Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Substrate-surface-structure tuned electrical and magnetic properties of PrCoO3/CaCoO2.5 superlattices†
Xiaomin
Jia
a,
Yanbin
Chen
a,
Ce-Wen
Nan
 a,
Jing
Ma
*a and
Chonglin
Chen
a,
Jing
Ma
*a and
Chonglin
Chen
 *b
*b
aSchool of Materials Science and Engineering, Tsinghua University, Beijing 100084, China. E-mail: ma-jing@mail.tsinghua.edu.cn
bDepartment of Physics and Astronomy & Advanced Materials Engineering Program, University of Texas at San Antonio, San Antonio, Texas 78249, USA. E-mail: chonglin.chen@utsa.edu
First published on 20th January 2025
Abstract
Interface engineering using substrate surface structures, especially the surface steps, terraces, and facets, is an effective way of tuning the physical properties of epitaxial films and superstructures. The superlattices comprising perovskite PrCoO3 and brownmillerite CaCoO2.5 were grown on three different surface structures, namely, the (001) SrTiO3 substrate with clear surface-step-terraces, the (001) (LaAlO3)0.3(Sr2AlTaO6)0.7 (LSAT) substrate with poorly defined surface-step-terraces, and the (001) LaAlO3 substrate with zig-zag surface facets. The superlattices on LaAlO3 substrates not only exhibit superior ferromagnetic properties but also greater electrical conductivity, with their room-temperature resistivities at the most four orders of magnitude smaller than those on SrTiO3 and LSAT substrates. The antiphase domain boundaries that extend all the way from the interface to the surface can be formed at the edges of the surface-step-terraces, which impede the pathways for charge carrier hopping and magnetic exchange interactions, thereby leading to the well-disciplined ferromagnetic insulating properties of the superlattices on SrTiO3 and LSAT substrates. These results provide new insights into the understanding of the correlation between substrate surface structures and physical properties of the superlattice system.
1. Introduction
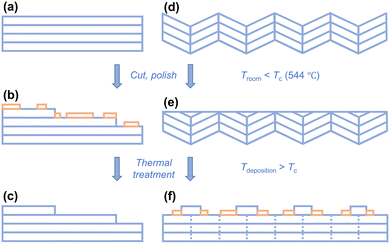
Substrate surface structures, i.e., surface terminations and step heights, have received considerable research attention because of their significant impact on the different functionalities of highly epitaxial oxide thin films.1–8 There are various steps, terraces, and other defects on the crystalline surfaces.8–12 For instance, on the (001) SrTiO3 (STO) substrate, the TiO2 termination is more stable than the SrO termination at the deposition temperatures for most transition metal oxides and many surface-step-terraces appear (Fig. 1a).13–15 Another representative morphology of perovskite substrates is the facetted surfaces on the (001) LaAlO3 (LAO) substrate. The LAO substrate undergoes a ferroelastic phase transition at 544 °C.16,17 The twinned rhombohedral microstructure at room temperature leads to periodic corrugation with zig-zag facets on the (001) LAO surfaces (Fig. 1b).18–20 However, the surface structure of the (001) (LaAlO3)0.3(Sr2AlTaO6)0.7 (LSAT) substrate lies intermediate between that of the (001) STO and (001) LAO substrates. Although there are no twins in the LSAT substrate, the annealing temperature is required to exceed 1100 °C to achieve clear and defined terraces on the LSAT surfaces. The LSAT surfaces usually exhibit poorly defined steps, terraces, and mounds at the film deposition temperature.21 Therefore, the epitaxial quality of the oxide thin films is highly dependent upon the substrate surface structures.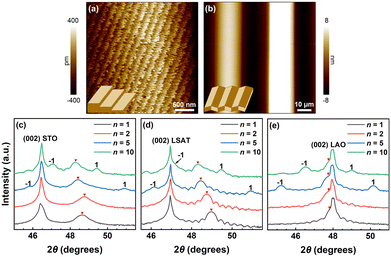 | ||
| Fig. 1 (a) and (b) Typical atomic force microscopy images of commercially available perovskite substrates with surface-step-terraces [(001) STO substrate] and surface facets [(001) LAO substrate], respectively. The insets show a schematic drawing of the surface morphology of these substrates. (c)–(e) X-ray diffraction θ–2θ patterns of the as-grown SLs around the (002) peaks of the substrates. The main (002) peaks and satellite peaks of the SLs are indicated by red triangles and numbers, respectively. For easy comparison, (c) is replotted from ref. 22. | ||
The step heights, terrace dimensions, and edge/facet distributions on the substrate surface play a key role in governing the microstructures, including both conservative and non-conservative antiphase domain boundaries (APBs), and the physical properties of the epitaxial films.2,23 Furthermore, the mismatch between the film unit-cell arrangement and the substrate surface-step-terrace dimensions results in additional strain energy stored in the films, named “residual matching”.24 For example, the electronic transport and magnetic properties of the LaBaCo2O5.5+δ films can be strongly regulated by the dimension of surface terraces,13,25,26 and the superconducting properties of the YBa2Cu3O7−δ films are highly dependent on the surface facets of the twinned substrate.27,28 Recently, we found that the substrate surface structures are crucial not only for the single-layer cobaltate films but also for their superlattices (SLs).22 Specifically, the APBs and electronic transport properties of the SLs comprising perovskite PrCoO3 (PCO) and brownmillerite CaCoO2.5 (CCO) have extraordinary sensitivity to the surface-step-terraces on the (001) STO substrate. However, most research studies on substrate surface structures focus on single-layer oxide films, and no systematic study has been conducted to investigate the effects of various substrate surface structures on oxide SLs.
To systematically investigate the substrate effects on the physical properties of highly epitaxial films and heterostructures, we extended the SLs composed of perovskite PCO and brownmillerite CCO on the (001) STO substrates with a well-defined surface-step-terrace structure to other substrates, i.e., the (001) LSAT substrates with an unwell-defined surface-step-terrace structure, and the (001) LAO substrates with a twinned zig-zag facet structure. Our results indicate that the room-temperature resistivity of the SLs on STO and LSAT substrates is at the most four orders of magnitude larger than that on LAO substrates. While the saturation magnetization on LAO substrates is greater than that on the other two substrates, which is nearly twice that on LSAT substrates. The enhanced insulating and reduced ferromagnetic properties on STO and LSAT substrates can be attributed to the APBs that formed at the edges of terraces continuing throughout the SLs, which induce additional scattering of charge carriers and break the paths of the magnetic exchange interactions across the APBs. These findings enable one to design and explore new emergent phenomena in multifunctional oxide SLs by the surface engineering technique.
2. Results and discussion
The [(PCO)n/(CCO)n]m SLs, simplified as (PCO)n/(CCO)n SLs (n = 1, 2, 5, and 10, denotes the number of unit cells in a period; m = 40, 20, 8, and 4, respectively, denotes the number of periods in a SL) with a total thickness of 32 nm were fabricated by pulsed laser deposition (PLD). Details of the epitaxial growth of SLs are given in the ESI.† The X-ray diffraction (XRD) patterns of the PCO/CCO bilayer films are shown in Fig. S1.† The half-order (0 0 1/2) peaks in the XRD spectra indicate the brownmillerite structure of the CCO films. Fig. 1c–e show the XRD θ–2θ scans of the (PCO)n/(CCO)n SLs around the (002) peaks of the substrates. The satellite peaks of the n = 1 and n = 2 SLs exhibit a very low diffraction intensity and are located at considerable angular distances from the main peaks due to their small period thicknesses, as seen in Fig. S2.† The calculated period thicknesses of all the SLs from the angle distance between adjacent satellite peaks are summarized in Fig. S3,† which align well with the designed period thicknesses, indicating precise control over the growth of the SLs. The reciprocal space mapping (RSM) of the (103) reflection shows that these SLs are fully strained by the LSAT and LAO substrates, but can partially relieve the strain when grown on STO substrates, as seen in Fig. S4.†We then measured the electrical transport properties of the (PCO)n/(CCO)n SLs. Fig. 2a–c show the temperature dependence of the resistivity of the SLs on different substrates and the room-temperature resistivities in the insets. Obviously, the resistivity is highly dependent upon the type of the substrate, especially for the short-period SLs with n = 1 and 2. For the SLs on STO and LSAT substrates with surface-step-terraces, the resistivity generally decreases as n is reduced from 10 to 2, with the exception of a slight increase for the n = 2 SL near room temperature, as seen in the insets of Fig. 2a and b. The SL with n = 1 has a significantly larger resistivity than other samples, with its room-temperature resistivity about three orders of magnitude larger than that of the n = 5 SL. Furthermore, the resistivity of the n = 1 SL on the STO substrate is nearly twice that on the LSAT substrate despite the presence of steps and terraces on the surfaces of both substrates. In contrast, for the SLs deposited on LAO substrates with surface facets, the resistivity monotonically decreases with decreasing n over the entire range of measured temperatures, as shown in Fig. 2c. The room-temperature resistivity of the n = 1 SL on LAO substrates is four orders of magnitude smaller than that on STO and LSAT substrates.
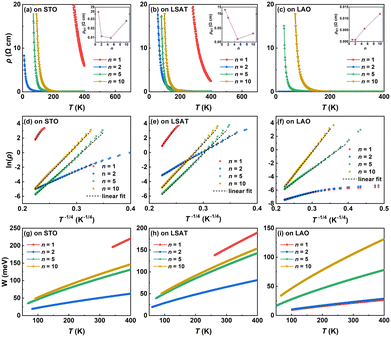 | ||
Fig. 2 Electrical transport characteristics of the (PCO)n/(CCO)n SLs. (a)–(c) Temperature-dependent resistivity of the SLs on the (001) STO, (001) LSAT, and (001) LAO substrates, respectively. The insets show the variation of the room-temperature (RT) resistivity with n. (d)–(f) Fitting the resistivity data using Mott's variable range hopping (VRH) model (ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ρ ∝ T−1/4). (g)–(i) The hopping energy as a function of temperature. For easy comparison, (a) is replotted from ref. 22. ρ ∝ T−1/4). (g)–(i) The hopping energy as a function of temperature. For easy comparison, (a) is replotted from ref. 22. | ||
To elucidate the origin of the distinct electrical transport characteristics on different substrates, we first employed X-ray photoelectron spectroscopy (XPS) measurements to analyze the atomic states of these SLs. Fig. S5† shows the Co 2p core peaks of the SLs on various substrates. A mixed Co valence state of +3 and +4 is found in all the SLs on these three substrates, and the Co4+/Co3+ ratio increases with decreasing n, as seen in Fig. S5d.† Since the Co ions in stoichiometric bulk PCO and CCO exhibit a valence state of +3, the increase in the nominal valence state of Co ions implies a reduction in the oxygen vacancy concentration within the SL system to maintain charge conservation. The variation of oxygen stoichiometry may originate from the PCO/CCO interfaces, where the oxygen vacancy formation in the tetrahedral sub-layers of the brownmillerite CCO on one side of the interface would be suppressed by adjacent octahedral sub-layers of the perovskite PCO on the other side to maintain the continuity of the interfacial structure, and this suppression effect is greatly magnified in short-period SLs.29 Therefore, we observed a continuous rise in the valence state of Co ions as the interfacial density increases (or n decreases). Then, the increased conductivity with decreased n for the SLs on LAO substrates and for the longer-period SLs (n = 2, 5, and 10) on STO and LSAT substrates can be ascribed to the enhanced charge carrier hopping from Co3+ to Co4+.
The significantly large resistivity of the short-period SLs on STO substrates is closely related to the APBs formed at the edges of surface terraces, which we have extensively studied in our previous research.22 The (001) STO substrates are commonly treated with cutting and polishing, and exhibit a mixed termination of SrO and TiO2 (Fig. 3b). However, the TiO2 termination is more stable than the SrO termination after thermal treatment. Therefore, the (001) STO substrates show the TiO2 surface termination and consist of many surface-step-terraces with each step height of one unit cell before growing the SLs (Fig. 3c).13 For the n = 1 SL, the thickness of each constituent layer is ∼0.4 nm, which is close to the step height on the (001) STO surfaces. With continuous layer-by-layer growth, perpendicular APBs would be formed at the edges of surface terraces and penetrate the entire SL thickness (Fig. S6†). These APBs act as additional scattering centers of charge carriers and reduce the mobility of charge carriers across the step edges, leading to the good insulating behavior of the n = 1 SL. For the n = 2 SL, the thickness of each PCO and CCO layer is ∼0.8 nm, which is twice the step height of the (001) STO surface. Only a small number of discontinuous APBs can be formed in the SLs (see Fig. S6†), providing pathways for charge carriers traversing across the step edges. Therefore, its resistivity is significantly lower than that of the n = 1 SL. For the SLs with n ≥ 5, the thickness of each PCO and CCO layer is much larger, so the carrier scattering effect induced by the APBs becomes negligible.
The (001) LSAT substrates also show a surface-step-terrace morphology, but the steps and terraces are poorly defined compared to those on STO surfaces since they require a temperature exceeding 1100 °C to achieve clear and defined step-terrace structures.30 This implies that the shape of the surface-step-terrace edges on the LSAT substrate deviates from straightness and becomes irregular at the deposition temperature. Furthermore, the LSAT surfaces show mixed A-site and B-site terminations with the step height not confined to one unit cell,21 leading to fewer APBs formed on the LSAT surfaces. The less APB structures will result in less carrier scattering at the boundaries in the n = 1 SL. Therefore, the resistivity of the n = 1 SL on the LSAT substrate is almost half of that on the STO substrate. Unlike the (001) STO and (001) LSAT surfaces, the (001) LAO surface always consists of zig-zag surface facets due to its twinned rhombohedral microstructure at the temperature below 544 °C (Fig. 3d).11 After cutting and polishing, the LAO substrate can show a macroscopic (001) surface (Fig. 3e). It is important to know that on the (001) LAO substrate, the surface termination is mainly a LaO top layer after annealing at ∼850 °C but becomes an AlO2 top layer for annealing at a temperature higher than 900 °C.11 Since the film was deposited at the temperature of 750 °C, higher than the phase transition temperature (544 °C) of LAO from the pseudocubic rhombohedral R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c phase to the cubic Pm3m phase,11 the wedge-shaped morphology should have reappeared in the substrate by the partial reconstruction of the surface steps, terraces, and kinks, as seen in Fig. 3f. Therefore, the penetrating APBs that resulted from the step-and-terrace structure on STO and LSAT substrates are absent in the SLs on LAO substrates, leading to their higher conductivity.
c phase to the cubic Pm3m phase,11 the wedge-shaped morphology should have reappeared in the substrate by the partial reconstruction of the surface steps, terraces, and kinks, as seen in Fig. 3f. Therefore, the penetrating APBs that resulted from the step-and-terrace structure on STO and LSAT substrates are absent in the SLs on LAO substrates, leading to their higher conductivity.
To determine the mechanism of the transport behavior, the temperature-dependent resistivity data were fit to different models.31–33 We found that Mott's variable range hopping (VRH) model best fits the data near room temperature, as shown in Fig. 2d–f. The VRH model is usually used in various disordered systems where charge carriers move by hopping between localized electronic states.34 As discussed earlier, the Co4+ ions in the SLs originated from the variation of oxygen stoichiometry at the PCO/CCO interfaces. Therefore, the charge transfer between Co4+ and Co3+ ions is mainly localized in small regions near the interfaces. The electrical transport of these SLs is then dominated by hopping in the conduction channels at a single interface or between conduction channels at different interfaces when the distance between adjacent interfaces is very small, such as in some short-period SLs.35,36 We estimated the hopping energy (W) based on the slope of the fitted curves.37Fig. 2g–i show the hopping energy as a function of temperature. For the SLs on LAO substrates, the hopping energy decreases monotonically with decreasing n due to the increasing interfacial density and thus interfacial charge transfer regions. While for the SLs on STO and LSAT substrates, the hopping energy of the n = 1 SL is substantially enhanced, possessing the highest value among all samples. This is due to the fact that the charge carriers have to overcome the APBs to hop from one domain to another in the n = 1 SL, resulting in their increased hopping difficulty. Furthermore, the n = 1 SL on the STO substrate has a larger hopping energy than that on the LSAT substrate since vertically penetrating APBs are more readily formed on the STO surfaces with clear steps and terraces.
Apart from the electrical transport properties, the substrate surface structures can also strongly modulate the magnetic properties of the SLs, as seen in Fig. 4. For comparison, the magnetic hysteresis loops of the cation-disordered Pr0.5Ca0.5CoO3−δ (PCCO) films are also displayed. We found that the hysteresis loop of the PCCO film on the LAO substrate is a combination of two loops, which should be attributed to the nanoscale-ordered and disordered PCCO phases in the film.26 The nanoscale-ordered phase is dominant in the film with small strain, whereas the disordered phase is dominant with large strain.2 The smaller lattice mismatch between PCCO and LAO (+0.41%)38 may lead to more nanoscale-ordered phases in the film on the LAO substrate and thus its double-loop structure. All the SLs exhibit ferromagnetism, with short-period SLs possessing larger saturation magnetization. The ratio of Co4+/Co3+ shows a rise with the decrease of n (Fig. S5†), so the magnetic exchange interactions between Co3+ and Co4+ may account for the emergence of ferromagnetism in the SLs.
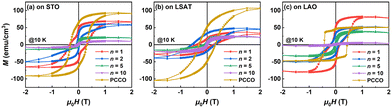 | ||
| Fig. 4 Magnetic-field dependence of the magnetization curves of the (PCO)n/(CCO)n SLs and PCCO films measured at 10 K on the (a) (001) STO, (b) (001) LSAT, and (c) (001) LAO substrates, respectively. For easy comparison, (a) is replotted from ref. 22. | ||
The n = 1 SL can be regarded as a cation-ordered PCCO film where the Pr3+ and Ca2+ ions are separated by the interfaces of the SLs. Considering the promoting effect of cation ordering on magnetic exchange interactions,39,40 the n = 1 SL is expected to exhibit stronger magnetism than the disordered PCCO films. The n = 1 SL on the LAO substrate indeed has larger magnetization than the PCCO films, but on STO and LSAT substrates has remarkably smaller magnetization. Moreover, the n = 1 SL on the LAO substrate demonstrates notably superior magnetic properties than that on other substrates, with its saturation magnetization being approximately twice that on the LSAT substrate. These phenomena are probably related to the substrate-induced strain and the defects in the SLs. On the one hand, the compressive strain from the LAO substrate (see Fig. 1 and S4†) could induce the shortening of the Co–O–Co bond length, making the magnetic exchange easier and increasing the magnetic moment, which is also found in our previous studies.26 On the other hand, the SLs on STO and LSAT substrates have greater strain than those on LAO substrates, which may lead to the formation of more lattice defects, such as dislocations, to relieve the strain energy. Furthermore, the APBs can nucleate at the edges of the steps on STO and LSAT surfaces. Therefore, the probability of spin scattering can be greatly increased owing to the obstacles from APBs, dislocations, and other defects in the SLs, resulting in the weakening of magnetism.41 Nevertheless, the SLs on various substrates demonstrate magnetic moments of the same magnitude though their resistivities differ by orders of magnitude, exhibiting a great potential for tunable ferromagnetic insulators.
3. Conclusions
In summary, epitaxial (PCO)n/(CCO)n SLs were epitaxially grown on (001) STO, (001) LSAT, and (001) LAO substrates to systematically investigate the effect of substrate surface structures on the electronic transport and magnetic properties. The APBs were formed at the edges of the surface-step-terraces on STO and LSAT substrates, which impede the migration of charge carriers from one domain to another and disrupt the magnetic exchange pathways, leading to increased resistivity and diminished ferromagnetism of the SLs. The room-temperature resistivity of the n = 1 SL on the LAO substrate is four orders of magnitude smaller than that on the other two substrates, while its saturation magnetization is at the most twice that on the other two substrates. The greater modulation capability of the substrate surface structures on insulating properties than on magnetic properties paves a new way for designing and fabricating artificial materials with excellent ferromagnetic insulating properties.Data availability
The data that support the findings of this study are available in the ESI† of this article.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 52421001, 52388201, and 52027817).Notes and references
- Y. Lin and C. Chen, Interface effects on highly epitaxial ferroelectric thin films, J. Mater. Sci., 2009, 44, 5274–5287 CrossRef.
- C. Ma, M. Liu, J. Liu, G. Collins, Y. Zhang, H. Wang, C. Chen, Y. Lin, J. He, J. Jiang, E. I. Meletis and A. J. Jacobson, Interface effects on the electronic transport properties in highly epitaxial LaBaCo2O5.5+δ films, ACS Appl. Mater. Interfaces, 2014, 6, 2540–2545 CrossRef.
- F. Sanchez, C. Ocal and J. Fontcuberta, Tailored surfaces of perovskite oxide substrates for conducted growth of thin films, Chem. Soc. Rev., 2014, 43, 2272–2285 RSC.
- X. N. Zhu, T. T. Gao, X. Xu, W. Z. Liang, Y. Lin, C. Chen and X. M. Chen, Piezoelectric and dielectric properties of multilayered BaTiO3/(Ba,Ca)TiO3/CaTiO3 thin films, ACS Appl. Mater. Interfaces, 2016, 8, 22309–22315 CrossRef.
- A. Biswas, C.-H. Yang, R. Ramesh and Y. H. Jeong, Atomically flat single terminated oxide substrate surfaces, Prog. Surf. Sci., 2017, 92, 117–141 CrossRef.
- Y. Yang, L. Wang, H. Huang, C. Kang, H. Zong, C. Zou, Y. Lu, X. Li, B. Hong and C. Gao, Controlling metal-insulator transition in (010)-VO2/(0001)-Al2O3 epitaxial thin film through surface morphological engineering, Ceram. Int., 2018, 44, 3348–3355 CrossRef.
- H. Wang, Z. Wang, Z. Ali, E. Wang, M. Saghayezhian, J. Guo, Y. Zhu, J. Tao and J. Zhang, Surface termination effect of SrTiO3 substrate on ultrathin SrRuO3, Phys. Rev. Mater., 2024, 8, 013605 CrossRef.
- C. Chen and T. T. Tsong, Behavior of Ir atoms and clusters on Ir surfaces, Phys. Rev. B, 1990, 41, 12403–12412 CrossRef.
- Y. J. Chang and S.-h. Phark, Atomic-scale visualization of initial growth of perovskites on SrTiO3 (001) using scanning tunneling microscope, Curr. Appl. Phys., 2017, 17, 640–656 CrossRef.
- C. L. Chen, Y. Cao, Z. J. Huang, Q. D. Jiang, Z. Zhang, Y. Y. Sun, W. N. Kang, L. M. Dezaneti, W. K. Chu and C. W. Chu, Epitaxial SrRuO3 thin films on (001) SrTiO3, Appl. Phys. Lett., 1997, 71, 1047–1049 CrossRef.
- H.-J. Gao, C. L. Chen, B. Rafferty, S. J. Pennycook, G. P. Luo and C. W. Chu, Atomic structure of Ba0.5Sr0.5TiO3 thin films on LaAlO3, Appl. Phys. Lett., 1999, 75, 2542–2544 CrossRef.
- J. H. Chen, C. L. Lia, K. Urban and C. L. Chen, Unusual lattice distortion in a Ba0.5Sr0.5TiO3 thin film on a LaAlO3 substrate, Appl. Phys. Lett., 2002, 81, 1291–1293 CrossRef.
- Q. Zou, M. Liu, G. Wang, H. Lu, T. Yang, H. Guo, C. Ma, X. Xu, M. Zhang, J. Jiang, E. I. Meletis, Y. Lin, H. Gao and C. Chen, Step terrace tuned anisotropic transport properties of highly epitaxial LaBaCo2O5.5+δ thin films on vicinal SrTiO3 substrates, ACS Appl. Mater. Interfaces, 2014, 6, 6704–6708 CrossRef PubMed.
- R. Eason, Pulsed laser deposition of thin films: applications-led growth of functional materials, John Wiley & Sons Ltd., Hoboken, USA & Chichester, UK, 2006 Search PubMed.
- G.-B. Cho, M. Yamamoto and Y. Endo, Surface features of self-organized SrTiO3 (001) substrates inclined in [100] and [110] directions, Thin Solid Films, 2004, 464–465, 80–84 CrossRef.
- Z. Hussain, D. Kumar and V. R. Reddy, Effect of ferro-elastic twin domains of LaAlO3 substrate on the magnetic anisotropy and the magnetization reversal process of cobalt thin film, J. Magn. Magn. Mater., 2019, 477, 408–414 CrossRef.
- S. Bueble, K. Knorr, E. Brecht and W. W. Schmahl, Influence of the ferroelastic twin domain structure on the {100} surface morphology of LaAlO3 HTSC substrates, Surf. Sci., 1998, 400, 345–355 CrossRef.
- C. Li, Y. Zhang, Q. Ji, J. Shi, Z. Chen, X. Zhou, Q. Fang and Y. Zhang, Substrate effect on the growth of monolayer dendritic MoS2 on LaAlO3 (100) and its electrocatalytic applications, 2D Mater., 2016, 3, 035001 CrossRef.
- O. I. Lebedev, G. V. Tendeloo, S. Amelinckx, H. L. Ju and K. M. Krishnan, High-resolution electron microscopy study of strained epitaxial La0.7Sr0.3MnO3 thin films, Philos. Mag. A, 2000, 80, 673–691 Search PubMed.
- A. A. Burema, J. J. L. van Rijn and T. Banerjee, Temperature dependence of the magnetization of La0.67Sr0.33MnO3 thin films on LaAlO3, J. Vac. Sci. Technol., A, 2019, 37, 021103 CrossRef.
- J. H. Ngai, T. C. Schwendemann, A. E. Walker, Y. Segal, F. J. Walker, E. I. Altman and C. H. Ahn, Achieving A-site termination on La0.18Sr0.82Al0.59Ta0.41O3 substrates, Adv. Mater., 2010, 22, 2945–2948 CrossRef.
- X. Jia, Y. Chen, C.-W. Nan, J. Ma and C. Chen, Engineering antiphase domain boundaries boosted tunable ferromagnetic insulation, Appl. Phys. Lett., 2024, 125, 151605 CrossRef.
- J. Jiang, Y. Lin, C. Chen, C. Chu and E. Meletis, Microstructures and surface step-induced antiphase boundaries in epitaxial ferroelectric Ba0.6Sr0.4TiO3 thin film on MgO, J. Appl. Phys., 2002, 91, 3188–3192 CrossRef.
- C. Ma, M. Liu, C. Chen, Y. Lin, Y. Li, J. S. Horwitz, J. Jiang, E. I. Meletis and Q. Zhang, The origin of local strain in highly epitaxial oxide thin films, Sci. Rep., 2013, 3, 3092 CrossRef PubMed.
- C. Ma, M. Liu, G. Collins, H. Wang, S. Bao, X. Xu, E. Enriquez, C. Chen, Y. Lin and M.-H. Whangbo, Magnetic and electrical transport properties of LaBaCo2O5.5+δ thin films on vicinal (001) SrTiO3 surfaces, ACS Appl. Mater. Interfaces, 2013, 5, 451–455 CrossRef.
- M. Liu, S. Ren, J. Lu, C. Ma, X. Xu and C. Chen, Surface-step-terrace tuned magnetic properties of epitaxial LaBaCo2O5.5+δ thin films on vicinal (La,Sr)(Al,Ta)O3 substrates, CrystEngComm, 2015, 17, 8339–8344 RSC.
- Z.-X. Ye, Q. Li, Y. Hu, W. Si, P. Johnson and Y. Zhu, Enhanced flux pinning in YBa2Cu3O7-δ films by nanoscaled substrate surface roughness, Appl. Phys. Lett., 2005, 87, 122502 CrossRef.
- Y. Zuxin, L. Qiang, W. D. Si and P. D. Johnson, Critical current density enhancement in YBa2Cu3O7-δ thin films by twin domains of LaAlO3 substrates, IEEE Trans. Appl. Supercond., 2005, 15, 3013–3015 CrossRef.
- S. Cook, T. K. Andersen, H. Hong, R. A. Rosenberg, L. D. Marks and D. D. Fong, Engineering the oxygen coordination in digital superlattices, APL Mater., 2017, 5, 126101 CrossRef.
- P. Pranav Pradeep, P. C. Shyni, V. Gopal, S. G. Bhat and P. S. A. Kumar, LSAT (001) termination: an investigation on the influence of annealing parameters on topography, Phys. B, 2022, 640, 414092 CrossRef.
- T. T. M. Palstra, B. Batlogg, L. F. Schneemeyer and J. V. Waszczak, Thermally activated dissipation in Bi2.2Sr2Ca0.8Cu2O8+δ, Phys. Rev. Lett., 1988, 61, 1662–1665 CrossRef PubMed.
- A. L. Efros and B. I. Shklovskii, Coulomb gap and low temperature conductivity of disordered systems, J. Phys. C:Solid State Phys., 1975, 8, L49 CrossRef.
- N. F. Mott, Conduction in glasses containing transition metal ions, J. Non-Cryst. Solids, 1968, 1, 1–17 CrossRef.
- S. Bao, S. Pang, W. Wang, J. Chen, M. Chen, J. Ma, C.-W. Nan and C. Chen, Ca doping effect on the magnetic and electronic transport properties in double perovskite PrBaCo2O5+δ films, Appl. Phys. Lett., 2017, 111, 232406 CrossRef.
- A. Bhattacharya, S. J. May, S. G. te Velthuis, M. Warusawithana, X. Zhai, B. Jiang, J. M. Zuo, M. R. Fitzsimmons, S. D. Bader and J. N. Eckstein, Metal-insulator transition and its relation to magnetic structure in (LaMnO3)2n/(SrMnO3)n superlattices, Phys. Rev. Lett., 2008, 100, 257203 CrossRef.
- G. Wang, R. Du, D. Wu and A. Li, Magnetic and transport characteristics of long-period [(LaMnO3)n/(SrMnO3)n]m (n ≥ 3) superlattices, J. Appl. Phys., 2012, 112, 103917 CrossRef.
- J. Kurian and R. Singh, Electron spin resonance and resistivity studies of charge-ordered Bi(1−x)SrxMnO3, J. Alloys Compd., 2011, 509, 5127–5136 CrossRef.
- S. Tsubouchi, T. Kyômen, M. Itoh, P. Ganguly, M. Oguni, Y. Shimojo, Y. Morii and Y. Ishii, Simultaneous metal-insulator and spin-state transitions in Pr0.5Ca0.5CoO3, Phys. Rev. B, 2002, 66, 052418 CrossRef.
- S. J. May, P. J. Ryan, J. L. Robertson, J. W. Kim, T. S. Santos, E. Karapetrova, J. L. Zarestky, X. Zhai, S. G. te Velthuis, J. N. Eckstein, S. D. Bader and A. Bhattacharya, Enhanced ordering temperatures in antiferromagnetic manganite superlattices, Nat. Mater., 2009, 8, 892–897 CrossRef.
- S. Noh, G. Ahn, J. Seo, Z. Gai, H. N. Lee, W. S. Choi and S. J. Moon, (LaCoO3)n/(SrCoO2.5)n superlattices: tunable ferromagnetic insulator, Phys. Rev. B, 2019, 100, 064415 CrossRef.
- J. Wu, R. Guzman, Y. Zhang, H. Chen, Y. Chen, S. Bao, D. Yi, C.-W. Nan, W. Zhou, C. Chen and J. Ma, Lateral magnetic anisotropy modulated by antiphase domain boundaries in PrBaCo2O5+δ thin films, Acta Mater., 2023, 247, 118760 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ce01166j |
| This journal is © The Royal Society of Chemistry 2025 |