Salinity alters the toxicity of copper nanoparticles to anammox consortia through modulating extracellular polymeric substances and membrane permeability†
Ya-Fei
Cheng‡
b,
Meng
Li‡
b,
Hai-Tian
Xu
b,
Shu-Yang
Fang
b,
Yu
Zhang
a,
Zheng-Zhe
Zhang
*a and
Ren-Cun
Jin
 *ab
*ab
aSchool of Engineering, Hangzhou Normal University, Hangzhou 310018, China. E-mail: zhangzhengzhe@126.com; rcjin@hznu.edu.cn; jrczju@aliyun.com; Fax: +86 571 28865333; Tel: +86 571 28861265
bSchool of Life and Environmental Sciences, Hangzhou Normal University, Hangzhou 311121, China
First published on 11th October 2024
Abstract
Among numerous engineered nanoparticles (NPs), CuNPs have been identified as a kind of high-risk inhibitor to anammox bacteria; however, the potential effects of salinity on the toxicity of CuNPs to anammox consortia remain unclear. Their short-term and long-term effects on anammox consortia were investigated by batch assays and continuously-fed bioreactors. The addition of 5.0–7.4 g L−1 NaCl immediately shielded the acute inhibition of 2.0–4.6 mg L−1 CuNPs on anammox activity. However, the coexistence of 5.0 g L−1 NaCl significantly aggravated the inhibitory effect of 3.0 mg L−1 CuNPs on anammox activity after exposure of about one month through reducing the content of extracellular polysaccharides. Even, the membrane permeability was significantly increased with the further increase of NaCl to 8.0 g L−1. Although the relative abundance of anammox bacteria at the DNA level was relatively higher, most of the anammox cells may not be able to perform metabolic functions normally due to membrane damage. Thus, appropriate salinity would attenuate the adverse impacts caused by the short-term shock of CuNPs, while pre-treatment is required to avoid the synergistic stress of high CuNPs when treating high salt wastewater.
Environmental significanceAs the use of ENPs expands across various sectors, their inevitable release into aquatic ecosystems poses potential ecological risks, particularly to advanced biological nitrogen removal systems like anammox. By investigating the effect of salinity on the toxicity of CuNPs to the anammox process, this study filled the knowledge gap regarding the effect of CuNPs on the anammox process in the presence of salinity. The findings aim to contribute to safer and more efficient wastewater management practices, especially in coastal regions facing high salinity levels, thereby protecting water resources and sustaining environmental health. This study underscores the environmental significance of understanding the complex interactions between engineered nanoparticles (ENPs), specifically CuNPs, and salinity in wastewater treatment processes. |
1. Introduction
Engineered nanoparticles (ENPs) have unique physical and chemical properties that enable their wide application in various fields, such as electronics, magnetism and optoelectronics, biomedicine, pharmaceutics, energy, catalysis and materials.1,2 However, the extensive use of ENPs inevitably leads to their release into the environment, which may pose potential threats to ecosystems.3–5 Notably, most of the released ENPs into wastewater eventually accumulate in the activated sludge of wastewater treatment plants.6 Due to their biotoxicity, the adverse effects of ENPs have been widely reported on biological processes, including nitrification, denitrification, and anaerobic digestion of waste sludge.7Anaerobic ammonium oxidation (anammox) is an innovative biological nitrogen removal process that offers the benefits of lower energy consumption and carbon source demand, and it has attracted considerable attention due to its promising application in wastewater treatment in recent years.8,9 However, due to the slow growth rate and high susceptibility of anammox bacteria to variations in environmental conditions, the implementation of the anammox process has been restricted by numerous factors inherent in wastewater, such as heavy metals, ENPs, antibiotics, salinity, phosphates, and sulfides.10,11 Notably, among numerous ENPs, CuNPs exhibited significant toxicity to anammox bacteria by releasing copper ions, thereby inhibiting cellular metabolic activity.12–14 However, current research has predominantly focused on the individual impact, but neglected the complexity of actual wastewater compositions, which often contain substances capable of altering the dispersion and ion release behavior of CuNPs. For example, sulfidation or phosphates may weaken the toxicity of CuNPs to anammox bacteria or denitrifying bacteria.15,16 Notably, ammonium-rich wastewater produced from petroleum, textile, pharmaceutical, leather and other industries often contains a certain level of salinity.17 For example, the salinity of a landfill leachate (1500–2400 mgN L−1) was about 13–30 g L−1.18 Thus, salinity is also a significant factor that cannot be ignored because the increase of salinity may affect the content of extracellular polymeric substances (EPS), membrane fluidity and mechanical properties.19–21
In practice, some high-salt industrial wastewater is often diluted with sewage for co-treatment.22 In addition, there is a growing reliance on seawater for flushing toilets and municipal water due to the lack of freshwater resources in coastal cities, which inevitably leads to the elevated salinity levels in wastewater treatment.23,24 For instance, the salinity and ammonium concentration of the municipal wastewater of Hongkong (a seawater toilet-flushing city) are about 5 g L−1 and 40–100 mgN L−1, respectively.17 Due to the differences in the salinity tolerance and osmoadaptation strategies in four anammox species (Ca. Brocadia, Ca. Jettenia, Ca. Kuenenia, and Ca. Scalindua), the halotolerant “Ca. Kuenenia” is more suitable for treating most of the saline ammonium-rich wastewater mentioned above, which could be tolerant to 60 g L−1 salinity with optimum growth at 2.5–15 g L−1.25 However, to the best of our knowledge, the potential effects of salinity on the toxicity of ENPs to the halotolerant anammox consortia remain unclear.
In this study, we hypothesized that the regulation role of salinity in EPS and/or cell membranes' properties might modify the toxicity of ENPs to anammox biomass. Therefore, CuNPs were selected as the model toxic ENPs to evaluate their effects on halotolerant anammox consortia with the variation of salinity. First, their acute impact on anammox activity was investigated by batch assays using the central composite design response surface method (CCD-RSM). Second, the chronic response of nitrogen removal capacity to the exposure to CuNPs and salinity was compared using continuously-fed bioreactors. Furthermore, the evolution of physiological characteristics including the specific anammox activity (SAA), membrane permeability and EPS was also focused on. Meanwhile, the succession of the anammox community was tracked by high-throughput sequencing. Overall, the obtained results would shed light on the double-edged effects of salinity on the toxicity of CuNPs to anammox consortia.
2. Materials and methods
2.1. Origin of anammox biomass and CuNPs
Mature anammox biomass dominated by a halotolerant anammox species (Ca. Kuenenia) was collected from a laboratory-scale up-flow anaerobic sludge blanket (UASB) reactor with a volume of 2.0 L. This reactor maintained stable operation under thermostatic conditions (35 ± 1 °C) for a period exceeding one year. CuNPs with a commercial origin (10–30 nm, purity of 99.9%) were procured from Aladdin Reagent Co. Ltd. (Shanghai, China). The initial suspension underwent homogenization in an ultrasonic bath (25 °C, 40 kHz, 250 W) to disperse aggregates before being added into the influent of the reactors. Dynamic light scattering (DLS) analysis conducted with a Malvern Zetasizer NanoZS (Malvern Instruments, UK) revealed an average particle size of approximately 85 nm for the CuNPs in the stock suspension.2.2. Experimental design of batch assays
So far, there is no precise data on the actual concentration of NPs in wastewater. The predicted environmental exposures to NPs were mainly derived using modelling techniques, and the estimated amount of NPs in wastewater varied from μg L−1 to mg L−1.26 Accordingly, the effects of CuNPs at environmentally relevant levels (1.0 mg L−1) on anammox sludge were investigated in this study. Since their high affinity for sludge may cause NPs to accumulate, the amounts of NPs in most previous studies ranged from 1 to 100 mg L−1 or mg g−1 suspended solid (SS).26–28 Given that some scientists believe that the concentration of CuNPs entering wastewater treatment plants is unlikely to exceed 10 mg L−1,29 the potential effects of higher loads of CuNPs (10 mg L−1) were also investigated to reach a final conclusion. Given the salinity tolerance of Ca. Kuenenia, levels higher than 15 g L−1 NaCl would not be tested in this study. Subsequently, a CCD-RSM approach was used to evaluate the interactive effects of CuNPs and salinity on anammox activity. Based on preliminary experiments, the half-maximal inhibitory concentration (IC50) of CuNPs or NaCl on normalized anammox activity (NAA, %) was calculated using an improved non-competitive inhibition model. Experimental groups were designed using Design-Expert 10.0 (STAT-EASE Inc., Minneapolis, USA). Five different coded levels of CuNPs and NaCl (−α, −1, 0, +1, +α) were evaluated, leading to a series of 13 batch assays as depicted in Table 1. According to the design, batch experiments were conducted at various concentrations of CuNPs and NaCl at fixed initial substrate levels. Detailed procedures for the measurement of the specific anammox activity are provided in ESI† Text S1. ANOVA was employed to examine the correlation between factors and response values, where p < 0.05 indicated significant effects. The degree of fit of the fitted model was assessed based on the coefficient of determination (R2), and the statistical significance was determined using the F-test, while the significance of correlation coefficients was assessed using the t-test. The joint effects consisting of four main types (independent effect, additive effect, synergistic effect and antagonistic effect) were assessed using the isobole plot method.| Run order | NaCl (g L−1) | CuNPs (mg L−1) | NAA (%) | |
|---|---|---|---|---|
| Experimental value | Model predicted | |||
| 1 | 12.0 | 10.0 | 22.35 | 27.72 |
| 2 | 13.4 | 6.0 | 41.82 | 42.08 |
| 3 | 12.0 | 2.0 | 65.55 | 61.46 |
| 4 | 8.5 | 6.0 | 81.58 | 75.83 |
| 5 | 8.5 | 11.7 | 32.19 | 22.69 |
| 6 | 8.5 | 6.0 | 80.35 | 75.83 |
| 7 | 8.5 | 6.0 | 75.11 | 75.83 |
| 8 | 8.5 | 6.0 | 72.36 | 75.83 |
| 9 | 8.5 | 6.0 | 69.76 | 75.83 |
| 10 | 5.0 | 10.0 | 38.80 | 48.51 |
| 11 | 5.0 | 2.0 | 114.00 | 114.25 |
| 12 | 3.6 | 6.0 | 100.00 | 94.12 |
| 13 | 8.5 | 0.3 | 89.16 | 93.04 |
2.3. Setup and operation of bioreactors
To investigate the chronic response of nitrogen removal capacity, continuous-flow experiments were conducted in two UASB reactors (marked as CR and SR) with an initial biomass concentration of 10 gVSS L−1 and a hydraulic retention time of 1.3 h. Each reactor had an inner diameter of 5 cm and an effective volume of 2.0 L. The two reactors were placed in a dark artificial climate chamber at 35 ± 1 °C to provide an optimal cultivation environment for anammox consortia. The components of synthetic wastewater are detailed in Table S1.†30 The parameters of six phases are shown in Table S2.† In the phase of P0, the two reactors were operated in parallel without addition of CuNPs and NaCl to achieve the stable state. Subsequently, the concentration of CuNPs gradually increased from 1.0 to 3.0 mg L−1 in the two reactors, while 5.0 g L−1 NaCl was introduced to SR in P1–P3 in order to explore the interaction between CuNPs and salinity at low levels. In P4, the concentration of CuNPs remained at 3.0 mg L−1 in the two reactors, while the level of NaCl increased to 8.0 g L−1 in SR in order to further explore the effect of salinity on the toxic effects of CuNPs. Finally, the addition of CuNPs and salinity was terminated to assess the recovery of reactor performance in P5. Meanwhile, the levels of influent substrates were reduced to avoid the inhibition of free ammonia and free nitrous acid on the damaged biomass.31 Whenever the level of effluent nitrite was below 20 mgN L−1, the influent substrate concentration was increased in steps of 70 mgN L−1. This empirical strategy for recovery has been proven to be highly effective in our previous research. At the end of each stage, the increased biomass was discharged from the reactors to maintain a relatively stable biomass concentration of 10 gVSS L−1. The sludge retention time was controlled at ∼60 days and the excess sludge was used to determine physicochemical characteristics and microbial sequencing.2.4. Characterization of physiological properties
At the end of each stage, sludge samples were collected from the CR and SR reactors for SAA analysis. EPS were extracted using a previously reported heat-extraction method.32 Polysaccharides (PS) were quantified using the anthrone method with glucose as the standard, while proteins (PN) were quantified using the phenol reagent method with bovine serum albumin as the standard. The excitation–emission matrix (EEM) fluorescence spectra of the sludge samples were scanned using a fluorescence spectrophotometer (Hitachi F4600, Japan). The EEM fluorescence data were analyzed using MATLAB R2020a software. To assess membrane permeability, the activity of extracellular lactate dehydrogenase was determined as described in the ESI.†2.5. Microbial community analysis
A total of 11 biomass samples were collected from the two reactors at the end of P0–P5, which were labeled F, C1, C2, C3, C4, C5, S1, S2, S3, S4 and S5. The DNA of each sample was extracted using a Power Soil DNA Kit (MoBio Laboratories, USA), and then the 16S rRNA gene was amplified using universal primers 341F/805R targeting the V3–V4 region. Subsequently, paired-end sequencing (2 × 250) was conducted on the Illumina MiSeq PE250 platform in Majorbio Technology (Shanghai, China). Clustering of operational taxonomic units (OTUs) was performed on high-quality sequences with a similarity threshold of ≥97%. The taxonomy of each 16S rRNA gene sequence was analyzed using the RDP Classifier against the Silva (SSU123) 16S rRNA database with a confidence threshold of 70%. Alpha diversity metrics (Chao, ACE, Simpson, and Shannon indices) for the samples were calculated using Mothur software (v1.31.2). The raw data are available in the National Center for Biotechnology Information-Sequence Read Archive (NCBI-SRA) database under accession number PRJNA1153362.2.6. Other analytical methods
The determination of pH, NH4+-N, NO2−-N, NO3−-N, SS and VSS was conducted according to standard methods.23 Each experiment was repeated three times to ensure that the standard deviation was less than 10% of the mean value. Significance analysis was performed using t-tests in SPSS version 20.0 (SPSS Inc., USA). Principal component analysis (PCA) was employed to evaluate the variability of microbial communities, while Mantel tests and Pearson's correlation analysis were conducted to elucidate the correlation between microbial communities and environmental variables.3. Results
3.1. Acute response of anammox activity
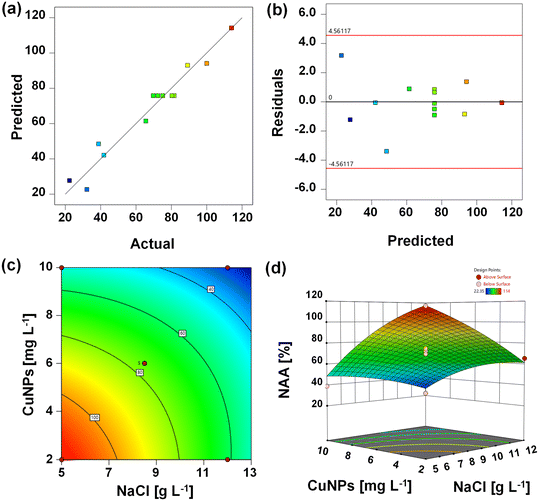
The individual effects of CuNPs and NaCl on anammox activity were investigated through batch assays. The responses of SAA to these substances were effectively fitted using a modified non-competitive inhibition model. The IC50 values of CuNPs and NaCl were estimated to be 7.1 mg L−1 (R2 = 0.988) and 11.2 g L−1 (R2 = 0.989), respectively (Fig. S1†). Building upon these values, thirteen experiments were conducted to examine the combined effects of CuNPs and salinity on anammox activity (Table 1). The response of normalized specific anammox activity (NAA, %) to variations of CuNPs and NaCl was shown as the following equation (eqn (1)).| NAA = 143.95 − 3.32 × [NaCl] − 4.34 × [CuNPs] + 0.57 × [NaCl] × [CuNPs] − 0.32 × [NaCl]2 − 0.56 × [CuNPs]2 | (1) |
The parameters of the quadratic model for NAA are shown in Table 2. The model's F-value of 31.19 indicated significant reliability (p = 0.0001). The “Lack of Fit F-value” of 3.62 (p = 0.1230) suggested that the present model was suitable for describing the relationships among the studied parameters. As a result, a good convergence was observed between the experimental and predictive values because of the high regression coefficient (0.9570) (Fig. 1a). Verification of the constant variance assumption was essential, as indicated by the random distribution of residuals, which suggested homogeneous error variances across the observations (Fig. 1b). The two-dimensional contour plots and three-dimensional response surface are shown in Fig. 1c and d. Obviously, the co-presence of 5.0–7.4 g L−1 NaCl immediately shielded the inhibition of 2.0–4.6 mg L−1 CuNPs. This might be attributed to the promotion effect of NaCl on the dominant species Ca. Kuenenia, which showed optimum activity at low levels of salinity. With the increased levels of CuNPs, its directly interactive effects with NaCl shifted from independent to antagonistic (Fig. S2†).
| Source | Sum of squares | df | Mean square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 8525.13 | 5 | 1705.03 | 31.19 | 0.0001 | Significant |
| A-NaCl | 2707.60 | 1 | 2707.60 | 49.52 | 0.0002 | |
| B-CuNPs | 4948.82 | 1 | 4948.82 | 90.52 | <0.0001 | |
| AB | 256.07 | 1 | 256.07 | 4.68 | 0.0672 | |
| A2 | 103.94 | 1 | 103.94 | 1.90 | 0.2104 | |
| B2 | 561.30 | 1 | 561.30 | 10.27 | 0.0150 | |
| Residual | 382.71 | 7 | 54.67 | |||
| Lack of fit | 279.66 | 3 | 93.22 | 3.62 | 0.1230 | Not significant |
| Pure error | 103.05 | 4 | 25.76 | |||
| Cor total | 8907.84 | 12 | ||||
| Std. dev. | 7.39 | R 2 | 0.9570 | |||
| Mean | 67.93 | R 2adj | 0.9263 | |||
| C.V. % | 10.89 | R 2pred | 0.7587 | |||
| PRESS | 2149.72 | Adeq precision | 18.2272 |
3.2. Chronic response of nitrogen removal capacity
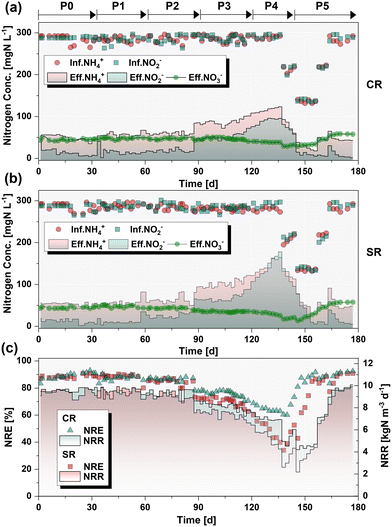
Given the cumulative toxicity of CuNPs to anammox granules, the chronic response of nitrogen removal capacity in the presence of NaCl was compared using continuously-fed bioreactors. Prior to the addition of CuNPs and NaCl (P0: 1–32 d), the excellent nitrogen removal efficiency (NRE) of 89.0 ± 2.4% was achieved at an initial nitrogen loading rate (NLR) of 10.8 kgN m−3 d−1 (Fig. 2). Moreover, the ratio of ammonium consumption to nitrite consumption (RS = 1.21 ± 0.04) and the ratio of ammonium consumption to nitrate production (RP = 0.20 ± 0.01) were close to the theoretical values of the anammox reaction (RS = 1.32 and RP = 0.26) (Fig. S3†), reflecting the dominance of the anammox reaction in the two reactors. In P1 (33–60 d), 1.0 mg L−1 CuNPs were introduced into CR, while 1.0 mg L−1 CuNPs and 5.0 g L−1 NaCl were added to SR. The NRE of CR (87.5 ± 1.8%) and SR (88.9 ± 0.8%) remained similar to those at the initial level. Subsequently, the concentration of CuNPs was increased to 2.0 mg L−1 in the two reactors, while the NaCl concentration was unchanged in SR (P2: 60–89 d). No visible difference in the NRE was observed when compared with P0 (p > 0.05, t test). However, with the increased concentration of CuNPs to 3.0 mg L−1 in P3 (90–118 d), the nitrogen removal performance of the two reactors showed a downward trend. The NRE significantly decreased to 76.1% and 70.4% in CR and SR, respectively. These results indicated that the presence of 5.0 g L−1 NaCl slightly exacerbated the deterioration of performance under the stress of 3.0 mg L−1 CuNPs. To amplify this effect of salinity, the NaCl concentration was elevated to 8.0 g L−1 in SR, while the CuNPs remained at 3.0 mg L−1 in P4 (119–138 d). Inevitably, the NRE plummeted from 62.7% to 39.9% in SR, and it showed a significantly worse performance compared to CR (p < 0.05, t test). The results verified that high concentration of salinity could weaken the tolerance of anammox bacteria to CuNPs. It was noticed that the effluent NO2−-N accumulated sharply to >100 mg L−1. In order to avoid the adverse effects of NO2− inhibition and to test the feasibility of performance recovery, the addition of CuNPs and NaCl was stopped and the influent substrate concentrations were decreased in P5 (139–180 d). Afterwards, the performance of the two reactors gradually recovered to the initial level by incrementally increasing the influent substrate concentration.3.3. Physiological properties of anammox biomass
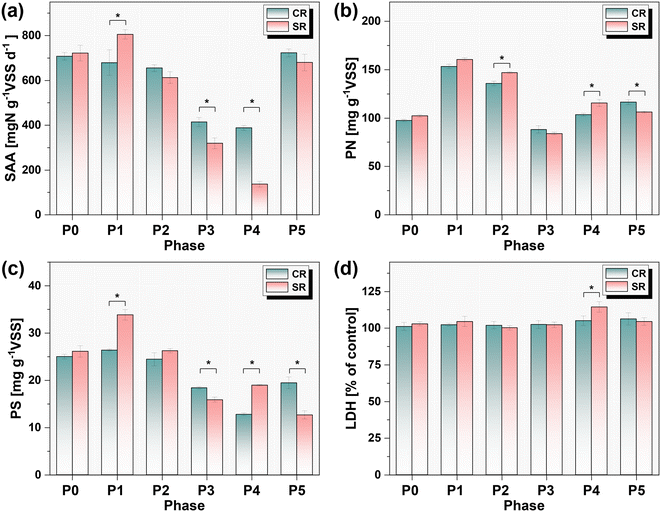
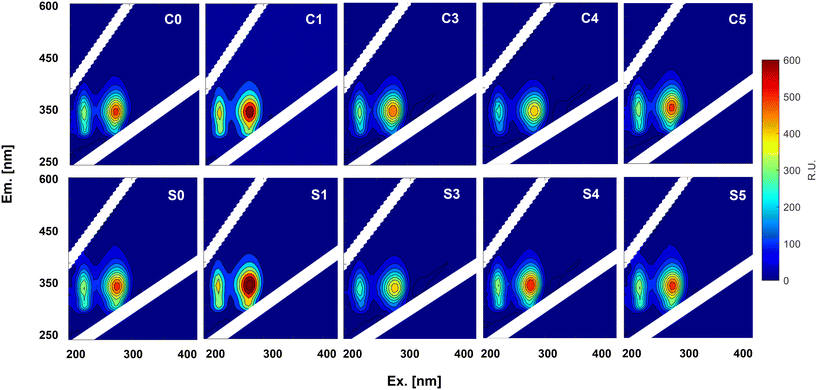
3.4. Evolution of extracellular polymeric substances
3.5. Dynamics of microbial communities
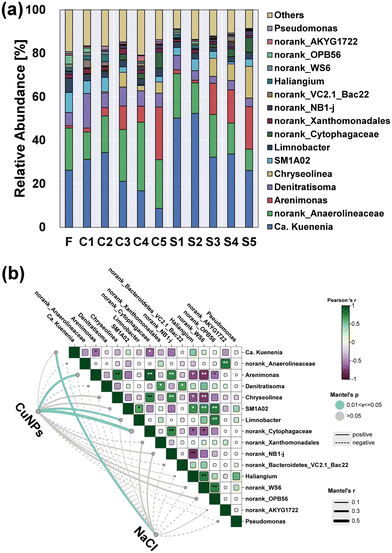
The changes in bacterial communities in response to CuNPs and salinity were monitored using 16S rRNA gene high-throughput sequencing. According to the α-diversity indices (Table 3), the richness and diversity of microbial communities were reduced with the increase of CuNPs and salinity. Most of the population in the two reactors belonged to Proteobacteria, Planctomycetes, Chloroflexi and Bacteroidetes (Fig. 5a), and they accounted for 95.2% of the total variability of the bacterial community at the phylum level (Fig. 5b). Their contribution to the total variability followed the order Planctomycetes > Chloroflexi > Proteobacteria > Bacteroidetes. With the elevated levels of CuNPs in CR, the most abundant phylum shifted from Planctomycetes to Proteobacteria. Conversely, the addition of NaCl to SR significantly increased the relative abundance of Planctomycetes from 35.9% to 58.8% when exposed to low levels of CuNPs, which explained the separation of S1 and S2 communities from others.| Samples | Sobs | Shannon | Simpson | Ace | Chao | Coverage |
|---|---|---|---|---|---|---|
| F | 399 | 3.4770 | 0.0918 | 484.29 | 505.95 | 0.9980 |
| C1 | 359 | 3.2649 | 0.1195 | 428.02 | 427.05 | 0.9983 |
| C2 | 346 | 3.2303 | 0.1343 | 416.25 | 425.03 | 0.9984 |
| C3 | 316 | 3.2922 | 0.0816 | 395.62 | 399.60 | 0.9983 |
| C4 | 339 | 3.4849 | 0.0637 | 397.09 | 398.44 | 0.9985 |
| C5 | 259 | 3.2103 | 0.0901 | 314.92 | 312.20 | 0.9986 |
| S1 | 301 | 2.5512 | 0.2630 | 400.37 | 416.16 | 0.9980 |
| S2 | 348 | 2.5941 | 0.2838 | 410.68 | 402.38 | 0.9986 |
| S3 | 251 | 2.8707 | 0.1422 | 292.62 | 302.13 | 0.9988 |
| S4 | 263 | 2.8254 | 0.1507 | 313.67 | 301.68 | 0.9987 |
| S5 | 226 | 2.7054 | 0.1375 | 288.56 | 285.40 | 0.9985 |
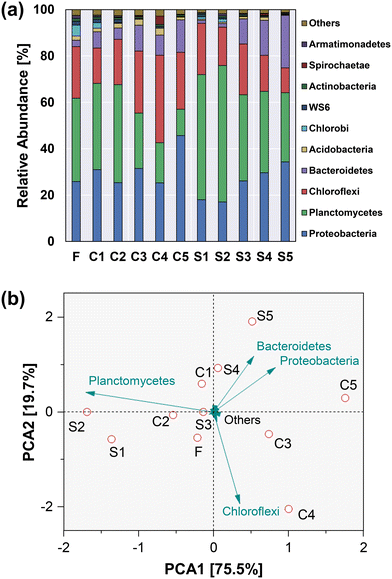 | ||
| Fig. 5 Microbial community structure of the anammox biomass at the phylum level (a). PCA analysis showing the differentiation of microbial communities at the phylum level (b). | ||
Notably, Ca. Kuenenia was the dominant anammox bacterium in Planctomycetes, and its abundance showed a positive correlation with the presence of NaCl (Fig. 6). In particular, the relative abundance of Ca. Kuenenia in the presence of 5.0 mg L−1 NaCl was increased to 52.4% (S2), which was 52.3% higher than that without the addition of NaCl (C2). Even though the level of CuNPs was subsequently increased to 3.0 mg L−1, its abundance in S4 (33.7%) was still twice as high as that in C4 (16.8%). These results suggested an important role of salinity in the maintenance of Ca. Kuenenia under the stress of CuNPs. The variation of norank_Anaerolineaceae belonging to Chloroflexi showed an inverse trend with the increase of CuNPs to 3.0 mg L−1, which was associated with the hydrolytic acidification of microbial products secreted by active anammox bacteria or released from decaying biomass.40 Notably, its relative abundance without the addition of NaCl (C4) was increased to 31.5%, which was 118% higher than that in S4. This result indicated that the presence of NaCl limited the function of hydrolytic acidification although the release of intracellular components was enhanced.
In addition to fermentative bacteria and anammox bacteria, denitrifying bacteria were also one of the major functional groups. Denitratisoma is an autotrophic denitrifying bacterium, which is a relatively common flanking population in the anammox community. The negative correlations between its variation with the addition of CuNPs and NaCl indicated its susceptibility to the interference of CuNPs or salinity. Notably, the addition of CuNPs showed positive correlations with Arenimonas and Chryseolinea. In particular, Chryseolinea was initially undetected when the level of CuNPs was lower than 3.0 mg L−1, whereas its relative abundance increased to 6.8% in C3 and 8.3% in S3, which might use the microbial products as the carbon sources for nitrite reduction.41 Moreover, the abundances of Arenimonas and Chryseolinea exhibited an opposite trend to the changes of Ca. Kuenenia, indicating the enhanced function potential of heterotrophic denitrification and the weakened potential of anammox under the stress of CuNPs. And the addition of 8 g L−1 NaCl further increased their total abundances from 9.7% in C4 to 22.8% in S4. In addition, Limnobacter also negatively correlated with the addition of CuNPs, which was associated with the function of sulfur-driven nitrite reduction to ammonium.
4. Discussion
4.1. Disparate effects of salinity on the acute and chronic toxicity of CuNPs
The results of batch assays showed that the addition of 5.0–7.4 g L−1 NaCl immediately shielded the inhibition of 2.0–4.6 mg L−1 CuNPs on anammox activity due to the stimulation effect of salinity on the dominant anammox species Ca. Kuenenia. Moreover, its directly interactive effects shifted from independent to antagonistic with the increased levels of CuNPs. However, the presence of 5.0–8.0 g L−1 NaCl significantly aggravated the inhibitory effect of 3.0 mg L−1 CuNPs on anammox activity after long-term exposure of approximately one month. Several reasons should be considered to understand these disparate effects of salinity on the acute and chronic toxicity of CuNPs. First, the toxicity of CuNPs was mainly dependent on the amount of active copper ions reaching the anammox cells. However, the EPS of anammox biomass are often the first barrier of anammox cells and the amido-I of proteins and the C–O–C in polysaccharides contributed to the bonding of EPS with CuNPs, which would delay or even block the migration of copper ions. After saturation of binding sites, copper ions will continuously dissociate from the EPS layer, causing chronic toxicity to anammox cells.42 Due to this accumulation and delay effects, its interactive effects of salinity with CuNPs on highly aggregated biomass with high content of EPS may be underrated during several hours of exposure. Thus, long-term testing would be still necessary in practice for evaluating the toxicity of wastewater to anammox sludge.4.2. Roles of salinity in the chronic toxicity of CuNPs to anammox consortia
Notably, the relative abundance of anammox bacteria in the presence of 8.0 g L−1 NaCl was more than twice that exposed to 3.0 mg L−1 CuNPs, whereas the SAA was only approximately one-third of it. It is still an interesting question why the presence of 5.0–8.0 g L−1 NaCl significantly maintained the relative abundance of anammox bacteria but aggravated the inhibitory effect of 3.0 mg L−1 CuNPs on the SAA of anammox consortia. Although the relative abundance of anammox bacteria at the DNA level was relatively higher, most of the anammox cells may not be able to perform metabolic functions normally due to membrane damage.19 This asynchronous change of abundance and activity might be attributed to the inhibition of high salinity on the proliferation of saprophytic population in the community, which used the substances released from the damaged anammox cells as their nutrients.43 Therefore, future research needs to pay more attention to the impact of microbial communities at the transcriptional and protein levels in order to accurately assess the combined effects of salinity and ENPs.In addition, long-term addition of 5.0 g L−1 NaCl greatly increased the content of PS, thereby improving the SAA under the exposure to 1.0 mg L−1 CuNPs. This up-regulation of PS in response to the increased salinity was consistent with the results of previous studies.44,45 The potential reason may be due to the osmoadaptation strategies of Ca. Kuenenia dominant in anammox biomass, which increased the synthesis of trehalose to enhance the extent of salinity tolerance.25 However, this stimulation effect was invisible with the increase of CuNPs. In particular, the content of PS was significantly decreased under the stress of 3.0 mg L−1 CuNPs and the presence of 5.0 g L−1 NaCl exacerbated the inhibition of CuNPs on SAA. Notably, the further increase of NaCl to 8.0 g L−1 caused a significant increase in cell membrane permeability and the release of intracellular substances into the extracellular space, which induced the abnormal increase in extracellular PS and PN content. It has been reported that anammox bacteria would increase membrane fluidity and decrease mechanical properties under the stress of salinity shock via shortening the ladderane fatty acid chain length of the anammoxosome, which resulted in the destruction of proton dynamics driving ATP synthesis and the slowing down of energy metabolism activity.19 Even, higher salt content exceeding their tolerance threshold would destroy the cell membrane and enzymes in bacteria, causing death.46 Indeed, 65% of the SAA was lost when compared with the individual exposure to 3.0 mg L−1 CuNPs. These results verified that high concentration of salinity could weaken the tolerance of anammox biomass to CuNPs by reducing EPS and inducing membrane damage.
5. Conclusion
The obtained results shed light on the double-edged effects of salinity on the toxicity of CuNPs to anammox consortia. The addition of appropriate salinity would attenuate the adverse impacts caused by the short-term shock of CuNPs through stimulating the PS production, which might shield the released toxic copper ions. However, after long-term exposure to CuNPs, the increase of salinity significantly aggravated the inhibitory effect through reducing the PS content. Even, most of the SAA was lost due to membrane damage with the further increase of salinity, which might pose osmotic stress. Thus, pre-treatment is required to avoid the synergistic stress of CuNPs when treating high-salt wastewater.Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.Author contributions
Ya-Fei Cheng: conceptualization; investigation; methodology; writing – original draft. Meng Li: conceptualization; writing – original draft; visualization. Hai-Tian Xu: writing – original draft; writing – review & editing. Shu-Yang Fang: writing – original draft; writing – review & editing. Yu Zhang: writing – review & editing. Zheng-Zhe Zhang: writing – review & editing; supervision; resources. Ren-Cun Jin: writing – review & editing; supervision; resources.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work was financially supported by the Natural Science Foundation of China (No. 52070061) and the Zhejiang Provincial Natural Science Foundation of China (No. LR20E080001). Ya-Fei Cheng acknowledges the support of the Shanghai Tongji Gao Tingyao Environmental Science and Technology Development Foundation.References
- P. Biswas and C. Y. Wu, Nanoparticles and the environment, J. Air Waste Manage. Assoc., 2005, 55, 708–746 CrossRef CAS.
- H. Mu, X. Zheng, Y. Chen, H. Chen and K. Liu, Response of Anaerobic Granular Sludge to a Shock Load of Zinc Oxide Nanoparticles during Biological Wastewater Treatment, Environ. Sci. Technol., 2012, 46, 5997–6003 CrossRef CAS PubMed.
- D. Wang and Y. Chen, Critical review of the influences of nanoparticles on biological wastewater treatment and sludge digestion, Crit. Rev. Biotechnol., 2016, 36, 816–828 CrossRef CAS PubMed.
- Y. Zheng, L. Hou, M. Liu, S. E. Newell, G. Yin, C. Yu, H. Zhang, X. Li, D. Gao, J. Gao, R. Wang and C. Liu, Effects of silver nanoparticles on nitrification and associated nitrous oxide production in aquatic environments, Sci. Adv., 2017, 3, e1603229 CrossRef.
- X. Zhang, D. Wei, H. Zhang, Y. He, S. Zhang, J. Dai and X. Wen, Comprehensive analysis of the impacts of iron-based nanoparticles and ions on Anammox process, Biochem. Eng. J., 2022, 180, 108371 CrossRef CAS.
- Q. Abbas, B. Yousaf, M. U. Amina, M. A. M. Ali, A. Munir, J. Rinklebe El-Naggar and M. Naushad, Transformation pathways and fate of engineered nanoparticles (ENPs) in distinct interactive environmental compartments: A review, Environ. Int., 2020, 138, 105646 CrossRef CAS PubMed.
- M. W. Peng, X. L. Yu, Y. Guan, P. Liu, P. Yan, F. Fang, J. Guo and Y. P. Chen, Underlying Promotion Mechanism of High Concentration of Silver Nanoparticles on Anammox Process, ACS Nano, 2019, 13, 14500–14510 CrossRef CAS.
- S. W. H. Van Hulle, H. J. P. Vandeweyer, B. D. Meesschaert, P. A. Vanrolleghem, P. Dejans and A. Dumoulin, Engineering aspects and practical application of autotrophic nitrogen removal from nitrogen rich streams, Chem. Eng. J., 2010, 162, 1–20 CrossRef CAS.
- A. Joss, D. Salzgeber, J. Eugster, R. König, K. Rottermann, S. Burger, P. Fabijan, S. Leumann, J. Mohn and H. Siegrist, Full-Scale Nitrogen Removal from Digester Liquid with Partial Nitritation and Anammox in One SBR, Environ. Sci. Technol., 2009, 43, 5301–5306 CrossRef CAS PubMed.
- R. C. Jin, G. F. Yang, Q. Q. Zhang, C. Ma, J. J. Yu and B. S. Xing, The effect of sulfide inhibition on the ANAMMOX process, Water Res., 2013, 47, 1459–1469 CrossRef CAS PubMed.
- G. F. Li, B. C. Huang, Z. Z. Zhang, Y. F. Cheng, N. S. Fan and R. C. Jin, Recent advances regarding the impacts of engineered nanomaterials on the anaerobic ammonium oxidation process: performances and mechanisms, Environ. Sci.: Nano, 2019, 6, 3501–3512 RSC.
- Z. Z. Zhang, Y. F. Cheng, L. Z. Xu, J. Wu, Y. H. Bai, J. J. Xu, Z. J. Shi and R. C. Jin, Discrepant effects of metal and metal oxide nanoparticles on anammox sludge properties: A comparison between Cu and CuO nanoparticles, Bioresour. Technol., 2018, 266, 507–515 CrossRef CAS.
- Z. Z. Zhang, J. J. Xu, Z. J. Shi, Y. F. Cheng, Z. Q. Ji, R. Deng and R. C. Jin, Short-term impacts of Cu, CuO, ZnO and Ag nanoparticles (NPs) on anammox sludge: CuNPs make a difference, Bioresour. Technol., 2017, 235, 281–291 CrossRef CAS PubMed.
- Z. Z. Zhang, Y. F. Cheng, L. Z. J. Xu, Y. H. Bai, J. J. Xu, Z. J. Shi, Y. Y. Shen and R. C. Jin, Evaluating the effects of metal oxide nanoparticles (TiO2, Al2O3, SiO2 and CeO2) on anammox process: Performance, microflora and sludge properties, Bioresour. Technol., 2018, 266, 11–18 CrossRef CAS PubMed.
- Z. Z. Zhang, Y. Zhang, Y. F. Cheng and R. C. Jin, Sulfidation attenuates the adverse impacts of metallic nanoparticles on anammox from the perspective of chronic exposure, Environ. Sci.: Nano, 2020, 7, 1681–1691 RSC.
- J. Lv, S. Zhang, L. Luo, W. Han, J. Zhang, K. Yang and P. Christie, Dissolution and microstructural transformation of ZnO nanoparticles under the influence of phosphate, Environ. Sci. Technol., 2012, 46, 7215–7221 CrossRef CAS PubMed.
- L. Lin, S. Pratt, Z. Li and L. Ye, Adaptation and evolution of freshwater Anammox communities treating saline/brackish wastewater, Water Res., 2021, 207, 117815 CrossRef CAS.
- A. Magrí, M. Ruscalleda, A. Vilà, T. R. V. Akaboci, M. D. Balaguer, J. M. Llenas and J. Colprim, Scaling-Up and Long-Term Operation of a Full-Scale Two-Stage Partial Nitritation-Anammox System Treating Landfill Leachate, Processes, 2021, 9, 800 CrossRef.
- Y. T. Xiong, X. W. Liao, J. S. Guo, F. Fang, Y. P. Chen and P. Yan, Potential Role of the Anammoxosome in the Adaptation of Anammox Bacteria to Salinity Stress, Environ. Sci. Technol., 2024, 58, 6670–6681 CrossRef CAS.
- H. Etesami, H. Fatemi and M. Rizwan, Interactions of nanoparticles and salinity stress at physiological, biochemical and molecular levels in plants: A review, Ecotoxicol. Environ. Saf., 2021, 225, 112769 CrossRef CAS PubMed.
- J. Wu, R. Jiang, W. Lin and G. Ouyang, Effect of salinity and humic acid on the aggregation and toxicity of polystyrene nanoplastics with different functional groups and charges, Environ. Pollut., 2019, 245, 836–843 CrossRef CAS.
- C. Cortés-Lorenzo, M. Rodríguez-Díaz, C. López-Lopez, M. Sánchez-Peinado, B. Rodelas and J. González-López, Effect of salinity on enzymatic activities in a submerged fixed bed biofilm reactor for municipal sewage treatment, Bioresour. Technol., 2012, 121, 312–319 CrossRef.
- D. S. Yu, Y. Z. Peng and K. Zhang, Effects of seawater salinity on nitrite accumulation in short-range nitrification to nitrite as end product, J. Environ. Sci., 2004, 16, 247–251 CAS.
- Z. Huang, Y. Wang, L. Jiang, B. Xu, Y. Wang, H. Zhao and W. Zhou, Mechanism and performance of a self-flocculating marine bacterium in saline wastewater treatment, Chem. Eng. J., 2018, 334, 732–740 CrossRef CAS.
- S. Okabe, A. Kamizono, L. Zhang, S. Kawasaki, K. Kobayashi and M. Oshiki, Salinity Tolerance and Osmoadaptation Strategies in Four Genera of Anammox Bacteria: Brocadia, Jettenia, Kuenenia, and Scalindua, Environ. Sci. Technol., 2024, 58, 5357–5371 CrossRef CAS PubMed.
- N. Musee, M. Thwala and N. Nota, The antibacterial effects of engineered nanomaterials: implications for wastewater treatment plants, J. Environ. Monit., 2011, 13, 1164–1183 RSC.
- H. Mu, X. Zheng, Y. Chen, H. Chen and K. Liu, Response of Anaerobic Granular Sludge to a Shock Load of Zinc Oxide Nanoparticles during Biological Wastewater Treatment, Environ. Sci. Technol., 2012, 46, 5997–6003 CrossRef CAS.
- C. Walden and W. Zhang, Biofilms Versus Activated Sludge: Considerations in Metal and Metal Oxide Nanoparticle Removal from Wastewater, Environ. Sci. Technol., 2016, 50, 8417–8431 CrossRef CAS PubMed.
- Y. Chen, D. Wang, X. Zhu, X. Zheng and L. Feng, Long-Term Effects of Copper Nanoparticles on Wastewater Biological Nutrient Removal and N2O Generation in the Activated Sludge Process, Environ. Sci. Technol., 2012, 46, 12452–12458 CrossRef CAS.
- Z. Z. Zhang, Y. F. Cheng, L. Z. J. Xu, Y. H. Bai and R. C. Jin, Anammox granules show strong resistance to engineered silver nanoparticles during long-term exposure, Bioresour. Technol., 2018, 259, 10–17 CrossRef CAS.
- Z. Z. Zhang, Y. F. Cheng, L. Z. J. Xu, Y. H. Bai, J. J. Xu, Z. J. Shi, Q. Q. Zhang and R. C. Jin, Transient disturbance of engineered ZnO nanoparticles enhances the resistance and resilience of anammox process in wastewater treatment, Sci. Total Environ., 2018, 622–623, 402–409 CrossRef CAS.
- C. Yin, F. Meng and G.-H. Chen, Spectroscopic characterization of extracellular polymeric substances from a mixed culture dominated by ammonia-oxidizing bacteria, Water Res., 2015, 68, 740–749 CrossRef CAS.
- M. Zhang, F. Han, H. Chen, J. Yao, Q. Li, Z. Li and W. Zhou, The effect of salinity on ammonium-assimilating biosystems in hypersaline wastewater treatment, Sci. Total Environ., 2022, 829, 154622 CrossRef CAS.
- Z. Z. Zhang, R. Deng, Y. F. Cheng, Y. H. Zhou, X. Buayi, X. Zhang, H. Z. Wang and R. C. Jin, Behavior and fate of copper ions in an anammox granular sludge reactor and strategies for remediation, J. Hazard. Mater., 2015, 300, 838–846 CrossRef CAS.
- D. Q. Cao, W. Y. Yang, Z. Wang and X. D. Hao, Role of extracellular polymeric substance in adsorption of quinolone antibiotics by microbial cells in excess sludge, Chem. Eng. J., 2019, 370, 684–694 CrossRef CAS.
- T. T. Chen, P. Zheng and L. D. Shen, Growth and metabolism characteristics of anaerobic ammonium-oxidizing bacteria aggregates, Appl. Microbiol. Biotechnol., 2012, 97, 5575–5583 CrossRef.
- W. Chen, P. Westerhoff, J. A. Leenheer and K. Booksh, Fluorescence Excitation−Emission Matrix Regional Integration to Quantify Spectra for Dissolved Organic Matter, Environ. Sci. Technol., 2003, 37, 5701–5710 CrossRef CAS PubMed.
- G. F. Li, W. J. Ma, Y. F. Cheng, S. T. Li, J. W. Zhao, J. P. Li, Q. Liu, N. S. Fan, B. C. Huang and R. C. Jin, A spectra metrology insight into the binding characteristics of Cu2+ onto anammox extracellular polymeric substances, Chem. Eng. J., 2020, 393, 124800 CrossRef CAS.
- Z. Z. Zhang, J. J. Xu, Z. J. Shi, Y. F. Cheng, Z. Q. Ji, R. Deng and R. C. Jin, Combined impacts of nanoparticles on anammox granules and the roles of EDTA and S(2−) in attenuation, J. Hazard. Mater., 2017, 334, 49–58 CrossRef CAS.
- Q. Y. Hu, D. Kang, R. Wang, A. Q. Ding, G. Abbas, M. Zhang, L. Qiu, H. F. Lu, H. J. Lu and P. Zheng, Characterization of oligotrophic AnAOB culture: morphological, physiological, and ecological features, Appl. Microbiol. Biotechnol., 2018, 102, 995–1003 CrossRef CAS PubMed.
- A. Mandel, I. Zekker, M. Jaagura and T. Tenno, Enhancement of anoxic phosphorus uptake of denitrifying phosphorus removal process by biomass adaption, Int. J. Environ. Sci. Technol., 2019, 16, 5965–5978 CrossRef CAS.
- Z. Z. Zhang, H. Y. Hu, J. J. Xu, Z. J. Shi, Y. Y. Shen, M. L. Shi and R. C. Jin, Susceptibility, resistance and resilience of anammox biomass to nanoscale copper stress, Bioresour. Technol., 2017, 241, 35–43 CrossRef CAS.
- M. Naufal and J. H. Wu, Stability of microbial functionality in anammox sludge adaptation to various salt concentrations and different salt-adding steps, Environ. Pollut., 2020, 264, 114713 CrossRef CAS PubMed.
- H. Lu, Y. Li, X. Shan, G. Abbas, Z. Zeng, D. Kang, Y. Wang, P. Zheng and M. Zhang, A holistic analysis of ANAMMOX process in response to salinity: From adaptation to collapse, Sep. Purif. Technol., 2019, 215, 342–350 CrossRef CAS.
- A. Zhang, S. Wang, M. Yang, H. Li, H. Wang, F. Fang and J. Guo, Influence of NaCl salinity on the aggregation performance of anammox granules, J. Water Process Eng., 2021, 39, 101687 CrossRef.
- Q. Wang, S. He, W. Yang, J. Zhu, W. Zhang, R. Xue and L. Liu, The effects of salinity changes on anammox performance: The response rule and tolerance mechanism, Water Environ. Res., 2022, 94, e10789 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4en00688g |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |