Synergistic effect of foliar exposure to TiO2 nanoparticles and planting density modulates the metabolite profile and transcription to alleviate cadmium induced phytotoxicity to wheat (Triticum aestivum L.)†
Min
Wang
a,
Junxiao
Luo
a,
Hongbo
Li
 a,
Chenghao
Ge
a,
Feng
Jing
a,
Jingxia
Guo
a,
Qingya
Zhang
b,
Xuezhen
Gao
b,
Cheng
Cheng
*ac and
Dongmei
Zhou
a,
Chenghao
Ge
a,
Feng
Jing
a,
Jingxia
Guo
a,
Qingya
Zhang
b,
Xuezhen
Gao
b,
Cheng
Cheng
*ac and
Dongmei
Zhou
 *a
*a
aState Key Laboratory of Pollution Control and Resource Reuse, School of the Environment, Nanjing University, Nanjing 210023, Jiangsu, P.R. China. E-mail: dmzhou@nju.edu.cn
bJiangsu DDBS Environmental Remediation CO., LTD, Nanjing 210012, Jiangsu, P.R. China
cSchool of Ecology and Applied Meteorology, Nanjing University of Information Science & Technology, Nanjing 210044, Jiangsu, P.R. China. E-mail: chengcheng918@nuist.edu.cn
First published on 18th November 2024
Abstract
A reasonable planting density is vital for wheat resource efficiency and yield enhancement. However, systematic research on the impact of spraying TiO2-NPs on wheat growth, metabolism, and stress tolerance cultivated in cadmium (Cd)-contaminated soil is limited, especially in integration with planting density, requiring a deeper understanding. Our study showed that spraying with 3.1 mg per plant TiO2-NPs (in pots) and 21.6 mg m−2 TiO2-NPs combined with high planting densities (in the field) both significantly reduced the Cd content in wheat grains by 27.9 and 35.7%, respectively. Immobilization of subcellular water-soluble Cd and the conversion of Cd into inactive plant components in leaves were the primary reasons for this reduction. Metabolomics further revealed the up-regulation of metabolites related to antioxidant activity, plant stress resistance, growth promotion, and the tricarboxylic acid (TCA) cycle, which promotes plant growth, enhances wheat antioxidant enzyme activity, and alleviates oxidative stress. Transcriptomic analysis validated the association between these responses and improved plant stress resistance, with genes such as MYB, WRKY, P450, and Cd membrane transport-related genes like ABCG2 and ABCC3 contributing to the decrease in Cd levels in wheat. Importantly, the Cd-associated human health risk index was also reduced via foliar TiO2-NPs application. Overall, foliar spraying of TiO2-NPs combined with high plant density was beneficial in alleviating Cd levels in wheat grains, limiting the risk of Cd exposure to human health via the food chain.
Environmental significanceCadmium contamination is becoming a considerable and urgent concern to ensuring food safety and quality. Suitable usage of nanomaterials is an effective approach for reducing heavy metal stress and increasing the mineral element content of plants due to their unique physicochemical characteristics. The present study first proved that foliar application of 3.1 mg per plant TiO2-NPs in pots and 21.6 mg m−2 TiO2-NPs combined with high planting densities under field conditions was effective in minimizing Cd contents in wheat grains, thereby mitigating the risk of Cd exposure to human health through the food chain. |
Introduction
Cadmium (Cd) is a hazardous heavy metal that poses severe negative impact on human health, even at low doses.1,2 A notable example is the notorious Itai–itai disease, which is caused by prolonged ingestion of Cd-contaminated rice.3 Although Cd levels in agricultural soil are typically trace amounts, human activities, including mining, metal smelting, air depositing, sewage irrigation, and the application of pesticides or fertilizers containing metals, may raise soil Cd concentrations and threaten food safety.1,2,4,5 The soil–plant–human body food chain serves as a critical pathway for Cd to affect human health,6 with crops like wheat and rice playing crucial roles.7,8 Consequently, reducing Cd content in crops is crucial to prevent its entry into the human body through the food chain.1,9 Various strategies have been implemented, including screening for low Cd-accumulating varieties,10,11 adding passivation agents,12 modulating soil conditions like pH,13 and water management.14 However, effectively reducing Cd accumulation in wheat remains a challenge, primarily due to wheat's stringent standard value (0.1 mg kg−1 in China)15 and the difficulties in modifying its growth cycle and altering soil characteristics.A wide range of nanotechnology applications, including engineered nanoparticles (NPs), nano-fertilizers, and nano-pesticides, have displayed remarkable utility across various sectors such as agriculture, food production, biomedicine, electronics, and renewable energy.16 These applications have yielded promising results in enhancing delivery mechanisms, improving nutrient use efficiency, and effectively managing diseases and crop yields in the agricultural sector. Titanium dioxide nanoparticles (TiO2-NPs) are a commonly used type of NPs, with an annual production exceeding 5000 tons, primarily finding their way into personal care products.17 Recently, research on TiO2-NPs has primarily focused on their uptake and transport mechanisms in plants. For instance, the utilization of μ-XRF and μ-XANES techniques has facilitated the demonstration of TiO2-NPs transportation in cucumbers without biological transformation, suggesting potential entry into the food chain.18 Additionally, spICP-MS analysis has revealed varying size distributions of internalized TiO2-NPs among NPs of different sizes.19 Previous studies have indicated that TiO2-NPs exhibit toxicity to wheat20 and rice,21 resulting in growth inhibition, altered soil enzyme activity, and interference with plant antioxidant defense and metabolic systems. However, contrasting findings suggest that TiO2-NPs may not be toxic to plants at most concentrations, with reports indicating non-toxicity up to 5000 mg kg−1 for tomatoes22 and 4000 mg L−1 for rape plants23 in soil, respectively. These contradictory findings highlight the significant influence of plant species and concentrations on the toxicity of TiO2-NPs.
The application of TiO2-NPs can significantly reduce the accumulation of lead24 and arsenic25 in rice seedlings, as well as tetracycline26 in Arabidopsis thaliana, and Cd in maize seedlings.27 Interestingly, while foliar TiO2-NPs application shows promise in reducing Cd content in maize, root application has actually promoted Cd uptake.27 This suggests that foliar application of TiO2-NPs could effectively reduce Cd content in plants, including wheat. One investigation which integrated transcriptomic and metabolomic techniques delved into the harmful effects of TiO2-NPs–Cd on rice, uncovering noteworthy up-regulation of 14 differentially expressed genes (DEGs). These include asparaginyl-tRNA synthetase and methionyl-tRNA formyltransferase, genes pivotal to aminoacyl-tRNA biosynthetic processes, with their heightened expression intimately linked to alterations in associated metabolic compounds.28 However, it is crucial to note that the soil Cd content used in this study reached 30 mg kg−1, significantly exceeding the typical actual background level of Cd pollution in soil.29 To date, there exists a notable lack of extensive research endeavors examining the impact of TiO2-NPs spraying on Cd levels in wheat grains, particularly within real field conditions. The field-based investigation conducted hitherto has demonstrated the efficacy of TiO2-NPs, derived from plant extracts, in reducing Cd accumulation in wheat grains upon foliar application, thereby alleviating potential health risks posed by Cd via the food chain, as reported by Irshad et al. (2021).30 However, to ascertain the optimal concentration for TiO2-NPs application, additional research endeavors remain imperative.
Planting density and spacing significantly affect crop yield. Appropriately reducing planting spacing can effectively mitigate the accumulation of heavy metals, notably Cd, within crops.31 Additionally, an increase in planting density correlates positively with an increase in food production.32,33 However, excessively high or low planting densities often fail to optimize yield, resulting in resource wastage. Therefore, fine-tuning the planting density is crucial for improving grain yield and resource utilization efficiency.33 Currently, there is limited research exploring the influence of planting density on Cd accumulation in wheat, especially when considering the integration of NPs. It is crucial to determine whether these combinations can effectively mitigate Cd accumulation in grains. Meanwhile, sequestration of Cd within plant subcellular compartments serves as a vital detoxification mechanism against heavy metal accumulation.34 However, previous studies have primarily focused on dividing subcellular compartments into cell walls, organelles, and water-soluble fractions, without delving into the precise distribution of organelles.35,36 Furthermore, the speciation of Cd within plants is a pivotal factor determining the plant's tolerance to Cd stress.37 Hence, a comprehensive analysis of the influences that NPs spraying exerts on the subcellular distribution and chemical forms of Cd in wheat is paramount. This comprehensive approach is essential for developing effective strategies to mitigate Cd accumulation and enhance the resilience of wheat crops.
Metabolomics and transcriptomics association analysis is a potent technique to identify the mechanism of activity of pollutants.25 Current research on wheat primarily focuses on its response to contaminants through changes in metabolites. For instance, exposure to TiO2-NPs altered the amino acid metabolism, glycerolipid biosynthesis, and citrate and glyoxylate metabolism in wheat.38 Based on these findings, this study aims to achieve three main objectives: firstly, to identify the optimal concentration of TiO2-NPs for spraying on wheat grown in Cd-contaminated soil in order to promote its growth. Secondly, to employ metabolomics and transcriptomics techniques to elucidate the fundamental mechanisms underlying the reduced Cd accumulation in wheat grains with TiO2-NPs. Finally, to verify the effectiveness of TiO2-NPs spraying in mitigating Cd content in wheat grains by incorporating varying planting densities in field experiments.
Materials and methods
Nanoparticles
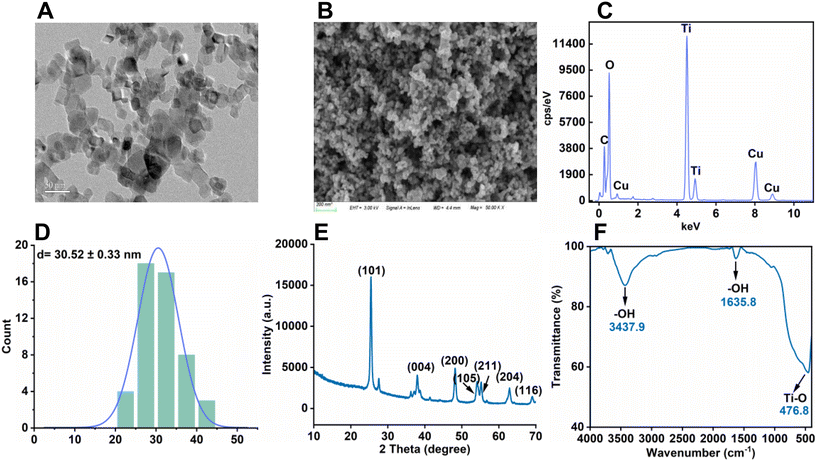
TiO2-NPs (99.9% purity) were procured from Tianjin Baima Inc., Tianjin, China. For a comprehensive understanding of the TiO2-NPs, a multifaceted approach was utilized for their characterization, as detailed in Text S1.† The TiO2-NPs exhibited a particle size of 30.52 nm (Fig. 1), with an average hydrodynamic diameter of 318 ± 15.4 nm, and a corresponding zeta potential value of 5.90 ± 0.70 mV.Foliar pot and field experiment
An experiment was conducted in the greenhouse of Nanjing University, spanning the period from November 2022 to June 2023, and located at the geographical coordinates of 118°57′E and 32°07′N. To prevent fungal contamination, the surface of wheat (Triticum aestivum L.) seeds (XN-979) was sterilized by immersion in a 5% (v/v) H2O2 solution for a duration of 30 min. Afterward, the seeds were thoroughly rinsed three times with ultrapure water and soaked overnight in ultrapure water at 20 °C in a dark environment.The agricultural soil was procured from the top 20 cm layer of soil at experimental stations located in Southern Jiangsu Province, China. The procured soil underwent air-drying, homogenization, grinding, and screening to ensure a particle size of 5 mm or finer before being utilized. The comprehensive soil properties are outlined in Table S1.† Plastic containers were filled with 5 kg of this prepared agricultural soil. To ensure adequate nutrient supply during cultivation, the soil was amended with 0.60 g of CO(NH2)2 as an N fertilizer and 0.132 g of KH2PO4 as a source of P and K fertilizer. Additional N fertilizer [0.15 g kg−1 CO(NH2)2] was applied to the wheat at the jointing stage.
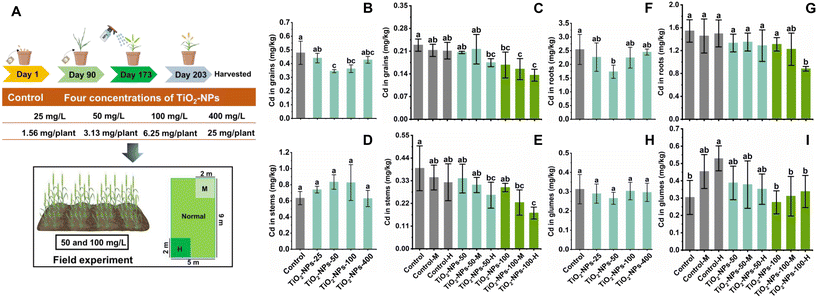
Five treatments were established: a control (without NPs) and four additional treatments involving foliar application of TiO2-NPs at concentrations of 25, 50, 100, and 400 mg L−1, respectively. The specific preparation method involved weighing the TiO2-NPs (0.0025, 0.005, 0.01, and 0.04 g) and dissolving them in ultrapure water (100 mL), with the addition of 0.1% surfactant (Tween 80). Prior to spraying, the freshly formulated NPs suspension was sonicated for 30 min at 20 °C. Three replicate pots were grown for each treatment, with a total of eight plants per pot. The foliar application experiment was conducted during the pre-filling stage (one week after the wheat flowering stage) of plant growth, with 100 mL sprays each time, with a one day interval between each spray, resulting in a total of five applications. The application rate of TiO2-NPs was 1.56, 3.13, 6.25, and 25 mg per plant, respectively. On the fifth day subsequent to the completion of spraying, fresh flag leaves in each pot were preserved at −80 °C for subsequent exhaustive evaluations, including physiological parameters, metabolomic profiling, and transcriptomic analysis. A month after the completion of the spraying stages, the wheat including roots, stems, glumes, and grains was harvested. During harvesting, the height of the wheat plants and the number of effective panicles were recorded. Thereafter, the samples underwent a drying process: firstly in an oven at 100 °C for 30 min, subsequently continuing at a reduced temperature of 70 °C for three days. This drying process facilitated the accurate measurement of the dry weight of the grains. After harvest, the ear weight, yield, and hundred-grain weight were recorded for each wheat sample and were subsequently analyzed.
Based on the physiological results of the pot experiments, subsequent foliar field trials in Southern Jiangsu Province, China (located at 31°23′N, 121°5′E) were conducted, from November 2022 to June 2023. The goal of these field trials was to uncover more about the benefits of spraying TiO2-NPs on wheat under practical conditions. Three distinct treatments were implemented: a control (no NPs) and two groups sprayed with TiO2-NPs concentrations of 50 and 100 mg L−1. Tween 80 (0.1%) was added as a surfactant. During the pre-filling stage, the TiO2-NPs were sprayed twice using an electric sprayer, with each spray application at 4 L per pot (Fig. 2A). Consequently, the application doses were 10.8 and 21.6 mg m−2, respectively. To further explore the combined effects of planting density and TiO2-NPs spraying on wheat yield and fundamental traits, we established three planting densities within each plot: normal (0.0255 kg m−2), medium (M) (0.03825 kg m−2), and high (H) (0.051 kg m−2) (Fig. 2A). The wheat was harvested one month after the spraying stages, with harvest indexes aligning with those observed in the pot experiments.
Physiological indicators of wheat
The wheat leaves underwent a thorough rinsing process, initially with tap water for 5 min, followed by ultrapure water. The leaves were washed, dried using Kimwipes, and then ground into a fine powder using liquid nitrogen. To determine the photosynthetic pigments and total phenolic content in the wheat leaves, we used the methodology by Singleton and Rossi.39 Additionally, the total antioxidant capacity was assessed as described by Benzie and Strain.40 Detailed information regarding these assays can be found in Text S2.† Simultaneously, the wheat leaves were extracted in a chilled phosphate buffer solution (0.05 mol L−1, pH adjusted to 7.8), the entire process maintained within an ice bath to preserve integrity. Subsequently, the extracted mixture was centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm (4 °C) for a duration of 10 min. The resultant supernatant was used for assessing the enzymatic functionalities of catalase (CAT), superoxide dismutase (SOD), peroxidase (POD), and polyphenol oxidase (PPO), as well as to measure the malonaldehyde (MDA) content. Additionally, quantitative measurements of protein levels and hydrogen peroxide (H2O2) concentrations were carried out using specialized assay kits procured from Nanjing Jiancheng Bioengineering Institute.
000 rpm (4 °C) for a duration of 10 min. The resultant supernatant was used for assessing the enzymatic functionalities of catalase (CAT), superoxide dismutase (SOD), peroxidase (POD), and polyphenol oxidase (PPO), as well as to measure the malonaldehyde (MDA) content. Additionally, quantitative measurements of protein levels and hydrogen peroxide (H2O2) concentrations were carried out using specialized assay kits procured from Nanjing Jiancheng Bioengineering Institute.
Element content determination by ICP-MS
Wheat roots, stems, glumes, and grains powder underwent a digestion process using a 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume ratio mixture of HNO3 (7.2–7.55 mol L−1) and H2O2 on a graphite digester at 105 °C, adhering to the guidelines outlined in US EPA Method 3050B.41 Upon completion of digestion and subsequent cooling of the samples, the digestion solution was diluted with ultrapure water and filtered meticulously through a 0.22 μm filter membrane. For accurate quantification of the Cd concentration, inductively coupled plasma mass spectrometry (ICP-MS) was employed, specifically utilizing the NexION 2000 system from PerkinElmer. Quality control measures were implemented by selecting the GBW10010a sample, which demonstrated a commendable recovery rate, falling within the prescribed limits of 94.9% to 100.4%.
1 volume ratio mixture of HNO3 (7.2–7.55 mol L−1) and H2O2 on a graphite digester at 105 °C, adhering to the guidelines outlined in US EPA Method 3050B.41 Upon completion of digestion and subsequent cooling of the samples, the digestion solution was diluted with ultrapure water and filtered meticulously through a 0.22 μm filter membrane. For accurate quantification of the Cd concentration, inductively coupled plasma mass spectrometry (ICP-MS) was employed, specifically utilizing the NexION 2000 system from PerkinElmer. Quality control measures were implemented by selecting the GBW10010a sample, which demonstrated a commendable recovery rate, falling within the prescribed limits of 94.9% to 100.4%.
Cadmium subcellular distribution and speciation in wheat leaves
Since the NPs are directly in contact with the leaves, the subcellular distribution and speciation of Cd in wheat leaves were assessed. The subcellular fractionation method of Wu et al.42 was used with minor adjustments. Leaves were weighed, homogenized in chilled buffer mixed with sucrose, Tris-HCl, and dithioerythritol, then filtered. The cell-wall fraction (FCW) remained on the filter cloth. The filtrate was centrifuged for plastids (FP), then the supernatant was centrifuged for the nucleus (FN), followed by the mitochondria (FM). The final supernatant was the soluble fraction (FS). All fractions were dried at 70 °C, digested with mixed acids, and the Cd concentrations were determined by ICP-MS.Cadmium speciation in wheat leaves was evaluated following the method of Wang et al.43 with modifications. The procedure involved overnight immersion of 1.00 g of wheat leaves in 10 mL of solution at 30 °C, followed by centrifugation at 5000g for 10 min. The pellet was resuspended twice in extraction solution, then subjected to three additional 2 h extractions with fresh solvent. The four extraction solutions were combined, representing the total Cd content of the analyzed component. For a visual representation of the entire extraction process, refer to Text S3† for the detailed flowchart.
Metabolite profiling in wheat leaves
To elucidate the molecular underpinnings of the differential mechanism of foliar application of TiO2-NPs (50 mg L−1) treatment in the pot experiment, given that metabolites serve as the ultimate outcomes of biological processes, the metabolome of TiO2-NPs-50-treated leaves was initially explored. Metabolite profiling of wheat leaves was conducted using gas chromatography-mass spectrometry (GC-MS) for a nontargeted approach. TiO2-NPs (50 mg L−1) treated and untreated wheat leaves were snap-frozen in liquid nitrogen, ground, and stored at −80 °C prior to extraction. Extraction employed chilled methanol, an internal standard, and derivatization with methoxyamine hydrochloride and BSTFA + 1% TMCS. The Agilent 80B GC-5977A mass detector system was used to analyze samples on a DB-5MS column, identifying and quantifying metabolites based on retention index, mass spectra, and peak height, assigning BinBase numbers. For the metabolomic data interpretation, samples were subjected to partial least-squares discriminant analysis (PLS-DA) in R 4.2.3, assessing distribution and stability. The variable importance of projection (VIP) scores derived from OPLS-DA ranked variables for group differentiation, identifying significant metabolites (VIP > 1.00, p < 0.05). GC-MS methodology and sample derivatization protocols are detailed in previous work.44Transcriptomics profiling in wheat leaves
RNA-seq analysis was conducted in wheat leaves treated with 50 mg L−1 TiO2-NPs foliar treatment and untreated controls. Total RNA was extracted using the FastPure Universal Plant RNA Kit (Invitrogen, CA, USA), adhering strictly to the protocols. RNA quality was verified by the Agilent 2100 Bioanalyzer (Agilent Technologies, CA, USA). OE Biotech Co., Ltd. (Shanghai, China) prepared RNA-seq libraries using the VAHTS kit and sequenced on an Illumina HiSeq xten/NovaSeq 6000 platform. DESeq2 was employed to identify DEGs with q < 0.05 and >2-fold or <0.5-fold change. Gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses were performed on these DEGs. Visualizations and insights were facilitated by R (v3.2.0). Sequencing yielded 37.4 M to 47.0 M reads per sample, averaging 43.3 M, with a Q30 base average of 95.5% (Table S2†). These findings attest to the suitability of the sequencing data for subsequent analyses.Assessment of human health risk
To evaluate the Cd-related human health risk index (HHRI), the daily intake of Cd (DIC) was calculated and compared against the oral reference dose (RFD) of 0.001 mg kg−1.45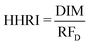 | (1) |
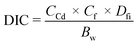 | (2) |
Statistical analysis
A one-way ANOVA was performed in SPSS 22.0, setting significance at p < 0.05. Data are reported as mean ± SD (n = 3). Histograms were generated with Origin 2023 and refined in Adobe Illustrator 2021.Results and discussion
Growth of wheat
Compared with the control, there were insignificant (p > 0.05) differences in plant height, effective panicle number, panicle length, etc. (Fig. S1 and S2†). However, when spraying 400 mg L−1 TiO2-NPs, the grain yield was decreased compared with the control (Fig. S1A†). This indicated that spraying TiO2-NPs at concentrations of 25–100 mg L−1 showed no adverse effect on wheat growth, aligning with prior findings of improved growth at concentrations below 100 mg L−1.48,49Cadmium content of wheat
The application of TiO2-NPs via foliar spray showed a significant (p < 0.05) impact on the uptake and accumulation of Cd in wheat, as observed in both pot and field studies (Fig. 2). Notably, in the pot experiment, the utilization of 50 mg L−1 TiO2-NPs spray led to a significant (p < 0.05) decrease in Cd levels within wheat grains, achieving a reduction of 27.9% from the control level of 0.48 to 0.35 mg kg−1 (Fig. 2B). Furthermore, in the field experiment, foliar application of 100 mg L−1 TiO2-NPs, combined with normal, medium, and high planting densities, significantly (p < 0.05) reduced the Cd content in wheat grains by 26.8, 27.2, and 35.8%, respectively, compared with the control (Fig. 2C). Meanwhile, a significant (p < 0.05) 35.8% reduction in Cd content was also observed in HHRI via foliar spraying of 100 mg L−1 TiO2-NPs combined with high planting densities (Fig. S3†). The observed results indicate that a specific concentration of TiO2-NPs exhibits a potent capacity to impede the translocation of Cd from the roots to shoots, and ultimately into the grains of wheat. Consistent with our research, previous studies have shown that foliar application of TiO2-NPs, as well as a combination of biochar and TiO2-NPs, reduced Cd contents in wheat grains compared to the control group.30,47 Nonetheless, recent studies have shown a notable decrease in both the height of rice plants and the dry weight of their roots within the TiO2-NPs–Cd-treated group, as opposed to the unexposed control group.28 Consequently, it is plausible to hypothesize that the influence of TiO2-NPs on the absorption and accumulation of Cd in agricultural produce varies according to the specific crop species involved.Noticeably, although no significant (p > 0.05) difference in Cd content in stems was observed in the pot experiment, compared to the control group, application of 50 mg L−1 TiO2-NPs (0.83 mg kg−1) resulted in a 24.9% increase in Cd content (Fig. 2D). Previous research has also demonstrated that the incorporation of TiO2-NPs leads to an increase in the accumulation of Cd within wheat stems;50 this increase in Cd concentration within the stems might subsequently lead to a reduction of Cd in wheat grains, making it easier to remove Cd by discarding stems and other wheat parts after harvesting. In terms of anatomical structure and functionality, the xylem within the base of each wheat grain displays discontinuous features, thereby rendering the phloem the sole continuous conduit responsible for transporting Cd towards the maturing grains.51 Therefore, maintaining a higher Cd concentration in the stems could function as a protective mechanism, preventing Cd from accumulating in the grains.50
Conversely, a decreasing trend was observed in the Cd content of wheat roots and glumes (Fig. 2F–I). Specifically, in the pot experiment, the application of 50 mg L−1 TiO2-NPs (1.73 mg kg−1) significantly (p < 0.05) reduced the Cd content in wheat roots by 31.9%, compared to the control (Fig. 2F). Additionally, in field trials, the incorporation of 100 mg L−1 TiO2-NPs along with an elevated planting density led to a notable (p < 0.05) decrease in the Cd content of glumes, achieving a reduction of 36.2% when compared to the control (Fig. 2I), which was related to the increased photosynthesis (Fig. S4†). We speculate two possible reasons to explain the decrease in Cd uptake in wheat caused by NPs spraying. Firstly, NPs can influence the expression of transport genes in root and leaves, thereby influencing the absorption and transport of Cd by plants. For instance, the application of selenium (Se) and silicon NPs in wheat can down-regulate Cd intracellular transporter protein and up-regulate Cd transporter protein, ultimately inhibiting Cd transport.52 Secondly, NPs may lead to an increase in root secretions, such as changes in the abundance of growth-promoting rhizobia in the rhizosphere and increasing nitrogen-related metabolites. These changes may contribute to reduced Cd transport from the roots to the grains.53,54 Meanwhile, spraying NPs led to a decrease in organic acids in the rhizosphere, thereby reducing the bioavailability of Cd in rhizosphere soil.53 Hence, it is imperative to conduct additional studies to delve into the effects that the application of composite NPs via foliage has on the alterations occurring within the crop rhizosphere and the transcriptome mechanism in Cd-contaminated soil, as this holds immense significance in addressing the issue of hidden hunger.
MDA content and antioxidant enzyme activities of wheat
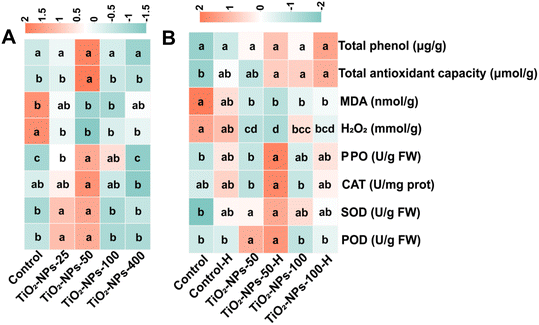
Although no differences (p > 0.05) in the phenolic content and antioxidant capacity of the leaves were evident among all the TiO2-NPs treatments (Fig. 3), pot-grown wheat exposed to 50 mg L−1 of TiO2-NPs and field-grown wheat managed with a high plant density and also treated with 50 mg L−1 TiO2-NPs displayed decreased levels of hydrogen peroxide (H2O2) alongside heightened activity of antioxidant enzymes. Specifically, in the pot experiment, compared to the control, wheat sprayed with 50 mg L−1 TiO2-NPs showed a significant (p < 0.05) reduction (35.1%) in H2O2 content, while PPO, CAT, SOD, and POD enzyme activities significantly (p < 0.05) increased by 49.6, 18.2, 17.2, and 18.0%, respectively. Cadmium may cause plant physiological disorders by generating reactive oxygen species (ROS), leading to membrane lipid peroxidation and protein/lipid damage.1 Similar to Mohammadi et al.,55 foliar applying of TiO2-NPs increased the antioxidant enzyme activity, reducing lipid peroxidation. ROS overproduction enhances the lipid peroxidation process, increasing MDA content in Cd-stressed plants. Thus, the reduced H2O2 production/accumulation suggests that TiO2-NPs alleviate Cd toxicity in wheat.On the other hand, under Cd stress, plants possess the capacity to eliminate ROS via diverse antioxidative mechanisms, including the activation of antioxidative enzymes.49 As a primary defense enzyme, SOD functions by transforming superoxide radicals (O2−) into H2O2. Additionally, CAT activity plays a crucial role in detoxifying higher levels of H2O2 by converting H2O2 into water in peroxisomes, effectively mitigating its deleterious effects.44 Our findings indicated a significant (p < 0.05) increase in the activity of antioxidant enzymes (Fig. 3). Interestingly, the optimal concentration for increasing enzyme activity in plants was 50 mg L−1 combined with high plant density in field trials. This finding is intriguing as it differs from the most effective concentration of 100 mg L−1 with high plant density treatment for Cd reduction, indicating a potential link with plant density. Therefore, the foliar application of TiO2-NPs results in a substantial decrease in the Cd content present within wheat grains, attributed to the oxidative suppression and the enhanced enzymatic antioxidant defenses, such as SOD, CAT, PPO, and POD, resulting from the NPs' uptake.
Subcellular distribution and speciation of Cd in wheat leaves
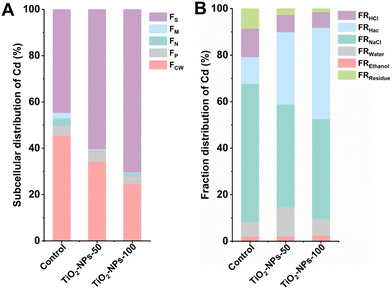
Plants have the capability to detoxify heavy metal ions that enter them by accumulating them in various plant tissues or organelles, and/or by chelating or converting them into non-toxic forms, thus neutralizing their harmful effects.42 In order to delve deeper into the underlying processes responsible for the mitigation of Cd levels in wheat grains via foliar application of TiO2-NPs, we examined the subcellular distribution of Cd and Cd speciation in pot-grown wheat (Fig. 4). Regardless of the treatments, the Cd distribution in the leaves followed a pattern of FS > FCW > FP > FN > FM when compared to the control. Remarkably, the application of TiO2-NPs-100 spray led to a noteworthy increase of 25.8% in the FS levels within the treatment group, compared to the control. Additionally, the organelle fraction (FP + FN + FM) decreased by 5% in TiO2-NPs-100 spray, with a specific reduction of 2.1% in FS, relative to control treatment. Cadmium fixation in the water-soluble part of wheat leaves has been achieved through the spraying of SeNPs/inorganic Se.35,44 Furthermore, numerous investigations have revealed that the translocation of harmful metals within certain plant species is effectively impeded by the complexation process involving metallothioneins (MTs) and phytochelatins (PCs).56 Consequently, the localization and subcellular distribution of heavy metals within plant tissues are intricately linked to their toxicity and tolerance. Moreover, it was found that FRNaCl was the primary speciation in leaves, either control or TiO2-NPs-50/100 treatment. Interestingly, FRHac increased by 27.8% after the application of TiO2-NPs-100 spray, while FRNaCl and FRHCl decreased by 16.4 and 5.5%, respectively, relative to control treatment. It is conceivable that the transformation of Cd speciation, shifting from its active forms to inactive ones, could be a contributing factor to the observed reduction of Cd levels in wheat grains.Wheat leaf metabolome reprogramming
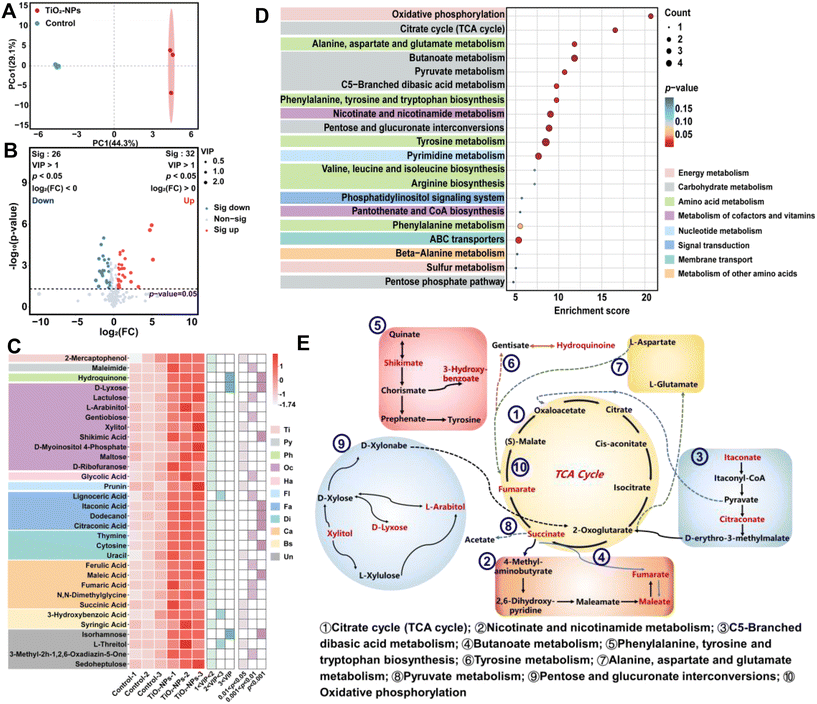
Through the application of GC-MS metabolomics techniques, we were able to identify and partially quantify a comprehensive set of 328 distinct metabolites, as illustrated in Fig. 5. To assess the metabolomic variations between the control and TiO2-NPs-50 treatment, a OPLSDA model was employed. The distinct separation observed in the OPLSDA score plot along PC1 (Fig. 5A) suggests the presence of notable metabolic variations between the two wheat leaves. Our findings revealed that TiO2-NPs-50 treatment significantly up-regulated 32 metabolites and down-regulated 26 metabolites among the 58 differentiated metabolites compared to the control (Fig. 5B). Based on the fact that leaves are the part where NPs come into direct contact with plants, and their physiological properties are improved after the application of TiO2-NPs-50, we hypothesize the existence of a correlation between the reduced Cd concentration in the leaves and the observed alterations in metabolite profiles.Firstly, the application of foliar TiO2-NPs-50 significantly up-regulated antioxidant-related metabolites, compared to the control (Fig. 5). Specifically, D-lyxose (33.6 fold), L-arabinitol (0.9 fold), N,N-dimethylglycine (0.6 fold), ferulic acid (1.22 fold), itaconic acid (1.3 fold), hydroquinone (29.9 fold), prunin (0.5 fold), xylitol (0.7 fold), and syringic acid (0.5 fold) were significantly up-regulated (Fig. 5C; S5†). For instance, D-lyxose and L-arabinitol, being natural sugar alcohols and monosaccharides respectively, can directly react with free radicals, mitigating the oxidative stress-induced damage to cells and exhibiting potent antioxidant effects.57,58 Additionally, ferulic acid and syringic acid are phenolic antioxidants widely present in plants, primarily functioning as antioxidants in leaves. Ferulic acid also plays a crucial role as a functional phenolic acid in plant cell walls. Both compounds exhibit significant antioxidant and free radical scavenging activities.59,60 Consequently, the increased antioxidant capacity of plants may underlie the reduction in Cd concentration observed in wheat grains following the foliar application of TiO2-NPs-50.
Additionally, the intermediates of the tricarboxylic acid (TCA) cycle, specifically fumaric acid (1.3 fold) and succinic acid (0.6 fold), were significantly increased in the foliar TiO2-NPs-50 application compared to the control group (Fig. 5C; S6†). Fumaric acid, an intermediate in the TCA cycle, can be metabolized alongside starch and soluble sugars to generate energy and a carbon skeleton.61 Alternatively, succinic acid occupies a pivotal position in modulating the development and growth processes of crops.62 Specifically, succinic acid is a precursor of dicarboxylic growth hormones that arise as intermediates of the TCA cycle and can be converted into hormones such as indoleacetic acid and abscisic acid. The activation of the TCA cycle in the foliar TiO2-NPs-50 application increased, potentially stimulating the production of defensive proteins. The acceleration of the TCA cycle promotes protein synthesis in two ways: by provisioning the prerequisites for ATP production and by providing the fundamental amino acids necessary for protein biosynthesis,63 the TCA cycle plays a pivotal role. For example, it disseminates metabolites into the cytoplasm, which then act as essential constituents in the assembly of diverse macromolecules, encompassing lipids and nucleotides. Additionally, Fig. 5D depicts an upregulation of the TCA cycle. As a result, the augmented generation of defensive macromolecules, which encompass amino acids, lipids, and nucleotides, can be attributed directly to the enhanced performance of the TCA cycle.
Furthermore, a noteworthy increase in metabolites associated with plant stress resistance and growth promotion was observed in wheat leaves treated with TiO2-NPs-50 spray, compared to the control, including shikimic acid (0.6 fold), maltose (0.6 fold), lignoceric acid (3.7 fold), gentiobiose (0.6 fold), and maleic acid (1.8 fold) (Fig. S7†). Shikimic acid, a crucial intermediate in plant metabolism, plays a pivotal role in the biosynthesis of numerous secondary metabolites. The shikimate acid (SHA) pathway serves as a central hub for the production of compounds crucial for plant functions, including defense mechanisms, such as flavonoids, lignin, indole derivatives, and numerous aromatic alkaloids.64 Maltose and maleic acid, on the other hand, are plant growth regulators that enhance plant growth, stress resistance, and yield.65,66 Gentiobiose, a rare disaccharide, serves as a dormancy release signal for Gentian OWBs in the ascorbate–glutathione (AsA–GSH) cycle. The accumulation of gentiobiose may contribute to stress resistance in plants.67 Hence, the observed elevation in defensive and specialized metabolites in wheat leaves treated with TiO2-NPs-50 under Cd stress could be a contributing factor to the systemic decline in H2O2 levels. In summary, the metabolomics analysis provides a detailed understanding of the metabolic transformations elicited by the exposure of leaves to TiO2-NPs-50 (Fig. 5E). To foster the production of antioxidants and signaling metabolites, the TiO2-NPs-50 application substantially boosts the TCA cycle, marking a significant departure from the control group. This metabolic surge prepares wheat for Cd stress, thus highlighting the remarkable potential of foliar TiO2-NPs-50 application in mitigating Cd accumulation in wheat.
Wheat leaf transcriptome reprogramming
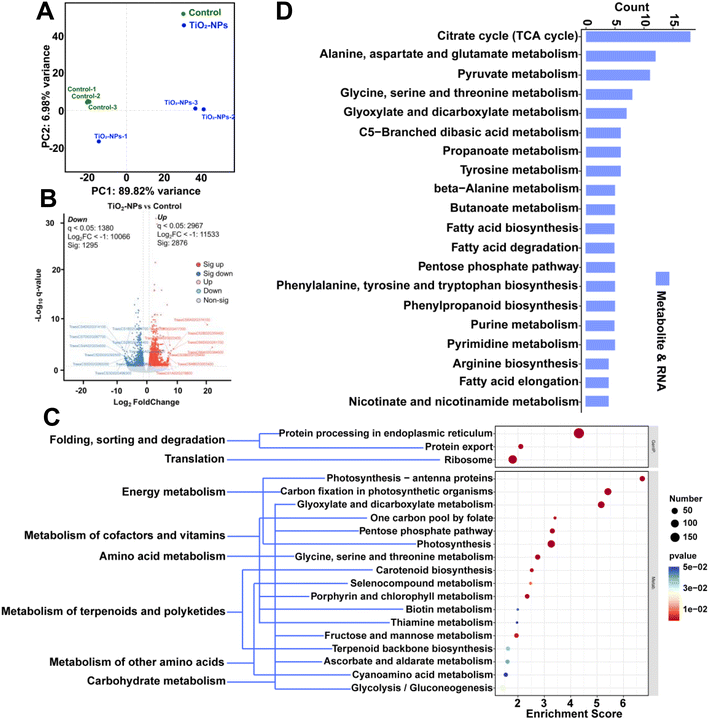
To gain a comprehensive understanding of the modified molecular pathways responsible for the observed physiological and metabolic changes, we utilized RNA-seq technology to conduct transcriptome profiling. This profiling also aided in elucidating the mechanisms by which foliar application of TiO2-NPs-50 alleviates Cd stress. Based on principal component analysis (PCA), two distinct groups were clearly separated along both PC1 and PC2 (Fig. 6A). This suggests that foliar application of TiO2-NPs-50 results in extensive transcriptional reprogramming in wheat leaves. Specifically, TiO2-NPs-50 up-regulated numerous transcriptional changes, with 2876 DEGs identified (Fig. 6B). This response pattern aligns with the metabolomics data (Fig. 5B), indicating that the most significant molecular reprogramming in wheat leaves was triggered by the foliar treatment of TiO2-NPs-50.Based on the KEGG pathway analysis (comparing control vs. TiO2-NPs-50, top 20), a notable abundance of distinct DEGs was observed in critical processes including photosynthetic organisms, photosynthesis, photosynthesis – antenna proteins, pentose phosphate pathway, and photosynthesis (Fig. 6C). This unequivocally underscores the profound effect of TiO2-NPs-50 foliar-applied on the energy metabolism dynamics within wheat leaves. Furthermore, our combined metabolomics and transcriptomics analysis revealed that the TCA cycle pathway was the most significantly affected by these changes (Fig. 6D). The acceleration of the TCA cycle facilitates protein synthesis in two primary ways: by providing the necessary precursors for ATP generation and by supplying essential amino acids for protein synthesis. Consequently, we postulate that the enhanced plant growth observed after spraying with TiO2-NPs-50 is primarily attributed to the stimulation of the TCA cycle, providing additional energy for plant growth, ultimately leading to improved antioxidant enzyme activities. The foliar application of TiO2-NPs-50 elicited the expression patterns of diverse stress-responsive transcription factors (TFs), notably within the WRKY and MYB gene families in the leaves (Fig. S8†). WRKY1, alternatively known as zinc reactive transcriptional activator ZAP1, is the inaugural member of the WRKY gene family identified in Arabidopsis thaliana and belongs to group I. This gene is believed to be implicated in the salicylic acid (SA) signaling cascade, regulating stomatal movements in guard cells, a crucial aspect of salt stress tolerance.68 Furthermore, WRKY1 influences stress-responsive mechanisms such as secondary metabolism and nitrogen metabolism in plants. MYB TFs serve as pivotal regulators of both biological and abiotic stress responses in plants. These factors not only modulate plant cold tolerance but also play a significant role in orchestrating the synthesis of specialized metabolites, including anthocyanin.69 The expression of defense-related genes is also under the control of these transcription factors. It is worth mentioning that the cytochrome P450 monooxygenase (P450) gene family occupies a paramount position in the biosynthesis of numerous specialized metabolites.63 These P450 enzymes not only produce defense-oriented specialized metabolites such as flavonoids, phytohormones, tropane alkaloids, cutin, and cuticular wax, but also regulate and accelerate their biosynthetic processes. Collectively, these findings suggest that the spraying of TiO2-NPs enhances the resilience of plants against heavy metal stress.
There are no specific transporters designed to take up or transport Cd due to its toxicity to plants. The elemental composition of Cd exhibits similarities to those of essential nutrients, including iron (Fe), zinc (Zn), and manganese (Mn).2 Certain metal transporters have also been significantly affected by Cd (Fig. S8†). Flavonoids, a widely occurring class of natural products in higher plants, have been reported to exhibit an inhibitory effect on the activity of ABC transporter G family member 2 (ABCG2). Numerous in vitro studies, utilizing various flavonoid classes combined with cytotoxic drugs, have confirmed their ability to inhibit ABCG2. Consequently, the down-regulation of this gene reflects an enhanced antioxidant capacity in plants.70 Cytoplasmic Cd can be sequestered by phytochelatins (PCs), yielding a robust PC–Cd complex instrumental in the vital process of Cd detoxification. Cd-induced expression of AtABCC3 enhances phytochelatin-mediated cadmium tolerance in Arabidopsis thaliana. Our study suggests that the down-regulation of TaABCC3 may contribute to the reduced Cd stress observed in plants across different species.71 PCs are synthesized by PC enzymes using GSH as a substrate. The binding of PCs to cytoplasmic Cd creates a stable PC–Cd complex, which is essential for Cd detoxification. The up-regulation of glutathione synthetase (GSHB) accounts for the increased synthesis of PCs and facilitates their transfer to the vacuole for detoxification purposes (Fig. S9†).71
Multiple kinases, including pyruvate kinase (PKM), protein phosphatase 1A (PPM1A), and serine/threonine-protein kinase (PLL), exhibited either inhibitory or activating responses in wheat leaves treated with foliar TiO2-NPs-50 application. PKM is involved in redirecting glycolytic substrates towards anabolic processes and can also function as a protein kinase, regulating diverse plant growth mechanisms (Fig. S10†).72 PPM1A, belonging to the Ser/Thr protein phosphatase family, necessitates magnesium for the execution of its biological function. This enzyme has the ability to bind to and dephosphorylate various proteins, thus playing a pivotal role in regulating a range of physiological processes. Its significance extends to transcriptional regulation, cell proliferation, and apoptosis. Furthermore, studies have implicated PPM1A in the onset and progression of cancers affecting organs such as the lung, bladder, and breast (Fig. S10†).73 Conversely, pll genes have been implicated in cellular processes pertaining to cell wall formation, notably in the context of pectin breakdown. They maintain the integrity of the cell wall and contribute to establishing barriers that aid in plant defense mechanisms (Fig. S10†).74 Ferrithionein serves as a crucial electron transporter in organisms, with ferredoxin being a notable example in chloroplasts, where it plays a fundamental role in photosynthesis (Fig. S11†).75 Another noteworthy protein is the zinc finger protein ZAT6, which has been demonstrated to be involved in plants exhibiting enhanced tolerance to Cd;76 the up-regulation of ZPR1 may also related to the enhanced tolerance to Cd (Fig. S11†). In conclusion, the application of foliar TiO2-NPs-50 has the potential to activate multiple stress defense signaling pathways.
Conclusions
The effects of TiO2-NPs on wheat grains grown in Cd-contaminated soil have been rarely reported, particularly studies that combine pot and field experiments. Planting density shows a significant impact on crop yield, and adjusting the planting density is vital for enhancing both grain yield and resource utilization efficiency. We identified the optimal concentration of TiO2-NPs combined with plant density, for spraying in wheat grown on Cd-contaminated soil, and explored the mechanisms via metabolomics and transcriptomics. Our findings demonstrated that administering 3.1 mg per plant of TiO2-NPs in pots and employing 21.6 mg m−2 of TiO2-NPs with high planting density in field trials significantly decreased the Cd concentration in wheat grains. This notable decrease was primarily facilitated by the enhanced activity of antioxidant enzymes in plants, the sequestration of water-soluble Cd within subcellular compartments, and the transformation of Cd into non-toxic plant components. Metabolomics analysis further corroborated that the physiological maintenance observed after TiO2-NPs exposure was primarily due to the up-regulation of metabolites related to antioxidant activity, plant stress resistance, growth promotion, and the tricarboxylic acid (TCA) cycle. Transcriptomic analysis reinforced the link between these responses and the enhancement of plant stress resistance, with genes such as MYB, WRKY, P450, and membrane transport-related genes like ABCG2 and ABCC3 contributing to the decrease in Cd levels in wheat.Previous reports have often portrayed TiO2-NPs as a pollutant, discussing their toxicity to plants or their absorption and transport within plants. However, at appropriate concentrations, TiO2-NPs can be viewed as a beneficial agent for agricultural soil remediation and this research underscores the immense potential of TiO2-NPs as a valuable resource for promoting safe and sustainable wheat cultivation in soils moderately contaminated with harmful metals like Cd. It is imperative to delve deeper into the long-term behavior of TiO2-NPs under the intricate and fluctuating environments of soils, as well as their efficacy and lifecycle implications on non-target organisms and agroecosystems through large-scale field studies. Since our material is directly purchased, future investigations may delve deeper into elucidating the impacts of diversely synthesized TiO2-NPs on plant physiology. Such endeavors are imperative to fully tap into the prospects for nanotechnology-driven agricultural development, thereby fostering a more encouraging perspective on global food safety.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available due to proprietary restrictions but are available from the authors on reasonable request.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was supported by grants from National Key Research and Development Program of China (2021YFC1809101) and Key Project of Provincial Key Research and Development Program (Social Development) (BE2021717). We also thank Ruicheng Shi for his help with this experiment.References
- T. El Rasafi, A. Oukarroum, A. Haddioui, H. Song, E. E. Kwon, N. Bolan, F. M. G. Tack, A. Sebastian, M. N. V. Prasad and J. Rinklebe, Cadmium stress in plants: A critical review of the effects, mechanisms, and tolerance strategies, Crit. Rev. Environ. Sci. Technol., 2020, 52(5), 675–726 CrossRef.
- F. J. Zhao, Z. Tang, J. J. Song, X. Y. Huang and P. Wang, Toxic metals and metalloids: Uptake, transport, detoxification, phytoremediation, and crop improvement for safer food, Mol. Plant, 2022, 15(1), 27–44 CrossRef CAS PubMed.
- H. Horiguchi, H. Teranlshi, K. Nilya, K. Aoshima, T. Katoh, N. Sakuragawa and M. Kasuya, Hypoproduction of erythropoietin contributes to anemia in chronic cadmium intoxication: clinical study on Itai-itai disease in Japan, Arch. Toxicol., 1994, 68, 632–636 CrossRef CAS PubMed.
- E. Yu, W. Wang, N. Yamaji, S. Fukuoka, J. Che, D. Ueno, T. Ando, F. Deng, K. Hori, M. Yano, R. F. Shen and J. F. Ma, Duplication of a manganese/cadmium transporter gene reduces cadmium accumulation in rice grain, Nat. Food, 2022, 3(8), 597–607 CrossRef CAS.
- C. Ge, Y. Wang, W. Ma, H. A. Ahmad, C. Cheng, H.-B. Li and D. Zhou, The potential ability of fungi in preventing cadmium accumulation in wheat seedlings grown in weakly alkaline soils: Evidence from the application of fungicide, Appl. Soil Ecol., 2024, 198, 105359 CrossRef.
- P. Wang, H. Chen, P. M. Kopittke and F. J. Zhao, Cadmium contamination in agricultural soils of China and the impact on food safety, Environ. Pollut., 2019, 249, 1038–1048 CrossRef CAS.
- L. Zhang, C. Gao, C. Chen, W. Zhang, X. Y. Huang and F. J. Zhao, Overexpression of rice OsHMA3 in wheat greatly decreases cadmium accumulation in wheat grains, Environ. Sci. Technol., 2020, 54(16), 10100–10108 CrossRef CAS.
- C. Ge, Y. Wang, W. Ma, H. A. Ahmad, L. Zhao, C. Cheng, H. Li and D. Zhou, Effect of seed priming with cations on cadmium accumulation in wheat seedings under cadmium-contaminated weakly alkaline soil, ACS Agric. Sci. Technol., 2024, 4(4), 478–489 CrossRef CAS.
- J. Yang, J. Wang, X. Liao, H. Tao and Y. Li, Chain modeling for the biogeochemical nexus of cadmium in soil-rice-human health system, Environ. Int., 2022, 167, 107424 CrossRef CAS.
- L. Bai, S. Ding, X. Huang, X. Chen, Y. Chen, X. Cao, X. Wang, X. Yu and J. Dai, Prediction of the cadmium content in grains of low-accumulating wheat cultivars and soil cadmium threshold for safe production, J. Cleaner Prod., 2023, 417, 138081 CrossRef CAS.
- C. Liu, M. J. Guttieri, B. M. Waters, K. M. Eskridge, A. Easterly and P. Stephen Baenziger, Cadmium concentration in terminal tissues as tools to select low-cadmium wheat, Plant Soil, 2018, 430(1–2), 127–138 CrossRef CAS.
- X. Fang, J. Wang, H. Chen, I. Christl, P. Wang, R. Kretzschmar and F. J. Zhao, Two-year and multi-site field trials to evaluate soil amendments for controlling cadmium accumulation in rice grain, Environ. Pollut., 2021, 289, 117918 CrossRef CAS.
- J. Wang, P. M. Wang, Y. Gu, P. M. Kopittke, F. J. Zhao and P. Wang, Iron-manganese (oxyhydro)oxides, rather than oxidation of sulfides, determine mobilization of Cd during soil drainage in paddy soil systems, Environ. Sci. Technol., 2019, 53(5), 2500–2508 CrossRef CAS.
- S. Zhong, X. Li, F. Li, T. Liu, F. Huang, H. Yin, G. Chen and J. Cui, Water management alters cadmium isotope fractionation between shoots and nodes/leaves in a soil-rice system, Environ. Sci. Technol., 2021, 55(19), 12902–12913 CAS.
- EU, Commission Regulation (EU) 2021/1323 of 10 August 2021 amending Regulation (EC) No 1881/2006 as regards maximum levels of cadmium in certain foodstuffs, 2022 Search PubMed.
- G. V. Lowry, A. Avellan and L. M. Gilbertson, Opportunities and challenges for nanotechnology in the agri-tech revolution, Nat. Nanotechnol., 2019, 14(6), 517–522 CrossRef CAS.
- A. Weir, P. Westerhoff, L. Fabricius, K. Hristovski and N. von Goetz, Titanium dioxide nanoparticles in food and personal care products, Environ. Sci. Technol., 2012, 46(4), 2242–2250 CrossRef CAS.
- A. D. Servin, H. Castillo-Michel, J. A. Hernandez-Viezcas, B. C. Diaz, J. R. Peralta-Videa and J. L. Gardea-Torresdey, Synchrotron micro-XRF and micro-XANES confirmation of the uptake and translocation of TiO(2) nanoparticles in cucumber (Cucumis sativus) plants, Environ. Sci. Technol., 2012, 46(14), 7637–7643 CrossRef CAS PubMed.
- Y. Deng, E. J. Petersen, K. E. Challis, S. A. Rabb, R. D. Holbrook, J. F. Ranville, B. C. Nelson and B. Xing, Multiple method analysis of TiO(2) nanoparticle uptake in rice (Oryza sativa L.) plants, Environ. Sci. Technol., 2017, 51(18), 10615–10623 CrossRef CAS PubMed.
- W. Du, Y. Sun, R. Ji, J. Zhu, J. Wu and H. Guo, TiO2 and ZnO nanoparticles negatively affect wheat growth and soil enzyme activities in agricultural soil, J. Environ. Monit., 2011, 13, 822–828 RSC.
- B. Wu, L. Zhu and X. C. Le, Metabolomics analysis of TiO(2) nanoparticles induced toxicological effects on rice (Oryza sativa L.), Environ. Pollut., 2017, 230, 302–310 CrossRef CAS.
- U. Song, H. Jun, B. Waldman, J. Roh, Y. Kim, J. Yi and E. J. Lee, Functional analyses of nanoparticle toxicity: a comparative study of the effects of TiO2 and Ag on tomatoes (Lycopersicon esculentum), Ecotoxicol. Environ. Saf., 2013, 93, 60–67 CrossRef CAS PubMed.
- J. Li, M. S. Naeem, X. Wang, L. Liu, C. Chen, N. Ma and C. Zhang, Nano-TiO2 is not phytotoxic As revealed by the Oilseed Rape growth and photosynthetic apparatus ultra-structural response, PLoS One, 2015, 10(12), e0143885 CrossRef PubMed.
- F. Cai, X. Wu, H. Zhang, X. Shen, M. Zhang, W. Chen, Q. Gao, J. C. White, S. Tao and X. Wang, Impact of TiO2 nanoparticles on lead uptake and bioaccumulation in rice (Oryza sativa L.), NanoImpact, 2017, 5, 101–108 CrossRef.
- X. Wu, J. Hu, F. Wu, X. Zhang, B. Wang, Y. Yang, G. Shen, J. Liu, S. Tao and X. Wang, Application of TiO(2) nanoparticles to reduce bioaccumulation of arsenic in rice seedlings (Oryza sativa L.): A mechanistic study, J. Hazard. Mater., 2021, 405, 124047 CrossRef CAS PubMed.
- H. Liu, C. Ma, G. Chen, J. C. White, Z. Wang, B. Xing and O. P. Dhankher, Titanium dioxide nanoparticles alleviate tetracycline toxicity to Arabidopsis thaliana (L.), ACS Sustainable Chem. Eng., 2017, 5(4), 3204–3213 CrossRef CAS.
- J. Lian, L. Zhao, J. Wu, H. Xiong, Y. Bao, A. Zeb, J. Tang and W. Liu, Foliar spray of TiO(2) nanoparticles prevails over root application in reducing Cd accumulation and mitigating Cd-induced phytotoxicity in maize (Zea mays L.), Chemosphere, 2020, 239, 124794 CrossRef CAS PubMed.
- L. Qiang, N. Zhao, K. Liao, X. Sun, Q. Wang and H. Jin, Metabolomics and transcriptomics reveal the toxic mechanism of Cd and nano TiO(2) coexposure on rice (Oryza sativa L.), J. Hazard. Mater., 2023, 453, 131411 CrossRef CAS PubMed.
- G. Q. Su, F. Li, J. S. Lin, C. F. Liu and G. R. Shi, Peanut as a potential crop for bioenergy production via Cd-phytoextraction: A life-cycle pot experiment, Plant Soil, 2012, 365(1–2), 337–345 Search PubMed.
- M. A. Irshad, M. Z. U. Rehman, M. Anwar-Ul-Haq, M. Rizwan, R. Nawaz, M. B. Shakoor, L. Wijaya, M. N. Alyemeni, P. Ahmad and S. Ali, Effect of green and chemically synthesized titanium dioxide nanoparticles on cadmium accumulation in wheat grains and potential dietary health risk: A field investigation, J. Hazard. Mater., 2021, 415, 125585 CrossRef CAS PubMed.
- C. Wu, Y. Wu, F. Li, X. Ding, S. Yi, S. Hang, F. Ge and M. Zhang, Reducing the accumulation of cadmium and phenanthrene in rice by optimizing planting spacing: Role of low-abundance but core rhizobacterial communities, Sci. Total Environ., 2024, 926, 171856 CrossRef CAS.
- Y. Assefa, P. Carter, M. Hinds, G. Bhalla, R. Schon, M. Jeschke, S. Paszkiewicz, S. Smith and I. A. Ciampitti, Analysis of long term study indicates both agronomic optimal plant density and increase maize yield per plant contributed to yield gain, Sci. Rep., 2018, 8(1), 4937 CrossRef PubMed.
- Z. Du, L. Yang, D. Zhang, T. Cui, X. He, T. Xiao, H. Li, S. Xing and C. Xie, Optimizing maize planting density based on soil organic matter to achieve synergistic improvements of yield, economic benefits, and resource use efficiency, Sci. Total Environ., 2024, 906, 167597 CrossRef CAS PubMed.
- X. Wang, Y. Liu, G. Zeng, L. Chai, X. Song, Z. Min and X. Xiao, Subcellular distribution and chemical forms of cadmium in Bechmeria nivea (L.) Gaud, Environ. Exp. Bot., 2008, 62(3), 389–395 CrossRef CAS.
- X. Di, R. Jing, X. Qin, Y. Wei, X. Liang, L. Wang, Y. Xu, Y. Sun and Q. Huang, Transcriptome analysis reveals the molecular mechanism of different forms of selenium in reducing cadmium uptake and accumulation in wheat seedlings, Chemosphere, 2023, 340, 139888 CrossRef CAS PubMed.
- M. Wang, Y. Wang, C. Ge, H. Wu, F. Jing, S. Wu, H. Li and D. Zhou, Foliar selenium nanoparticles application promotes the growth of maize (Zea mays L.) seedlings by regulating carbon, nitrogen and oxidative stress metabolism, Sci. Hortic., 2023, 311, 111816 CrossRef CAS.
- J. Xu, Z. Bao, J. Yang, H. Liu and W. Song, Chemical forms of Pb, Cd and Cu in crops (in Chinese), Yingyong Shengtai Xuebao, 1991, 2(3), 244–248 Search PubMed.
- S. Silva, T. P. Ribeiro, C. Santos, D. Pinto and A. M. S. Silva, TiO(2) nanoparticles induced sugar impairments and metabolic pathway shift towards amino acid metabolism in wheat, J. Hazard. Mater., 2020, 399, 122982 CrossRef CAS.
- V. Singleton and J. A. Rossi, Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents, Am. J. Enol. Vitic., 1964, 16(3), 144–158 CrossRef.
- I. F. F. Benzie and J. J. Strain, The ferric reducing ability of plasma (FRAP) as a measure of “Antioxidant Power”: The FRAP assay, Anal. Biochem., 1996, 239, 70–76 CrossRef CAS PubMed.
- USEPA Method 3050B, Acid digestion of sediments, sludges, and soils, 1996 Search PubMed.
- Z. Wu, K. McGrouther, D. Chen, W. Wu and H. Wang, Subcellular distribution of metals within Brassica chinensis L. in response to elevated lead and Chromium Stress, J. Agric. Food Chem., 2013, 61(20), 4715–4722 CrossRef CAS.
- Y.-M. Wang, D.-D. Tang, X.-H. Zhang, M. Uchimiya, X.-Y. Yuan, M. Li and Y.-Z. Chen, Effects of soil amendments on cadmium transfer along the lettuce-snail food chain: Influence of chemical speciation, Sci. Total Environ., 2019, 649, 801–807 CrossRef CAS PubMed.
- M. Wang, C. Mu, Y. Li, Y. Wang, W. Ma, C. Ge, C. Cheng, G. Shi, H. Li and D. Zhou, Foliar application of selenium nanoparticles alleviates cadmium toxicity in maize (Zea mays L.) seedlings: Evidence on antioxidant, gene expression, and metabolomics analysis, Sci. Total Environ., 2023, 899, 165521 CrossRef CAS.
- A. Mahmood and R. N. Malik, Human health risk assessment of heavy metals via consumption of contaminated vegetables collected from different irrigation sources in Lahore, Pakistan, Arabian J. Chem., 2014, 7(1), 91–99 CrossRef CAS.
- F. J. Zhao, J. L. Stroud, T. Eagling, S. J. Dunham, S. P. McGrath and P. R. Shewry, Accumulation, distribution, and speciation of arsenic in wheat grain, Environ. Sci. Technol., 2010, 44(14), 5464–5468 CrossRef CAS.
- F. Chen, Y. Li, M. A. Irshad, A. Hussain, R. Nawaz, M. F. Qayyum, J. Ma, M. Zia-Ur-Rehman, M. Rizwan and S. Ali, Effect of titanium dioxide nanoparticles and co-composted biochar on growth and Cd uptake by wheat plants: A field study, Environ. Res., 2023, 231(Pt 1), 116057 CrossRef CAS.
- Y. Weng, X. Bai, M. Kang, Y. Huang, Y. Ji, H. Wang and Z. Hua, Comparative analysis of chemically and green synthesized titanium dioxide nanoparticles for the regulation of photosynthesis in Lactuca sativa L, Environ. Sci.: Nano, 2024, 11(1), 161–174 RSC.
- H. Mohammadi, Z. Mousavi, S. Hazrati, A. Aghaee, F. Bovand and M. Brestic, Foliar applied titanium dioxide nanoparticles (TiO2 NPs) modulate growth, physiological and phytochemical traits in Melissa officinalis L. under various light intensities, Ind. Crops Prod., 2023, 197, 116642 CrossRef CAS.
- J. Lian, L. Cheng, X. Zhai, R. Wu, W. Liu, J. Pan, M. J. I. Shohag, X. Xin, Z. He and X. Yang, Foliar spray of combined metal-oxide nanoparticles alters the accumulation, translocation and health risk of Cd in wheat (Triticum aestivum L.), J. Hazard. Mater., 2022, 440, 129857 CrossRef CAS.
- C. Kamaral, S. M. Neate, N. Gunasinghe, P. J. Milham, D. J. Paterson, P. M. Kopittke and S. Seneweera, Genetic biofortification of wheat with zinc: Opportunities to fine-tune zinc uptake, transport and grain loading, Physiol. Plant., 2022, 174(1), e13612 CrossRef CAS.
- J. Zhou, C. Zhang, B. Du, H. Cui, X. Fan, D. Zhou and J. Zhou, Soil and foliar applications of silicon and selenium effects on cadmium accumulation and plant growth by modulation of antioxidant system and Cd translocation: Comparison of soft vs. durum wheat varieties, J. Hazard. Mater., 2021, 402, 123546 CrossRef CAS.
- M. Wang, C. Mu, X. Lin, W. Ma, H. Wu, D. Si, C. Ge, C. Cheng, L. Zhao, H. Li and D. Zhou, Foliar application of nanoparticles reduced cadmium content in wheat (Triticum aestivum L.) grains via long-distance “leaf-root-microorganism” regulation, Environ. Sci. Technol., 2024, 58, 6900–6912 CrossRef CAS PubMed.
- Y. Liu, X. Cao, L. Yue, C. Wang, M. Tao, Z. Wang and B. Xing, Foliar-applied cerium oxide nanomaterials improve maize yield under salinity stress: Reactive oxygen species homeostasis and rhizobacteria regulation, Environ. Pollut., 2022, 299, 118900 CrossRef CAS.
- H. Mohammadi, Z. Kazemi, A. Aghaee, S. Hazrati, R. Golzari Dehno and M. Ghorbanpour, Unraveling the influence of TiO(2) nanoparticles on growth, physiological and phytochemical characteristics of Mentha piperita L. in cadmium-contaminated soil, Sci. Rep., 2023, 13(1), 22280 CrossRef CAS PubMed.
- C. S. Cobbett, Phytochelatins and their roles in heavy metal detoxification, Plant Physiol., 2000, 123, 825–832 CrossRef CAS PubMed.
- Z. Hricovíniová, Š. Mascaretti, J. Hricovíniová, A. Čížek and J. Jampílek, New unnatural gallotannins: A way toward green antioxidants, antimicrobials and antibiofilm agents, Antioxidants, 2021, 10, 1288 CrossRef.
- T. Kotake, Y. Yamanashi, C. Imaizumi and Y. Tsumuraya, Metabolism of L-arabinose in plants, J. Plant Res., 2016, 129(5), 781–792 CrossRef CAS.
- E. J. Calabrese, E. Agathokleous and V. Calabrese, Ferulic acid and hormesis: Biomedical and environmental implications, Mech. Ageing Dev., 2021, 198, 111544 CrossRef CAS.
- E. Adams, T. Miyazaki, J. Y. Moon, Y. Sawada, M. Sato, K. Toyooka, M. Y. Hirai and R. Shin, Syringic acid alleviates cesium-induced growth defect in arabidopsis, Int. J. Mol. Sci., 2020, 21, 9116 CrossRef CAS.
- D. Chia, T. Yoder, W. D. Reiter and S. I. Gibson, Fumaric acid: an overlooked form of fixed carbon in Arabidopsis and other plant species, Planta, 2000, 211, 743–751 CrossRef CAS.
- Z. Cui, Y. Zhong, Z. Sun, Z. Jiang, J. Deng, Q. Wang, J. Nielsen, J. Hou and Q. Qi, Reconfiguration of the reductive TCA cycle enables high-level succinic acid production by Yarrowia lipolytica, Nat. Commun., 2023, 14(1), 8480 CrossRef CAS PubMed.
- S. Chen, Z. Pan, W. Zhao, Y. Zhou, Y. Rui, C. Jiang, Y. Wang, J. C. White and L. Zhao, Engineering climate-resilient rice using a nanobiostimulant-based “stress training” strategy, ACS Nano, 2023, 17(11), 10760–10773 CrossRef CAS PubMed.
- E. Scalabrin, M. Radaelli and G. Capodaglio, Simultaneous determination of shikimic acid, salicylic acid and jasmonic acid in wild and transgenic Nicotiana langsdorffii plants exposed to abiotic stresses, Plant Physiol. Biochem., 2016, 103, 53–60 CrossRef CAS.
- N. G. J. Ming, S. Binte Mostafiz, N. S. Johon, N. S. Abdullah Zulkifli and A. Wagiran, Combination of plant growth regulators, maltose, and partial desiccation treatment enhance somatic embryogenesis in selected malaysian rice cultivar, Plants, 2019, 8, 144 CrossRef CAS PubMed.
- J. Yeon, A. R. Park, H. T. T. Nguyen, H. Gwak, J. Kim, M. K. Sang and J. C. Kim, Inhibition of oomycetes by the mixture of maleic acid and copper sulfate, Plant Dis., 2022, 106(3), 960–965 CrossRef CAS PubMed.
- H. Takahashi, T. Imamura, N. Konno, T. Takeda, K. Fujita, T. Konishi, M. Nishihara and H. Uchimiya, The gentio-oligosaccharide gentiobiose functions in the modulation of bud dormancy in the herbaceous perennial gentiana, Plant Cell, 2014, 26(10), 3949–3963 CrossRef CAS.
- X. Wu, J. Xu, X. Meng, X. Fang, M. Xia, J. Zhang, S. Cao and T. Fan, Linker histone variant HIS1-3 and WRKY1 oppositely regulate salt stress tolerance in Arabidopsis, Plant Physiol., 2022, 189(3), 1833–1847 CrossRef CAS.
- J. P. An, X. F. Wang, X. W. Zhang, H. F. Xu, S. Q. Bi, C. X. You and Y. J. Hao, An apple MYB transcription factor regulates cold tolerance and anthocyanin accumulation and undergoes MIEL1-mediated degradation, Plant Biotechnol. J., 2019, 18(2), 337–353 CrossRef.
- M. A. Versiani, T. Diyabalanage, R. Ratnayake, C. J. Henrich, S. E. Bates, J. B. McMahon and K. R. Gustafson, Flavonoids from eight tropical plant species that inhibit the multidrug resistance transporter ABCG2, J. Nat. Prod., 2011, 74(2), 262–266 CrossRef CAS PubMed.
- P. Brunetti, L. Zanella, A. De Paolis, D. Di Litta, V. Cecchetti, G. Falasca, M. Barbieri, M. M. Altamura, P. Costantino and M. Cardarelli, Cadmium-inducible expression of the ABC-type transporter AtABCC3 increases phytochelatin-mediated cadmium tolerance in Arabidopsis, J. Exp. Bot., 2015, 66(13), 3815–3829 CrossRef CAS PubMed.
- N. S. Kejiou, L. Ilan, S. Aigner, E. Luo, T. Tonn, H. Ozadam, M. Lee, B. Cole, G. I. Rabano, N. Rajakulendran, B. A. Yee, S. Najafabadi Hamed, F. Moraes, T. S. Angers, W. Y. Gene, C. Cenik and F. Palazzo Alexander, Pyruvate Kinase M (PKM) binds ribosomes in a poly-ADP ribosylation dependent manner to induce translational stalling, Nucleic Acids Res., 2023, 51(12), 6461–6478 CrossRef CAS.
- M. Li, X. Xu, Y. Su, X. Shao, Y. Zhou and J. Yan, A comprehensive overview of PPM1A: From structure to disease, Exp. Biol. Med., 2022, 247(6), 453–461 CrossRef CAS.
- Y. Zheng, J. Yan, S. Wang, M. Xu, K. Huang, G. Chen and Y. Ding, Genome-wide identification of the pectate lyase-like (PLL) gene family and functional analysis of two PLL genes in rice, Mol. Genet. Genomics, 2018, 293(6), 1317–1331 CrossRef CAS PubMed.
- C. Mignee, R. Mutoh, A. Krieger-Liszkay, G. Kurisu and P. Setif, Gallium ferredoxin as a tool to study the effects of ferredoxin binding to photosystem I without ferredoxin reduction, Photosynth. Res., 2017, 134(3), 251–263 CrossRef CAS.
- J. Chen, L. Yang, X. Yan, Y. Liu, R. Wang, T. Fan, Y. Ren, X. Tang, F. Xiao, Y. Liu and S. Cao, Zinc-finger transcription factor ZAT6 positively regulates cadmium tolerance through the glutathione-dependent pathway in Arabidopsis, Plant Physiol., 2016, 171(1), 707–719 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Text S1–S3. Characterization of nanoparticles; total antioxidant capacity assays; extraction of Cd form from plants. Fig. S1–12. Basic growth index of wheat; height and effective panicle number of wheat; HHRI in the field experiment; chlorophyll content; antioxidant differential metabolites; differential metabolites associated with the tricarboxylic acid cycle; differential metabolites associated with stress resistance; expression of various stress-responsive transcription factors; expression of various glutathione transcription factors; expression of various serine and phosphatase transcription factors; expression of various zinc finger proteins and iron related proteins. Tables S1 and S2. Physicochemical properties of the experimental soil; statistics of sequencing data. See DOI: https://doi.org/10.1039/d4en00763h |
| This journal is © The Royal Society of Chemistry 2025 |