Amphiphilic engineering of MoS2–g–C3N4 nanocomposites into a mangrove-inspired cascade system for sustainable drinking water production†
Sichen
Liu‡
a,
Haotian
Wang‡
a,
Yumeng
Xiao
a,
David G.
Calatayud
b,
Boyang
Mao
c,
Gaoqi
Zhang
d,
Chenhui
Yang
d,
Lidong
Wang
 a and
Meng
Li
a and
Meng
Li
 *a
*a
aHebei Key Lab of Power Plant Flue Gas Multi-Pollutants Control, Department of Environmental Science and Engineering, North China Electric Power University, Baoding, 071003, P. R. China. E-mail: mlincepu@hotmail.com
bDepartment of Electroceramics, Instituto de Ceramica y Vidrio CSIC, Kelsen 5, Campus de Cantoblanco, Madrid, 28049, Spain
cDepartment of Engineering, University of Cambridge, Cambridge, CB3 0FA, UK
dKey Laboratory of Bio-based Material Science and Technology of Ministry of Education, Material Science and Engineering College, Northeast Forestry University, Hexing Road 26, Harbin, 150040, P. R. China
First published on 16th October 2024
Abstract
Drinking water contamination and water shortages are seriously exacerbated by industrial wastewater discharge. However, due to the high complexity of wastewater treatment systems, effective high-concentration pollutant removal and simplified wastewater recycling remain major challenges. Inspired by mangrove interconnected purification mechanisms, a novel cascade water treatment system has been developed using MoS2–g–C3N4 (MoG), an amphiphilic material, as the main and single component to directly produce drinking water from wastewater with high efficiency. This cascade system integrates membrane filtration and solar-powered water evaporation processes to produce clean water, while also overcoming the requirement for less polluted source water that is typically required for standalone solar evaporation-based clean water production. The MoG membrane, featuring an amphiphilic platform, exhibits a high removal rate for organic and heavy metal contaminants and achieves a water flow of 966 L m−2 h−1 bar−1 and an 80% efficiency in pollutant removal. The MoG-based aerogel enables nano- and micro-channels and exhibits a clean water production rate of 1.48 kg m−2 h−1 under 1 sun irradiation. The compact cascade system for practical use can produce drinking water that meets WHO standards from heavily polluted wastewater with an average hourly water production rate of 1.39 kg m−2 h−1. Life cycle assessment confirms that the cascade system displays significant environmental profile improvement with reduced CO2 equivalent (CO2e) levels with only 1/25 of that observed in conventional water treatment systems.
Environmental significanceWater contamination and scarcity are exacerbated by the discharge of industrial wastewater. Heavy metal and organic pollution cause serious issues to public health and ecological environment. Nevertheless, the high cost and complexity of current wastewater treatment systems pose challenges to effective pollutant removal and simplified water resource recycling. Through emulating the ecosystem of mangroves, we innovatively integrated MoS2–g–C3N4 (MoG) membrane filtration and MoG-based aerogel water evaporation, developing an efficient, energy-saving, and environmentally friendly cascaded wastewater treatment system. This innovative approach holds significant importance in tackling the scarcity of global potable water resources, providing a new solution to the global water crisis. |
1. Introduction
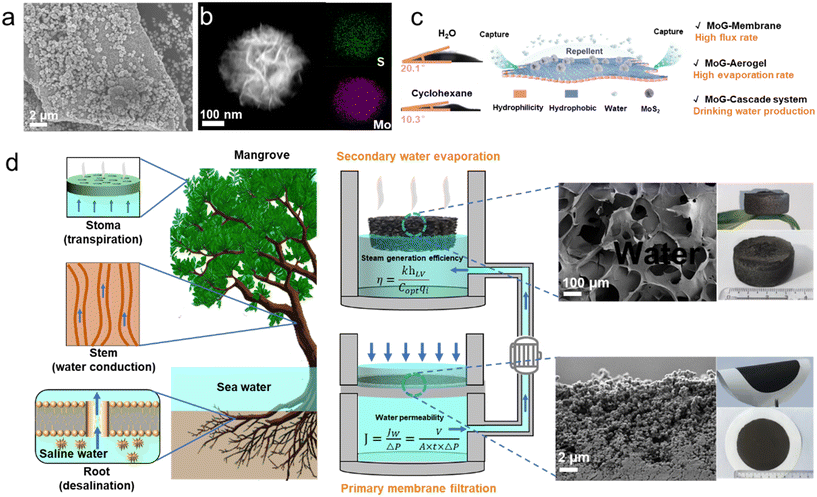
Increased global industrialization and population have elevated the need for reliable drinking water resources.1 Recycling wastewater into drinking water could be an efficient and long-term solution to this problem.2 However, due to the high complexity of the conventional wastewater treatment system, there is a pressing need for a simplified and affordable new treatment method. Looking to nature for inspiration is usually helpful when tackling challenging issues.3 For instance, mangroves, which thrive in brackish coastal waters, use a cascade system that has evolved to be very adaptable and resilient to deal with high-salinity environments.4,5 Mangrove roots and leaves cooperate to remove the salt from saline water to produce clean water: the mangrove roots, featuring a membrane architecture, act as a barrier to stop salt intrusion while the water evaporates from the leaves, generating freshwater droplets.6–8 Compared with a variety of traditional and current water treatment processes, solar driven water evaporation interface technology shows significant low energy consumption and low carbon emission characteristics, this advantage not only meets the current global urgent demand for energy conservation and emission reduction, but also indicates its broad application prospects in the future field of water resources management. Most importantly, the technology does not rely on any fossil fuels or external power sources, and is powered entirely by clean, renewable solar energy, thus achieving an unprecedented level of autonomy and sustainability in energy supply.9Inspired by the transpiration seen in mangrove leaves, solar evaporation offers a promising alternative for wastewater purification that could circumvent the need for complex and energy-intensive membranes.10–12 However, solar evaporation techniques necessitate the use of a stock wastewater source with a limited pollutant concentration.13 For instance, high pollutant-containing wastewater (normally refers to heavy metal content exceeding 50 mg L−1) can result in a failure of solar evaporation purification process, as the evaporated water may still contain harmful amounts of the contaminant due to the limited rejection rate of the photothermal materials.14 Thus, solar evaporation may be appropriate for saltwater desalination, but it is not ideal for sewage and industrial wastewater treatment. Previous studies on optimizing solar evaporation have typically focused on altering a single element, such as enhancing absorptivity or reducing heat loss. For instance, Li et al. demonstrated that optimizing the absorptive properties of the evaporative surface significantly increases desalination efficiency but does little to address the chemical and biological contaminants found in sewage.15 Similarly, Liu et al. highlighted how modifications to thermal insulation could improve performance in controlled conditions, yet the system remains inadequate for diverse industrial effluents.16 Leading to this poignant question: is it wise to only use a single step to achieve our water purification goals? Here, we merge membrane filtration and water evaporation technologies into one integrated system to manufacture drinking water cooperatively, mimicking the Mangrove roots and leaves cooperate to remove pollutants for freshwater production.
Unlike a simple one-plus-one combination strategy, we utilize a single nanomaterial, MoS2–g–C3N4 (MoG), as the main component to develop a cascade system and achieve high energy efficiency. MoG was synthesized by decorating MoS2 nanospheres onto exfoliated g-C3N4 flakes using a hydrothermal method. Due to its 2-dimensional (2D) nature, the MoG can be used as the basic building block to form a well-orientated laminate structure. This laminate structure is applied as the first step in the cascade wastewater treatment system. Thanks to the amphiphilic character of g-C3N4, the MoG membrane can allow both aqueous and organic compounds to access its nano and micro-channels to achieve efficient purification.17 Additionally, because MoS2 exhibits excellent light absorption capacity (absorbs ∼96% of sunlight), MoG-based aerogels are ideal for effective solar evaporation and clean water production.18
Herein, we present a novel cascade system inspired by the water purification mechanisms observed in mangroves. Specifically, the cascade system incorporates a MoG membrane to mimic the mangroves roots as the first step in treatment. The MoG membrane can achieve a high-water flux of 966 L m−2 h−1 bar−1 and a 70% efficiency in pollutant removal. The MoG-based aerogel also serves for secondary treatment, facilitating water transport and achieving a water evaporation rate of 1.48 kg m−2 h−1 under one Sun irradiation. We confirmed that this cascade system is effective for high concentration contaminant removal and water sterilization, achieving removal efficiencies of 99.9% for heavy metals and 100% for organic dyes. The produced drinking water has contamination levels well below the standards set by the World Health Organization (WHO).19 Our system sacrifices some of the primary membrane's rejection rate to achieve a high flux rate and overcome the limitations of bulk water requirements in solar evaporation to directly recycle wastewater into drinking water with high efficiency. Moreover, the cascade treatment system significantly reduces energy consumption, providing distinct advantages over conventional treatment processes such as ion exchange, chemical oxidation, and magnetic separation.20 When comparing this system with other evaporators and conventional wastewater treatment systems, the cascade system's CO2 equivalence (CO2e) levels are much lower than those of typical treatment systems, generating 1/25 that of conventional water treatment systems.
2. Experimental section
2.1 Materials and chemicals
Mercuric acetate was purchased from Jiang yan Global Reagent Factory. The melamine was purchased from TCI. The Sodium hydroxide (NaOH) was purchased from RON reagent. Chitosan (CS high viscosity >400 mPa s), potassium chloride (KCl), LB Broth Agar, chromium nitrate tetrahydrate (Cd(NO3)2·4H2O), lead nitrate (Pb(NO3)2), cupric nitrate (Cu(NO3)2) was purchased from Shanghai Aladdin Bio-Chem Technology Co., Ltd. Acetate acid (C2H4O2, >99.8%) was purchased from Shanghai Macklin Biochemical Co., Ltd. Thioacetamide (C2H5NS), glutaraldehyde (50%), ammonium molybdate tetrahydrate ((NH4)6Mo7O24·4H2O), congo red (CR) and rhodamine B (RhB) were analytical-pure, nitric acid (HNO3) were superior grade pure, and all purchased from Tianjin Kemio Chemical Reagent Co., Ltd. Nylon ultrafiltration membrane supports with 0.22 μm pores were obtained from Zhejiang Yibo Separator Company.2.2 Materials characterizations
SEM images were acquired using an S-4800 field-emission scanning electron microscope (Hitachi, Japan) at magnifications ranging from 2500 to 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. The morphology and structure of the specimen were examined using a JEM-2100 transmission electron microscope (TEM) operating at an acceleration voltage of 200 kV. Analysis of surface elements and functional groups on the adsorbent was conducted using X-ray photoelectron spectroscopy (XPS, ESCALAB 250Xi) with resolutions of 0.48 eV (Ag 3d5/2) and 0.68 eV (C 1s), respectively. UV-visible absorption spectra were recorded using the Beijing Puxi T6 New Century UV-visible spectrophotometer. AFS-933 atomic fluorescence photometer and ICP-OES were used to measure the Hg2+, Cu2+, Pb2+, Cd2+ concentration in the solution.
000. The morphology and structure of the specimen were examined using a JEM-2100 transmission electron microscope (TEM) operating at an acceleration voltage of 200 kV. Analysis of surface elements and functional groups on the adsorbent was conducted using X-ray photoelectron spectroscopy (XPS, ESCALAB 250Xi) with resolutions of 0.48 eV (Ag 3d5/2) and 0.68 eV (C 1s), respectively. UV-visible absorption spectra were recorded using the Beijing Puxi T6 New Century UV-visible spectrophotometer. AFS-933 atomic fluorescence photometer and ICP-OES were used to measure the Hg2+, Cu2+, Pb2+, Cd2+ concentration in the solution.
2.3 Preparation of MoG-based materials
3. Result and discussion
3.1 Construction of a cascade water treatment system based on amphiphilic MoG
MoS2 nanospheres are produced by a hydrothermal method and decorated on exfoliated g-C3N4 flakes to fabricate an amphiphilic material MoS2–g–C3N4 (MoG) for enhanced pollutants removal in wastewater. Negatively charged g-C3N4 nanosheets facilitate the binding and reassembly of molybdenum precursors onto their surface, resulting in the formation of MoS2 nanospheres.23 Fig. S1† illustrates a general heterogeneous nucleation and diffusion-controlled growth process of MoG. The SEM image reveals that MoS2 nanospheres, each with a diameter of approximately 0.6 μm, are uniformly dispersed on g-C3N4 nanosheets, which have a lateral size of about 15 μm (Fig. 1a). Simultaneously, energy-dispersive spectroscopy (EDS) mapping analysis shows that S and Mo are evenly distributed on the g-C3N4 surface (Fig. 1b). The specific surface area of MoG can reach ∼40.1 m2 g−1 (Fig. S2†), and the pore size is generally less than 5 nm, indicating a high porosity and nanochannel-containing structure. X-ray photoelectron spectroscopy (XPS) was used to determine the component elements and chemical valence states of MoG, and the resulting spectra are given in Fig. S3a–d.† The results confirm the successful integration of MoS2 nano spheres and g-C3N4, as well as the coexistence of 1T-phase and 2H-phase MoS2. In addition, because the superior adsorption capability of 1T-phase MoS2 and the pronounced electronic and optical response of 2H-phase MoS2, the MoG nanocomposite exhibits distinct advantages for both pollutant removal and photothermal absorption.24,25Fig. 1c shows the amphiphilicity of MoG materials. The contact angle experiment confirm that MoG nanomaterials display amphiphilic behavior towards water and oil. This amphiphilicity of MoG arises from the hydrophilic properties of the edge groups in g-C3N4 and the hydrophobic characteristics of MoS2. The designed cascade system utilizing amphiphilic MoG material exhibits impressive efficiency in removing pollutants and producing clean water.Based on the amphiphilicity of MoG nanomaterials, the primary aim of our cascade wastewater treatment strategy is to mimic the essential properties of the mangrove ecosystems. Fig. 1d illustrates the key elements of a mangrove, with desalination at the roots and water conduction and transpiration in the stem and leaf domains. For natural mangrove ecosystems, the wood vascular system connects the leaves and roots, facilitating the transport of desalinated water to the leaves while establishing negative pressure at the roots. To simulate the high-water flux and efficient salt exclusion observed in the roots of mangroves, our cascaded wastewater treatment system employs a MoG based membrane. Aqueous solutions permeate through the MoG membrane under a pressure of 0.8 bar; the subsequent water flux is determined using eqn (1):
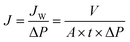 | (1) |
Mangroves undergo transpiration through the stomata on their leaf surfaces, enabling the release of water vapor into the atmosphere through these microscopic pores. To achieve higher rates of water evaporation, a porous MoG-based aerogel was used as an evaporator to mimic the mangroves' stems and leaves. Due to the amphiphilic nature and porous structure of aerogels, water and organic pollutants can be continuously transported to the aerogel's surface through capillary action. Subsequently, it is heated on the surface and evaporates through small pores. The efficiency of water evaporation can be calculated using eqn (2):26
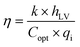 | (2) |
3.2 Characterization and adsorption capacities of amphiphilic MoG
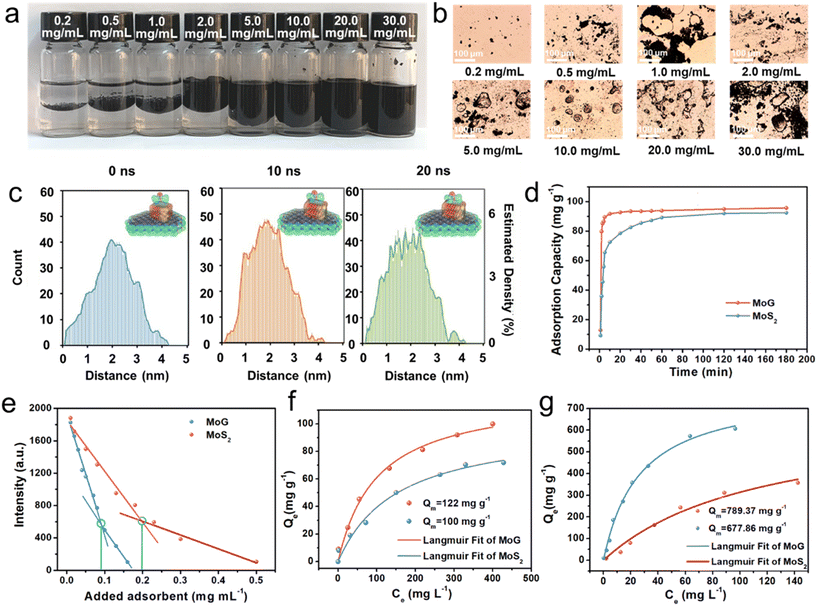
Research indicates that amphiphilic nanomaterials can disperse evenly throughout oil–water mixtures. To showcase the amphiphilic characteristics of MoG, we incorporated it into a cyclohexane/water mixture with equal volumes of each phase. The results revealed that, MoG flakes dispersed in the cyclohexane's nonpolar phase, enabling the creation of an emulsion in the vial through simple manual shaking (Fig. 2a).27,28Fig. 2b presents the stabilized droplets as seen under an optical microscope. The microscope image clearly shows the droplets, whose sizes are influenced by the MoG concentration.In addition, molecular dynamics (MD) simulations were performed to explore the interaction between MoS2 (2-layer)/g-C3N4 (2-layer) and RhB. As illustrated in Fig. 2c, S4 and S5,† RhB initially distanced from the surfaces of g-C3N4, MoS2 and MoG, and gradually adsorbed to these surfaces. Notably, at approximately 10 ns into the simulation, RhB established contact with the surfaces of g-C3N4, MoS2 and MoG. Subsequently, RhB remained attached to these surfaces until the conclusion of the simulation. Considering the conformational dynamics throughout this process, the transition state for the interactions was determined to be 10 ns. If we look at the histogram of distances, we can observe that the interaction between MoS2 (2-layer)/g-C3N4 (2-layer) and RhB presents a narrower histogram centered at smaller distances at both 10 and 20 ns compared to MoS2 and g-C3N4. This suggests that this composite presents a superior capacity for the adsorption of RhB, characterized by a more efficient process with a higher adsorption strength and specificity, with smaller interaction distances with a narrower interaction distribution. In light of these results, the compound exhibits pronounced amphiphilic characteristics, attributed not only to the active sites on g-C3N4 but also to the modulating role of MoS2, which exerts its influence through additional active sites. To explore the disparity in RhB adsorption rates between MoG and MoS2, their effects on the adsorption capacity of RhB over varied contact times were examined. Illustrated in Fig. 2d, MoG demonstrated a notably quicker adsorption rate, achieving equilibrium in merely 10 minutes, whereas MoS2 required 60 minutes to reach the same state. This observation highlights MoG's superior efficiency in adsorption, attributed to its amphiphilic nature, suggesting its advantageous utility in adsorption processes involving organic cationic dyes.
To further obtain insight into the amphipathic superiority of MoG towards the adsorption of pollutant. The adsorption properties of MoG and MoS2 for RhB were evaluated using fluorescence titration experiments (Fig. S6a and b†). The emission of RhB at 580 nm decreases significantly upon the addition of MoG, while the fluorescence intensity of RhB did not decrease much even when additional MoS2 was used. As shown in Fig. 2e, when MoG and MoS2 were added into the RhB solution, RhB was adsorbed onto the MoS2–g–C3N4 and MoS2, and the fluorescence intensity decreased continuously continued to decrease until reaching a minimum level. At the intersection, adsorption equilibrium was reached, indicating RhB has been completely adsorbed by MoG and MoS2. Furthermore, as shown in Fig. 2f compared to pure MoS2, MoG exhibits superior performance in the adsorption other dyes (the maximum adsorption capacity is about 20% higher). Additionally, MoG exhibits good adsorption capabilities for a wide range of dyes with more than 85% removal efficiency under both low and high concentration conditions (Fig. S7†).
Industrial wastewater always contains not only organic dyes but also a complex mixture of heavy metal ions coexisting with the dyes. Therefore, it is necessary for MoG to exhibit effective adsorption of both dyes and heavy metals.29Fig. 2g presents the initial investigation into the adsorption capacities of MoG and MoS2 for Hg2+. The findings revealed that MoG possesses a superior adsorption capacity for Hg2+, demonstrating its effectiveness in capturing this specific ion.
3.3 Removal performance of MoG membranes for pollutants
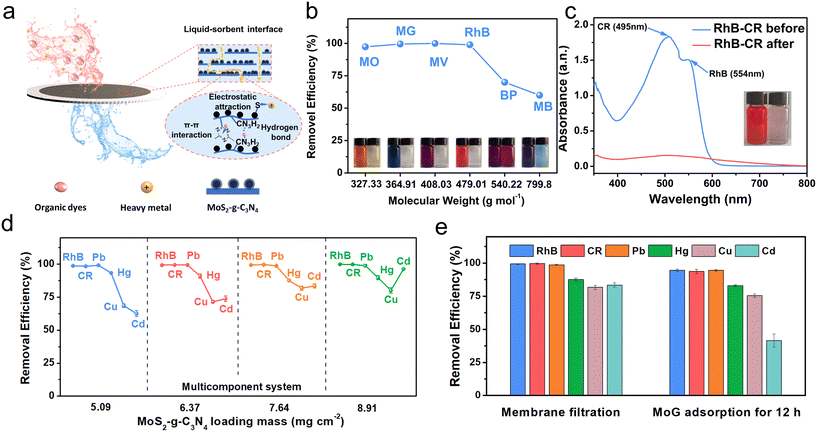
The effective filtering performance of the MoG membrane depends on the adsorption performance of MoG. Moreover, amphiphilicity can improve membrane purification by increasing the hydrophobic attraction between the membrane and aromatic dye molecules, which boosts the effectiveness of removal. The channel's hydrophilic areas make it easier for water to access; this encourages increased water flow.17,30 The MoG adsorption capability for RhB and Hg2+ was investigated using isotherm adsorption models and kinetic models (Fig. S8–S13†). The adsorption behavior of MoG for RhB and Hg2+ adheres to the Langmuir model, underscoring the attainment of monolayer coverage. The adsorption capacity of MoG for Hg2+ decreases with increasing temperature. This could be ascribed to the attenuation of interactions between the active sites of the adsorbents and adsorbate species, as well as among neighboring molecules within the adsorbed phases.31 Furthermore, a series of adsorption experiments have substantiated the concurrent adsorption capabilities of MoG nanomaterials for various pollutants (Fig. S14 and S15†).Due to the exceptional removal ability, MoG was used to fabricate MoG membranes to act as the primary step in the cascade system. The nanochannel-containing MoG materials are deposited onto a porous nylon substrate through vacuum filtration (Fig. S16†). Through fine tuning of the MoG dispersion volume, the loading mass of MoG can be precisely controlled within the range of 5.09 to 8.91 g cm−2. Fig. 3a, illustrates a schematic representation of a nanocomposite membrane used for the removal of pollutants from an aqueous solution, which provides insights into the interaction mechanisms at play between the membrane and the pollutants, including electrostatic attraction, π–π interactions, and hydrogen bonds, which are instrumental in capturing the pollutants. This underscores the potential application of the material in various pollution scenarios, particularly in water treatment technologies that require efficient filtration of multiple types of contaminants.
RhB was used as an organic pollutant model to test the filtration performance of the membrane. Due to variations in transit channel distance, the loading mass of MoG has a significant impact on both water permeability and rejection rate.32 As illustrated in Fig. S17,† the rejection rate increased from 86% to 99% with increased MoG loading mass, whereas water flux decreased from 1190 to 837 L m−2 h−1 bar−1. An optimized loading mass of approximately 7.64 mg cm−2 was chosen for further performance assessment which exhibited a water permeability at 940 L m−2 h−1 bar−1 with a RhB rejection of 97.52%. Additionally, a range of dye molecules with different weights, (ranging from 327.33 to 799.8 g mol−1), were selected for comparison, including methyl orange (MO), malachite green (MG), methyl violet (MV), rhodamine B (RhB), bromocresol purple (BP), and methyl blue (MB). The chemical compositions and surface charge attributes for each molecule are shown in Table S5.† As shown in Fig. 3b, the MoG membranes consistently exhibit rejection rates surpassing 95% for MO, MG, MV, and RhB, irrespective of the solute charge characteristics. From Table S5,† we can see that BP, and MB have larger molecular sizes and accumulated charge centers compared to MO. The larger molecular size may create a spatial barrier effect, which is likely the reason for a different removal rate.33–35
Moreover, to assess the viability of mixed molecule separation, binary mixture separation trials were executed utilizing the MoG membrane (Fig. 3c and S18†). The experiment was conducted at 0.8 bar with a combined concentration of 100 ppm for the RhB-CR binary feed solution. In the UV-vis spectra, the mixed feed solution showcases two absorbance peaks corresponding to RhB and CR, the resultant permeates solutions exhibit a solitary peak attributed to the smaller CR molecules post-filtration. This result illustrates the effective separation of the majority of smaller RhB molecules from the larger CR molecules using the MoG membrane. As shown in Fig. S19,† the filtration efficiency of the MoG membrane for Hg2+ was also evaluated under different loading conditions. As with dye molecules, the loading mass of MoG has an impact on the removal efficiency for Hg2+. As the loading mass increases, the water flux of the membrane experiences a gradual reduction, accompanied by a progressive enhancement in removal efficiency.36
A synthetic wastewater was prepared to mimic the real application scenario to evaluate further the membrane's purification ability (methods S6†).37 This synthetic wastewater was used as a solvent to prepare model dye effluent containing 50 mg L−1 of Cu2+, Cd2+, Pb2+, Hg2+, RhB and CR. The removal efficiency of the prepared membranes was assessed through membrane filtration experiments using MoG membranes with simulated wastewater (Fig. 3d). It was found that, as the loading capacity increases, the removal efficiency of dyes and Pb2+ remains relatively stable, while the removal efficiency for Cu2+ and Cd2+ gradually increases. The stability of dye and Pb2+ removal efficiency can be primarily attributed to the minimal hydration ion radius of Pb2+ (40.1 pm) and the various adsorption mechanisms of dyes. These factors result in the rapid migration of both dyes and Pb2+ to adsorption sites, thereby maintaining their stability. The improved removal rates for Cu2+ and Cd2+ can be linked to the heightened availability of MoG adsorption sites within the membrane.38,39 However, there is a slight reduction in the removal efficiency of Hg2+. This phenomenon can likely be attributed to heightened competition for adsorption sites between additional heavy metal ions and mercury ions. In addition, due to the confined space and the capillary force, the removal efficiency of the MoG membrane is superior to that of using only MoG powder for adsorption (Fig. 3e).
3.4 Solar evaporation performance of MoG-based aerogel
We then evaluated the evaporation capacity of the MoG-based aerogel, as the secondary step in the cascade system. As shown in Fig. 4a, under the irradiation of one sun, the water evaporation rate of MoS2-based aerogel is 0.78 kg m−2 h−1, Mog-based aerogel is 1.48 kg m−2 h−1, 1 sun which exceeds the evaporation rate of pure water (0.29 kg m−2 h−1, 1 sun). Compared with MoS2, MoG shows better photohot water evaporation performance. To comprehensively assess the efficacy of the MoG evaporation system in solar steam generation under 1 sun, a detailed analysis was performed to compare the system's heat loss and energy gain (calculated using eqn (S4) and (S5), ESI†).40 The results indicated that, the top and side energy losses of the MoG evaporative system were calculated to be 0.0839 W, while the environmental energy gain, attributed to the lower temperature of the MoG-based aerogel compared to the ambient temperature, was 0.1108 W. This net effect of the disparity confirms a significant energy gain of 0.0269 W from the environment, effectively enhancing the overall water evaporation rate of the system. In addition, we use infrared cameras to capture real-time temperature changes. Fig. 4b illustrates that the surface temperature of MoG enhanced from 12.3 to 36.3 °C within 20 minutes and sustained equilibrium. In contrast, the temperature variation for pure water was minimal. This solar evaporation performance can be attributed to the extensive spectral absorption and hydrophilic channel surface of MoG. To date, one of the critical challenges in the practical application of solar water evaporation technology is its ability to address diverse and complex water quality environments.41Fig. 4c illustrates the water evaporation rates of MoG-based aerogel for different solutions (wastewater, RhB, pure water, acid, and alkali solutions: under 1 sun irradiation). The evaporation rates for wastewater, RhB, acid, and alkali solutions were 1.47, 1.30, 1.48, and 1.29 kg m−2 h−1, respectively, which are close to the water evaporation rate in deionized water (1.41 kg m−2 h−1). This indicates that the aerogels exhibit adaptability to various challenging water quality environments. Furthermore, simulated wastewater was formulated through controlled indoor experiments to assess the evaporation efficiency of MoG for wastewater treatment. During the evaporation process, although the concentrations of the four major ions notably decreased (rejection rate >95%), they still fell short of the required drinking water standards (Fig. S20†).It is noteworthy that although the MoG membrane can effectively remove most pollutants from simulated wastewater, the purified water obtained after just one purification process, still does not meet the WHO drinking water standards. In addition, in the presence of excessively high pollutants and/or salt content in wastewater, the performance of standalone solar water evaporation systems is also significantly affected. This is because the excessive impurities in water increase the enthalpy of evaporation, leading to a reduction in the evaporation rate, and certain impurities can be carried into the distilled water along with water vapor during the evaporation process.42,43 Therefore, to solve this problem, drawing inspiration from mangroves, with this research we explored the integration of a membrane filtration system and a water evaporation system to form a two-stage cascade system, with the goal of achieving energy and cost efficient, high-quality and the rapid production of drinking water. Fig. S21† presents the schematic diagram of the cascade system, the simulant wastewater is filtered once using vacuum suction, and then the primary purified water is pumped into the evaporation system using a peristaltic pump. To verify the wastewater treatment capability of the cascade system, simulated wastewater was prepared,37 containing heavy metal ions Cu2+, Hg2+, Pb2+, Cd2+ at a concentration of 50 mg L−1, along with 50 mg L−1 of RhB and CR dyes. In June 2023, using an outdoor cascade system running experiments were conducted from 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 00 to 17
00 to 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 00 in Baoding, China. Fig. 4d illustrates the operational photographs of the cascade system under real sunlight. It was observed that by 17
00 in Baoding, China. Fig. 4d illustrates the operational photographs of the cascade system under real sunlight. It was observed that by 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 00, condensed water droplets appeared on the inner walls of the system, and the collection area had accumulated a significant amount of distilled water. After treating with the cascade system, no peak corresponding to RhB and CR was detected in the collected water, confirming complete removal of organic pollutants (Fig. S22†). In addition the concentration of Hg2+, Cu2+, Pb2+ and Cd2+ in the produced purified water was reduced to 0.92 μg L−1, 16.7 μg L−1, 9.8 μg L−1 and 4.6 μg L−1, which was below the WHO standards (Table S7†) for drinking water (Fig. 4e).44 In addition, following the WHO drinking water standards Escherichia coli (E. coli) should not be detected in the water. We further evaluated the antibacterial activity of the cascade system by inoculating with E. coli. After the bacteria solution was treated by the cascade system under 1 sun illumination, no colonies were found in the collected condensed water on the solid medium, indicating excellent removal of bacteria by the evaporation system. At the same time, the effect of light on the antibacterial effect of MoG-based aerogel was also evaluated. As shown in Fig. 4f, for MoG-based aerogel samples without light treatment, a large number of colonies can be seen on the solid media. However, after light treatment, significant inhibition zones were observed for the MoG-based aerogel indicating excellent bacterial purification ability. The inhibitory effect of MoG-based aerogel on bacteria may be due to the high temperature generated. As shown in Fig. S21,† the infrared images of MoG-based aerogel were also obtained, and the surface temperature of MoG-based aerogel increased rapidly from 27.5 to 70.2 °C within 40 min and then remained stable. This temperature could effectively kill bacteria in conventional wastewater.45,46 The excellent anti-bacterial behavior is also attributed to the visible light induced antibacterial action of MoS2. When MoS2 was irradiated with visible light, it can catalyze the production of reactive oxygen species (ROS) containing, ·OH and ·O2− to accelerate the inactivation of bacteria, therefore exhibiting excellent antibacterial effects.47,48 To validate the stability of the cascade system, it was operated continuously for 30 days under real sunlight, for eight hours each day. The results indicated that the solar energy system exhibited excellent stability (Fig. 4g).
00, condensed water droplets appeared on the inner walls of the system, and the collection area had accumulated a significant amount of distilled water. After treating with the cascade system, no peak corresponding to RhB and CR was detected in the collected water, confirming complete removal of organic pollutants (Fig. S22†). In addition the concentration of Hg2+, Cu2+, Pb2+ and Cd2+ in the produced purified water was reduced to 0.92 μg L−1, 16.7 μg L−1, 9.8 μg L−1 and 4.6 μg L−1, which was below the WHO standards (Table S7†) for drinking water (Fig. 4e).44 In addition, following the WHO drinking water standards Escherichia coli (E. coli) should not be detected in the water. We further evaluated the antibacterial activity of the cascade system by inoculating with E. coli. After the bacteria solution was treated by the cascade system under 1 sun illumination, no colonies were found in the collected condensed water on the solid medium, indicating excellent removal of bacteria by the evaporation system. At the same time, the effect of light on the antibacterial effect of MoG-based aerogel was also evaluated. As shown in Fig. 4f, for MoG-based aerogel samples without light treatment, a large number of colonies can be seen on the solid media. However, after light treatment, significant inhibition zones were observed for the MoG-based aerogel indicating excellent bacterial purification ability. The inhibitory effect of MoG-based aerogel on bacteria may be due to the high temperature generated. As shown in Fig. S21,† the infrared images of MoG-based aerogel were also obtained, and the surface temperature of MoG-based aerogel increased rapidly from 27.5 to 70.2 °C within 40 min and then remained stable. This temperature could effectively kill bacteria in conventional wastewater.45,46 The excellent anti-bacterial behavior is also attributed to the visible light induced antibacterial action of MoS2. When MoS2 was irradiated with visible light, it can catalyze the production of reactive oxygen species (ROS) containing, ·OH and ·O2− to accelerate the inactivation of bacteria, therefore exhibiting excellent antibacterial effects.47,48 To validate the stability of the cascade system, it was operated continuously for 30 days under real sunlight, for eight hours each day. The results indicated that the solar energy system exhibited excellent stability (Fig. 4g).
3.5 Life-cycle assessment
To date, there has been limited research on the global warming potential (GWP) associated with solar evaporation technologies, especially within the context of wastewater purification systems. Nonetheless, for a comprehensive validation of these systems, it is essential to assess their environmental impact—specifically their GWP—and conduct a comparative analysis with traditional wastewater treatment methods. Fig. 5a illustrates a comparative analysis between the cascade wastewater treatment system and conventional wastewater treatment methodologies. The process delineated on the left is markedly streamlined, presenting an advantageous approach for environments where energy efficiency and water scarcity are critical concerns. Its ease of operation, reduced energy requirements, and sustainable nature make it an optimal solution for resource-constrained settings. As shown in Fig. 5b, the cascade system achieves the same level of treatment effect (more than 99%) as that for conventional six-step treatment processes using only two steps, indicating significant simplification of processing when compared to the traditional industrial wastewater treatment process. A preliminary life cycle assessment (LCA) was conducted based on ISO Standard 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 040,49 utilizing lab- and pilot-scale data. Evaluating the CO2e produced by the raw materials needed for the system with that produced by other traditional processes, the environmental advantages of the cascade system were assessed. Given that the systems evaluated in this study are not present in the ecoinvent v3.5 database, we constructed and compared each system side by side using industry and literature data.50–55 As shown in Fig. 5c and methods S7,† when the water yield is 5000 L h−1, the CO2e of the raw material required for the cascade system is 3.95 × 103 kg, while the CO2e of the raw material in the production of conventional wastewater treatment process under the same conditions is 9.96 × 104 kg, which is 25 times that of the cascade system. Furthermore, the first pie chart within Fig. 5c illustrates the percentage CO2e contribution of raw materials used in the cascade system, with thioacetamide constituting more than half of the emissions at 50.57%. The second pie chart details the CO2e distribution for the traditional process, highlighting activated carbon as the predominant contributor at 95.95%. To further mitigate carbon dioxide emissions, strategic optimization of raw materials with the most significant emissions contribution is essential. For instance, substituting thioacetamide with raw materials that have a reduced carbon footprint could render the cascade system more eco-efficient. Fig. 5d shows the results of the IPCC 2013 20a method for each system. This method assesses the CO2e of the raw materials required to generate 1 L pure water from each evaporator. The CO2e produced by the cascade system is lower than other evaporators, which means that our cascade system is more environmentally friendly. In addition, the cascade system offers a relatively low-cost for materials in production and operation, estimated as $5.22. (see Table S8†). Over a stable operational period of 30 days, the average daily water production rate stands at 11.2 kg m−2, with daily production rates consistently ranging between 9.92–12.16 kg m−2. Therefore, this low-cost approach readily enables the production of small-scale systems suitable for household generation of drinking water, while also offering a promising concept for the development of more environmentally sustainable and cost-effective large-scale water purification facilities. As such, the cascade system proposed in this study holds significant potential for addressing water-related challenges, representing a sustainable and energy-efficient approach for real world applications.
040,49 utilizing lab- and pilot-scale data. Evaluating the CO2e produced by the raw materials needed for the system with that produced by other traditional processes, the environmental advantages of the cascade system were assessed. Given that the systems evaluated in this study are not present in the ecoinvent v3.5 database, we constructed and compared each system side by side using industry and literature data.50–55 As shown in Fig. 5c and methods S7,† when the water yield is 5000 L h−1, the CO2e of the raw material required for the cascade system is 3.95 × 103 kg, while the CO2e of the raw material in the production of conventional wastewater treatment process under the same conditions is 9.96 × 104 kg, which is 25 times that of the cascade system. Furthermore, the first pie chart within Fig. 5c illustrates the percentage CO2e contribution of raw materials used in the cascade system, with thioacetamide constituting more than half of the emissions at 50.57%. The second pie chart details the CO2e distribution for the traditional process, highlighting activated carbon as the predominant contributor at 95.95%. To further mitigate carbon dioxide emissions, strategic optimization of raw materials with the most significant emissions contribution is essential. For instance, substituting thioacetamide with raw materials that have a reduced carbon footprint could render the cascade system more eco-efficient. Fig. 5d shows the results of the IPCC 2013 20a method for each system. This method assesses the CO2e of the raw materials required to generate 1 L pure water from each evaporator. The CO2e produced by the cascade system is lower than other evaporators, which means that our cascade system is more environmentally friendly. In addition, the cascade system offers a relatively low-cost for materials in production and operation, estimated as $5.22. (see Table S8†). Over a stable operational period of 30 days, the average daily water production rate stands at 11.2 kg m−2, with daily production rates consistently ranging between 9.92–12.16 kg m−2. Therefore, this low-cost approach readily enables the production of small-scale systems suitable for household generation of drinking water, while also offering a promising concept for the development of more environmentally sustainable and cost-effective large-scale water purification facilities. As such, the cascade system proposed in this study holds significant potential for addressing water-related challenges, representing a sustainable and energy-efficient approach for real world applications.
4. Conclusions
Unlike the single-step process, herein, we investigated a system-level, nature inspired cascade system. Consisting of two systems: membrane filtration and water evaporation. The MoG membrane, featuring an amphiphilic platform, exhibits a high removal rate of organic and heavy metal contaminants and achieves a water flow of 966 L m−2 h−1 bar−1. The MoG-based aerogel exhibits nano and micro-channels and has a production rate of 1.48 kg m−2 h−1 under 1 sun irradiation. Using two steps, the cascade system can produce drinking water that meets WHO standards from heavily polluted water with an average hourly water production rate of 1.39 kg m−2 h−1. The ability to directly purify highly contaminated water into drinking water cannot be achieved by individual membrane filtration techniques or solely solar vapor purification approaches. From the LCA, our cascade system has significantly reduced CO2 equivalent (CO2e) levels in manufacturing, only 1/25 of that for conventional water treatment systems. Due to its facile design, low cost, consistent performance, and improved environmental profile, our cascade systems exhibit the potential to be a practical solution for wastewater treatment.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Sichen Liu: methodology, investigation, validation, data curation, formal analysis, writing – original draft. Haotian Wang: resources, investigation, validation, funding acquisition. Yumeng Xiao: software. David G. Calatayud: supervision, Boyang Mao: validation, Gaoqi Zhang: supervision, Chenhui Yang: data curation, Lidong Wang: validation, Meng Li: supervision, resources, writing – review & editing.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The present work is supported by the National Natural Science Foundation of China (Grant #: 52370110). This work was also supported by the Fundamental Research Funds for the Central Universities (Grant #: 2023MS146).References
- Q. Cheng, Q. Li, Z. Yuan, S. Li, J. H. Xin and D. Ye, Bifunctional regenerated cellulose/polyaniline/nanosilver fibers as a catalyst/bactericide for water decontamination, ACS Appl. Mater. Interfaces, 2021, 13, 4410–4418 CrossRef CAS.
- X. Zhang, J. Qian and B. Pan, Fabrication of novel magnetic nanoparticles of multifunctionality for water decontamination, Environ. Sci. Technol., 2016, 50, 881–889 CrossRef CAS.
- J. Zhang, Toward understanding the evolutionary histories and mechanisms of mangroves, Natl. Sci. Rev., 2017, 4, 737 CrossRef CAS.
- Z.-C. Xiong, Y.-J. Zhu, Z.-Y. Wang, Y.-Q. Chen and H.-P. Yu, Tree-inspired ultralong hydroxyapatite nanowires-based multifunctional aerogel with vertically aligned channels for continuous flow catalysis, water disinfection, and solar energy-driven water purification, Adv. Funct. Mater., 2022, 32, 2106978 CrossRef CAS.
- K. Kim, E. Seo, S.-K. Chang, T. J. Park and S. J. Lee, Novel water filtration of saline water in the outermost layer of mangrove roots, Sci. Rep., 2016, 6, 20426 CrossRef CAS.
- Y. Wang, J. Lee, J. R. Werber and M. Elimelech, Capillary-driven desalination in a synthetic mangrove, Sci. Adv., 2020, 6, eaax5253 CrossRef CAS PubMed.
- K. Kim, H. Kim, J. H. Lim and S. J. Lee, Development of a Desalination Membrane Bioinspired by Mangrove Roots for Spontaneous Filtration of Sodium Ions, ACS Nano, 2016, 10, 11428–11433 CrossRef CAS.
- P. Sivaperumal, K. Kamala, D. M. Ganapathy, G. Dharani, S. Sundarrajan and S. Ramakrishna, Fabrication of AgNPs mediated fibrous membrane from Rhizophora mucronata mangrove plant extract for biological properties, J. Drug Delivery Sci. Technol., 2023, 86, 104710 CrossRef CAS.
- Y. Zheng, R. A. Caceres Gonzalez, K. B. Hatzell and M. C. Hatzell, Large-scale solar-thermal desalination, Joule, 2021, 5, 1971–1986 CrossRef CAS.
- B. Yang, Z. Zhang, P. Liu, X. Fu, J. Wang, Y. Cao, R. Tang, X. Du, W. Chen, S. Li, H. Yan, Z. Li, X. Zhao, G. Qin, X. Q. Chen and L. Zuo, Flatband λ-Ti3O5 towards extraordinary solar steam generation, Nature, 2023, 12, 6461 Search PubMed.
- A. K. Menon, I. Haechler, S. Kaur, S. Lubner and R. S. Prasher, Enhanced solar evaporation using a photo-thermal umbrella for wastewater management, Nat. Sustain., 2020, 3, 144–151 CrossRef.
- Y. Guo, H. Lu, F. Zhao, X. Zhou, W. Shi and G. Yu, Biomass-derived hybrid hydrogel evaporators for cost-effective solar water purification, Adv. Mater., 2020, 32, 1907061 CrossRef CAS.
- A. K. Pandey, R. Reji Kumar, K. B, I. A. Laghari, M. Samykano, R. Kothari, A. M. Abusorrah, K. Sharma and V. V. Tyagi, Utilization of solar energy for wastewater treatment: challenges and progressive research trends, J. Environ. Manage., 2021, 297, 113300 CrossRef CAS.
- M. W. Higgins, A. R. Shakeel Rahmaan, R. R. Devarapalli, M. V. Shelke and N. Jha, Carbon fabric based solar steam generation for waste water treatment, Sol. Energy, 2018, 159, 800–810 CrossRef CAS.
- J. Zhou, X. Li, W. Xie, Q. Chen, Y. Jiang, X. Wu and L. Yang, Realizing efficient solar evaporation and rapid lithium sorption by the halloysite nanotubes-based hydrogel, J. Cleaner Prod., 2024, 437, 140523 CrossRef CAS.
- J. Sun, R. Teng, J. Tan, M. Xu, C. Ma, W. Li and S. Liu, An integrated cellulose aerogel evaporator with improved thermal management and reduced enthalpy of evaporation using a hierarchical coordinated control strategy, J. Mater. Chem. A, 2023, 11, 6248–6257 RSC.
- M. Li, Q. Li, M. Xu, B. Liu, D. G. Calatayud, L. Wang, Z. Hu, T. D. James and B. Mao, Amphiphilic engineering of reduced graphene oxides using a carbon nitride coating for superior removal of organic pollutants from wastewater, Carbon, 2021, 184, 479–491 CrossRef CAS.
- J. Li-Ying, Y. Ying-Ting, Y. Zao, Y. Hua, L. Zhi-You, S. Ju, Z. Zi-Gang, C. Xi-Fang and Y. You-Gen, A four-band perfect absorber based on high quality factor and high figure of merit of monolayer molybdenum disulfide, Acta Phys. Sin., 2021, 70, 128101 CrossRef.
- W. Li, M. C. Tekell, Y. Huang, K. Bertelsmann, M. Lau and D. Fan, Synergistic high-rate solar steaming and mercury removal with MoS2/C @ polyurethane composite sponges, Adv. Energy Mater., 2018, 8, 1802108 CrossRef.
- F. E. Titchou, H. Zazou, H. Afanga, J. El Gaayda, R. Ait Akbour, P. V. Nidheesh and M. Hamdani, Removal of organic pollutants from wastewater by advanced oxidation processes and its combination with membrane processes, Chem. Eng. Process., 2021, 169, 108631 CrossRef CAS.
- M. Li, T. Qi, R. Yang, H.-N. Xiao, Z. Fang, S. A. Hodge, T. D. James, L. Wang and B. Mao, Promoting magnesium sulfite oxidation via partly oxidized metal nanoparticles on graphitic carbon nitride (g-C3N4) in the magnesia desulfurization process, J. Mater. Chem. A, 2018, 6, 11296–11305 RSC.
- Y. Zhang, X. Yin, B. Yu, X. Wang, Q. Guo and J. Yang, Recyclable polydopamine-functionalized sponge for high-efficiency clean water generation with dual-purpose solar evaporation and contaminant adsorption, ACS Appl. Mater. Interfaces, 2019, 11, 32559–32568 CrossRef CAS.
- M. Li, J. Zhou, J. Zhou, C. Guo, Y. Han, Y. Zhu, G. Wang and Y. Qian, Ultrathin SnS2 nanosheets as robust polysulfides immobilizers for high performance lithium-sulfur batteries, Mater. Res. Bull., 2017, 96, 509–515 CrossRef CAS.
- X. Mu, X. Gao, H.-t. Zhao, M. George and T. Wu, Density functional theory study of the adsorption of elemental mercury on a 1T-MoS2 monolayer, J. Zhejiang Univ., Sci., A, 2018, 19, 60–67 CrossRef CAS.
- X. Zang, Y. Qin, T. Wang, F. Li, Q. Shao and N. Cao, 1T/2H Mixed phase MoS2 nanosheets integrated by a 3D nitrogen-doped graphene derivative for enhanced electrocatalytic hydrogen evolution, ACS Appl. Mater. Interfaces, 2020, 12, 55884–55893 CrossRef CAS.
- X. Zhang, L. Yang, B. Dang, J. Tao, S. Li, S. Zhao, W. Li, J. Li, Z. Chen and S. Liu, Nature-inspired design: p-toluenesulfonic acid-assisted hydrothermally engineered wood for solar steam generation, Nano Energy, 2020, 78, 105322 CrossRef CAS.
- Y. Wang, C. Jiang, Y. Le, B. Cheng and J. Yu, Hierarchical honeycomb-like Pt/NiFe-LDH/rGO nanocomposite with excellent formaldehyde decomposition activity, Chem. Eng. J., 2019, 365, 378–388 CrossRef CAS.
- A. W. Kuziel, K. Z. Milowska, P.-L. Chau, S. Boncel, K. K. Koziol, N. Yahya and M. C. Payne, The True Amphipathic Nature of Graphene Flakes: A Versatile 2D Stabilizer, Adv. Mater., 2020, 32, 2000608 CrossRef CAS.
- W. Zhao, H. Zhang, K. Feng, T. Wang, L. Han, H. Xing, F. Huang and W. Wang, Reconstruction of metal ions extracted from coal gangue to synthesize cost-effective adsorbent for highly efficient removal of pollutants, Chem. – Asian J., 2023, 18, e202300146 CrossRef CAS PubMed.
- J. Hu, Y. Chen, J. Lu, X. Fan, J. Li, Z. Li, G. Zeng and W. Liu, A self-supported gel filter membrane for dye removal with high anti-fouling and water flux performance, Polymer, 2020, 201, 122531 CrossRef CAS.
- Y. Li, J. Sun, Q. Du, L. Zhang, X. Yang, S. Wu, Y. Xia, Z. Wang, L. Xia and A. Cao, Mechanical and dye adsorption properties of graphene oxide/chitosan composite fibers prepared by wet spinning, Carbohydr. Polym., 2014, 102, 755–761 CrossRef CAS.
- W. Zhang, H. Xu, F. Xie, X. Ma, B. Niu, M. Chen, H. Zhang, Y. Zhang and D. Long, General synthesis of ultrafine metal oxide/reduced graphene oxide nanocomposites for ultrahigh-flux nanofiltration membrane, Nat. Commun., 2022, 13, 471 CrossRef CAS.
- B. Krishnappa, S. Saravu, J. M. Shivanna, M. Naik and G. Hegde, Fast and effective removal of textile dyes from the wastewater using reusable porous nano-carbons: a study on adsorptive parameters and isotherms, Environ. Sci. Pollut. Res., 2022, 29, 79067–79081 CrossRef CAS PubMed.
- M. Danish, T. Ahmad, R. Hashim, N. Said, M. N. Akhtar, J. Mohamad-Saleh and O. Sulaiman, Comparison of surface properties of wood biomass activated carbons and their application against rhodamine B and methylene blue dye, Surf. Interfaces, 2018, 11, 1–13 CrossRef CAS.
- M. Rabbani, Z. S. Seghatoleslami and R. Rahimi, Selective adsorption of organic dye methylene blue by Cs4H2PMo11FeO40·6H2O in presence of methyl orange and Rhodamine-B, J. Mol. Struct., 2017, 1146, 113–118 CrossRef CAS.
- Q. Guo, M. Xu, Q. Tang, Y. Liu, W. Zhang, C. Guo, X. Zhao, Y. Zhu, S. Ye, D. Liu, W. Lei and C. Chen, Advanced hybrid nanosheet membranes with stable nanochannels for ultrafast molecular separation, npj Clean Water, 2023, 6, 38 CrossRef CAS.
- F. Zhao, E. Repo, D. Yin, Y. Meng, S. Jafari and M. Sillanpää, EDTA-Cross-linked β-cyclodextrin: an environmentally friendly bifunctional adsorbent for simultaneous adsorption of metals and cationic dyes, Environ. Sci. Technol., 2015, 49, 10570–10580 CrossRef CAS PubMed.
- M. Min, L. Shen, G. Hong, M. Zhu, Y. Zhang, X. Wang, Y. Chen and B. S. Hsiao, Micro-nano structure poly(ether sulfones)/poly(ethyleneimine) nanofibrous affinity membranes for adsorption of anionic dyes and heavy metal ions in aqueous solution, Chem. Eng. J., 2012, 197, 88–100 CrossRef CAS.
- T. Sheela and Y. A. Nayaka, Kinetics and thermodynamics of cadmium and lead ions adsorption on NiO nanoparticles, Chem. Eng. J., 2012, 191, 123–131 CrossRef CAS.
- L. Zhang, Z. Xu, L. Zhao, B. Bhatia, Y. Zhong, S. Gong and E. N. Wang, Passive, high-efficiency thermally-localized solar desalination, Energy Environ. Sci., 2021, 14, 1771–1793 RSC.
- X. Zhou, F. Zhao, P. Zhang and G. Yu, Solar Water Evaporation Toward Water Purification and Beyond, ACS Mater. Lett., 2021, 3, 1112–1129 CrossRef CAS.
- J. Sun, T. Wu, H. Wu, W. Li, L. Li, S. Liu, J. Wang, W. J. Malfait and S. Zhao, Aerogel-based solar-powered water production from atmosphere and ocean: a review, Mater. Sci. Eng., R, 2023, 154, 100735 CrossRef.
- M. Peydayesh, T. Greber, I. Haechler, A. Armanious, X. Jia, M. Usuelli, M. Bagnani and R. Mezzenga, Renewable water harvesting by amyloid aerogels and sun, Adv. Sustainable Syst., 2022, 6, 2100309 CrossRef CAS.
- W. Li, M. C. Tekell, Y. Huang, K. Bertelsmann, M. Lau and D. Fan, Synergistic high-rate solar steaming and mercury removal with MoS2/C @ polyurethane composite sponges, Adv. Energy Mater., 2018, 8, 1802108 CrossRef.
- S.-G. Wang, Y.-C. Chen and Y.-C. J. N. Chen, Antibacterial gold nanoparticle-based photothermal killing of vancomycin-resistant bacteria, Nanomedicine, 2018, 13, 1405–1416 CrossRef CAS PubMed.
- H. Hu, H. Wang, Y. Yang, J.-F. Xu and X. Zhang, A bacteria-responsive porphyrin for adaptable photodynamic/photothermal therapy, Angew. Chem., Int. Ed., 2022, 61, e202200799 CrossRef CAS PubMed.
- S. K. Ray, D. Dhakal, J. Hur and S. W. Lee, Visible light driven MoS2/α-NiMoO4 ultra-thin nanoneedle composite for efficient staphylococcus aureus inactivation, J. Hazard. Mater., 2020, 385, 121553 CrossRef CAS.
- M. Zhang, K. Wang, S. Zeng, Y. Xu, W. Nie, P. Chen and Y. Zhou, Visible light-induced antibacterial effect of MoS2: effect of the synthesis methods, Chem. Eng. J., 2021, 411, 128517 CrossRef CAS.
- I. S. Arvanitoyannis, Waste management for the food industries, Academic Press, 2010 Search PubMed.
- X. Zhao, Y. Chen, Y. Yin, L. Zou, Q. Chen, K. Liu, P. Lin, H. Su and Y. Chen, Janus polypyrrole nanobelt@polyvinyl alcohol hydrogel evaporator for robust solar-thermal seawater desalination and sewage purification, ACS Appl. Mater. Interfaces, 2021, 13, 46717–46726 CrossRef CAS.
- Y. Guo, X. Zhao, F. Zhao, Z. Jiao, X. Zhou and G. Yu, Tailoring surface wetting states for ultrafast solar-driven water evaporation, Energy Environ. Sci., 2020, 13, 2087–2095 RSC.
- C. Li, S. Cao, J. Lutzki, J. Yang, T. Konegger, F. Kleitz and A. Thomas, A covalent organic famework/graphene dual-region hydrogel for enhanced solar-driven water generation, J. Am. Chem. Soc., 2022, 144, 3083–3090 CrossRef CAS PubMed.
- T. Xue, F. Yang, X. Zhao, F. He, Z. Wang, Q. Wali, W. Fan and T. Liu, Portable solar interfacial evaporator based on polyimide nanofiber aerogel for efficient desalination, Chem. Eng. J., 2023, 461, 141909 CrossRef CAS.
- T. Xue, F. Yang, X. Zhao, F. He, Z. Wang, Q. Wali, W. Fan and T. Liu, Portable solar interfacial evaporator based on polyimide nanofiber aerogel for efficient desalination, Chem. Eng. J., 2023, 461, 141909 CrossRef CAS.
- K. Yang, T. Pan, S. Dang, Q. Gan and Y. Han, Three-dimensional open architecture enabling salt-rejection solar evaporators with boosted water production efficiency, Nat. Commun., 2022, 13, 6653 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4en00633j |
| ‡ Indicates the co-first author. |
| This journal is © The Royal Society of Chemistry 2025 |