | Author | Ref. |
|---|---|---|---|---|---|---|---|
| Mechanical stimulation | Magnetic nanoparticles | Direct stimulation of magnetic-labeled cells | Endothelial cells | 3D microtissues | Angiogenesis | Yang et al. | 78 |
| Magnetic nanoparticles | Direct stimulation of magnetic-labeled cells | Human pluripotent stem cells | Magnetoid | Patterning through local mechanical forces | Abdel Fattah et al. | 79 | |
| Magnetic scaffolds | Deformation of magneto-responsive scaffold | Mesenchymal stem cells | Magnetic scaffold | Vascularization | Manjua et al. | 80 | |
| Magnetite nanoparticles | Deformation of magneto-responsive scaffold | Cartilage, bone tissue | Magnetic scaffold | Regeneration | Spangenberg et al. | 81 | |
| NdFeB particles | Deformation of magneto-responsive scaffold | Alveolar epithelial cells | Magnetic scaffold | Cytoskeletal reinforcement | Wei et al. | 82 | |
| Thermal stimulation | Magnetite nanoparticles | Magnetothermal drug release | Osteoblasts, chondrocytes | Magnetic scaffold | VEGF release | Meikle et al. | 84 |
| Magnetic nanoparticles | Magnetothermal drug release | Dorsal root ganglion neurons | Neural network | Axon growth | Rosenfeld et al. | 85 | |
| Magnetite nanoparticles | Magnetothermal gene expression activation | Cancer cells | Tumor model | Gene therapy | Yamaguchi et al. | 86 | |
| Electrical stimulation | CoFe2O4@BaTiO3 core–shell nanostructures | Wireless magnetoelectric stimulation with MENPs | Bone cells | Magnetoelectric scaffold | Osteogenic differentiation | Qi et al. | 88 |
| CoFe2O4@BaTiO3 core–shell nanostructures | Neural activity synchronization with MENPs | Hippocampal neurons | MENP-based scaffold | Neuronal activation | Zhang et al. | 89 | |
| Biochemical stimulation | MNP-incorporated microcarriers | Targeted growth factor delivery | Stem cells | Microcarrier System | Growth factor delivery | Yang et al. | 95 |
| SPIONs functionalized with poly(acrylic) acid and PEI | Magnetically enhanced gene delivery | Melanoma cells | Magnetic gene delivery | Transfection enhancement | Prosen et al. | 96 | |
| Lentiviral vectors with MNPs | Lentiviral transduction via magnetic targeting | Wound healing cells | Magnetic lentivirus | Angiogenesis | Heun et al. | 97 | |
| Gold yarn-dynamo with poly(S-nitrosoglutathione) | Magnetic stimulation-induced NO secretion | Neurons | Silk microneedle system | Nerve repair | Chiang et al. | 98 | |
| Magnetic nanoparticles | Magnetic stimulation-induced EV secretion | Mesenchymal stem cells | EV therapy system | Ischemic repair | Kim et al. | 99 | |
| Magnetic nanoparticles | Magnetic stimulation-induced EV secretion | Schwann cells | Magnetic hydrogel | Nerve regeneration | Xia et al. | 100 |
4. Conclusions and outlook
Here, we have thoroughly examined the principles, applications, and prospects of MagTE within the broader context of biofabrication. By exploring the magnetic properties of various materials, the mechanisms of magnetic actuation, and the diverse applications of MagTE in cell manipulation and stimulation, we have highlighted its pivotal role in advancing tissue engineering and regenerative medicine. Our discussion has emphasized the effectiveness of MagTE in generating complex 3D structures, including cell sheets, spheroids, and organoids, through precise magnetic labeling and manipulation. Case studies, such as the alignment of cardiomyocytes to enhance contractility and the creation of vascularized tissue structures using magnetic scaffolds, demonstrate MagTE's potential to replicate and improve upon the functional complexities of natural biological systems.One of MagTE's key strengths is its ability to simplify biofabrication processes while ensuring high precision in the arrangement of cells and scaffolds. Traditional biofabrication techniques often require labor-intensive and time-consuming steps to achieve desired cellular architectures. In contrast, MagTE utilizes magnetic fields to non-invasively and rapidly organize cells and biomaterials, significantly reducing production time and enhancing reproducibility. This streamlined approach is especially beneficial for large-scale tissue production and the fabrication of intricate 3D structures, facilitating the advancement of personalized regenerative therapies. Although the cost-effectiveness of MagTE has not yet been fully validated in practical settings, improvements in fabrication productivity and tissue quality are expected to secure significant economic benefits, further supporting the widespread adoption of MagTE-based approaches in the future. Furthermore, MagTE's versatility in providing mechanical, thermal, electrical, and biochemical stimulations via magnetic actuation expands its scope, enabling the creation of dynamic and responsive tissue environments that closely mimic in vivo conditions.
Despite these advancements, MagTE faces several challenges that hinder its broader clinical and industrial application. Most notably, concerns about the long-term biocompatibility and safety of magnetic particles remain significant. Although magnetic particles, such as SPIONs, are generally regarded as biodegradable and biocompatible, the risks of long-term accumulation and cytotoxic effects, particularly under large-scale application, remain unresolved.101 For clinical translation, meeting stringent regulatory standards is essential, including comprehensive safety evaluations for materials with magnetic properties.102 In addition to long-term biocompatibility and safety concerns, several technical bottlenecks also hinder the expansive development of MagTE technologies. A major limitation lies in the attenuation of magnetic force and torque with increasing distance, which presents a significant obstacle in scaling MagTE approaches from localized cellular to organ-level tissue constructs. The strength of a magnetic field decreases rapidly with distance; specifically, in the case of a dipole field, the magnetic field strength is inversely proportional to the cube of the distance (∝1/r3). This steep decline makes it extremely challenging to achieve uniform and sufficient magnetic manipulation across large and thick tissue constructs. As the size of the target tissue increases, the magnetic gradient becomes weaker and more heterogeneous, making it difficult to maintain precise control over cellular alignment and scaffold deformation throughout the entire structure. Furthermore, compensating for this distance-related attenuation by applying stronger external magnetic fields may introduce additional risks of mechanical and thermal stress to cells. High-intensity magnetic fields can directly exert mechanical stress on cells, negatively impacting their viability, adhesion, and differentiation processes. Moreover, excessive magnetic hyperthermia induced by AMF could compromise the biological integrity of the engineered tissues. These challenges underscore the need for continued research to improve the properties of magnetic materials, reduce cytotoxic effects, and develop robust magnetic control systems capable of managing larger and more complex tissue constructs.
To overcome these obstacles and fully realize MagTE's potential, it is crucial to integrate it more effectively with other emerging biofabrication technologies. For example, combining MagTE with 3D bioprinting could enhance the precision and complexity of tissue structures by enabling the simultaneous magnetic manipulation and layer-by-layer deposition of cells and biomaterials. Additionally, integrating microfluidic systems with MagTE could improve the nutrient and oxygen supply within large tissue constructs, addressing the critical challenge of vascularization. These synergistic integrations are essential to overcoming current technical limitations and enabling the fabrication of physiologically relevant and clinically applicable tissues and organs.
A particularly promising yet underexplored direction for expanding MagTE's capabilities involves the use of hard magnetic particles, which are widely employed in magnetic robotics.103,104 Unlike superparamagnetic particles that lose magnetization when the external field is removed, hard magnetic particles retain their magnetization, enabling persistent magnetic actuation even without continuous external field application.103,104 This persistent magnetization allows for real-time and sustained control over the spatial arrangement and structural properties of tissues, supporting the fabrication of highly functional and adaptable organ models. However, challenges remain: hard magnetic particles may present biocompatibility issues due to their persistent magnetization, potentially leading to undesirable interactions and cytotoxicity as previously discussed. Notably, unlike superparamagnetic materials—which generally exhibit minimal cytotoxicity when embedded in polymeric matrices—hard magnetic particles such as NdFeB have demonstrated concentration-dependent cytotoxic effects even without external magnetic field exposure. These adverse effects, however, were substantially mitigated by applying biocompatible polyelectrolyte surface coatings, emphasizing the critical role of interface engineering in minimizing unintended interactions with biological components and preserving tissue architecture.105 Additionally, integrating them into biofabrication workflows requires advanced magnetic control systems that can precisely guide and modulate these particles without compromising tissue integrity. The nonlinear dynamic behaviors of hard-magnetic structures—such as undesired vibrations or positional instability under time-varying magnetic fields—can compromise the spatial accuracy of cell patterning and alignment during biofabrication. These effects necessitate the integration of closed-loop control strategies, such as PID controllers, to ensure reliable and precise actuation throughout the tissue assembly process in biomedical applications.106 To address these challenges, future efforts should focus on the development of biocompatible hard magnetic materials, refinement of particle size and surface properties to reduce cytotoxicity, and the engineering of adaptive control systems optimized for organ-scale constructs. In parallel, comprehensive studies on their long-term behavior and safety in biological environments will be essential for advancing clinical translation.
In conclusion, MagTE has demonstrated remarkable capabilities in cellular manipulation and tissue formation, providing a strong foundation for next-generation biofabrication technologies. By addressing existing limitations through integration with complementary biofabrication tools and exploring the untapped potential of hard magnetic particles, MagTE is well-positioned to revolutionize the creation of complex, functional artificial tissues and organs. These advancements will not only address pressing clinical challenges but also drive the evolution of precision medicine, ultimately leading to the development of innovative therapeutic solutions and improved patient outcomes.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by Korean Fund for Regenerative Medicine funded by the Ministry of Science and ICT, and the Ministry of Health and Welfare (21A0104L1, Republic of Korea); and the National Research Foundation of Korea (NRF) grant funded by the Korea Government (MSIT) (no. 2021R1A2C2004981); and the Alchemist Project 1415180884 (20012378, Development of Meta Soft Organ Module Manufacturing Technology without Immunity Rejection and Module Assembly Robot System) funded by the Ministry of Trade, Industry & Energy (MOTIE, Korea).References
- J. P. Vacanti and R. Langer, Lancet, 1999, 354, S32–S34 CrossRef PubMed.
- N. Ashammakhi, S. Ahadian, C. Xu, H. Montazerian, H. Ko, R. Nasiri, N. Barros and A. Khademhosseini, Mater. Today Bio, 2019, 1, 100008 CrossRef CAS PubMed.
- V. Mironov, T. Boland, T. Trusk, G. Forgacs and R. R. Markwald, Trends Biotechnol., 2003, 21, 157–161 CrossRef CAS PubMed.
- J. H. Park, J. Jang, J.-S. Lee and D.-W. Cho, Tissue Eng. Regener. Med., 2016, 13, 612–621 CrossRef CAS PubMed.
- S. Cho and J. Jang, Front. Bioeng. Biotechnol., 2021, 9, 782333 CrossRef PubMed.
- D. G. Hwang, W. Kang, S.-M. Park and J. Jang, Biosens. Bioelectron., 2024, 260, 116420 CrossRef CAS PubMed.
- M. Kim and J. Jang, APL Bioeng., 2021, 5(4), 041506 CrossRef CAS PubMed.
- J. Jang, H.-J. Park, S.-W. Kim, H. Kim, J. Y. Park, S. J. Na, H. J. Kim, M. N. Park, S. H. Choi and S. H. Park, Biomaterials, 2017, 112, 264–274 Search PubMed.
- J. Yoon, G. Yoon and J. Jang, Organoid, 2024, 4, e8 Search PubMed.
- Y.-m. Choi, H. Lee, M. Ann, M. Song, J. Rheey and J. Jang, Biofabrication, 2023, 15, 034104 CrossRef CAS PubMed.
- G. Chen, Y. Qi, L. Niu, T. Di, J. Zhong, T. Fang and W. Yan, Biomed. Rep., 2015, 3, 749–757 Search PubMed.
- M. Li, J. Ma, Y. Gao and L. Yang, Cytotherapy, 2019, 21, 3–16 Search PubMed.
- J. Jang, H.-G. Yi and D.-W. Cho, ACS Biomater. Sci. Eng., 2016, 2, 1722–1731 CrossRef CAS PubMed.
- S. Das, H. Nam and J. Jang, APL Bioeng., 2021, 5(3), 031508 CrossRef CAS PubMed.
- J. Jang, J. Y. Park, G. Gao and D.-W. Cho, Biomaterials, 2018, 156, 88–106 CrossRef CAS PubMed.
- J. Yoon, N. K. Singh, J. Jang and D.-W. Cho, Appl. Phys. Rev., 2022, 9(4), 041408 CAS.
- S.-J. Kim and J. Jang, J. Cardiovasc. Intervention, 2025, 4(1), 1–28 CrossRef.
- D. B. Kolesky, R. L. Truby, A. S. Gladman, T. A. Busbee, K. A. Homan and J. A. Lewis, Adv. Mater., 2014, 26, 3124–3130 Search PubMed.
- Y. m. Choi, D. Na, G. Yoon, J. Kim, S. Min, H. G. Yi, S. J. Cho, J. H. Cho, C. Lee and J. Jang, Adv. Sci., 2025, 2411769 CrossRef CAS PubMed.
- J. E. Moon, Y. N. Lee, S. Jeong, H. R. Jun, M. H. Hoang, Y. Jo, J. Jang, I. K. Shim and S. C. Kim, Stem Cell Res. Ther., 2024, 15, 374 CrossRef CAS PubMed.
- J. Han, S. Najafi, Y. Byun, L. Geonzon, S.-H. Oh, J. Park, J. M. Koo, J. Kim, T. Chung and I. K. Han, Nat. Commun., 2024, 15, 6553 CrossRef CAS PubMed.
- M. M. De Santis, H. N. Alsafadi, S. Tas, D. A. Bölükbas, S. Prithiviraj, I. A. Da Silva, M. Mittendorfer, C. Ota, J. Stegmayr and F. Daoud, Adv. Mater., 2021, 33, 2005476 CrossRef CAS PubMed.
- J. J. Kim, J. Y. Park, V. V. T. Nguyen, M. Bae, M. Kim, J. Jang, J. Y. Won and D. W. Cho, Adv. Funct. Mater., 2023, 33, 2213649 Search PubMed.
- W. Park, M. Bae, M. Hwang, J. Jang, D.-W. Cho and H.-G. Yi, J. Visualized Exp., 2021, e61945 Search PubMed.
- H. Nam, Y.-m. Choi and J. Jang, Appl. Sci., 2020, 10, 900 CrossRef CAS.
- C. Colosi, S. R. Shin, V. Manoharan, S. Massa, M. Costantini, A. Barbetta, M. R. Dokmeci, M. Dentini and A. Khademhosseini, Adv. Mater., 2016, 28, 677–684 CrossRef CAS PubMed.
- D. G. Hwang, H. Choi, U. Yong, D. Kim, W. Kang, S. M. Park and J. Jang, Adv. Mater., 2024, 2400364 CrossRef CAS PubMed.
- L. Moroni, J. A. Burdick, C. Highley, S. J. Lee, Y. Morimoto, S. Takeuchi and J. J. Yoo, Nat. Rev. Mater., 2018, 3, 21–37 Search PubMed.
- Y.-H. P. Zhang, J. Sun and Y. Ma, J. Ind. Microbiol. Biotechnol., 2017, 44, 773–784 CrossRef CAS PubMed.
- A. M. Holmes, A. Charlton, B. Derby, L. Ewart, A. Scott and W. Shu, Biofabrication, 2017, 9, 033001 CrossRef PubMed.
- G. Gao, B. S. Kim, J. Jang and D.-W. Cho, ACS Biomater. Sci. Eng., 2019, 5, 1150–1169 CrossRef CAS PubMed.
- D. Kang, S. Hong, S.-J. Kim, H. Choi, K. Kim and J. Jang, Virtual Phys. Prototyping, 2024, 19, e2390484 CrossRef.
- Y. Jo, D. G. Hwang, M. Kim, U. Yong and J. Jang, Trends Biotechnol., 2023, 41, 93–105 CrossRef CAS PubMed.
- D. Kim, D. Kang, D. Kim and J. Jang, MRS Bull., 2023, 48, 657–667 CrossRef CAS.
- H. Kim, B. Kang, X. Cui, S. H. Lee, K. Lee, D. W. Cho, W. Hwang, T. B. Woodfield, K. S. Lim and J. Jang, Adv. Funct. Mater., 2021, 31, 2011252 CrossRef CAS.
- Y.-J. Choi, Y.-J. Jun, D. Y. Kim, H.-G. Yi, S.-H. Chae, J. Kang, J. Lee, G. Gao, J.-S. Kong and J. Jang, Biomaterials, 2019, 206, 160–169 CrossRef CAS PubMed.
- H. Kim, S.-J. Park, J.-H. Park, S. Lee, B.-W. Park, S. M. Lee, J.-W. Hwang, J.-J. Kim, B. Kang and W.-S. Sim, Exp. Mol. Med., 2022, 54, 1165–1178 CrossRef CAS PubMed.
- U. Yong, D. Kim, H. Kim, D. G. Hwang, S. Cho, H. Nam, S. Kim, T. Kim, U. Jeong and K. Kim, Adv. Mater., 2023, 35, 2208983 CrossRef CAS PubMed.
- J. Kim and J. Jang, Int. J. Bioprint., 2022, 9, 643 CrossRef PubMed.
- L. F. Santos, A. S. Silva and J. F. Mano, Adv. Healthcare Mater., 2023, 12, 2300605 CrossRef CAS PubMed.
- Z. Liu, J. Liu, X. Cui, X. Wang, L. Zhang and P. Tang, Front. Chem., 2020, 8, 124 CrossRef CAS PubMed.
- M. Zborowski, J. J. Chalmers and W. G. Lowrie, Microtechnology for Cell manipulation and Sorting, 2017, pp. 15–55 Search PubMed.
- J. Kolosnjaj-Tabi, C. Wilhelm, O. Clément and F. Gazeau, J. Nanobiotechnol., 2013, 11, 1–19 CrossRef PubMed.
- X. Wang, J. Law, M. Luo, Z. Gong, J. Yu, W. Tang, Z. Zhang, X. Mei, Z. Huang and L. You, ACS Nano, 2020, 14, 3805–3821 Search PubMed.
- X. Z. Chen, B. Jang, D. Ahmed, C. Hu, C. De Marco, M. Hoop, F. Mushtaq, B. J. Nelson and S. Pané, Adv. Mater., 2018, 30, 1705061 CrossRef PubMed.
- H. Zhou, C. C. Mayorga-Martinez, S. Pané, L. Zhang and M. Pumera, Chem. Rev., 2021, 121, 4999–5041 CrossRef CAS PubMed.
- R. Zhao, Y. Kim, S. A. Chester, P. Sharma and X. Zhao, J. Mech. Phys. Solids, 2019, 124, 244–263 CrossRef CAS.
- Y. Kim and X. Zhao, Chem. Rev., 2022, 122, 5317–5364 CrossRef CAS PubMed.
- R. V. Ramanujan, Biomed. Mater., 2009, 477–491 Search PubMed.
- Z. Karimi, L. Karimi and H. Shokrollahi, Mater. Sci. Eng., C, 2013, 33, 2465–2475 Search PubMed.
- S. Liu, B. Yu, S. Wang, Y. Shen and H. Cong, Adv. Colloid Interface Sci., 2020, 281, 102165 CrossRef CAS PubMed.
- D. Schieber, Electromagnetic induction phenomena, Springer Science & Business Media, 2012 Search PubMed.
- Y. Sun, B. Wang, H. Wang and J. Jiang, J. Colloid Interface Sci., 2007, 308, 332–336 CrossRef CAS PubMed.
- Z. Gao, Q. Zhang, Y. Cao, P. Pan, F. Bai and G. Bai, J. Chromatogr., A, 2009, 1216, 7670–7676 Search PubMed.
- M. Longmire, P. L. Choyke and H. Kobayashi, Nanomedicine, 2008, 3, 703–717 Search PubMed.
- I. V. Zelepukin, A. V. Yaremenko, I. N. Ivanov, M. V. Yuryev, V. R. Cherkasov, S. M. Deyev, P. I. Nikitin and M. P. Nikitin, ACS Nano, 2021, 15, 11341–11357 Search PubMed.
- S. Richard, A. K. Silva, G. Mary, H. Ragot, J. E. Perez, C. Ménager, F. Gazeau, I. Boucenna, O. Agbulut and C. Wilhelm, ACS Appl. Bio Mater., 2020, 3, 6802–6810 CrossRef CAS PubMed.
- N. Matsuda, T. Shimizu, M. Yamato and T. Okano, Adv. Mater., 2007, 19, 3089–3099 CrossRef CAS.
- D. Hu, C. Gao, J. Li, P. Tong and Y. Sun, Stem Cell Res. Ther., 2024, 15, 326 CrossRef PubMed.
- M. Ishii, R. Shibata, Y. Numaguchi, T. Kito, H. Suzuki, K. Shimizu, A. Ito, H. Honda and T. Murohara, Arterioscler., Thromb., Vasc. Biol., 2011, 31, 2210–2215 Search PubMed.
- J. A. Kim, J.-H. Choi, M. Kim, W. J. Rhee, B. Son, H.-K. Jung and T. H. Park, Biomaterials, 2013, 34, 8555–8563 Search PubMed.
- W. M. Guo, X. J. Loh, E. Y. Tan, J. S. Loo and V. H. Ho, Mol. Pharm., 2014, 11, 2182–2189 Search PubMed.
- V. H. Ho, K. H. Müller, A. Barcza, R. Chen and N. K. Slater, Biomaterials, 2010, 31, 3095–3102 Search PubMed.
- A. M. Bratt-Leal, K. L. Kepple, R. L. Carpenedo, M. T. Cooke and T. C. McDevitt, Integr. Biol., 2011, 3, 1224–1232 Search PubMed.
- B. R. Whatley, X. Li, N. Zhang and X. Wen, J. Biomed. Mater. Res., Part A, 2014, 102, 1537–1547 Search PubMed.
- N. Luciani, V. Du, F. Gazeau, A. Richert, D. Letourneur, C. Le Visage and C. Wilhelm, Acta Biomater., 2016, 37, 101–110 CrossRef CAS PubMed.
- D. A. Bowser and M. J. Moore, Biofabrication, 2019, 12, 015002 CrossRef PubMed.
- C. Adine, K. K. Ng, S. Rungarunlert, G. R. Souza and J. N. Ferreira, Biomaterials, 2018, 180, 52–66 Search PubMed.
- S. Liu, C. Gao and F. Peng, Mater. Today Adv., 2022, 16, 100281 Search PubMed.
- Y. Yu, J. Guo, Y. Wang, C. Shao, Y. Wang and Y. Zhao, ACS Appl. Mater. Interfaces, 2020, 12, 16097–16103 CrossRef CAS PubMed.
- S. Jeon, S. Kim, S. Ha, S. Lee, E. Kim, S. Y. Kim, S. H. Park, J. H. Jeon, S. W. Kim and C. Moon, Sci. Robot, 2019, 4, eaav4317 Search PubMed.
- M. Takeuchi, M. Iriguchi, M. Hattori, E. Kim, A. Ichikawa, Y. Hasegawa, Q. Huang and T. Fukuda, Biomed. Mater., 2020, 15, 055001 CrossRef CAS PubMed.
- V. A. Parfenov, E. V. Koudan, E. A. Bulanova, P. A. Karalkin, F. D. Pereira, N. E. Norkin, A. D. Knyazeva, A. A. Gryadunova, O. F. Petrov and M. M. Vasiliev, Biofabrication, 2018, 10, 034104 CrossRef PubMed.
- K. K. Moncal, S. Yaman and N. G. Durmus, Adv. Funct. Mater., 2022, 32, 2204092 CrossRef CAS.
- N. Wang, J. Phys. D: Appl. Phys., 2017, 50, 233002 CrossRef PubMed.
- T. D. Brown, J. Biomech., 2000, 33, 3–14 CrossRef CAS PubMed.
- Y. Zhang and P. Habibovic, Adv. Mater., 2022, 34, 2110267 Search PubMed.
- G. Yang, F. Jiang, Y. Lu, S. Lin, C. Liu, A. Li, D. L. Kaplan, S. Zhang, Y. He and C. Huang, Biofabrication, 2021, 13, 035040 Search PubMed.
- A. R. Abdel Fattah, N. Kolaitis, K. Van Daele, B. Daza, A. G. Rustandi and A. Ranga, Nat. Commun., 2023, 14, 5281 Search PubMed.
- A. C. Manjua, J. M. Cabral, C. A. Portugal and F. C. Ferreira, Sci. Technol. Adv. Mater., 2021, 22, 461–480 CrossRef CAS PubMed.
- J. Spangenberg, D. Kilian, C. Czichy, T. Ahlfeld, A. Lode, S. Günther, S. Odenbach and M. Gelinsky, ACS Biomater. Sci. Eng., 2021, 7, 648–662 Search PubMed.
- K. Wei, A. Roy, S. Ejike, M. K. Eiken, E. M. Plaster, A. Shi, M. Shtein and C. Loebel, Cell. Mol. Bioeng., 2024, 1–11 CAS.
- D. Chen, W. Tian, Y. Li, W. Tang and C. Zhang, Biochem. Biophys. Res. Commun., 2012, 424, 176–181 Search PubMed.
- S. Meikle, Y. Piñeiro, M. B. López, J. Rivas and M. Santin, Acta Biomater., 2016, 40, 235–242 CrossRef CAS PubMed.
- D. Rosenfeld, H. Field, Y. J. Kim, K. K. L. Pang, K. Nagao, F. Koehler and P. Anikeeva, Adv. Funct. Mater., 2022, 32, 0224558 Search PubMed.
- M. Yamaguchi, A. Ito, A. Ono, Y. Kawabe and M. Kamihira, ACS Synth. Biol., 2014, 3, 273–279 Search PubMed.
- S. Kopyl, R. Surmenev, M. Surmeneva, Y. Fetisov and A. Kholkin, Mater. Today Bio, 2021, 12, 100149 Search PubMed.
- F. Qi, X. Gao, Y. Shuai, S. Peng, Y. Deng, S. Yang, Y. Yang and C. Shuai, Composites, Part B, 2022, 237, 109864 Search PubMed.
- E. Zhang, M. Abdel-Mottaleb, P. Liang, B. Navarrete, Y. A. Yildirim, M. A. Campos, I. Smith, P. Wang, B. Yildirim and L. Yang, Brain Stimul., 2022, 15, 1451–1462 CrossRef CAS PubMed.
- M. Chen, W. Guo, S. Gao, C. Hao, S. Shen, Z. Zhang, Z. Wang, Z. Wang, X. Li and X. Jing, BioMed Res. Int., 2018, 2018, 8472309 Search PubMed.
- N. Nayerossadat, T. Maedeh and P. A. Ali, Adv. Biomed. Res., 2012, 1, 27 CrossRef PubMed.
- M. Karimi, A. Ghasemi, P. S. Zangabad, R. Rahighi, S. M. M. Basri, H. Mirshekari, M. Amiri, Z. S. Pishabad, A. Aslani and M. Bozorgomid, Chem. Soc. Rev., 2016, 45, 1457–1501 Search PubMed.
- J.-E. Kim, J.-Y. Shin and M.-H. Cho, Arch. Toxicol., 2012, 86, 685–700 CrossRef CAS PubMed.
- S. Bucak, B. Yavuztürk and A. D. Sezer, Recent Adv. Novel Drug Carrier Syst., 2012, 2, 165–200 Search PubMed.
- L. Yang, W. Yang, W. Xu, Y. Zhao and L. Shang, Chem. Eng. J., 2023, 476, 146797 Search PubMed.
- L. Prosen, S. Prijic, B. Music, J. Lavrencak, M. Cemazar and G. Sersa, BioMed Res. Int., 2013, 2013, 209452 Search PubMed.
- Y. Heun, K. Pogoda, M. Anton, J. Pircher, A. Pfeifer, M. Woernle, A. Ribeiro, P. Kameritsch, O. Mykhaylyk and C. Plank, Mol. Ther., 2017, 25, 1616–1627 Search PubMed.
- M. R. Chiang, Y. H. Lin, W. J. Zhao, H. C. Liu, R. S. Hsu, T. C. Chou, T. T. Lu, I. C. Lee, L. D. Liao and S. H. Chiou, Adv. Sci., 2023, 10, 2303566 Search PubMed.
- H. Y. Kim, T. J. Kim, L. Kang, Y.-J. Kim, M. K. Kang, J. Kim, J. H. Ryu, T. Hyeon, B.-W. Yoon and S.-B. Ko, Biomaterials, 2020, 243, 119942 Search PubMed.
- B. Xia, X. Gao, J. Qian, S. Li, B. Yu, Y. Hao, B. Wei, T. Ma, H. Wu and S. Yang, Adv. Mater., 2024, 36, 2305374 CrossRef CAS PubMed.
- M. Colombo, S. Carregal-Romero, M. F. Casula, L. Gutiérrez, M. P. Morales, I. B. Böhm, J. T. Heverhagen, D. Prosperi and W. J. Parak, Chem. Soc. Rev., 2012, 41, 4306–4334 RSC.
- S. E. Barry, Int. J. Hyperthermia, 2008, 24, 451–466 CrossRef CAS PubMed.
- Z. Yang and L. Zhang, Adv. Intell. Syst., 2020, 2, 2000082 CrossRef.
- S. Lucarini, M. Hossain and D. Garcia-Gonzalez, Compos. Struct., 2022, 279, 114800 CrossRef.
- V. Iacovacci, G. Lucarini, C. Innocenti, N. Comisso, P. Dario, L. Ricotti and A. Menciassi, Biomed. Microdevices, 2015, 17, 1–7 Search PubMed.
- Z. Xing and H. Yong, Int. J. Smart Nano Mater., 2021, 12, 429–449 CrossRef.
| This journal is © The Royal Society of Chemistry 2025 |


