Open Access Article
Open Access ArticleNano-scaled advanced materials for antimicrobial applications – mechanistic insight, functional performance measures, and potential towards sustainability and circularity
Benjamin
Punz
 a,
Constantin
Christ
a,
Alrun
Waldl
a,
Su
Li
ab,
Yingnan
Liu
ab,
Litty
Johnson
a,
Vanessa
Auer
a,
Olavo
Cardozo
d,
Patricia M. A.
Farias
d,
Arnaldo C. D. S.
Andrade
e,
Andreas
Stingl
f,
Guocheng
Wang
a,
Constantin
Christ
a,
Alrun
Waldl
a,
Su
Li
ab,
Yingnan
Liu
ab,
Litty
Johnson
a,
Vanessa
Auer
a,
Olavo
Cardozo
d,
Patricia M. A.
Farias
d,
Arnaldo C. D. S.
Andrade
e,
Andreas
Stingl
f,
Guocheng
Wang
 c,
Yang
Li
c,
Yang
Li
 b and
Martin
Himly
b and
Martin
Himly
 *a
*a
aDept. Biosciences & Medical Biology, Paris Lodron University of Salzburg, Austria. E-mail: martin.himly@plus.ac.at
bLaboratory of Immunology and Nanomedicine & China-Italy Joint Laboratory of Pharmacobiotechnology for Medical Immunomodulation, Laboratory of Inflammation and Vaccines, Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences, Shenzhen, China
cResearch Center for Human Tissues and Organs Degeneration, Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences, Shenzhen, China
dPost-graduate Program on Materials Science, Federal University of Pernambuco, Brazil
ePetribu Mill S.A., Lagoa de Itaenga, Pernambuco, Brazil
fPhornano Holding GmbH, Korneuburg, Austria
First published on 16th January 2025
Abstract
About 13.7 million people died worldwide from infectious diseases in 2019, which accounts for one fifth of all annual deaths. Infectious diseases are caused by microbes (i.e. bacteria, fungi, viruses) predominantly targeting the respiratory system, bloodstream, gastrointestinal region and urinary tract, which can lead to severe health problems. Microbes can naturally adapt and develop antimicrobial resistance to conventional medication. Health systems are concerned by the overuse of antibiotics in the medical, agricultural, and food industries. This leads to bacterial multidrug resistance, causing more than half a million deaths annually. In consequence, research and innovation have focused on nano-scaled advanced materials to explore their potential to reinforce antimicrobial treatments. Advanced materials are complex composites that achieve superior, combined functionalities with an optimized safety, sustainability, and circularity profile. They often contain nano-scaled materials, which are highly versatile, organic, or inorganic materials that can adopt different sizes, compositions, topographies, and surface modifications. All these properties need to be carefully defined using physicochemical characterization techniques and should be considered when selecting the most efficient nanomaterials against widespread microbes. In this review, we cover (i) potential candidates of engineered nanomaterials and their physicochemical characteristics, and demonstrate their efficacy in antimicrobial action; (ii) the mechanisms of action against microbes specific to nanomaterials; (iii) well-established methods and highlight methodological advancements; (iv) the potential improvements in sustainability and circularity; (v) the current and future fields of application and ongoing development in the medical, agricultural, high-tech, textile, and food industries. For the first time, nano-scaled advanced materials produced by green synthesis methods are discussed with respect to their gain in sustainability and circularity and a comprehensive set of methodologies for safety, sustainability, and circularity assessment are described in detail.
Environmental significanceThe current overuse of antibiotics, such as in the fields of healthcare, agriculture, food production, or water remediation, creates a big demand for future materials research and innovation. Innovative advanced materials, with many of them being based on the incorporation of nano-scaled materials, open new opportunities, combining improved functional performance while being safer and more sustainable, as recently postulated by the Advanced Materials Initiative 2030 and the European Commission's recommendation for Safe-and-Sustainable-by-Design production. Moreover, circularity measures demand for greener synthesis methods for nanomaterials which may pave the way forward towards improved safety, less exploitation of non-renewable and critical raw materials, optimized energy consumption, and better environmental footprint. |
1. Introduction
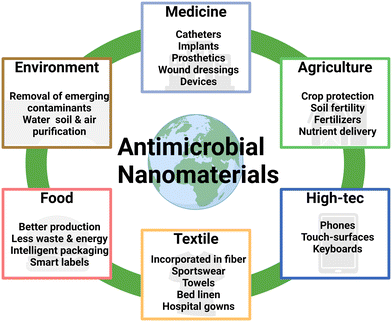
One fifth of all annual deaths are caused by infectious diseases, which accumulates to more than 13 million people per year globally.1 It became evident that modern society encounters difficulties in handling ravaging pathogens, which was depicted impressively during the SARS-CoV-2 pandemic with countless nationwide lockdowns between 2020 and 2022. It can be seen quite easily that lockdowns are a versatile & powerful tool to contain the spread of pathogens during pandemics when being held active for at least 10 days, even more so for 20 days.2 The effectiveness is also diminished if lockdowns cannot be enforced properly, may this be for private, socio-economic or political reasons.3 Hence, additional aid is needed to prevent the spread and infection in the first place. Most frequently prescribed medications, like antibiotics against bacteria, are used in an attempt to handle pathogens, when it is already too late, i.e. when microbes have managed to enter their host's body surpassing the immune system and cause noticeable negative effects. Additional problems may occur when broad-spectrum medicine is used commonly or prescribed for minor conditions that could be treated with conventional methods, allowing pathogens to develop resistances and rendering the medication useless such as in the case of multi-drug resistant strains.4 The emergence of multidrug resistance makes it more difficult to treat bacterial, fungal, and viral infections effectively. This phenomenon poses a threat to public health because it leads to higher rates of morbidity and mortality, increased spending on the development of new medications, and a heavier load on public health systems.4 Moreover, the increasingly roaming threat of viruses (like SARS-CoV-2 or monkeypox virus) as well as potentially emerging fungal infections (due to climate change) puts a high pressure on current state-of-the-art medication.5 A recent 2024 WHO report highlights a critical shortage of new antibiotics.6 Only 32 urgently needed novel antibiotics are currently being developed, and 4 of these are effective against at least one of the WHO's “critical pathogens”. Gram-negative bacteria pose a significant challenge due to their rapid resistance development and adaptability. Additionally, the current drug development process often focuses on a broad range of pathogens, hindering the development of targeted treatments. This is further complicated by the lack of fast, affordable, and reliable diagnostic tools. However, there is hope on alternatives, like bacteriophages. Their use in the clinic started already in the early 20th century, where not much was known about the so-called “bacterial-eaters”.7 Phage therapy offers a promising approach to combat antibiotic-resistant bacteria. Phages specifically target bacteria with complementary receptors, making them highly effective, with the involvement of CRISPR-Cas9, but also limiting their host range.8,9 While these preclinical investigations have indicated their potential, regulatory approval for human use in the EU and US is still pending. Concerns about immunomodulatory effects, the host range, and horizontal gene transfer necessitate further research to fully harness their therapeutic potential.10,11 Alternatively, antibody-based therapy approaches provide another avenue, leveraging the body's immune response to target bacterial toxins.12 This approach offers several advantages, including the ability to target unique bacterial antigens, avoiding the selection pressure that drives antibiotic resistance.13 However, it requires precise identification of the causative pathogen, making rapid and accurate diagnosis crucial for effective treatment.14 Further promising approaches deal with anti-virulence-, immune-modulating- and microbiome-modulating agents, where research is still at earlier stages. One of these alternative and promising candidates that require attention is the use of innovative advanced materials enabling sanitation at antimicrobial surfaces (priority area for research & innovation advocated by AMI2030 in Materials 2030 Manifesto),15 before encounter with humans. This allows the prevention of resistance development and reduces the necessity to costly discover new antibiotics. Against common belief, pathogens are also able to develop resistances to nanomaterials when exposed to non-lethal doses over extended time.16 But the development of resistances in the application of extracorporeal devices serves the single purpose of diminishing and inhibiting the growth of microbes, reducing the risk of infections. These materials can be applied or attached to surfaces of many sorts. Selection hereby depends on the mode of action that is employed by the antimicrobial materials, for instance, surfaces in sensitive areas, electronic displays, door handles, face masks, gloves, etc. Fast decaying materials, due to particle dissolution via the main mode of action (ion release), may find a more suitable application in single use items, whereas long-lasting candidates can be applied on surfaces that need not be replaced frequently.This review provides an overview of the utility of advanced engineered nanomaterials as candidates for preventing the spread of microbes on highly affected areas or equipment used for personal protection. First, several suitable nanomaterial candidates are identified and their physicochemical properties and various other traits (size, shape, topography, solubility, and crystallinity) which influence their effectiveness as suitable antimicrobial agents are discussed. Next, we introduce readers to the various modes of action and how nanomaterials can actively prevent the growth of pathogens. These mechanisms are categorized into three big groups, being (1) membrane interaction/agglomeration, (2) ion release and lastly (3) reactive oxygen species (ROS) generation. In this section, it becomes evident that different kinds of particles also exert different combinations of mechanisms, where details and data may be used to train machine learning tools and utilize artificial intelligence to be able to predict the effectiveness of nanomaterials. Subsequently, we highlight current state-of-the-art methodologies and assays to investigate the growth delay and inhibition that different types of nano-scaled materials employ against bacteria, fungi, and viruses by explaining how the assays work and naming examples indicating the functional performance measures and readouts, thus, highlighting the effectiveness of the antimicrobial particles from various studies over recent years. In the final section, safety, sustainability and circularity considerations are discussed. Novel approaches to synthesize these nanomaterials in a green, renewable, safe, and sustainable manner are conceptually introduced aiming at reducing the negative impact on the environment and alleviating the exploitation of the planet's resources by finding non-toxic and better sustainable substitutes. Altering well-established production protocols requires profound characterization and batch-to-batch analyses to confirm similar performance whilst providing a better environmental footprint and safety profile. This chapter also covers potential economic fields where these materials and technologies can be applied. We propose a new viewpoint, by highlighting alternative methods and approaches in the context of antimicrobial nanomaterial synthesis, and describe a wide array of testing methods available to verify their respective antimicrobial effectiveness for a broad range of pathogens, with potential worldwide application, ready for use in real life.
2. Nano-scaled materials displaying antimicrobial functionality
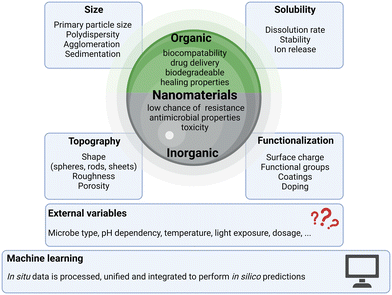
The utilization of nano-scaled materials including nanoparticles (NPs), nanorods, and nano-structured surfaces in combating microbial threats has emerged as a cutting-edge approach with far-reaching implications in various fields, including medicine, agriculture, and environmental sciences. These minuscule composites, typically with 1–3 dimensions measuring between 1 and 100 nanometers,17 exhibit exceptional antimicrobial properties, offering a powerful arsenal against microorganisms. To harness their potential effectively, appropriate characterization of the material needs to be conducted investigating all associated traits, as depicted in Fig. 1, which will further feed into development of predictive models based on machine learning approaches, certainly still being dependent on the different fields of application and numerous external factors as additional variables to be considered for design of antimicrobial materials. In general, NPs can be categorized into two major classes: organic and inorganic, and each of them can be distinguished by selective properties and applications in microbial control. Moreover, this categorization of NPs into organic and inorganic forms sets the stage for a comprehensive exploration of their respective advantages, disadvantages, mechanisms of action, and specific applications in the realm of microbial control. Hence, the choice between organic and inorganic NPs depends on the specific requirements of the application, including biocompatibility, stability, drug loading capacity, and the desired physical and chemical properties. Often, a combination of both types of NPs, known as hybrid NPs, is used to leverage the advantages of each while mitigating their respective disadvantages. This chapter will highlight some representatives from the organic and inorganic classes and show how physicochemical properties like size, topography and functionalization may impact the antimicrobial effects.Organic NPs, often derived from biopolymers like chitosan or synthetic polymers, offer biocompatibility and versatility.18 They are suitable for drug delivery and wound healing applications.19 These NPs are generally biocompatible and less likely to induce adverse reactions in biological systems. Organic NPs excel in drug delivery systems, allowing for precise control over the release of antimicrobial agents or therapeutics, minimizing side effects and optimizing treatment efficacy. They have shown promise in wound healing applications, promoting tissue regeneration and reducing infection risk.20 Additionally, many of them are biodegradable, reducing long-term environmental concerns.21 However, also downsides have been reported for organic NPs. Their antimicrobial efficacy can be variable and dependent on factors like the choice of polymer and formulation, which may limit their effectiveness against certain microbes.22 The production of organic NPs using biological methods has major advantages such as non-toxicity and environmental friendliness.23 They may also have a more limited spectrum of antimicrobial activity compared to some inorganic NPs.24
Inorganic NPs, exemplified by materials like Ag, Cu, and ZnO, are known for their potent and broad-spectrum antimicrobial activity.25 They are effective against a wide range of microorganisms and can provide a long-lasting antimicrobial effect due to their slow release of antimicrobial ions.26 As highlighted and summarized by Kamat et al., under the right circumstances pathogens may also be able to develop resistances against NPs.27 They can be incorporated into various materials, such as coatings and textiles, to impart antimicrobial properties.28 However, inorganic NPs do have their downsides as well. They can raise concerns about potential toxicity to human cells and the environment, depending on factors like size, concentration, and surface chemistry.29 The release of inorganic NPs into the environment can have adverse effects on ecosystems and aquatic life, posing environmental risks.30 Additionally, the production of high-quality, multifunctional inorganic NPs can be expensive, limiting their widespread use in some applications.31 The regulatory landscape for inorganic NPs is still evolving, posing challenges in ensuring their safe and effective use in various products.32
The choice between organic and inorganic NPs depends on specific application requirements, and industrial research and innovation need to consider benefits and downsides in their decision-making processes.
2.1. Physicochemical properties with impact on antimicrobial action
The antimicrobial effectiveness of NPs is strongly influenced by their physicochemical properties. Various characteristics can affect their interactions with microorganisms and, consequently, their antimicrobial activity. Table 1 depicts a multitude of NPs with their respective observed modes of action, which can be substantially different depending on differences in their physicochemical properties.| NP type | Pathogen | Mode of action | Ref. |
|---|---|---|---|
| Au | Escherichia coli | Induction of apoptotic-like death phenotypes: membrane depolarization induction, DNA fragmentation, caspase activation, and imbalance in the redox status | 33 |
| Au | Escherichia coli | Visible surface damage, disruption of the cell membrane and cell loss integrity | 34 |
| Staphylococcus aureus | |||
| Pseudomonas aeruginosa | |||
| Au | 30 unique clinical carbapenem-resistant Enterobacteriaceae strains | Elevated bacterial reactive oxygen species (ROS) generation and ROS accumulation within bacteria; enhancement of inner membrane permeability | 35 |
| Ag | Pseudomonas aeruginosa | Membrane deformation or rupture, with vacuoles, and nucleoplasm agglutination; imbalance in the redox status with higher ROS production | 36 |
| Ag | Escherichia coli | Disruption of the cell membrane with loss of integrity of the cell membrane through the effect of silver ions on the cell membrane stability; depolarization of the bacterial membrane and alteration of permeability | 37 |
| Salmonella typhimurium | |||
| Bacillus subtilis | Depolarization of the bacterial membrane and alteration of permeability with no changes in the membrane visible appearance | ||
| Staphylococcus aureus | |||
| Ag | Candida glabrata | Abnormal morphology, some pores, and distorted membrane | 38 |
| Ag-modified CeO2 | Coronavirus OC43 | Disruption in virus–receptor interactions through induction of virion aggregation | 39 |
| Parainfluenza virus type 5 | Disruption in virus–receptor interactions through induction of virion aggregation | ||
| CuO | Escherichia coli | Rupture of the cell wall and a decrease in electron density with a high concentration of small particles in the cell wall and part of the cytoplasm | 40 |
| Bacillus cereus | Cell lysis and leakage of cytoplasmic fluid, which leads to total shrinking of the cytoplasmic membrane | ||
| Staphylococcus aureus | Membrane damage that seems to have ruptured which leads to leakage of intracellular contents, with further cellular damage and full cell deformation | ||
| CuO | Tested with 2,2-diphenyl-1-picrylhydrazyl (DPPH) | Inhibition of antioxidants which might have an effect on bacterial viability through ROS | 41 |
| Farnesol-loaded polymer | Staphylococcus aureus | Membrane damage, which leads to leakage of the cytoplasmic contents as well as a disordered cytoplasmic structure, and death | 42 |
| SARS-CoV-2 | Intercalation of NPs into the double lipid layer of the viral envelope, causing membrane fluidity alterations, and inhibition of virus attachment and further intracellular penetration into the host cell | ||
| Fe0 | Bacillus subtilis | Induction of ROS response, resulting in decreased redox sensor activity, with subsequent oxidation to Fe2O3 | 43 |
| Bacillus thuringiensis | |||
| FeXOY – chitosan coated | Bacillus subtilis | Enhanced production of ROS with membrane depolarization | 44 |
| Escherichia coli | |||
| Se | Candida glabrata | Dramatic change of fungal morphology from a cylindrical to distorted cell structure with the breakdown of the cell membranes | 38 |
| Se – chitosan coated | Porcine reproductive and respiratory syndrome virus | Promotion of GSH production and inhibition of H2O2 synthesis; inhibition of ORF5 gene expression and viral titer; inhibition of ROS generation | 45 |
| ZnO | Hepatitis E virus | Binding and entrapping of virus and therefore prevention of its entry into the host cell; inhibition of the viral replication step | 46 |
| Hepatitis C virus | |||
| ZnO | Botrytis cinerea | Interference of cell function and deformation of fungal hyphae | 47 |
| Penicillium expansum | Prevention of conidiophores and conidia development | ||
| ZnO | Penicillium expansum | Deformation of the structure of fungal hyphae and thereby growth inhibition | 38 |
| dC/dt = D × A(Cs − C)/h | (1) |
D … diffusion coefficient of the material
A … effective surface area
h … thickness of the diffusion layer around each particle
Cs … saturation solubility of the material in solution
C … concentration of the material
All parameters, besides A and C, in the equation can be considered as a constant. Increasing A, for instance by reducing the particle size, results in an increase in the dissolution rate.66 The process of dissolution irreversibly reduces the NPs' integrity, and consequently diminishes their long-term antimicrobial effectiveness.
2.2. Computational approaches for antimicrobial efficacy prediction
Taking all previously discussed physicochemical properties into consideration and attempting to correlate all dependencies into a prediction model are expected to substantially cut down costs and increase antimicrobial efficacy. Thus, for material design, predictive technologies by means of either data-driven or physics-based in silico modeling are turning out to be an essential research and development domain complementing experimental assessment of already existing materials. Notably, an enormous time advantage can be generated through modeling as materials do not require prior synthesis. Recent studies have increasingly incorporated predictive tools, starting from the initial stages of synthesis and data generation in the laboratory. Machine learning techniques, such as Extremely Random Trees (ERT) and XGBoosting, are frequently employed to predict factors like the dose-time dependency of antimicrobial effectiveness. These methods have demonstrated strong correlations and minimal error in various studies.67–70In silico models aiming to predict the antimicrobial efficacy of certain selected nanomaterials are getting more refined and precise. As described above, small changes in the physicochemical properties can have detrimental influences on the antimicrobial effectiveness. Due to the batch-to-batch variations during the production and the overall vastness of available nanomaterials, a huge effort must be invested to develop and further adapt suitable in silico predictive models and tools for shaping the effectiveness of particles and their respective desirable traits. As a prime example for a data-driven model for antimicrobial nanomaterial design, tools can operate like the one from Mirzaei et al., Nanomaterials, 2021.71 Here, data were first acquired from 60 peer-reviewed research articles entailing observed antimicrobial efficacy, NP characterization data (specific surface area, hydrodynamic size, zeta potential, core size) and time-dependent dosages. Essential characterization data like specific surface area, hydrodynamic diameter and zeta potential were missing entirely in 90% of the investigated articles and were thus excluded from evaluation. The data were then processed and unified to, for instance, simplify all the different possible bacterial coatings observed by using a binary decision and identifying them by either “coated” or “uncoated” types. The study further emphasizes that many observed studies have poorly reported characterization data, generating an ever-so harder time to establish unifiable in silico assessment methods and proving the necessity of executing and reporting studies following the FAIR data principles.72,73 It is undeniable that a well-defined set of minimum characterization requirements must be established and adopted in future publications. Without proper reporting, model design cannot be effectively performed, increasing the risk of overlooking critical correlations.3. Mechanisms of antimicrobial action induced by engineered nanomaterials
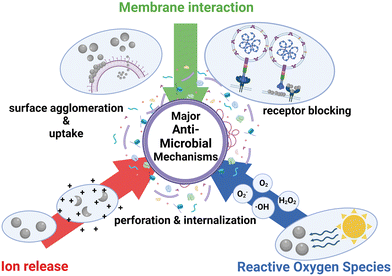
The bactericidal, fungicidal and virucidal effects are exerted by engineered nanomaterials via many different mechanisms of antimicrobial action. Some of these mechanisms include the production of ROS, accumulation of the NPs at the microbes' surface or inside upon uptake, electrostatic interactions, or release of toxic ions. These mechanisms can cause their effects either from the outside or upon uptake within the bacteria, fungi, and viruses. The specific mechanisms will be described in more detail but can be generally categorized into groups as illustrated in Fig. 2.3.1. Damage to the cell wall and cell membrane or interference with the viral surface
Contact of NPs with bacterial and fungal cells can lead to membrane damage caused by NP adsorption and sometimes subsequent penetration into the cells.74–77 Adsorption to the cell wall and a following breakdown of the aforementioned have been shown by many studies to be the main mechanism of toxicity.74,77–79 Metallic NPs and the membrane of microbes including bacteria, fungi, and viruses are electrostatically attracted to each other because the negatively charged functional groups in the microbial membrane attract metal cations. Therefore, the interaction with positively charged NPs is favored.80,81 In contrast, some fungal membranes are positively charged,82 which makes them less attractive to positively charged NPs. This can lead to a lower antifungal effect of metal NPs regarding specific fungal species. Among bacterial pathogens, there is a difference in the cell wall structures of Gram-positive and Gram-negative bacteria. Gram-positive bacteria are made of a thick cell wall containing peptidoglycan and teichoic acids while the cell wall of Gram-negative bacteria is built up of a thin peptidoglycan layer with an additional outer membrane consisting of lipopolysaccharides. Many studies stated that there is a better interaction of NPs with Gram-positive bacteria, rather than with Gram-negative bacteria, because NPs are only readily attracted by the presence of negative charges carried by lipopolysaccharides in the outer membrane of Gram-negative bacteria.83 Furthermore, the bilayer membrane acts as a selective physical barrier against hydrophobic compounds such as detergents and antibiotics. Even with a thick peptidoglycan layer, Gram-positive bacteria are more permeable because a monolayer membrane is insufficient to block the entry of foreign molecules. In addition, the cell wall has a higher negative charge than the one of Gram-negative bacteria,84 which is determined by the properties of peptidoglycan and teichoic acid structures, which strongly attract NPs, resulting in membrane damage and cell death.85 Ag, Au, ZnO, and TiO2 NPs can be attracted to cell walls through electrostatic attraction,86 van der Waals forces, and hydrophobic interactions,87 leading to changes in the morphology, function, and permeability of the microbial cells. Adsorption of NPs leads to depolarization of the cell wall, changing the normally negative charge on the wall and making it more permeable. Confocal laser scanning microscopy has been reported to show bacterial cell walls becoming blurred, indicating cell wall degradation.88 The pores in the membranes of bacteria are in the nanometer size range, which means that smaller particles have increased chances of being able to enter the microbes rather than bigger particles. Additionally, NPs accumulate in the cell wall and create pits, which leads to a release of lipopolysaccharides, membrane proteins and intracellular factors. The damage of the cell wall also leads to inhibition of the electron transport chain. Additionally, the mechanism of cell wall damage is related to the interruption of the replication of ATP and the DNA of the bacteria which leads to the death of the bacteria.81,89 Ag NPs have been found to adhere to the cell wall, degrade it, and increase ion passage to the cytosol, according to several studies.77,90,91 Ninganagouda et al. (2014), for instance, showed the capability of Ag NPs to adhere to bacterial surfaces and effectively kill bacteria by rupturing cell membranes and leaking intracellular components.92 Other studies showed that MgO NPs and Mg(OH)2 NPs can kill cells without entering the cell by electrostatically adhering to the cell wall.79,93 Additionally, CuO NPs are able to cross the cell membrane through pores of microbes in the micron range without any hindrance.94 In a few studies, the antimicrobial activity of silicon is assigned to the mechanical damage of the bacterial membrane,95–97 although Smirnov et al. (2018) have not reported any mechanical damage of the bacterial membrane when using Si NPs.98Every species of fungi has a cell wall and membrane. The wall contains mannoproteins, beta-glucan-chitin, beta-glucan, and mannoproteins once more, and the membrane is made up of phospholipids. Therefore, before the NPs are able to reach the phospholipids, integral proteins, peripheral proteins, and ionic channels, they must first interact with all of these macromolecules.99 When NPs are released and come into contact with fungal cells, they bind to these certain membrane proteins and thus affect their function. They also have an impact on the cell permeability. A study using electron microscopy revealed that Ag NPs caused damage to the cell wall and membrane, thus penetrating the cells, damaging organelles like the mitochondria and ribosomes, and causing chromatin to condense and marginate, a sign of apoptotic cell death.80
Considering viruses, NPs first exert their effects, by interfering with the viral replication cycle's initial stages. The NPs attach to the surface of the virus to prevent its attachment to the host cell.100 Ag NPs display their antiviral activity by the inhibition of the interaction of the viral spike membrane protein gp120 with target cell membrane receptors. This mode of antiviral action enables Ag NPs to inhibit HIV-1 infection regardless of viral tropism or the resistance profile, to bind to gp120 in a manner that prevents CD4-dependent virion binding, fusion, and infectivity, and to block HIV-1 cell-free and cell-associated infection, acting as a virucidal agent. Additionally, it was shown by Zhang et al. 2024 that small enough (i.e. 3 nm) CeO2 NPs could effectively block ACE2 and SARS-CoV2 spike protein receptor binding, whereas the bigger 30 nm counterparts were unable to do so. As a result, Ag NPs are known as efficient virucides because they render HIV particles inactive quickly and exert their activity at both the entry and post-entry stages of viral replication, which are the earliest stages of viral replication.101 In analogy, highly monodispersed Au NPs interfere with HSV-1 surface molecules and, thus, make it impossible for the virus cell to attach to the target cell inhibiting viral infection.102
3.2. Ion release
Another antimicrobial activity of nanomaterials has been shown to be based on the release of ions.77,103–107 The release of silver ions from Ag NPs, for example, has proven to mediate a high antibacterial, antifungal and antiviral activity, making them useful for a variety of antimicrobial treatment applications.80 When metal ions in solution come into contact with bacterial cells, they are evenly distributed around the bacterial cells without specific localization. In contrast, NPs interacting with the bacterial cell wall create a focal ion source that releases ions continuously and causes greater toxicity to the cell.77 Suggestion: when metal ions pass through the cell membrane, they can directly interact with functional groups of proteins and nucleic acids, such as sulfhydryl (–SH), amino (–NH), and carboxyl (–COOH) groups. These interactions can disrupt enzyme activity, alter the cell structure, and affect normal physiological processes, ultimately inhibiting microorganisms. The concentration of NPs directly affects toxicity; higher concentrations of NPs release more ions,108,109 and this effect increases over time.110 This correlates with findings that longer incubation times reduce microbial activity. For example, released Zn2+ ions interfere with metabolic processes and actively disturb enzymatic systems. This effect strongly depends on the concentration of the released Zn ions combined with the time of exposure. The Zn ions thus affect two mechanisms in parallel. Firstly, they directly interfere with microbial membranes leading to enhanced permeability and destabilization, and secondly, they get in direct interaction with nucleic acids followed by the deactivation of enzymes of the respiratory system.111–113 For Ag NPs, the main bactericidal activity is considered to be the release of toxic Ag+ ions into the aquatic system which then leads to cell functioning damage of the microbiota by binding to thiol containing biomolecules and disrupting their function, affecting membrane permeability leading to cell lysis and also cell death as well as oxidative stress by the production of ROS.114,115 However, during the antibacterial process of a metal oxide suspension, the effect of metal ions on the pH in the lipid vesicles is small, and the antibacterial activity is weak. Therefore, dissolved metal ions are not considered to be the main antibacterial mechanism of metal oxide NPs.116 CuI NPs have shown to display high antiviral activity against feline calicivirus due to the production of Cu+ ions, which was then followed by ROS production and capsid protein oxidation.1173.3. ROS generation
Reactive oxygen species have the capacity to induce oxidative stress, which is one of the most important antimicrobial mechanisms of engineered nanomaterials.81,118 ROS is a general term for molecules and active intermediates with a strong positive redox potential, and different types of NPs generate different types of ROS by reducing oxygen molecules. The four ROS types are superoxide radicals (O2−), hydroxyl radicals (·OH), hydrogen peroxide (H2O2), and singlet oxygen (O2), which have different kinetics and reactivity. Different metal oxide NPs generate different types of ROS, which have an impact on the activity against the microbes. For example, CaO and MgO NPs can generate O2− while ZnO NPs as well as TiO2 NPs generate three types of ROS (superoxide radical, hydroxyl radical and singlet oxygen), thus the formation of ROS plays a very important role in these particles. Meanwhile, CuO NPs can generate all four types of ROS. Studies have shown that O2− and H2O2 produce less acute stress responses and can be neutralized by endogenous antioxidants such as superoxidase and catalase, whereas ·OH and O2 can cause acute microbial death.119 Li et al. (2012) reported that CeO2 NPs generate only one type of ROS (O2−) under UV irradiation,118 while Zhuo, Ma and Quan (2021) found that ROS formation is not the main reason for the damage of the microbes considering CeO2 NPs.120 Ag NPs inhibit respiratory enzymes, which leads to the formation of all four types of ROS. This formation leads to several types of damage to the cell including cell membrane damage, leakage of cellular materials, and loss of respiratory activity as well as DNA damage which finally leads to the death of the cell.121ROS generation can be enhanced by light. For instance, ZnO NPs can be activated either by UV light or by visible light which leads to creation of electron–hole pairs. The holes are able to split H2O molecules into superoxide radical anions, hydroxyl radicals and molecules of H2O2. The superoxide radicals and the hydroxyl anions are positively charged and therefore are not able to penetrate into the cell membrane of the bacteria, but H2O2 particles in contrast are able to penetrate into the cell and are responsible for the induction of apoptosis.122 Yamamoto (2001) stated that the concentration of H2O2 generated from the ZnO NPs increases with decreasing particle size, because the number of ZnO particles per unit volume increases with decreasing particle size. An increase in the generation of H2O2 leads to a higher antimicrobial activity, but the effect on different bacteria depends on their sensitivity towards the ROS.123 Also, there is evidence that a longer storage of the particles leads to a higher production of H2O2 which again leads to a higher antimicrobial activity.123,124 ZnO NPs producing hydroxyl radicals and singlet oxygen showed high antifungal activity against C. albicans. The smaller the ZnO NPs used, the higher their antifungal activity.125
Diverging reports exist regarding the potential of Au NPs to exert their antimicrobial action via the ROS production; however, it can be seen that the production of ROS is for sure the main and essential mechanism for many NPs.126 The authors reported that Au NPs, when tested in E. coli, exerted other mechanisms, including the change in membrane potential with subsequent ATP synthase inhibition and inhibitory binding of the tRNA-binding ribosome subunit, which resulted in a collapse of biological processes, thus already effective enough, while ROS levels were not altered. This could allow the development of Au NP-based antibacterial agents that target the energy-metabolism and specific transcription of bacteria without triggering the ROS-mediated reactions. However, also the oxidation state of the metals in NPs contributes to the bactericidal activity. For example, Cu2O NPs showed higher antibacterial activity than CuO NPs, suggesting that the oxidation state of the metal may play a role in toxicity. When consumed O2 reacts with Cu2O to form Cu2+, the resulting cation can react with O2−, which causes persistent oxidative stress. These superoxide molecules can reduce Cu2+ to Cu+ ions, which then produce H2O2, which can react with Cu to form OH−. OH concentrations were measured in cells exposed to CuO NPs compared to Cu2O NPs; however, intracellular proteins interacted more intensely with Cu2O than with CuO.127 Due to the generation of ROS, FeXOY NPs were reported to effectively induce lipid peroxidation in the viral envelope of influenza A viruses leading to additional production of radicals by the viral lipid envelope causing damage to nearby proteins.128 Another study reported that the production of ROS contributes to the antiviral effect of FeXOY NPs against SARS-CoV-2.129 Lee et al. determined that the antifungal activity of Ag NPs against Candida albicans (C. albicans) was dependent on the generation of ROS. In contrast, ROS production did not affect the fungal species Saccharomyces cerevisiae (S. cerevisiae). These results led to the conclusion that the antifungal activity of Ag NPs, in the case being mediated via ROS production, depends on the fungal species.130
3.4. Intracellular responses in microorganisms
If the NPs are small enough to penetrate the cell membrane, this can lead to many different intracellular responses, rendering cellular uptake another important mechanism of antimicrobial action.131 As already mentioned before, the size of the particles plays an important role considering the cellular uptake.81 Au NPs have two main ways to carry out their antimicrobial activity. The first is that they are able to collapse the membrane potential by inhibiting ATPase activities whereby the ATP level is decreased. This mechanism leads to a general decline in metabolism of the microbes. The second operates by inhibition of the subunit of ribosomes through binding tRNA which leads to a collapse of biological processes. In an early-phase reaction, the Au NPs also enhance chemotaxis.126 CuO NPs are able to cross the cell membrane through pores of microbes in the micron range without any hindrance. Inside the bacteria or fungi, they form stable complexes with vital enzymes which hinders cellular functioning leading to cell death.94 Additionally, due to their tiny size, Ag NPs have the potential to attach to cell surfaces, enter cells without harming the cell wall, and lastly killing cells.804. Measuring functional performance of antimicrobial nanomaterials
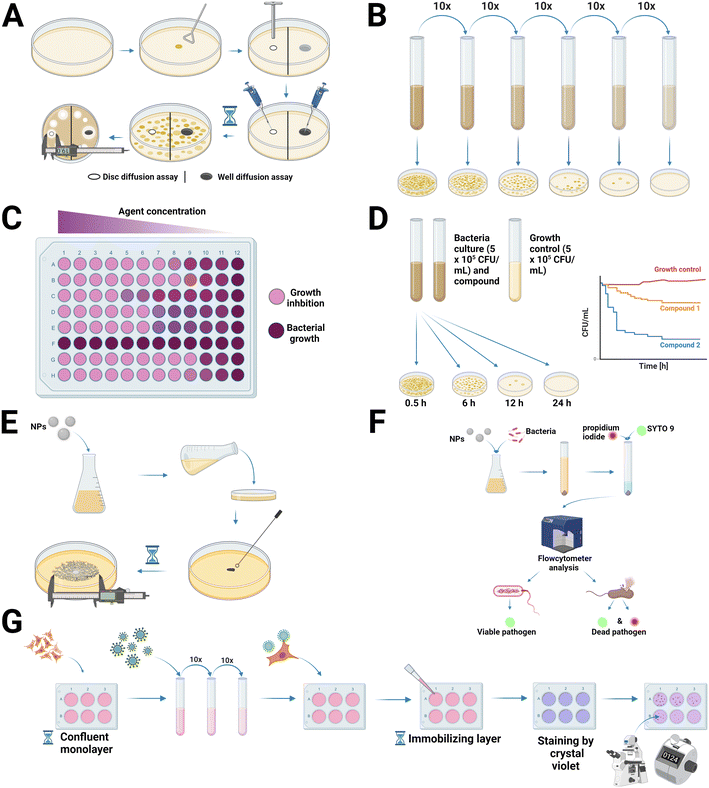
The antimicrobial activity can be monitored utilizing a wide range of well-reported methods, as depicted in Fig. 3. Discussed are well-established and -reported assays that have been slightly modified and adapted for assessing the antimicrobial efficiency of nanomaterials. More notably, as of writing this article, there were no nanomaterial-specific state-of-the-art assays tailored to the aforementioned antimicrobial testing methods. Specifically for testing the antimicrobial properties of nano-scaled materials, disc- and well-diffusion methods and broth dilution methods are the most used techniques. Additionally, time-kill tests in combination with plate count methods, known as total viable count methods, are well reported.132 The two most important readouts for antimicrobial testing are the minimum inhibitory concentration (MIC) and the minimum bactericidal concentration (MBC). The lowest dose of an antimicrobial agent that will suppress the visible growth of microbes after overnight incubation is known as the MIC, while the lowest concentration of an antimicrobial agent that prevents the growth of an organism after subculture onto antibiotic-free media is represented by the MBC.133Table 2 lists a broad selection of available studies reporting the antimicrobial activity of nanomaterials with their key results and assays applied.| NP type | Size [nm] | Antimicrobial test | Pathogen | Key result(s) | Ref. |
|---|---|---|---|---|---|
| Ag | 40–50 | Disc diffusion | Escherichia coli | 4.8 mm zone of inhibition | 134 |
| Ag | 6 | Broth microdilution | In vivo rabbit mouth | 9.8 wt% Ag NP mouthwash significantly reduced the number of bacteria in the oral cavity (P < 0.001) from 101.40 to 5.43 CFU after 4 days | 135 |
| Ag | 30–50 | Broth microdilution | Candida albicans | MBC: 48.0 ± 5.47 μg mL−1 | 136 |
| Candida albicans | MIC: 2.82 ± 0.68 μg mL−1 | ||||
| Streptococcus mutans | MBC: 18.5 ± 0.67 μg mL−1 | ||||
| Streptococcus mutans | MIC: 60.00 ± 22.36 μg mL−1 | ||||
| Ag | 33 | Disc diffusion | Escherichia coli | 18.7 mm zone of inhibition | 137 |
| Staphylococcus aureus | 17.7 mm zone of inhibition | ||||
| Bacillus cereus | 17.7 mm zone of inhibition | ||||
| Pseudomonas aeruginosa | 10.3 mm zone of inhibition | ||||
| Broth microdilution | Escherichia coli | MIC: 780 μg mL−1 | |||
| Staphylococcus aureus | MIC: 390 μg mL−1 | ||||
| Pseudomonas aeruginosa | MIC: 390 μg mL−1 | ||||
| Bacillus cereus | MIC: 780 μg mL−1 | ||||
| Ag | 10 | Plaque | Respiratory syncytial virus | HEp-2 cells: 78% reduction in replication | 138 |
| A549 cells: 79% reduction in replication | |||||
| Ag | 15 | MTT-assay | Herpes simplex virus | Decrease of 61.7 ± 6.6% of replication of virus | 139 |
| Ag | 5–18 | Poisson assay | Alternaria alternata | Mycelial diameter by 52% at 20 ppm | 140 |
| Pyricularia oryzae | Mycelial growth was reduced by 68% at 20 ppm | ||||
| Ag | 30 | Broth dilution method | Candida glabrata (12 strains) | MIC ranged from: 0.125 to 0.5 μg mL−1 | 42 |
| Ag modified CeO2 | 30–40 | TCID50 assays | Coronavirus OC43 | Treatment with 0.2 mg mL−1 for 4 h led to effectively inactivated infectivity to below the detectable limits | 43 |
| Parainfluenza virus type 5 | Treatment with 0.2 mg mL−1 for 4 h resulted in complete virus inactivation | ||||
| Combination of Ag, Cu, and WC (tungsten carbide) | 10–20 | Broth microdilution | Pseudomonas aeruginosa | Complete inhibition at 0.25 w/v% concentration | 141 |
| Live/dead staining | Staphylococcus aureus | 0.25 (w/v%) showed 98% of non-viable bacterial cells | |||
| Au | 30 | Broth microdilution | Escherichia coli | MIC: 16 μg mL−1 | 37 |
| Au | 14–50 | Disc diffusion | Escherichia coli | 8.5 mm zone of inhibition | 38 |
| Pseudomonas aeruginosa | 20.5 mm zone of inhibition | ||||
| Staphylococcus aureus | 16.5 mm zone of inhibition | ||||
| Time kill | Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus | 0.2 and 0.4 μg of NP reduction to zero population | |||
| Au | 6 | Plaque | Measles virus | Reduction of 84% of PFU at 3 h of incubation and 92% at 6 h of incubation | 142 |
| Real-time PCR | Viral load reduced by 95% at 3 h and 97% at 6 h of incubation | ||||
| Au | 10–16 | Virus pretreatment assay | Herpes simplex virus type 1 | 4 h pretreatment: 100-fold decrease of the HSV-1 load | 143 |
| CeO2 | 5 | Disc diffusion | Streptococcus mutans | 10 mm zone of inhibition | 144 |
| Staphylococcus aureus | 11 mm zone of inhibition | ||||
| Enterococcus faecalis | 9 mm zone of inhibition | ||||
| Candida albicans | 9 mm zone of inhibition | ||||
| CuO | 42 | Broth microdilution | Bacillus cereus | MIC = 0.62 mg mL−1 | 45 |
| Staphylococcus aureus | MIC = 0.16 mg mL−1 | ||||
| CuO | 4–50 | Well diffusion | Klebsiella oxytoca | 14 ± 0.31 mm zone of inhibition | 44 |
| Escherichia coli | 16 ± 0.53 mm zone of inhibition | ||||
| Staphylococcus aureus | 11 ± 0.57 mm zone of inhibition | ||||
| Bacillus cereus | 10 ± 0.57 mm zone of inhibition | ||||
| Microdilution assays | Klebsiella oxytoca | MIC = 6.25 μg mL−1 | |||
| Escherichia coli | MIC = 3.12 μg mL−1 | ||||
| Staphylococcus aureus | MIC = 12.5 μg mL−1 | ||||
| Bacillus cereus | MIC = 25 μg mL−1 | ||||
| Time-kill kinetics | Klebsiella oxytoca | 2 h with 4 × MIC: 45% | |||
| Escherichia coli | 2 h with 4 × MIC: 63% | ||||
| Staphylococcus aureus | 2 h with 4 × MIC: 49% | ||||
| Bacillus cereus | 2 h with 4 × MIC: 59% | ||||
| Cu0 | 20–30 | Broth microdilution | Pseudomonas aeruginosa | c of 400 ppm of NPs: 94.9% | 145 |
| c of 800 ppm of NPs: 100% | |||||
| Inhibition | |||||
| Staphylococcus aureus | c of 800 ppm of NPs: 40% inhibition | ||||
| FeXOY | 10 | Broth microdilution | Bacillus subtilis | Viability reduction of 30% in the presence of 50 μM NPs | 48 |
| Live/dead staining | 50 μM NP treatment ∼10% of non-viable bacterial cells | ||||
| FeXOY – chitosan coated (positive surface charge) | 11 | Broth microdilution | Escherichia coli | Viability reduced by 70% in the presence of 50 μM NPs | |
| Live/dead staining | 50 μM NP treatment showed 90% of non-viable bacterial cells | ||||
| Fe3O4 – Cinnamomum verum functionalized | 10 | Viable cell count assay | Staphylococcus aureus | Biofilm: 4-fold inhibition for initial biofilms and 3-fold inhibition for mature biofilms compared to the control | 146 |
| Escherichia coli | Inhibition ranged from 2.5-fold for initial biofilms to up to 2-fold for mature biofilms compared to the control | ||||
| ZnO | 30–92 | Neutralization assays | SARS-CoV-2 virus (delta) | Virus concentrations in the cell culture supernatant of infected cells were reduced by more than 106 times | 147 |
| Immunohistochemistry | Pre-treatment with 20 mg mL−1 showed no infected cells | ||||
| ZnO | 30–70 | Clear zone technique | Herpes simplex virus | 83% inhibition | 148 |
| Disc diffusion | Candida albicans | 20 mm zone of inhibition | |||
| ZnO | 70 | Agar dilution method | Penicillium expansum | Reduction rate of fungal growth at 91% (12 mmol L−1) | 51 |
| ZnO | 50 | Broth microdilution | Candida Albicans | MIC = 0.25 mg mL−1 | 149 |
| Escherichia coli | MIC = 0.5 mg mL−1 |
4.1. Diffusion methods
This type of traditional antimicrobial assay is derived from the antibiotic discovery and depends on the diffusion of the antimicrobial agent in the growth medium, as schematically illustrated in Fig. 3A. To carry out the disc diffusion method, sterile paper discs are dipped in a NP suspension and are placed on an agar plate that is inoculated with microbes afterwards. An inhibition zone appears around the discs after overnight incubation if the nanomaterial has an antimicrobial activity to the tested microbes. The extent of the inhibition zone represents the measurement parameter for the antimicrobial effect of the NPs. The size of the inhibition zone is influenced by the size of the NPs and their rate of diffusion in combination with the agar's porosity and charge interactions between the NPs and the agar.81,132 For the disk diffusion method, many different media are available, but Mueller-Hinton agar (MHA) (pH 7.2–7.4) is considered the best for routine antimicrobial testing of using simple and robust bacterial and yeast strains.81,150,151 Mueller-Hinton agar used for antifungal testing should be supplemented with glucose to a final concentration of 2% and methylene blue dye should be added to a final concentration of 0.5 μg mL−1. These two additional steps are useful for the growth of the fungi as well as to enhance zone edge definition.150 Spherical Ag NPs were tested against yeast, E. coli and S. aureus using the disk diffusion method and effectively inhibited bacterial and fungal growth. The particles showed a strong antimicrobial activity against yeast and E. coli, whereas the activity against S. aureus was mild. The lower efficacy of the Ag NPs against S. aureus could be explained by differences in the membrane structures of Gram-negative and Gram-positive bacteria.103 In addition, Pop et al. (2020) reported that CeO2 NPs showed differing antimicrobial activities against Gram-positive vs. Gram-negative bacteria due to differences in their membrane structures. The antibacterial activity of CeO2 NPs showed that the same concentration of NPs had different inhibition zone diameters for E. coli and Salmonella typhimurium (S. typhimurium), which were 9 and 10 mm, respectively. Gram-positive pathogens showed the strongest inhibition effects, with the highest being exerted by Listeria monocytogenes (L. monocytogenes), followed by Bacillus cereus (B. cereus) and S. aureus. The MBC results showed that for S. typhimurium and L. monocytogenes, the highest sensitivity observed was only at a concentration of 1.07 g L−1 CeO2 NPs.152The well diffusion method works quite similar to the disc diffusion method, but instead of paper discs, wells are dug into an inoculated agar plate. The NPs are then loaded into the wells and if the NPs have an antimicrobial activity against the used microbe, an inhibition zone appears, which makes it possible to determine the antimicrobial effect.132 Au NPs were tested for the Gram-negative bacteria E. coli, Pseudomonas aeruginosa (P. aeruginosa) and S. typhimurium using the well diffusion method and a strong antibacterial activity was found; meanwhile for the Gram-positive bacteria B. subtilis, S. aureus and Streptococcus pyogenes (S. pyogenes), they only showed moderate effects.153 Chandrasekaran et al. (2016) reported that Ag NPs showed the highest antibacterial effect against Gram-negative bacteria, using the well diffusion method.154 Urnukhsaikhan, Bold and Gunbileg (2021), however, examined that Ag NPs both have efficient antibacterial activity against Gram-positive (Micrococcus luteus [M. luteus]) and Gram-negative (E. coli) bacterial strains.155 Arujo et al. (2012) tested three different types of new synthesis methods for Ag NPs against S. aureus, Listeria innocua (L. innocua), Salmonella enterica (formerly known as Salmonella choleraesuis, S. choleraesuis), P. aeruginosa and B. cereus and discovered that all three types exhibited high antimicrobial activity. There was no difference in the antimicrobial action between the Ag NPs and the sodium chloride treated Ag treatments, whereas the concentrated Ag NPs were the most effective.156 CuO NPs showed antimicrobial activity against S. aureus, E. coli, and P. aeruginosa when assessed by disc- and well diffusion assays.157 In another study, ZnO NPs were tested against S. aureus, E. coli, Shigella sonnei (S. sonnei) and S. enterica as well as the yeast C. albicans. The NPs were completely ineffective against E. coli, S. enterica and S. sonnei (Gram-negative) as well as against C. albicans but they had a strong effect against the Gram-positive bacteria S. aureus.158 Bulk, green and chemically synthesized ZnO NPs were tested against four different pathogenic fungal strains (Aspergillus flavus (A. flavus), Aspergillus nidulans (A. nidulans), Trichoderma harzianum (T. harzianum), and Rhizopus stolonifer (R. stolonifer)) using the disc and the well diffusion method showing antifungal activity against all four strains. The higher activity of green synthesized particles could be attributed to their smaller particle size. The correlation between small particle sizes and the high surface to volume ratio was identified as a key property for antimicrobial suitability, but more details on the differences between the green and chemically synthesized ZnO NPs will be given further below.159
4.2. Dilution methods
The broth dilution method is carried out by inoculating containers holding identical volumes of broth with a known number of a test organism. The broths contain antimicrobial solutions with different concentrations which increase incrementally, as depicted in Fig. 3B and C. There is also the possibility of performing the broth dilution method in microdilution plates with a capacity of ≤500 μL per well, which is called the broth microdilution method. The most used medium for broth dilution methods for bacteria is Mueller-Hinton broth,160 and for fungi a synthetic medium is recommended by the Clinical & Laboratory Standards Institute (CLSI, formerly NCCLS).150 After incubation, the MIC can be determined and the results can be analyzed.151 The broth-dilution method is often used in conjunction with the dynamic contact method (ASTM E2149-10 guide), in which different concentrations of NPs are exposed to a solution containing known concentrations of microorganisms for a specified period of time. Therefore, after NPs exert antimicrobial activity in liquid medium, they can be further inoculated onto agar-filled Petri dishes and cultured under specific growth conditions customized for the target microorganisms.151,161,162 The activity of ZnO NPs was tested using the NCCLS-recommended broth dilution method for two different fungal species, A. flavus and Aspergillus fumigatus (A. fumigatus). The NPs showed high antifungal activity against both fungal strains. The ZnO particles were also tested against S. aureus and S. typhimurium using the broth dilution method, and a significant antibacterial effect against them was reported.163A variant suitable for higher throughput is the microtiter plate-based method, which is often used for antimicrobial susceptibility testing. To perform this method, a 96-well plate is taken and 100 μL of the test material in 10% DMSO or sterile water is pipetted into the first row of the plate. All other plates are filled up with 50 μL of nutrient broth or saline. Afterwards, a serial dilution is performed, so that each well ultimately contains 50 μL of the testing material in serially decreasing concentrations. Also, 10 μL of resazurin indicator solution, 30 μL of so-called “iso-sensitized broth”, which is a pH-buffered variant giving better reproducible results, and 10 μL of a bacterial suspension (5 × 106 CFU mL−1) are added. This has to be done to achieve a final concentration of 5 × 105 CFU mL−1 of the bacterial suspension. Finally, the plates are wrapped with a cling film to make sure that the bacteria are not dehydrated. Each plate also contains a column for the positive control, a column with all solutions except the test compound and a column with all solutions except the bacterial solution as a control. After overnight incubation, a color change can be used to interpret the result of the test. A color change from purple to pink or colorless is recorded as positive and the MIC value is considered as the lowest concentration at which a color change occurs.164 ZnO NPs immobilized with antibiotics, non-immobilized ZnO NPs and zinc ions were tested against E. coli, Staphylococcus epidermidis (S. epidermidis) and Klebsiella pneumoniae (K. pneumoniae) using the microtiter plate-based method. Zinc ions were the least effective against all tested bacteria, whilst ZnO NPs successfully inhibited the microorganism growth and showed similar minimal inhibitory concentrations when compared to the immobilized-antibiotic counterparts.165
4.3. Time-kill assay
The time-kill assay gives information about the dynamic interaction between the antimicrobial agent and the microbes (bacteria or fungi). It either shows a time-dependent or a concentration-dependent antimicrobial activity. This test has been well standardized for bacteria and is performed using three tubes that contain a 5 × 105 colony forming units per milliliter (CFU mL−1) bacteria suspension, as depicted in Fig. 3D. The first two tubes contain the testing substance and the third is used as a growth control. After exposing the testing substance to the bacteria, the percentage of dead cells over a specific time period is calculated relative to the growth control. Therefore, the living cells (CFU mL−1) are counted using the plate count method,166 which is a method that demonstrates the number of bacteria that survived after an overnight interaction between the antimicrobial and the bacteria used.167 The Clinical and Laboratory Standards Institute (CLSI) defines significant bactericidal activity as a ≥3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 colony-forming unit (CFU mL−1) reduction in the number of colonies grown on agar plates over time compared to the original inoculum, whereas the antibacterial activity corresponds to <3
log10 colony-forming unit (CFU mL−1) reduction in the number of colonies grown on agar plates over time compared to the original inoculum, whereas the antibacterial activity corresponds to <3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 CFU mL−1.166,168,169 For antifungal susceptibility testing, the time-kill assay requires some modifications. It is recommended to use a starting inoculum of 104–106 CFU mL−1 and the growth medium should be RPMI 1640 buffered to a pH of 7.0 using 3-(N-morpholino-)propane sulfonic acid (MOPS). Time-kill samples should be agitated while incubating at 35 °C. Prior to implementation, sampling techniques should be assessed for their impact on the antifungal carryover and sampling needs to last at least 24 h.170 The time-kill method is a quite appropriate method determining the antimicrobial activity of different substances.166 CuO-based nanomaterials have been shown to effectively kill E. coli, S. aureus, P. aeruginosa, methicillin-resistant S. aureus (MRSA), and Proteus spp. when using time-kill assay formats in vitro.104,152 When assessing NP toxicity, the choice of medium used in the time-kill method can affect the results. For example, the presence of proteins, salts, and glucose increases the aggregation of diamond-type nanomaterials, leading to a decrease in their antibacterial activity.171 For this reason, phosphate-buffered saline (PBS) which lacks the nutrients that bacteria need to grow, but maintains pH and provides a stable, inert environment for bacteria, was used in some time-kill studies, instead of nutrient-buffered saline, to evaluate the antibacterial activity of tested agents.171,172 Pop et al. (2020) tested CeO2 NPs against E. coli, S. typhimurium, L. monocytogenes, S. aureus and B. cereus. The inhibitory effect could be observed from the first hour on, and both Gram-positive and Gram-negative bacteria were affected by the particles.152 In addition, using time-kill tests it was shown that CuO NPs exhibited antimicrobial properties against S. aureus, E. coli, P. aeruginosa, and S. epidermidis.173 S NPs coated with chitosan were tested against bacteria (E. coli, S. aureus) and fungi (A. flavus, C. albicans) using the time-kill method. The S NPs showed antimicrobial activity against a large array of bacteria and fungi, with a higher activity against bacteria than fungi. The S NPs showed the highest activity against E. coli.103
log10 CFU mL−1.166,168,169 For antifungal susceptibility testing, the time-kill assay requires some modifications. It is recommended to use a starting inoculum of 104–106 CFU mL−1 and the growth medium should be RPMI 1640 buffered to a pH of 7.0 using 3-(N-morpholino-)propane sulfonic acid (MOPS). Time-kill samples should be agitated while incubating at 35 °C. Prior to implementation, sampling techniques should be assessed for their impact on the antifungal carryover and sampling needs to last at least 24 h.170 The time-kill method is a quite appropriate method determining the antimicrobial activity of different substances.166 CuO-based nanomaterials have been shown to effectively kill E. coli, S. aureus, P. aeruginosa, methicillin-resistant S. aureus (MRSA), and Proteus spp. when using time-kill assay formats in vitro.104,152 When assessing NP toxicity, the choice of medium used in the time-kill method can affect the results. For example, the presence of proteins, salts, and glucose increases the aggregation of diamond-type nanomaterials, leading to a decrease in their antibacterial activity.171 For this reason, phosphate-buffered saline (PBS) which lacks the nutrients that bacteria need to grow, but maintains pH and provides a stable, inert environment for bacteria, was used in some time-kill studies, instead of nutrient-buffered saline, to evaluate the antibacterial activity of tested agents.171,172 Pop et al. (2020) tested CeO2 NPs against E. coli, S. typhimurium, L. monocytogenes, S. aureus and B. cereus. The inhibitory effect could be observed from the first hour on, and both Gram-positive and Gram-negative bacteria were affected by the particles.152 In addition, using time-kill tests it was shown that CuO NPs exhibited antimicrobial properties against S. aureus, E. coli, P. aeruginosa, and S. epidermidis.173 S NPs coated with chitosan were tested against bacteria (E. coli, S. aureus) and fungi (A. flavus, C. albicans) using the time-kill method. The S NPs showed antimicrobial activity against a large array of bacteria and fungi, with a higher activity against bacteria than fungi. The S NPs showed the highest activity against E. coli.103
4.4. Colony diameter assay
Another method used for antifungal testing is the so-called colony diameter measurement, which is also known as the radial growth rate. It involves taking various time-stamped measurements of the diameter (or radius) of macroscopic colonies on solid media,174 as depicted in Fig. 3E. For this method, the final concentration of the NPs is mixed with melted agar. The mixture is poured into Petri dishes, which are then incubated before being inoculated in the center with a mycelia disc or plug with a diameter of 6–8 mm or a spore suspension.47 Ag NPs were tested against eighteen plant phytopathogenic fungal species using this method and showed antifungal properties against almost all fungi. A high inhibition effect was shown for most fungi at a 100 ppm concentration of the Ag NPs.175 Cu NPs (at various concentrations) were tested against Fusarium kuroshium (F. kuroshium) and were by more than 80% effective than the cupric hydroxide-based commercial fungicide used as a positive control.176 Besides using a growth medium for antimicrobial testing of CeO2 NPs, also the buffer conditions play a role; in this context NaCl and PBS were used comparatively to determine the antimicrobial effects of the NPs. Zhuo, Ma and Quan (2021) used this method by exposing functionalized CeO2 NPs to 0.9% NaCl solution or PBS for 6 h. These conditions were chosen instead of the growth medium because NaCl and PBS are extensively used in lots of scenarios.1204.5. Live/dead staining for bacterial and fungal viability assessment
Bacterial and fungal viability assays by live/dead staining form an alternative method to the described culture-based assays like the time-kill assay. A widely used kit is the LIVE/DEAD® BacLight™ Bacterial Viability Kit. The viability of bacterial or fungal cells is measured by membrane integrity with dual fluorescence staining. The fluorophores used are SYTO 9 and propidium iodide. SYTO 9 has its excitation/emission maxima at ∼480/500 nm with green emission and propidium iodide has its excitation/emission maxima at ∼490/635 nm with red emission. Both colors intercalate with nucleic acids; however, they differ in their membrane permeability properties. SYTO 9 can cross the membrane of both dead and living cells. Propidium iodide can only stain cells with a disrupted cell membrane. The viability is shown between cells that are stained green as live cells and red cells as dead cells. Therefore, an easy differentiation can be made between live and dead cells in a population when analyzed with fluorescence imaging, flow cytometry, or microplate assays. Briefly, the cells are incubated in a nutrition broth to grow. At desired time points, the cells can be treated with the concentrations of the substance to be analyzed. After incubation, the suspension is centrifuged, and the supernatant is discarded. The pellet is resuspended in 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer, and this step is repeated a few times, and after the last discarding step, the fluorochromes are added. After 15 min of incubation, the samples can be analyzed.44,177 The benefits of this method are that it is rapid and almost allows real time assessments. Cell death through any underlying process can be quantified directly, and results do not have to be reverse-calculated. When analyzed with fluorescence imaging, the loss of membrane integrity can be monitored over time. Therefore, this method can be used for over-time viability as well as concentration-dependent viability assessments. The impact of the treatment substance can be reported in percentage or the total number of dead cells (1×), with its experimental approach depicted in Fig. 3F.Using the LIVE/DEAD® BacLight™ Bacterial Viability Kit, chitosan-coated FeXOY NPs (positive surface charge) showed a dead cell population of 90% in the presence of 50 μM NPs against Bacillus subtilis (B. subtilis) and E. coli.44 Bankier et al. (2018) showed that a combination of tungsten carbide, Ag, and Cu0 NPs resulted in successful inhibition of P. aeruginosa and S. aureus upon NP treatment at a concentration of 0.25% (w/v), following a NP dose-dependent increase in growth inhibition.178 Another study reported that CuO NPs displayed higher antimicrobial activity than ZnO and WO3 NPs. From the different test strands (S. aureus, E. coli, MRSA and C. albicans, oral and vaginal), E. coli showed the highest sensitivity against CuO NPs. However, the best antimicrobial activity was achieved with a combination of the three NPs.179
4.6. Plaque assay formats for anti-viral functional performance testing
Plaque assays, which involve counting discrete plaques, i.e. infectious units and cellular dead zones, in the adherent cell culture, are the most precise techniques for the direct quantification of infectious virions or cellular susceptibility towards viruses and, hence, can be used for the determination of antiviral substances,180 as depicted in Fig. 3G. In a plaque assay, a confluent monolayer of host cells is exposed to an unknown concentration of a lytic virus that has been serially diluted to a countable range, usually between 5 and 100 virions per well. Then, to stop viral infection from dispersing randomly through the liquid medium during viral propagation, infected monolayers are covered with an immobilizing overlay medium. Meanwhile solid or semisolid overlays like agarose, methyl cellulose, or carboxymethyl cellulose have been replaced as the preferred covering method by novel liquid overlays like Avicel. After the virus initially infects the cells and an immobilizing layer is applied, individual plaques will emerge. This occurs as viral replication is restricted to the local area of the cell monolayer. Infected cells will continue the replication → lysis → re-infection cycle, spreading the infection further and causing plaques to become more distinct and discrete. A visible plaque formation will typically take 2 to 14 days, depending on the viral growth kinetics and host cell used. After that, cellular monolayers can be counted either using a bright field microscope, or by being fixed and counterstained with neutral red or crystal violet to make plaques easy to spot with the naked eye. Plaques are counted after the infected cellular monolayer has been fixed and stained to determine the titers of viral stock samples in the number of plaque-forming units (pfu) per milliliter. Between serial dilutions, a log drop should be noted, and, depending on the plate size, between 5 and 100 plaques should be counted with a reference of a negative control triplicate. According to statistics, samples will differ by 10% between replicates for every 100 plaques counted. Plaque assays are advantageous for determining viral titers because they can count the precise number of infectious viral particles present in the sample. Plaque titrations use the terminology of units rather than virions because multiple virions may potentially infect a single cell.181 Park et al. 2014 showed that their investigated Ag NPs maintained highly potent antiviral properties against several viruses including bacteriophage X174 and murine norovirus in various environmental settings without ecological risks.182 In another study, three antimicrobial NP types (Ag, CuO, and ZnO) were coated on porous air-filter materials (e.g., for face masks) as well as solid flat surfaces, and their activity against SARS-CoV-2 viability was tested using the plaque assay. Of the three investigated nanomaterials, Ag as a coating displayed the most potent antiviral activity, whereas CuO showed moderate activity and ZnO did not show any reduction of the virus load. The authors, thus, concluded that CuO and Ag are promising raw materials for use as antiviral coatings of solid surfaces or air-filters to reduce viral transmission and super-spreading events, and their data provide crucial guidance for the, at that time, ongoing and any upcoming pandemic mitigation efforts.1834.7. Assay standardization and assessment of antimicrobial surfaces
Standardization of antimicrobial tests suitable for surfaces was first established in the year 2000 with the Japanese Industrial Standard JIS Z 2801 (ref. 184) and was harmonized and re-released internationally in 2007 with the ISO 22196.185 The JIS Z 2801 first only covered the usage and testing of plastics, foams and textiles, and it was then extended with the ISO 22196 to cover all non-porous surfaces. In short, the surface is inoculated with defined concentrations of Gram-positive and -negative bacteria and covered with a film to prevent evaporation. After 24 h of incubation at 37 °C and 90% humidity, the combined assembly is thoroughly washed and seeded in a serial dilution on agar plates to estimate the growth inhibition compared to a reference material. Compared to the aforementioned antimicrobial testing methods, the ISO standards receive, however, little coverage in the scientific literature.5. Safety, sustainability & circularity considerations
Significant advancements have been made in understanding how and when engineered nano-scaled materials can be used for antimicrobial therapy. The toxicity of nanomaterials is generally mediated through dissolution, releasing toxic ions that can disrupt enzyme function, interact directly with DNA, or induce cellular oxidative stress by generating ROS.186,187 More effective and precise methodologies are now being applied to explore the impact of nanomaterials more extensively. This includes generating functional and mechanistic insights into their health effects, their potential release into the environment, their environmental fate, and the types of hazards they might create. These efforts aim to develop a comprehensive hazard rating and strategies for exposure mitigation.188–191 Depending on the type of application, nanomaterials may already enter the environment and ecosystems during production; however, they will inevitably do so during the use phase or disposal of nanomaterial-based products. There is a significant amount of research investigating the human health concerns but a comparatively negligible amount investigating the environmental impact. This disparity has, for instance, been highlighted by Bundschuh et al. 2018.192 In terms of optimizing sustainability of such materials, several options may be conceived. As described in detail above, the physical properties of nanomaterials significantly influence their antimicrobial action. The physical properties including size and shape can be controlled by adjusting chemical concentrations and reaction conditions including temperature and pH.193 However, in reality, the NPs synthesized by altering these conditions encounter several challenges, including instability in harsh environments, potential chronic toxicity upon bioaccumulation, complex analysis requirements, difficulties in device assembly, and issues with recycling and regeneration.194 To mitigate these issues, ‘green synthesis’ methods are gaining popularity, focusing on sustainable and eco-friendly processes. Green synthesis aims to minimize waste, reduce pollution, and use safer and better sustainable, bio-based solvents and reagents, as well as renewable resources.195 The production of antimicrobial nanomaterials, particularly Ag NPs, via green synthesis methods has emerged as a viable strategy for combating the growing issue of antibiotic-resistant bacterial diseases.196 Traditional synthesis methods frequently use toxic chemicals and solvents, raising environmental and health problems. Green synthesis provides an environmentally acceptable alternative by using biological agents such as plant extracts or microbial sources as reducing and capping agents.197 While this alternative synthesis approach offers numerous advantages, it remains essential to also consider potential drawbacks. A significant concern lies in the variability of phytochemicals extracted from plants. Factors such as regional conditions (light, temperature, rainfall), soil quality, and fertilizer use can significantly impact the composition and quantity of these compounds.198–200 Consequently, achieving batch-to-batch reproducibility may be challenging, especially when using plants sourced from different regions or harvested at different times of the year. Additionally, scaling up green synthesis approaches may present challenges due to the previously mentioned variability in plant materials and the difficulty in standardizing extraction and purification processes.201,202 There are several methods that are employed in the green synthesis of antimicrobial nano-scaled materials. One prominent method is the use of plant extracts to synthesize antimicrobial NPs, specifically Ag NP-based materials. Plant extracts, such as those from Heterotheca inuloides (H. inuloides), have shown promise in creating NPs with antibacterial properties. Briefly, H. inuloides leaves were collected, cleaned, dried, ground into powder, and boiled in distilled water. The filtered solution was mixed with a 10 mM silver nitrate solution in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 ratio to synthesize 16 nm spherical Ag NPs. This method for green synthesis is simple, eco-friendly and cost-effective.203 Another significant method involves the use of microorganisms such as bacteria, fungi, yeasts, and algae in the green synthesis of NPs. These microorganisms operate as bio-capping and bio-reducing agents, making it easier to create antimicrobial nanomaterials. Microorganisms with reductase enzymes can convert metallic salts into nanomaterials. The microbial synthesis strategy can be carried out via extracellular or intracellular pathways, allowing more variability and efficiency in NP production.204 Ensuring the long-term sustainability and minimizing the environmental impact of antimicrobial nanomaterials necessitate comprehensive life cycle assessment (LCA). LCA serves as a highly valuable tool for systematically evaluating the environmental impacts of nanomaterials across their entire life cycle, from production to disposal.205 While LCA studies on green synthesized antimicrobial nanomaterials are still relatively sparse, recent advancements have focused on evaluating their environmental sustainability. These studies aim to pinpoint environmental “hot spots” and compare the ecological performance of green synthesized nanomaterials against conventional or chemically synthesized alternatives.206 Despite the increasing interest in green synthesized antimicrobial nanomaterials, significant challenges persist in thoroughly assessing their sustainability and environmental impacts. The lack of comprehensive LCI (life cycle inventory) data for often insufficiently characterized nanomaterials and the rapidly evolving nature of nanotechnology complicate accurate LCA.207 Green synthesis, using plant extracts and microorganisms, minimizes toxic chemical use and promotes renewable resources. However, ensuring the long-term sustainability of nanomaterials with antimicrobial properties necessitates comprehensive LCA to evaluate their environmental impacts from production to disposal. Future research should address these gaps by incorporating nano-specific environmental effects and enhancing data availability, fostering sustainable practices in antimicrobial nanomaterial production and use. Ultimately, LCA is crucial for improving the environmental impact of green synthesized antimicrobial nanomaterials, ensuring their responsible and effective application. Achievement of a net-zero greenhouse gas emission economy by 2050 has been a central aspect worldwide, which has led to strategies that strongly promote innovation in developing safe and sustainable ways, fundamentally important for the production of nanomaterials on a large scale, avoiding the use of critical raw materials and fossil derived solvents as well as making use of closed circle manufacturing processes, in which the gases produced during the manufacturing process are used in the very same process as precursors. The implementation of circular economy principles,208 allied to Safe-and-Sustainable-by-Design (SSbD) methodologies,209 as well as the integration of advanced nano-scaled materials for antimicrobial applications, establishes a comprehensive framework focused on environmental health and resource efficiency.210 The circular economy emphasizes reducing, reusing, and recycling materials to create a closed-loop system that minimizes waste and energy consumption, thus significantly reducing the CO2 footprint. SSbD principles guide the design and development of materials, ensuring that environmental and human health impacts are considered throughout their entire lifecycle. By integrating these concepts, advanced nanomaterials play a crucial role in providing innovative solutions that enhance material performance, durability, recyclability, and antimicrobial effectiveness. Materials, particularly those engineered at the nanoscale, as discussed in this article, have shown significant antimicrobial properties due to their ability to interact with microbial membranes, generate ROS, and release metal ions that can disrupt cellular functions. Recent advances have also been made towards integrating inorganic nanomaterials with nano-structured bio-based materials and show promising potential for broad antimicrobial action.211 Natural materials and secondary metabolites derived from endophytic microorganisms, residing within the internal tissues of plants, have demonstrated virucidal, fungicidal, and bactericidal activity combined with pharmacological potential.212 Endophytic compounds can add further functionalities, such as disruption of the generation of cellular energy, damage to the synthesis of nucleic acids, disruption of protein synthesis, and modulations to key metabolic pathways. In combination with engineered nanomaterials, these mechanisms make nanomaterials highly effective in combating bacteria, fungi, and viruses, which is why they are increasingly utilized in antimicrobial coatings, surfaces, and healthcare applications.213 To fully understand and mitigate the environmental and
health impacts of advanced nanomaterials, particularly those designed with sustainability and safety in mind, an interdisciplinary approach is essential. Leveraging artificial intelligence (AI) and comprehensive data banks enables thorough assessments of nanomaterials across their entire lifecycle—from raw material extraction to waste recycling. This holistic approach is crucial, as it addresses both the beneficial applications of nanomaterials in industries such as cosmetics, textiles, healthcare, microelectronics, coatings, agriculture, and antimicrobial treatments, and the potential drawbacks associated with their production. Specifically, the significant energy consumption and emissions of gases like NOx and SOx during production present substantial environmental challenges. Therefore, integrating sustainable practices, advanced technologies, and rigorous evaluation frameworks is vital to advancing the safe and responsible use of nanomaterials, minimizing their ecological footprint while maximizing their benefits in various applications.214
2.5 ratio to synthesize 16 nm spherical Ag NPs. This method for green synthesis is simple, eco-friendly and cost-effective.203 Another significant method involves the use of microorganisms such as bacteria, fungi, yeasts, and algae in the green synthesis of NPs. These microorganisms operate as bio-capping and bio-reducing agents, making it easier to create antimicrobial nanomaterials. Microorganisms with reductase enzymes can convert metallic salts into nanomaterials. The microbial synthesis strategy can be carried out via extracellular or intracellular pathways, allowing more variability and efficiency in NP production.204 Ensuring the long-term sustainability and minimizing the environmental impact of antimicrobial nanomaterials necessitate comprehensive life cycle assessment (LCA). LCA serves as a highly valuable tool for systematically evaluating the environmental impacts of nanomaterials across their entire life cycle, from production to disposal.205 While LCA studies on green synthesized antimicrobial nanomaterials are still relatively sparse, recent advancements have focused on evaluating their environmental sustainability. These studies aim to pinpoint environmental “hot spots” and compare the ecological performance of green synthesized nanomaterials against conventional or chemically synthesized alternatives.206 Despite the increasing interest in green synthesized antimicrobial nanomaterials, significant challenges persist in thoroughly assessing their sustainability and environmental impacts. The lack of comprehensive LCI (life cycle inventory) data for often insufficiently characterized nanomaterials and the rapidly evolving nature of nanotechnology complicate accurate LCA.207 Green synthesis, using plant extracts and microorganisms, minimizes toxic chemical use and promotes renewable resources. However, ensuring the long-term sustainability of nanomaterials with antimicrobial properties necessitates comprehensive LCA to evaluate their environmental impacts from production to disposal. Future research should address these gaps by incorporating nano-specific environmental effects and enhancing data availability, fostering sustainable practices in antimicrobial nanomaterial production and use. Ultimately, LCA is crucial for improving the environmental impact of green synthesized antimicrobial nanomaterials, ensuring their responsible and effective application. Achievement of a net-zero greenhouse gas emission economy by 2050 has been a central aspect worldwide, which has led to strategies that strongly promote innovation in developing safe and sustainable ways, fundamentally important for the production of nanomaterials on a large scale, avoiding the use of critical raw materials and fossil derived solvents as well as making use of closed circle manufacturing processes, in which the gases produced during the manufacturing process are used in the very same process as precursors. The implementation of circular economy principles,208 allied to Safe-and-Sustainable-by-Design (SSbD) methodologies,209 as well as the integration of advanced nano-scaled materials for antimicrobial applications, establishes a comprehensive framework focused on environmental health and resource efficiency.210 The circular economy emphasizes reducing, reusing, and recycling materials to create a closed-loop system that minimizes waste and energy consumption, thus significantly reducing the CO2 footprint. SSbD principles guide the design and development of materials, ensuring that environmental and human health impacts are considered throughout their entire lifecycle. By integrating these concepts, advanced nanomaterials play a crucial role in providing innovative solutions that enhance material performance, durability, recyclability, and antimicrobial effectiveness. Materials, particularly those engineered at the nanoscale, as discussed in this article, have shown significant antimicrobial properties due to their ability to interact with microbial membranes, generate ROS, and release metal ions that can disrupt cellular functions. Recent advances have also been made towards integrating inorganic nanomaterials with nano-structured bio-based materials and show promising potential for broad antimicrobial action.211 Natural materials and secondary metabolites derived from endophytic microorganisms, residing within the internal tissues of plants, have demonstrated virucidal, fungicidal, and bactericidal activity combined with pharmacological potential.212 Endophytic compounds can add further functionalities, such as disruption of the generation of cellular energy, damage to the synthesis of nucleic acids, disruption of protein synthesis, and modulations to key metabolic pathways. In combination with engineered nanomaterials, these mechanisms make nanomaterials highly effective in combating bacteria, fungi, and viruses, which is why they are increasingly utilized in antimicrobial coatings, surfaces, and healthcare applications.213 To fully understand and mitigate the environmental and
health impacts of advanced nanomaterials, particularly those designed with sustainability and safety in mind, an interdisciplinary approach is essential. Leveraging artificial intelligence (AI) and comprehensive data banks enables thorough assessments of nanomaterials across their entire lifecycle—from raw material extraction to waste recycling. This holistic approach is crucial, as it addresses both the beneficial applications of nanomaterials in industries such as cosmetics, textiles, healthcare, microelectronics, coatings, agriculture, and antimicrobial treatments, and the potential drawbacks associated with their production. Specifically, the significant energy consumption and emissions of gases like NOx and SOx during production present substantial environmental challenges. Therefore, integrating sustainable practices, advanced technologies, and rigorous evaluation frameworks is vital to advancing the safe and responsible use of nanomaterials, minimizing their ecological footprint while maximizing their benefits in various applications.214
Assessing water ecotoxicity
The EU has set out to protect and restore the health of oceans, seas, and waters through research and innovation, citizen engagement and blue investments by significantly reducing their pollution with a tight timeline of until 2030, which was formulated in their implementation plan and a broad call for action in June 2022.215Daphnia magna, a small freshwater crustacean, has emerged as a pivotal model organism in assessing the ecotoxicity of nanomaterials in water. Its sensitivity towards environmental stressors and the availability of well-established and standardized OECD test guidelines (TGs) make it an ideal candidate for evaluating the potential risks associated with NP exposure. Recent studies have shed light on the complex interactions between nanomaterials and aquatic ecosystems. The tests are conducted following standardized guidelines, such as OECD TG 202, which outlines a 48 h acute immobilization test (OECD, 2004).216 These tests are performed under controlled laboratory conditions, typically involving glass beakers containing a defined volume of the test medium, maintained at a constant temperature (20–25 °C) and a photoperiod of 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 h light
8 h light![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dark cycle. The NPs are dispersed in the test medium using techniques such as sonication to ensure a homogeneous suspension, a critical step given the tendency of NPs to aggregate, which affects their bioavailability and toxicity. Exposure concentrations for these tests are determined through range-finding studies to identify the concentration range that causes observable effects. The primary endpoint in acute toxicity tests is the immobilization of Daphnia within 24 to 48 h of exposure. Immobilization is defined as the inability of Daphnia to swim within 15 seconds after gentle agitation. In chronic toxicity studies, longer-term exposures are conducted, with endpoints such as survival, reproduction (number of offspring), and growth being measured. Additionally, sublethal effects, including changes in behavior, feeding rates, and physiological stress markers, can be assessed to provide a more comprehensive understanding of the NPs' effects. A study by Mackevica et al. 2015 examined how food availability impacts the harmful effects of Ag NPs on Daphnia magna over a 21-day period. They found that more food reduced the negative effects of Ag NPs on survival, growth, and reproduction. This suggests that food intake influences how Daphnia magna is exposed to and processes the NPs upon uptake.217 Additionally, Hou et al. 2017 investigated the toxicity of Ag NPs based on their size and surface coating. It was found that citrate-coated Ag NPs were more toxic than PVP-coated ones, and smaller Ag NPs (40 nm) were more toxic than larger ones (110 nm). The increased toxicity of citrate-coated and smaller Ag NPs was linked to higher Ag+ ion release and greater biological impact, as evidenced by changes in gene expression, particularly in pathways related to ion binding and RNA polymerase.218 Liu et al. 2022 published a comprehensive review investigating the importance of Daphnia as a model organism to investigate the ecotoxicological impact of nanomaterials. As already discussed, many studies have shown that oxidative stress is a common mechanism of NP toxicity in Daphnia, yet there is still a need for further research to fully understand and fill the remaining gaps of these complex interactions.219
dark cycle. The NPs are dispersed in the test medium using techniques such as sonication to ensure a homogeneous suspension, a critical step given the tendency of NPs to aggregate, which affects their bioavailability and toxicity. Exposure concentrations for these tests are determined through range-finding studies to identify the concentration range that causes observable effects. The primary endpoint in acute toxicity tests is the immobilization of Daphnia within 24 to 48 h of exposure. Immobilization is defined as the inability of Daphnia to swim within 15 seconds after gentle agitation. In chronic toxicity studies, longer-term exposures are conducted, with endpoints such as survival, reproduction (number of offspring), and growth being measured. Additionally, sublethal effects, including changes in behavior, feeding rates, and physiological stress markers, can be assessed to provide a more comprehensive understanding of the NPs' effects. A study by Mackevica et al. 2015 examined how food availability impacts the harmful effects of Ag NPs on Daphnia magna over a 21-day period. They found that more food reduced the negative effects of Ag NPs on survival, growth, and reproduction. This suggests that food intake influences how Daphnia magna is exposed to and processes the NPs upon uptake.217 Additionally, Hou et al. 2017 investigated the toxicity of Ag NPs based on their size and surface coating. It was found that citrate-coated Ag NPs were more toxic than PVP-coated ones, and smaller Ag NPs (40 nm) were more toxic than larger ones (110 nm). The increased toxicity of citrate-coated and smaller Ag NPs was linked to higher Ag+ ion release and greater biological impact, as evidenced by changes in gene expression, particularly in pathways related to ion binding and RNA polymerase.218 Liu et al. 2022 published a comprehensive review investigating the importance of Daphnia as a model organism to investigate the ecotoxicological impact of nanomaterials. As already discussed, many studies have shown that oxidative stress is a common mechanism of NP toxicity in Daphnia, yet there is still a need for further research to fully understand and fill the remaining gaps of these complex interactions.219
Assessing soil ecotoxicity
The microbiome is crucial for soil quality and fertility and is vulnerable to environmental stress induced by contaminants.220 Quite similar to using Daphnia for monitoring water ecotoxicity, there is also a soil ecotoxicity standardized protocol available, i.e. OECD TG 222. This protocol is designed to evaluate the impact of chemicals on earthworms (Eisenia fetida or Eisenia andrei). Adult worms are exposed to varying levels of a test substance in soil for eight weeks. The goal is to identify the chemical concentration causing harmful effects on reproduction and overall health. Observations include changes in behavior, appearance, weight, and offspring production. Statistical analysis determines the chemical concentration causing no observable effects (NOEC) and those causing specific levels of reproductive decline, for instance in effective concentration for 50% reduction (EC50). To ensure accurate results, the test includes a control group and a range of chemical concentrations that effectively monitor the substance's toxicity.221 For instance, one study found that both ZnO and TiO2 NPs had limited harmful effects on earthworms exposed to high concentrations for extended periods. Moreover, ZnO NPs showed a negative impact on reproduction, especially in clay soil. Interestingly, low levels of TiO2 NPs appeared to stimulate reproduction in earthworms. These results suggested that the toxicity of ZnO NPs to earthworms can vary depending on soil conditions.222 Another study demonstrated that Ag NPs can impact the behavior of organisms at doses commonly found in the environment. Surprisingly, the organisms seemed to sense the NPs, causing them to avoid areas where they were present. This behavior could significantly affect how model ecosystems function under specific settings.223,224 While these studies provide valuable insights, it is essential to acknowledge the challenges associated with standardized test methodologies and the complexity of natural environments. In this regard, indoor mesocosm experiments can be used for elucidating the complex interactions of the soil microbiome with advanced materials under specific settings, such as, for instance, when Cu-based (nano-scaled and bulk) agrochemicals were being tested recently against conventional Cu-hydroxide pesticide.223 Future research should, thus, focus on developing robust protocols, considering multiple endpoints, and incorporating realistic exposure scenarios to improve risk assessment and management strategies for antimicrobial nano-scaled materials with an optimized safety and sustainability profile to enable circular and bio-based technologies for innovative antimicrobial surfaces based on advanced materials.6. Current and future applications
Within the European Union, nine so-called material innovation markets have been identified by the Advanced Materials Initiative,225 a broad initiative involving industry, research organizations, and academia. The sub-category advanced surfaces is represented in seven out of the nine material innovation markets, and it is evident that nano-scaled materials will provide a major opportunity in research and innovation. Material innovation markets entail fields like electronic appliances, new energy, home & personal care, sustainable construction, transport, agriculture and textiles. Even before the AMI2030, nano-scaled materials have facilitated a steady increase in applications, as depicted in Fig. 4. In this chapter, we will highlight some of these application fields and describe how nano-scaled advanced materials can be exploited in novel and innovative ways.Incorporating nanomaterials into medical devices (e.g., catheters, implants, wound dressings and prosthetics) aims to reduce the risk of spreading infections, associated with medical procedures. For instance, catheter-associated urinary tract infections (CAUTIs) are a common complication in patients with indwelling catheters. The use of catheters coated with Ag NPs has been shown to reduce the incidence of CAUTIs in clinical studies.226 Reducing the risk of infections with nanomaterials requires less use of antibiotics, hence, the development of antibiotic resistances is delayed, rendering them more effective when they are indeed needed, like in life-threatening scenarios. Additionally, a series of infections can lead to sepsis, which was the cause of 20% of worldwide accounted deaths in 2017.227 The use of nanomaterials in wound dressings can help prevent infections and promote wound healing by providing anti-inflammatory signals or increasing cell proliferation and angiogenesis.228 Nanomaterials, regardless of being organic or inorganic, such as TiO2 and ceramic NPs, have been demonstrated to mediate antimicrobial functionality in wound dressings.228,229 Overall, the use of nano-scaled materials in medical devices has the potential to improve patient well-being also indirectly by keeping antibiotics an effective instrument with reduced likelihood of resistances to emerge, additionally reducing the economic burden in coherence with healthcare-associated infections.
Nano-scaled advanced materials with antimicrobial properties can also be incorporated during the manufacturing of textiles. This is particularly useful in healthcare settings in cases of a high risk of contact-based infections with contaminated textiles for patients as well as for the staff. Antimicrobial textiles can be produced by incorporating Ag, Cu, or ZnO NPs into the fibers. These NPs can kill microorganisms by disrupting their cell membranes and interfering with their metabolic processes. Antimicrobial textiles are used in a variety of healthcare settings, including hospitals and nursing homes. These can be used in hospital gowns, bed linens, towels, and as previously mentioned wound dressings that come into regular contact with patients. Antimicrobial textiles also act as personal protective equipment like masks and gloves. Inevitably, NPs in textiles will diminish over time by either dissolving into ions, or by being washed out and, thus, quickly lose their effectiveness as antimicrobial agents in the products. In this regard, single- or limited-use products have a wider applicability as effective concentrations are better controlled than under repetitive use.
Another increasingly important field is sportswear. Many studies cover the generation of nanofibers that are woven into the fabric. These nanofibers can repel moisture and heat, offer protection from ultraviolet radiation whilst still being able to eliminate/reduce the microbial load and neutralize the smell of sweat.230,231
In the area of water treatment and air purification, nano-scaled advanced materials show various positive characteristics, facilitating high throughput, quick kinetics, targeted specificity, broad-spectrum photochemical efficacy, and strong antibacterial activity. Materials such as TiO2 NPs, including various composites thereof, are ideal candidates for water treatment to kill microbes and, thus, enhance the purification potential of the treatment plant. Materials can be used in filters, membranes, or as reagents in water treatment systems. It is essential to mention that NPs can hardly be removed if being added to wastewater. Thus, it is of high importance to use materials that neither show any toxic effects in humans nor display negative environmental effects. Quite similarly, ZnO NP-containing advanced materials can be applied in antimicrobial air filters. These filters aim to reduce the spread of airborne bacteria and other microorganisms in hospitals, public buildings, and crowded spaces.
Another major field is the application of nano-scaled advanced materials for food packaging resulting in less expensive items facilitating more effective production workflows, generating less waste, and consuming less energy.232 Potential candidates must be thoroughly tested, particularly in cases where packaging-derived abrasion may be released into the digestion tract. For packaging, mixing nano-scaled materials with compatible polymers results in enhanced mechanical strength and thermal stability.233 Further, nanomaterials can aid certain important features for “intelligent packaging”, enabling improved food security, extended storage life, better flavor and nutrient delivery, on top of standard packaging function, which is food protection, moisture control, and antioxidant function. Furthermore, intelligent packaging encompasses “smart” multifunctional labels containing sensors that inherently indicate safety, integrity and quality of the food. Some of these smart label functions are already used within cold chain indicators, for instance by Tempix AB (Gaevle, Sweden), which document whether a preset threshold temperature has ever been exceeded.234
The global population is projected to reach approximately 9 billion people by 2050. In order to sustain so many people, food production needs to be increased by about 50% to be able to satisfy the nutritional demands of the world.235,236 Using conventional farming methods, around one third of the crops get destroyed and nano-formulated agrochemicals are ideally able to alleviate the said issues. Commonly used fertilizers attempt to enhance crop production, but this comes also at a cost by decreasing soil health and fertility, disturbing the mineral balance and enhancing nitrogen loss into the atmosphere,237 adding to the already ongoing surpass of aerosol loading, resource consumption, biosphere integrity, and biogeochemical flows (anthropogenic reactive N and P release into ocean and land) beyond the planetary boundaries.238 Furthermore, to sustain the increased demand in food production more pesticides and fertilizers need to be produced, which is a highly energy- and cost-intensive procedure resulting in a highly negative carbon footprint. Nano-formulated agrochemicals could provide a solution for these problems, but still remain to be tested and experimented on a larger scale and in field trials, especially since most of the research and development have mostly been performed at the lab-scale. Nano-scaled advanced materials can, for once, be used as smart delivery systems to provide crops the necessary nutrients for ideal growth conditions, and depending on size, can also easily find a way into the plant cells.239 Agricultural waste products can be transformed into advanced nano-bio-composites, which then lead to enhanced plant growth and seed germination after the application. Antimicrobial coatings can be broadly applied to crops and seeds, which helps to prevent the growth of harmful bacteria and fungi.240,241
The above-described examples shall highlight some projections from the AMI2030, emphasizing that the utilization and implementation of nano-scaled advanced materials in a number of current product sectors is a fast flourishing and ongoing field of research and development. It can be expected that nanomaterial-containing products will be found in many sectors in the near future, and public acceptance and tolerance will have to consequently cope with them, demanding targeted activities in environmental and social governance involving all relevant stakeholders.242
7. Summary and outlook
Nano-scaled advanced materials offer a promising approach to proactively combat infections by preventing microbial contamination. Their diverse mechanisms of action can be combined to create highly effective antimicrobial solutions. By carefully selecting materials based on their specific properties, researchers can tailor these technologies to various applications. For instance, materials that release ions can be effective for short-term use, such as single-use face masks. Their dissolution properties often make them unsuitable for long-term applications, such as for surface coatings at the workplace, and frequent replacement would be required. Additionally, materials that need minimal maintenance are more practical and user-friendly for a wider range of applications. Recent studies have demonstrated the significant potential of nano-scaled advanced materials as a preventive measure against pathogen exposure. This could dramatically reduce the need for antibiotics and provide widespread protection in homes, workplaces, healthcare settings, and public areas. Potential innovations, such as the integration of machine learning and artificial intelligence, are endowed to significantly enhance the applicability and effectiveness of antimicrobial nanomaterials. In the face of emerging pandemics or seasonal flu outbreaks, these technologies can facilitate the rapid identification and selection of the most potent nanomaterials against specific pathogens. However, it is crucial to consider the potential environmental impact of these materials at the end of their lifecycle. Widespread global use could lead to increased levels of nanomaterial drain into ecosystems, potentially affecting flora, fauna, soil, water, and even animals. The urgent need for alternative antimicrobial therapies is undeniable. As antibiotic resistance grows, we must explore and utilize all potential agents, whether they inhibit (static) or kill (cidal) pathogens. This multifaceted approach will help mitigate the development of resistance and alleviate the global burden on healthcare systems.8. Glossary of microbes described in cited studies (alphabetical order)
| Full names of pathogens described | Short name |
| Alternaria alternata | A. alternata |
| Aspergillus flavus | A. flavus |
| Aspergillus fumigatus | A. fumigatus |
| Aspergillus nidulans | A. nidulans |
| Bacillus cereus | B. cereus |
| Bacillus subtilis | B. subtilis |
| Bacillus thuringiensis | B. thuringiensis |
| Botrytis cinerea | B. cinerea |
| Candida albicans | C. albicans |
| Candida glabrata | C. glabrata |
| Escherichia coli | E. coli |
| Enterococcus faecalis | E. faecalis |
| Fusarium kuroshium | F. kuroshium |
| Klebsiella pneumoniae | K. pneumoniae |
| Klebsiella oxytoca | K. oxytoca |
| Listeria innocua | L. innocua |
| Listeria monocytogenes | L. monocytogenes |
| Listeria monocytogenes | L. monocytogenes |
| Methicillin-resistant Staphylococcus aureus | MRSA |
| Micrococcus luteus | M. luteus |
| Multiple unnamed species in the Proteus family | Proteus spp. |
| Penicillium expansum | P. expansum |
| Pseudomonas aeruginosa | P. aeruginosa |
| Pyricularia oryzae | P. oryzae |
| Rhizopus stolonifera | R. stolonifera |
| Saccharomyces cerevisiae | S. cerevisiae |
| Salmonella choleraesuis/enterica | S. choleraesuis/enterica |
| Salmonella typhimurium | S. typhimurium |
| Shigella sonnei | S. sonnei |
| Staphylococcus aureus | S. aureus |
| Staphylococcus epidermidis | S. epidermidis |
| Streptococcus pyogenes | S. pyogenes |
| Streptococcus mutans | S. mutans |
| Trichoderma harzianum | T. harzianum |
| Heterotheca inuloides | H. inuloides |
| Daphnia magna | D. magna |
| Eisenia fetida | E. fetida |
| Eisenia andrei | E. andrei |
Data availability
No new data are presented in this tutorial review article.Author contributions
The conception, literature screening, drafting, design of display items, and preparation of the original manuscript were undertaken by BP, CC, AW, SL, YL, LJ, VA, OC, AA, AS, and MH. The review, editing, and supervision of the manuscript were performed by YL, PF, GW, and MH. All authors have carefully read and agreed to the final version of the manuscript.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The authors gratefully acknowledge financial support by the SmartCERIALS project supported by the Austrian Research Promotion Agency (FFG, grant 890610), the PINK project funded by the European Union's Horizon Europe Research and Innovation program (grant no. 101137809), the CAS International Partnership Program (grant No. 172644KYSB20210011), the DIAGONAL project supported by the European Union's Horizon 2020 Research and Innovation program (grant no. 953152), the NanoProCoV project funded by the Scientific & Technological Cooperation program of the Austrian Agency for Education and Internationalization (OeAD-WTZ, grant CN 06/2021), the National Natural Science Foundation of China (grant no. 82261138630), and the CAS President's International Fellowship Initiative (grant no. 2025PVB0045). The figures and graphical abstract were prepared using https://BioRender.com (accessed August 2024).References
- A. Gray and F. Sharara, Global and regional sepsis and infectious syndrome mortality in 2019: a systematic analysis, Lancet Glob. Health, 2022, 10, S2 CrossRef.
- V. Alfano and S. Ercolano, The Efficacy of Lockdown Against COVID-19: A Cross-Country Panel Analysis, Appl. Health Econ. Health Policy, 2020, 18, 509–517 CrossRef PubMed.
- S. Kharroubi and F. Saleh, Are Lockdown Measures Effective Against COVID-19?, Front. Public Health, 2020, 8, 549692 CrossRef PubMed.
- M. Terreni, M. Taccani and M. Pregnolato, New Antibiotics for Multidrug-Resistant Bacterial Strains: Latest Research Developments and Future Perspectives, Molecules, 2021, 26, 2671 CrossRef CAS PubMed.
- GrrlScientist, Climate Change Could Make Fungi More Dangerous To Humans, https://www.forbes.com/sites/grrlscientist/2024/06/24/climate-change-could-make-fungi-more-dangerous-to-humans/, (accessed 08/27, 2024).
- WHO, WHO releases report on state of development of antibacterials, https://www.who.int/news/item/14-06-2024-who-releases-report-on-state-of-development-of-antibacterials, (accessed 12, 2024).
- N. Chanishvili, Phage therapy—history from Twort and d'Herelle through Soviet experience to current approaches, Adv. Virus Res., 2012, 83, 3–40 CrossRef CAS PubMed.
- D. Rakhuba, E. Kolomiets, E. S. Dey and G. Novik, Bacteriophage receptors, mechanisms of phage adsorption and penetration into host cell, Pol. J. Microbiol., 2010, 59, 145 CAS.
- I. Yosef, M. Manor, R. Kiro and U. Qimron, Temperate and lytic bacteriophages programmed to sensitize and kill antibiotic-resistant bacteria, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 7267–7272 CrossRef CAS PubMed.
- M. Colomer-Lluch, J. Jofre and M. Muniesa, Antibiotic resistance genes in the bacteriophage DNA fraction of environmental samples, PLoS One, 2011, 6, e17549 CrossRef CAS PubMed.
- S. R. Modi, H. H. Lee, C. S. Spina and J. J. Collins, Antibiotic treatment expands the resistance reservoir and ecological network of the phage metagenome, Nature, 2013, 499, 219–222 CrossRef CAS PubMed.
- K. Kharga, L. Kumar and S. K. S. Patel, Recent Advances in Monoclonal Antibody-Based Approaches in the Management of Bacterial Sepsis, Biomedicines, 2023, 11, 765 CrossRef CAS PubMed.
- L. Zhang, E. Liang, Y. Cheng, T. Mahmood, F. Ge, K. Zhou, M. Bao, L. Lv, L. Li and J. Yi, Is combined medication with natural medicine a promising therapy for bacterial biofilm infection?, Biomed. Pharmacother., 2020, 128, 110184 CrossRef CAS PubMed.
- A. M. Seixas, S. A. Sousa and J. H. Leitão, Antibody-based immunotherapies as a tool for tackling multidrug-resistant bacterial infections, Vaccines, 2022, 10, 1789 CrossRef CAS PubMed.
- A. Cremonesi, Materials 2030 Manifesto, EC Group, 2022 Search PubMed.
- O. McNeilly, R. Mann, M. Hamidian and C. Gunawan, Emerging Concern for Silver Nanoparticle Resistance in Acinetobacter baumannii and Other Bacteria, Front. Microbiol., 2021, 12, 652863 CrossRef PubMed.
- J. Potočnik, European-Commission, ed. Eu, Official Journal of the European Union, 2011, 38–40 Search PubMed.
- E. I. Rabea, M. E.-T. Badawy, C. V. Stevens, G. Smagghe and W. Steurbaut, Chitosan as antimicrobial agent: applications and mode of action, Biomacromolecules, 2003, 4, 1457–1465 CrossRef CAS PubMed.
- B. Sarmento, A. Ribeiro, F. Veiga, D. Ferreira and R. Neufeld, Oral bioavailability of insulin contained in polysaccharide nanoparticles, Biomacromolecules, 2007, 8, 3054–3060 CrossRef CAS PubMed.
- J. Tian, K. K. Wong, C. M. Ho, C. N. Lok, W. Y. Yu, C. M. Che, J. F. Chiu and P. K. Tam, Topical delivery of silver nanoparticles promotes wound healing, ChemMedChem, 2007, 2, 129–136 CrossRef CAS PubMed.
- S. Kaur and G. S. Dhillon, The versatile biopolymer chitosan: potential sources, evaluation of extraction methods and applications, Crit. Rev. Microbiol., 2014, 40, 155–175 CrossRef CAS PubMed.
- M. Hosseinnejad and S. M. Jafari, Evaluation of different factors affecting antimicrobial properties of chitosan, Int. J. Biol. Macromol., 2016, 85, 467–475 CrossRef CAS PubMed.
- G. Karunakaran, K. G. Sudha, S. Ali and E.-B. Cho, Biosynthesis of Nanoparticles from Various Biological Sources and Its Biomedical Applications, Molecules, 2023, 28, 4527 CrossRef CAS PubMed.
- V. A. Spirescu, C. Chircov, A. M. Grumezescu, B. Ş. Vasile and E. Andronescu, Inorganic Nanoparticles and Composite Films for Antimicrobial Therapies, Int. J. Mol. Sci., 2021, 22, 4595 CrossRef CAS PubMed.
- G. A. Sotiriou and S. E. Pratsinis, Antibacterial activity of nanosilver ions and particles, Environ. Sci. Technol., 2010, 44, 5649–5654 CrossRef CAS PubMed.
- V. Tsikourkitoudi, B. Henriques-Normark and G. A. Sotiriou, Inorganic nanoparticle engineering against bacterial infections, Curr. Opin. Chem. Eng., 2022, 38, 100872 CrossRef.
- S. Kamat and M. Kumari, Emergence of microbial resistance against nanoparticles: Mechanisms and strategies, Front. Microbiol., 2023, 14, 1102615 CrossRef PubMed.
- A. K. Yetisen, H. Qu, A. Manbachi, H. Butt, M. R. Dokmeci, J. P. Hinestroza, M. Skorobogatiy, A. Khademhosseini and S. H. Yun, Nanotechnology in Textiles, ACS Nano, 2016, 10, 3042–3068 CrossRef CAS PubMed.
- R. Foldbjerg, P. Olesen, M. Hougaard, D. A. Dang, H. J. Hoffmann and H. Autrup, PVP-coated silver nanoparticles and silver ions induce reactive oxygen species, apoptosis and necrosis in THP-1 monocytes, Toxicol. Lett., 2009, 190, 156–162 CrossRef CAS PubMed.
- T. M. Benn and P. Westerhoff, Nanoparticle silver released into water from commercially available sock fabrics, Environ. Sci. Technol., 2008, 42, 4133–4139 CrossRef CAS PubMed.
- Z. Cheng, A. Al Zaki, J. Z. Hui, V. R. Muzykantov and A. Tsourkas, Multifunctional Nanoparticles: Cost Versus Benefit of Adding Targeting and Imaging Capabilities, Science, 2012, 338, 903–910 CrossRef CAS PubMed.
- M. E. Vance, T. Kuiken, E. P. Vejerano, S. P. McGinnis, M. F. Hochella Jr, D. Rejeski and M. S. Hull, Nanotechnology in the real world: Redeveloping the nanomaterial consumer products inventory, Beilstein J. Nanotechnol., 2015, 6, 1769–1780 CrossRef CAS PubMed.
- H. Lee and D. G. Lee, Gold nanoparticles induce a reactive oxygen species-independent apoptotic pathway in Escherichia coli, Colloids Surf., B, 2018, 167, 1–7 CrossRef CAS PubMed.
- X. Zhang, H. Wu, Z. Geng, X. Huang, R. Hang, Y. Ma, X. Yao and B. Tang, Microstructure and cytotoxicity evaluation of duplex-treated silver-containing antibacterial TiO2 coatings, Mater. Sci. Eng., C, 2014, 45, 402–410 CrossRef CAS PubMed.
- H. Liu, Z. Huang, H. Chen, Y. Zhang, P. Yu, P. Hu, X. Zhang, J. Cao and T. Zhou, A potential strategy against clinical carbapenem-resistant Enterobacteriaceae: antimicrobial activity study of sweetener-decorated gold nanoparticles in vitro and in vivo, J. Nanobiotechnol., 2023, 21, 409 CrossRef CAS PubMed.
- S. Liao, Y. Zhang, X. Pan, F. Zhu, C. Jiang, Q. Liu, Z. Cheng, G. Dai, G. Wu and L. Wang, Antibacterial activity and mechanism of silver nanoparticles against multidrug-resistant Pseudomonas aeruginosa, Int. J. Nanomed., 2019, 1469–1487 CrossRef CAS PubMed.
- R. Vazquez-Muñoz, A. Meza-Villezcas, P. Fournier, E. Soria-Castro, K. Juarez-Moreno, A. Gallego-Hernández, N. Bogdanchikova, R. Vazquez-Duhalt and A. Huerta-Saquero, Enhancement of antibiotics antimicrobial activity due to the silver nanoparticles impact on the cell membrane, PLoS One, 2019, 14, e0224904 CrossRef PubMed.
- E. Lotfali, H. Toreyhi, K. M. Sharabiani, A. Fattahi, A. Soheili, R. Ghasemi, M. Keymaram, Y. Rezaee and S. Iranpanah, Comparison of antifungal properties of gold, silver, and selenium nanoparticles against amphotericin B-resistant Candida glabrata clinical isolates, Avicenna J. Med. Biotechnol., 2021, 13, 47 Search PubMed.
- C. R. Fox, K. Kedarinath, C. J. Neal, J. Sheiber, E. Kolanthai, U. Kumar, C. Drake, S. Seal and G. D. Parks, Broad-spectrum, potent, and durable ceria nanoparticles inactivate RNA virus infectivity by targeting virion surfaces and disrupting virus–receptor interactions, Molecules, 2023, 28, 5190 CrossRef CAS PubMed.
- A. M. Shehabeldine, B. H. Amin, F. A. Hagras, A. A. Ramadan, M. R. Kamel, M. A. Ahmed, K. H. Atia and S. S. Salem, Potential antimicrobial and antibiofilm properties of copper oxide nanoparticles: time-kill kinetic essay and ultrastructure of pathogenic bacterial cells, Appl. Biochem. Biotechnol., 2023, 195, 467–485 CrossRef CAS PubMed.
- A. G. Kaningini, T. Motlhalamme, G. K. More, K. C. Mohale and M. Maaza, Antimicrobial, antioxidant, and cytotoxic properties of biosynthesized copper oxide nanoparticles (CuO-NPs) using Athrixia phylicoides DC, Heliyon, 2023, 9, e15265 CrossRef CAS PubMed.
- A. Ivanova, K. Ivanova, L. Fiandra, P. Mantecca, T. Catelani, M. Natan, E. Banin, G. Jacobi and T. Tzanov, Antibacterial, antibiofilm, and antiviral farnesol-containing nanoparticles prevent Staphylococcus aureus from drug resistance development, Int. J. Mol. Sci., 2022, 23, 7527 CrossRef CAS PubMed.
- Y.-H. Hsueh, P.-H. Tsai, K.-S. Lin, W.-J. Ke and C.-L. Chiang, Antimicrobial effects of zero-valent iron nanoparticles on gram-positive Bacillus strains and gram-negative Escherichia coli strains, J. Nanobiotechnol., 2017, 15, 1–12 CrossRef PubMed.
- M. Arakha, S. Pal, D. Samantarrai, T. K. Panigrahi, B. C. Mallick, K. Pramanik, B. Mallick and S. Jha, Antimicrobial activity of iron oxide nanoparticle upon modulation of nanoparticle-bacteria interface, Sci. Rep., 2015, 5, 14813 CrossRef CAS PubMed.
- C. Shao, Z. Yu, T. Luo, B. Zhou, Q. Song, Z. Li, X. Yu, S. Jiang, Y. Zhou and W. Dong, Chitosan-coated selenium nanoparticles attenuate PRRSV replication and ROS/JNK-mediated apoptosis in vitro, Int. J. Nanomed., 2022, 3043–3054 CrossRef PubMed.
- J. Gupta, M. Irfan, N. Ramgir, K. Muthe, A. Debnath, S. Ansari, J. Gandhi, C. Ranjith-Kumar and M. Surjit, Antiviral activity of zinc oxide nanoparticles and tetrapods against the Hepatitis E and Hepatitis C viruses, Front. Microbiol., 2022, 13, 881595 CrossRef PubMed.
- L. He, Y. Liu, A. Mustapha and M. Lin, Antifungal activity of zinc oxide nanoparticles against Botrytis cinerea and Penicillium expansum, Microbiol. Res., 2011, 166, 207–215 CrossRef CAS PubMed.
- B. Punz, L. Johnson, M. Geppert, H.-H. Dang, J. Horejs-Hoeck, A. Duschl and M. Himly, Surface Functionalization of Silica Nanoparticles: Strategies to Optimize the Immune-Activating Profile of Carrier Platforms, Pharmaceutics, 2022, 14, 1103 CrossRef CAS PubMed.
- I. Hasenkopf, R. Mills-Goodlet, L. Johnson, I. Rouse, M. Geppert, A. Duschl, D. Maier, V. Lobaskin, I. Lynch and M. Himly, Computational prediction and experimental analysis of the nanoparticle-protein corona: Showcasing an in vitro-in silico workflow providing FAIR data, Nano Today, 2022, 46, 101561 CrossRef CAS.
- B. Sharma, U. Soni, L. O. Afonso and D. M. Cahill, Nanomaterial doping: Chemistry and strategies for agricultural applications, ACS Agric. Sci. Technol., 2022, 2, 240–257 CrossRef CAS.
- V. Albright, I. Zhuk, Y. Wang, V. Selin, B. van de Belt-Gritter, H. J. Busscher, H. C. van der Mei and S. A. Sukhishvili, Self-defensive antibiotic-loaded layer-by-layer coatings: Imaging of localized bacterial acidification and pH-triggering of antibiotic release, Acta Biomater., 2017, 61, 66–74 CrossRef CAS PubMed.
- J. S. Suk, Q. Xu, N. Kim, J. Hanes and L. M. Ensign, PEGylation as a strategy for improving nanoparticle-based drug and gene delivery, Adv. Drug Delivery Rev., 2016, 99, 28–51 CrossRef CAS PubMed.
- G. Bao, S. Mitragotri and S. Tong, Multifunctional Nanoparticles for Drug Delivery and Molecular Imaging, Annu. Rev. Biomed. Eng., 2013, 15, 253–282 CrossRef CAS PubMed.
- E. F. De Macedo, N. S. Santos, L. S. Nascimento, R. Mathey, S. Brenet, M. S. De Moura, Y. Hou and D. B. Tada, Interaction between Nanoparticles, Membranes and Proteins: A Surface Plasmon Resonance Study, Int. J. Mol. Sci., 2022, 24, 591 CrossRef PubMed.
- S. Behzadi, V. Serpooshan, W. Tao, M. A. Hamaly, M. Y. Alkawareek, E. C. Dreaden, D. Brown, A. M. Alkilany, O. C. Farokhzad and M. Mahmoudi, Cellular uptake of nanoparticles: journey inside the cell, Chem. Soc. Rev., 2017, 46, 4218–4244 RSC.
- M. A. Gatoo, S. Naseem, M. Y. Arfat, A. Mahmood Dar, K. Qasim and S. Zubair, Physicochemical Properties of Nanomaterials: Implication in Associated Toxic Manifestations, BioMed Res. Int., 2014, 2014, 1–8 CrossRef PubMed.
- R. Mills-Goodlet, L. Johnson, I. J. Hoppe, C. Regl, M. Geppert, M. Schenck, S. Huber, M. Hauser, F. Ferreira, N. Hüsing, C. G. Huber, H. Brandstetter, A. Duschl and M. Himly, The nanotopography of SiO2 particles impacts the selectivity and 3D fold of bound allergens, Nanoscale, 2021, 13, 20508–20520 RSC.
- S. Hofer, N. Hofstätter, B. Punz, I. Hasenkopf, L. Johnson and M. Himly, Immunotoxicity of nanomaterials in health and disease: Current challenges and emerging approaches for identifying immune modifiers in susceptible populations, WIREs Nanomed. Nanobi., 2022, 14, e1804 CrossRef CAS PubMed.
- P. Kaurani, A. D. Hindocha, R. M. Jayasinghe, U. Y. Pai, K. Batra and C. Price, Effect of addition of titanium dioxide nanoparticles on the antimicrobial properties, surface roughness and surface hardness of polymethyl methacrylate: A Systematic Review, F1000Research, 2023, 12, 577 CAS.
- I. Georgakopoulos-Soares, E. L. Papazoglou, P. Karmiris-Obratański, N. E. Karkalos and A. P. Markopoulos, Surface antibacterial properties enhanced through engineered textures and surface roughness: A review, Colloids Surf., B, 2023, 113584 CrossRef CAS PubMed.
- S. Perumal, R. Atchudan, S. Ramalingam, S. Aldawood, N. Devarajan, W. Lee and Y. R. Lee, Silver nanoparticles loaded graphene-poly-vinylpyrrolidone composites as an effective recyclable antimicrobial agent, Environ. Res., 2023, 216, 114706 CrossRef CAS PubMed.
- Y. Cao, S. M. Javadhesari, S. Mohammadnejad, A. Raise and M. Akbarpour, Microstructural characterization and antibacterial activity of carbon nanotube decorated with Cu nanoparticles synthesized by a novel solvothermal method, Ceram. Int., 2021, 47, 25729–25737 CrossRef CAS.
- M. Godoy-Gallardo, U. Eckhard, L. M. Delgado, Y. J. de Roo Puente, M. Hoyos-Nogués, F. J. Gil and R. A. Perez, Antibacterial approaches in tissue engineering using metal ions and nanoparticles: From mechanisms to applications, Bioact. Mater., 2021, 6, 4470–4490 CAS.
- Q. Zhang, Y. Hu, C. M. Masterson, W. Jang, Z. Xiao, A. Bohloul, D. Garcia-Rojas, H. L. Puppala, G. Bennett and V. L. Colvin, When function is biological: Discerning how silver nanoparticle structure dictates antimicrobial activity, iScience, 2022, 25, 104475 CrossRef CAS PubMed.
- M. Saliani, R. Jalal and E. Kafshadre, Goharshadi, Effects of pH and Temperature on Antibacterial Activity of Zinc Oxide Nanofluid Against E. coliO157:H7 and Staphylococcus aureus, Jundishapur J. Microbiol., 2015, 8(2), e17115 Search PubMed.
- K. Sigfridsson, A. J. Lundqvist and M. Strimfors, Particle size reduction and pharmacokinetic evaluation of a poorly soluble acid and a poorly soluble base during early development, Drug Dev. Ind. Pharm., 2011, 37, 243–251 CrossRef CAS PubMed.
- D. E. Navarro-López, Y. Perfecto-Avalos, A. Zavala, M. A. de Luna, A. Sanchez-Martinez, O. Ceballos-Sanchez, N. Tiwari, E. R. López-Mena and G. Sanchez-Ante, Unraveling the Complex Interactions: Machine Learning Approaches to Predict Bacterial Survival against ZnO and Lanthanum-Doped ZnO Nanoparticles, Antibiotics, 2024, 13, 220 CrossRef PubMed.
- Y. Perfecto-Avalos, D. E. Navarro-López, S. Martínez-Beltrán, D. E. Rojas-Torres, K. D. Suárez Ávila, T. I. Robles, A. Zavala, M. A. de Luna, A. Sanchez-Martinez, O. Ceballos-Sanchez, M. Sepúlveda-Villegas, G. Sanchez-Ante, N. Tiwari and E. R. López-Mena, Data-Driven Machine Learning to Predict Antibacterial Activity of Cerium-Doped Nanoparticles, ACS Appl. Nano Mater., 2023, 6, 20719–20730 CrossRef CAS.
- Z. Bian, T. Bao, X. Sun, N. Wang, Q. Mu, T. Jiang, Z. Yu, J. Ding, T. Wang and Q. Zhou, Machine Learning Tools to Assist the Synthesis of Antibacterial Carbon Dots, Int. J. Nanomed., 2024, 5213–5226 CrossRef PubMed.
- P. Mary and A. Mujeeb, A machine learning framework for the prediction of antibacterial capacity of silver nanoparticles, Nano Express, 2024, 5, 025022 CrossRef CAS.
- M. Mirzaei, I. Furxhi, F. Murphy and M. Mullins, A Machine Learning Tool to Predict the Antibacterial Capacity of Nanoparticles, Nanomaterials, 2021, 11, 1774 CrossRef CAS PubMed.
- M. D. Wilkinson, M. Dumontier, I. J. Aalbersberg, G. Appleton, M. Axton, A. Baak, N. Blomberg, J.-W. Boiten, L. B. Da Silva Santos, P. E. Bourne, J. Bouwman, A. J. Brookes, T. Clark, M. Crosas, I. Dillo, O. Dumon, S. Edmunds, C. T. Evelo, R. Finkers, A. Gonzalez-Beltran, A. J. G. Gray, P. Groth, C. Goble, J. S. Grethe, J. Heringa, P. A. C. 'T Hoen, R. Hooft, T. Kuhn, R. Kok, J. Kok, S. J. Lusher, M. E. Martone, A. Mons, A. L. Packer, B. Persson, P. Rocca-Serra, M. Roos, R. Van Schaik, S.-A. Sansone, E. Schultes, T. Sengstag, T. Slater, G. Strawn, M. A. Swertz, M. Thompson, J. Van Der Lei, E. Van Mulligen, J. Velterop, A. Waagmeester, P. Wittenburg, K. Wolstencroft, J. Zhao and B. Mons, The FAIR Guiding Principles for scientific data management and stewardship, Sci. Data, 2016, 3, 160018 CrossRef PubMed.
- V. I. Dumit, A. Ammar, M. I. Bakker, M. A. Bañares, C. Bossa, A. Costa, H. Cowie, D. Drobne, T. E. Exner, L. Farcal, S. Friedrichs, I. Furxhi, R. Grafström, A. Haase, M. Himly, N. Jeliazkova, I. Lynch, D. Maier, C. W. Noorlander, H. K. Shin, G. J. A. A. Soler-Illia, B. Suarez-Merino, E. Willighagen and P. Nymark, From principles to reality. FAIR implementation in the nanosafety community, Nano Today, 2023, 51, 101923 CrossRef.
- A. Thill, O. Zeyons, O. Spalla, F. Chauvat, J. Rose, M. Auffan and A. M. Flank, Cytotoxicity of CeO2 nanoparticles for Escherichia coli. Physico-chemical insight of the cytotoxicity mechanism, Environ. Sci. Technol., 2006, 40, 6151–6156 CrossRef CAS PubMed.
- D. A. Pelletier, A. K. Suresh, G. A. Holton, C. K. McKeown, W. Wang, B. Gu, N. P. Mortensen, D. P. Allison, D. C. Joy and M. R. Allison, Effects of engineered cerium oxide nanoparticles on bacterial growth and viability, Appl. Environ. Microbiol., 2010, 76, 7981–7989 CrossRef CAS PubMed.
- P. K. Stoimenov, R. L. Klinger, G. L. Marchin and K. J. Klabunde, Metal oxide nanoparticles as bactericidal agents, Langmuir, 2002, 18, 6679–6686 CrossRef CAS.
- J. S. McQuillan, H. Groenaga Infante, E. Stokes and A. M. Shaw, Silver nanoparticle enhanced silver ion stress response in Escherichia coli K12, Nanotoxicology, 2012, 6, 857–866 CrossRef CAS PubMed.
- A. Simon-Deckers, Size-, Composition- and Shape-Dependent Toxicological Impact of Metal Oxide Nanoparticles and Carbon Nanotubes toward Bacteria, Environ. Sci. Technol., 2009, 43, 8423–8429 CrossRef CAS PubMed.
- Y. H. Leung, A. M. C. Ng, X. Xu, Z. Shen, L. A. Gethings, M. T. Wong, C. M. N. Chan, M. Y. Guo, Y. H. Ng, A. B. Djurišić, P. K. H. Lee, W. K. Chan, L. H. Yu, D. L. Phillips, A. P. Y. Ma and F. C. C. Leung, Mechanisms of Antibacterial Activity of MgO: Non-ROS Mediated Toxicity of MgO Nanoparticles Towards Escherichia coli, Small, 2014, 10, 1171–1183 CrossRef CAS PubMed.
- Z.-K. Xia, Q.-H. Ma, S.-Y. Li, D.-Q. Zhang, L. Cong, Y.-L. Tian and R.-Y. Yang, The antifungal effect of silver nanoparticles on Trichosporon asahii, J. Microbiol., Immunol. Infect., 2016, 49, 182–188 CrossRef CAS PubMed.
- A. L. Vega-Jiménez, Nanoemulsions-Properties, Fabrications and Applications, ed. K. S. Koh, IntechOpen, London, UK, 2019, ch. 2, pp. 13–25, DOI:10.5772/intechopen.78812.
- Y. H. Kim, G. H. Kim, K. S. Yoon, S. Shankar and J.-W. Rhim, Comparative antibacterial and antifungal activities of sulfur nanoparticles capped with chitosan, Microb. Pathog., 2020, 144, 104178 CrossRef CAS PubMed.
- A. Bahrami, R. Delshadi and S. M. Jafari, Active delivery of antimicrobial nanoparticles into microbial cells through surface functionalization strategies, Trends Food Sci. Technol., 2020, 99, 217–228 CrossRef CAS.
- A. Sarwar, H. Katas, S. N. Samsudin and N. M. Zin, Regioselective Sequential Modification of Chitosan via Azide-Alkyne Click Reaction: Synthesis, Characterization, and Antimicrobial Activity of Chitosan Derivatives and Nanoparticles, PLoS One, 2015, 10, e0123084 CrossRef PubMed.
- Z. Liu, M. Zhang, X. Han, H. Xu, B. Zhang, Q. Yu and M. Li, TiO2 nanoparticles cause cell damage independent of apoptosis and autophagy by impairing the ROS-scavenging system in Pichia pastoris, Chem.-Biol. Interact., 2016, 252, 9–18 CrossRef CAS PubMed.
- H. Li, Q. Chen, J. Zhao and K. Urmila, Enhancing the antimicrobial activity of natural extraction using the synthetic ultrasmall metal nanoparticles, Sci. Rep., 2015, 5, 11033 CrossRef CAS PubMed.
- B. Luan, T. Huynh and R. Zhou, Complete wetting of graphene by biological lipids, Nanoscale, 2016, 8, 5750–5754 RSC.
- I. P. Mukha, A. M. Eremenko, N. P. Smirnova, A. I. Mikhienkova, G. I. Korchak, V. F. Gorchev and A. Y. Chunikhin, Antimicrobial activity of stable silver nanoparticles of a certain size, Appl. Biochem. Microbiol., 2013, 49, 199–206 CrossRef CAS.
- L. S. Dorobantu, C. Fallone, A. J. Noble, J. Veinot, G. Ma, G. G. Goss and R. E. Burrell, Toxicity of silver nanoparticles against bacteria, yeast, and algae, J. Nanopart. Res., 2015, 17, 172 CrossRef.
- C.-N. Lok, C.-M. Ho, R. Chen, Q.-Y. He, W.-Y. Yu, H. Sun, P. K.-H. Tam, J.-F. Chiu and C.-M. Che, Silver nanoparticles: partial oxidation and antibacterial activities, JBIC, J. Biol. Inorg. Chem., 2007, 12, 527–534 CrossRef CAS PubMed.
- S. Pal, Y. K. Tak and J. M. Song, Does the Antibacterial Activity of Silver Nanoparticles Depend on the Shape of the Nanoparticle? A Study of the Gram-Negative Bacterium Escherichia coli, Appl. Environ. Microbiol., 2007, 73, 1712–1720 CrossRef CAS PubMed.
- S. Ninganagouda, V. Rathod, D. Singh, J. Hiremath, A. K. Singh, J. Mathew and M. Ul-Haq, Growth Kinetics and Mechanistic Action of Reactive Oxygen Species Released by Silver Nanoparticles from Aspergillus niger on Escherichia coli, BioMed Res. Int., 2014, 2014, 1–9 CrossRef PubMed.
- X. Pan, Y. Wang, Z. Chen, D. Pan, Y. Cheng, Z. Liu, Z. Lin and X. Guan, Investigation of Antibacterial Activity and Related Mechanism of a Series of Nano-Mg(OH)2, ACS Appl. Mater. Interfaces, 2013, 5, 1137–1142 CrossRef CAS PubMed.
- O. Mahapatra, M. Bhagat, C. Gopalakrishnan and K. D. Arunachalam, Ultrafine dispersed CuO nanoparticles and their antibacterial activity, J. Exp. Nanosci., 2008, 3, 185–193 CrossRef CAS.
- E. P. Ivanova, J. Hasan, H. K. Webb, G. Gervinskas, S. Juodkazis, V. K. Truong, A. H. F. Wu, R. N. Lamb, V. A. Baulin, G. S. Watson, J. A. Watson, D. E. Mainwaring and R. J. Crawford, Bactericidal activity of black silicon, Nat. Commun., 2013, 4, 2838 CrossRef PubMed.
- D. P. Linklater, H. K. D. Nguyen, C. M. Bhadra, S. Juodkazis and E. P. Ivanova, Influence of nanoscale topology on bactericidal efficiency of black silicon surfaces, Nanotechnology, 2017, 28, 245301 CrossRef PubMed.
- J. S. Hartley, M. M. Hlaing, G. Seniutinas, S. Juodkazis and P. R. Stoddart, Black silicon as a platform for bacterial detection, Biomicrofluidics, 2015, 9, 061101 CrossRef PubMed.
- N. A. Smirnov, S. I. Kudryashov, A. A. Nastulyavichus, A. A. Rudenko, I. N. Saraeva, E. R. Tolordava, S. A. Gonchukov, Y. M. Romanova, A. A. Ionin and D. A. Zayarny, Antibacterial properties of silicon nanoparticles, Laser Phys. Lett., 2018, 15, 105602 CrossRef.
- J. A. Gutiérrez, S. Caballero, L. A. Díaz, M. A. Guerrero, J. Ruiz and C. C. Ortiz, High Antifungal Activity against Candida Species of Monometallic and Bimetallic Nanoparticles Synthesized in Nanoreactors, ACS Biomater. Sci. Eng., 2018, 4, 647–653 CrossRef PubMed.
- V. Cagno, P. Andreozzi, M. D'Alicarnasso, P. Jacob Silva, M. Mueller, M. Galloux, R. Le Goffic, S. T. Jones, M. Vallino, J. Hodek, J. Weber, S. Sen, E.-R. Janeček, A. Bekdemir, B. Sanavio, C. Martinelli, M. Donalisio, M.-A. Rameix Welti, J.-F. Eleouet, Y. Han, L. Kaiser, L. Vukovic, C. Tapparel, P. Král, S. Krol, D. Lembo and F. Stellacci, Broad-spectrum non-toxic antiviral nanoparticles with a virucidal inhibition mechanism, Nat. Mater., 2018, 17, 195–203 CrossRef CAS PubMed.
- H. H. Lara, N. V. Ayala-Nuñez, L. Ixtepan-Turrent and C. Rodriguez-Padilla, Mode of antiviral action of silver nanoparticles against HIV-1, J. Nanobiotechnol., 2010, 8, 1 CrossRef PubMed.
- A. Halder, S. Das, D. Ojha, D. Chattopadhyay and A. Mukherjee, Highly monodispersed gold nanoparticles synthesis and inhibition of herpes simplex virus infections, Mater. Sci. Eng., C, 2018, 89, 413–421 CrossRef CAS PubMed.
- J. S. Kim, E. Kuk, K. N. Yu, J.-H. Kim, S. J. Park, H. J. Lee, S. H. Kim, Y. K. Park, Y. H. Park, C.-Y. Hwang, Y.-K. Kim, Y.-S. Lee, D. H. Jeong and M.-H. Cho, Antimicrobial effects of silver nanoparticles, Nanomedicine, 2007, 3, 95–101 CrossRef CAS PubMed.
- G. Ren, D. Hu, E. W. C. Cheng, M. A. Vargas-Reus, P. Reip and R. P. Allaker, Characterisation of copper oxide nanoparticles for antimicrobial applications, Int. J. Antimicrob. Agents, 2009, 33, 587–590 CrossRef CAS PubMed.
- A. Ivask, A. Elbadawy, C. Kaweeteerawat, D. Boren, H. Fischer, Z. Ji, C. H. Chang, R. Liu, T. Tolaymat, D. Telesca, J. I. Zink, Y. Cohen, P. A. Holden and H. A. Godwin, Toxicity Mechanisms in Escherichia coli Vary for Silver Nanoparticles and Differ from Ionic Silver, ACS Nano, 2014, 8, 374–386 CrossRef CAS PubMed.
- L. Wang, H. He, Y. Yu, L. Sun, S. Liu, C. Zhang and L. He, Morphology-dependent bactericidal activities of Ag/CeO2 catalysts against Escherichia coli, J. Inorg. Biochem., 2014, 135, 45–53 CrossRef CAS PubMed.
- J. R. Morones, J. L. Elechiguerra, A. Camacho, K. Holt, J. B. Kouri, J. T. Ramírez and M. J. Yacaman, The bactericidal effect of silver nanoparticles, Nanotechnology, 2005, 16, 2346–2353 CrossRef CAS PubMed.
- J. Rousk, K. Ackermann, S. F. Curling and D. L. Jones, Comparative Toxicity of Nanoparticulate CuO and ZnO to Soil Bacterial Communities, PLoS One, 2012, 7, e34197 CrossRef CAS PubMed.
- S. Soltani Nezhad, M. Rabbani Khorasgani, G. Emtiazi, M. M. Yaghoobi and S. Shakeri, Isolation of copper oxide (CuO) nanoparticles resistant Pseudomonas strains from soil and investigation on possible mechanism for resistance, World J. Microbiol. Biotechnol., 2014, 30, 809–817 CrossRef CAS PubMed.
- L. A. Tamayo, P. A. Zapata, N. D. Vejar, M. I. Azócar, M. A. Gulppi, X. Zhou, G. E. Thompson, F. M. Rabagliati and M. A. Páez, Release of silver and copper nanoparticles from polyethylene nanocomposites and their penetration into Listeria monocytogenes, Mater. Sci. Eng., C, 2014, 40, 24–31 CrossRef CAS PubMed.
- A. Ali, A.-R. Phull and M. Zia, Elemental zinc to zinc nanoparticles: is ZnO NPs crucial for life? Synthesis, toxicological, and environmental concerns, Nanotechnol. Rev., 2018, 7, 413–441 CrossRef CAS.
- V. Stanić, S. Dimitrijević, J. Antić-Stanković, M. Mitrić, B. Jokić, I. B. Plećaš and S. Raičević, Synthesis, characterization and antimicrobial activity of copper and zinc-doped hydroxyapatite nanopowders, Appl. Surf. Sci., 2010, 256, 6083–6089 CrossRef.
- M. Fang, J. Chen, X. Xu, P. Yang and H. Hildebrand, Antibacterial activities of inorganic agents on six bacteria associated with oral infections by two susceptibility tests, Int. J. Antimicrob. Agents, 2006, 27, 513–517 CrossRef CAS PubMed.
- T. S. Peretyazhko, Q. Zhang and V. L. Colvin, Size-Controlled Dissolution of Silver Nanoparticles at Neutral and Acidic pH Conditions: Kinetics and Size Changes, Environ. Sci. Technol., 2014, 48, 11954–11961 CrossRef CAS PubMed.
- Z.-M. Xiu, J. Ma and P. J. J. Alvarez, Differential Effect of Common Ligands and Molecular Oxygen on Antimicrobial Activity of Silver Nanoparticles versus Silver Ions, Environ. Sci. Technol., 2011, 45, 9003–9008 CrossRef CAS PubMed.
- J. Yu, W. Zhang, Y. Li, G. Wang, L. Yang, J. Jin, Q. Chen and M. Huang, Synthesis, characterization, antimicrobial activity and mechanism of a novel hydroxyapatite whisker/nano zinc oxide biomaterial, Biomed. Mater., 2014, 10, 015001 CrossRef PubMed.
- N. Shionoiri, T. Sato, Y. Fujimori, T. Nakayama, M. Nemoto, T. Matsunaga and T. Tanaka, Investigation of the antiviral properties of copper iodide nanoparticles against feline calicivirus, J. Biosci. Bioeng., 2012, 113, 580–586 CrossRef CAS PubMed.
- Y. Li, W. Zhang, J. Niu and Y. Chen, Mechanism of Photogenerated Reactive Oxygen Species and Correlation with the Antibacterial Properties of Engineered Metal-Oxide Nanoparticles, ACS Nano, 2012, 6, 5164–5173 CrossRef CAS PubMed.
- E. Malka, I. Perelshtein, A. Lipovsky, Y. Shalom, L. Naparstek, N. Perkas, T. Patick, R. Lubart, Y. Nitzan, E. Banin and A. Gedanken, Eradication of Multi-Drug Resistant Bacteria by a Novel Zn-doped CuO Nanocomposite, Small, 2013, 9, 4069–4076 CrossRef CAS PubMed.
- M. Zhuo, J. Ma and X. Quan, Cytotoxicity of functionalized CeO2 nanoparticles towards Escherichia coli and adaptive response of membrane properties, Chemosphere, 2021, 281, 130865 CrossRef CAS PubMed.
- B. Ramalingam, T. Parandhaman and S. K. Das, Antibacterial Effects of Biosynthesized Silver Nanoparticles on Surface Ultrastructure and Nanomechanical Properties of Gram-Negative Bacteria viz. Escherichia coli and Pseudomonas aeruginosa, ACS Appl. Mater. Interfaces, 2016, 8, 4963–4976 CrossRef CAS PubMed.
- N. Padmavathy and R. Vijayaraghavan, Enhanced bioactivity of ZnO nanoparticles—an antimicrobial study, Sci. Technol. Adv. Mater., 2008, 9, 035004 CrossRef PubMed.
- O. Yamamoto, Influence of particle size on the antibacterial activity of zinc oxide, Int. J. Inorg. Mater., 2001, 3, 643–646 CrossRef CAS.
- L. Zhang, Y. Ding, M. Povey and D. York, ZnO nanofluids – A potential antibacterial agent, Prog. Nat. Sci., 2008, 18, 939–944 CrossRef CAS.
- A. Lipovsky, Y. Nitzan, A. Gedanken and R. Lubart, Antifungal activity of ZnO nanoparticles—the role of ROS mediated cell injury, Nanotechnology, 2011, 22, 105101 CrossRef PubMed.
- Y. Cui, Y. Zhao, Y. Tian, W. Zhang, X. Lü and X. Jiang, The molecular mechanism of action of bactericidal gold nanoparticles on Escherichia coli, Biomaterials, 2012, 33, 2327–2333 CrossRef CAS PubMed.
- S. Meghana, P. Kabra, S. Chakraborty and N. Padmavathy, Understanding the pathway of antibacterial activity of copper oxide nanoparticles, RSC Adv., 2015, 5, 12293–12299 RSC.
- T. Qin, R. Ma, Y. Yin, X. Miao, S. Chen, K. Fan, J. Xi, Q. Liu, Y. Gu, Y. Yin, J. Hu, X. Liu, D. Peng and L. Gao, Catalytic inactivation of influenza virus by iron oxide nanozyme, Theranostics, 2019, 9, 6920–6935 CrossRef CAS PubMed.
- M. L. Dediego, Y. Portilla, N. Daviu, D. López-García, L. Villamayor, V. Mulens-Arias, J. G. Ovejero, Á. Gallo-Cordova, S. Veintemillas-Verdaguer, M. P. Morales and D. F. Barber, Iron oxide and iron oxyhydroxide nanoparticles impair SARS-CoV-2 infection of cultured cells, J. Nanobiotechnol., 2022, 20(1), 352 CrossRef CAS PubMed.
- B. Lee, Silver nanoparticles induce reactive oxygen species-mediated cell cycle delay and synergistic cytotoxicity with 3-bromopyruvate in Candida albicans, but not in Saccharomyces cerevisiae, Int. J. Nanomed., 2019, 14, 4801–4816 CrossRef CAS PubMed.
- J. S. McQuillan and A. M. Shaw, Differential gene regulation in the Ag nanoparticle and Ag+-induced silver stress response in Escherichia coli: A full transcriptomic profile, Nanotoxicology, 2014, 8, 177–184 CrossRef CAS PubMed.
- J. Gopal, S. Chun, V. Anthonydhason, S. Jung, B. N. Mwang'Ombe, M. Muthu and I. Sivanesan, Assays Evaluating Antimicrobial Activity of Nanoparticles: A Myth Buster, J. Cluster Sci., 2018, 29, 207–213 CrossRef CAS.
- J. M. Andrews, Determination of minimum inhibitory concentrations, J. Antimicrob. Chemother., 2001, 48, 5–16 CrossRef CAS PubMed.
- J. Y. Cheon, S. J. Kim, Y. H. Rhee, O. H. Kwon and W. H. Park, Shape-dependent antimicrobial activities of silver nanoparticles, Int. J. Nanomed., 2019, 14, 2773–2780 CrossRef CAS PubMed.
- A. Moaddabi, P. Soltani, C. Rengo, S. Molaei, S. J. Mousavi, M. Mehdizadeh and G. Spagnuolo, Comparison of antimicrobial and wound-healing effects of silver nanoparticle and chlorhexidine mouthwashes: an in vivo study in rabbits, Odontology, 2022, 110, 577–583 CrossRef CAS PubMed.
- N. P. Panpaliya, P. T. Dahake, Y. J. Kale, M. V. Dadpe, S. B. Kendre, A. G. Siddiqi and U. R. Maggavi, In vitro evaluation of antimicrobial property of silver nanoparticles and chlorhexidine against five different oral pathogenic bacteria, Saudi Dent. J., 2019, 31, 76–83 CrossRef PubMed.
- G. Chinnasamy, S. Chandrasekharan, T. W. Koh and S. Bhatnagar, Synthesis, Characterization, Antibacterial and Wound Healing Efficacy of Silver Nanoparticles From Azadirachta indica, Front. Microbiol., 2021, 12, 611560 CrossRef PubMed.
- D. Morris, M. Ansar, J. Speshock, T. Ivanciuc, Y. Qu, A. Casola and R. Garofalo, Antiviral and Immunomodulatory Activity of Silver Nanoparticles in Experimental RSV Infection, Viruses, 2019, 11, 732 CrossRef CAS PubMed.
- M. Hasanin, M. A. Elbahnasawy, A. M. Shehabeldine and A. H. Hashem, Ecofriendly preparation of silver nanoparticles-based nanocomposite stabilized by polysaccharides with antibacterial, antifungal and antiviral activities, BioMetals, 2021, 34, 1313–1328 CrossRef CAS PubMed.
- V. F. Consolo, A. Torres-Nicolini and V. A. Alvarez, Mycosinthetized Ag, CuO and ZnO nanoparticles from a promising Trichoderma harzianum strain and their antifungal potential against important phytopathogens, Sci. Rep., 2020, 10, 20499 CrossRef CAS PubMed.
- C. Bankier, R. Matharu, Y. Cheong, G. Ren, E. Cloutman-Green and L. Ciric, Synergistic antibacterial effects of metallic nanoparticle combinations, Sci. Rep., 2019, 9, 16074 Search PubMed.
- M. A. Meléndez-Villanueva, K. Morán-Santibañez, J. J. Martínez-Sanmiguel, R. Rangel-López, M. A. Garza-Navarro, C. Rodríguez-Padilla, D. G. Zarate-Triviño and L. M. Trejo-Ávila, Virucidal Activity of Gold Nanoparticles Synthesized by Green Chemistry Using Garlic Extract, Viruses, 2019, 11, 1111 Search PubMed.
- E. Paradowska, M. Studzińska, A. Jabłońska, V. Lozovski, N. Rusinchuk, I. Mukha, N. Vitiuk and Z. J. Leśnikowski, Antiviral Effect of Nonfunctionalized Gold Nanoparticles against Herpes Simplex Virus Type-1 (HSV-1) and Possible Contribution of Near-Field Interaction Mechanism, Molecules, 2021, 26, 5960 Search PubMed.
- S. Maiti, N. Devi, D. Ganapathy and S. Rajeshkumar, Characterization and antimicrobial activity of cerium oxide nanoparticles synthesized using neem and ginger, J. Adv. Pharm. Technol. Res., 2022, 13, 491 Search PubMed.
- N. Jardón-Maximino, M. Pérez-Alvarez, G. Cadenas-Pliego, L. E. Lugo-Uribe, C. Cabello-Alvarado, J. M. Mata-Padilla and E. D. Barriga-Castro, Synthesis of Copper Nanoparticles Stabilized with Organic Ligands and Their Antimicrobial Properties, Polymer, 2021, 13, 2846 Search PubMed.
- A. Anghel, A. Grumezescu, M. Chirea, V. Grumezescu, G. Socol, F. Iordache, A. Oprea, I. Anghel and A. Holban, MAPLE Fabricated Fe3O4@Cinnamomum verum Antimicrobial Surfaces for Improved Gastrostomy Tubes, Molecules, 2014, 19, 8981–8994 Search PubMed.
- S. Wolfgruber, J. Rieger, O. Cardozo, B. Punz, M. Himly, A. Stingl, P. M. A. Farias, P. M. Abuja and K. Zatloukal, Antiviral Activity of Zinc Oxide Nanoparticles against SARS-CoV-2, Int. J. Mol. Sci., 2023, 24, 8425 Search PubMed.
- H. S. Hassan, D. Abol-Fotouh, E. Salama and M. F. Elkady, Assessment of antimicrobial, cytotoxicity, and antiviral impact of a green zinc oxide/activated carbon nanocomposite, Sci. Rep., 2022, 12, 8774 CrossRef CAS PubMed.
- E. Hannachi, F. Khan, Y. Slimani, S. Rehman, Z. Trabelsi, S. Akhtar and E. Al-Suhaimi, In Vitro Antimicrobial and Anticancer Peculiarities of Ytterbium and Cerium Co-Doped Zinc Oxide Nanoparticles, Biology, 2022, 11, 1836 Search PubMed.
- M. A. Pfaller, V. Chaturvedi, A. Espinel-Ingroff, M. A. Ghannoum, L. L. Gosey, F. C. Odds and D. W. Warnock, Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard—Second Edition, National Committee for Clinical Laboratory Standards, Clinical and laboratory standards institute, 2002 Search PubMed.
- F. R. Cockerill, M. A. Wikler, J. Alder, M. N. Dudley, G. M. Eliopoulos, M. J. Ferraro and J. B. Patel, Performance standards for antimicrobial disk susceptibility testing: approved standard National Committee for Clinical Laboratory Standards, Clinical and Laboratory Standards Institute, 2012 Search PubMed.
- O. L. Pop, A. Mesaros, D. C. Vodnar, R. Suharoschi, F. Tăbăran, L. Mageruşan, I. S. Tódor, Z. Diaconeasa, A. Balint, L. Ciontea and C. Socaciu, Cerium Oxide Nanoparticles and Their Efficient Antibacterial Application In Vitro against Gram-Positive and Gram-Negative Pathogens, Nanomaterials, 2020, 10, 1614 Search PubMed.
- S. Veena, T. Devasena, S. S. M. Sathak, M. Yasasve and L. A. Vishal, Green Synthesis of Gold Nanoparticles from Vitex negundo Leaf Extract: Characterization and In Vitro Evaluation of Antioxidant–Antibacterial Activity, J. Cluster Sci., 2019, 30, 1591–1597 Search PubMed.
- R. Chandrasekaran, S. Gnanasekar, P. Seetharaman, R. Keppanan, W. Arockiaswamy and S. Sivaperumal, Formulation of Carica papaya latex-functionalized silver nanoparticles for its improved antibacterial and anticancer applications, J. Mol. Liq., 2016, 219, 232–238 Search PubMed.
- E. Urnukhsaikhan, B.-E. Bold, A. Gunbileg, N. Sukhbaatar and T. Mishig-Ochir, Antibacterial activity and characteristics of silver nanoparticles biosynthesized from Carduus crispus, Sci. Rep., 2021, 11, 21047 Search PubMed.
- E. A. Araújo, N. J. Andrade, L. H. M. Da Silva, P. C. Bernardes, Á. V. N. de C Teixeira, J. P. N. De Sá, J. F. Q. Fialho and P. E. Fernandes, Antimicrobial Effects of Silver Nanoparticles against Bacterial Cells Adhered to Stainless Steel Surfaces, J. Food Prot., 2012, 75, 701–705 Search PubMed.
- R. Dadi, R. Azouani, M. Traore, C. Mielcarek and A. Kanaev, Antibacterial activity of ZnO and CuO nanoparticles against gram positive and gram negative strains, Mater. Sci. Eng., C, 2019, 104, 109968 Search PubMed.
- M. J. Klink, N. Laloo, A. Leudjo Taka, V. E. Pakade, M. E. Monapathi and J. S. Modise, Synthesis, Characterization and Antimicrobial Activity of Zinc Oxide Nanoparticles against Selected Waterborne Bacterial and Yeast Pathogens, Molecules, 2022, 27, 3532 CrossRef CAS PubMed.
- S. Gunalan, R. Sivaraj and V. Rajendran, Green synthesized ZnO nanoparticles against bacterial and fungal pathogens, Prog. Nat. Sci.: Mater. Int., 2012, 22, 693–700 CrossRef.
- EUCAST and ESCMID, Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by broth dilution, Clin. Microbiol. Infect., 2003, 9, ix–xv CrossRef.
- J. B. Patel, Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fifth Informational Supplement, Clinical and laboratory standards institute, 2015 Search PubMed.
- ASTM International, Standard Test Method for Determining the Antimicrobial Activity of Antimicrobial Agents Under Dynamic Contact Conditions, https://www.astm.org/e2149-20.html, (accessed 12/02, 2024) Search PubMed.
- G. R. Navale, M. Thripuranthaka, D. J. Late and S. S. Shinde, Antimicrobial Activity of ZnO Nanoparticles against Pathogenic Bacteria and Fungi, JSM Nanotechnology and Nanomedicine, 2015, 3(1), 1033 Search PubMed.
- S. D. Sarker, L. Nahar and Y. Kumarasamy, Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals, Methods, 2007, 42, 321–324 CrossRef CAS PubMed.
- A. Rogowska, V. Railean-Plugaru, P. Pomastowski, J. Walczak-Skierska, A. Król-Górniak, A. Gołębiowski and B. Buszewski, The Study on Molecular Profile Changes of Pathogens via Zinc Nanocomposites Immobilization Approach, Int. J. Mol. Sci., 2021, 22, 5395 CrossRef CAS PubMed.
- B. L. Arthur, Methods for determining bactericidal activity of antimicrobial agents: approved guideline: National Committee for Clinical Laboratory Standards Wayne, CLSI, M26-A edn., 1999.
- T. Gabriel, A. Vestine, K. D. Kim, S. J. Kwon, I. Sivanesan and S. C. Chun, Antibacterial Activity of Nanoparticles of Garlic (Allium sativum) Extract against Different Bacteria Such as Streptococcus mutans and Poryphormonas gingivalis, Appl. Sci., 2022, 12, 3491 CrossRef CAS.
- J.-H. Sim, N. S. Jamaludin, C.-H. Khoo, Y.-K. Cheah, S. N. B. A. Halim, H.-L. Seng and E. R. T. Tiekink, In vitro antibacterial and time-kill evaluation of phosphanegold(I) dithiocarbamates, R3PAu[S2CN(iPr)CH2CH2OH] for R = Ph, Cy and Et, against a broad range of Gram-positive and Gram-negative bacteria, Gold Bull., 2014, 47, 225–236 CrossRef CAS.
- I. Zadrazilova, S. Pospisilova, K. Pauk, A. Imramovsky, J. Vinsova, A. Cizek and J. Jampilek, In VitroBactericidal Activity of 4- and 5-Chloro-2-hydroxy-N-[1-oxo-1-(phenylamino)alkan-2-yl]benzamides against MRSA, BioMed Res. Int., 2015, 2015, 1–8 CrossRef PubMed.
- M. E. Klepser, E. J. Ernst, R. E. Lewis, M. E. Ernst and M. A. Pfaller, Influence of Test Conditions on Antifungal Time-Kill Curve Results: Proposal for Standardized Methods, Antimicrob. Agents Chemother., 1998, 42, 1207–1212 CrossRef CAS PubMed.
- M. Taran, M. Rad and M. Alavi, Antibacterial Activity of Copper Oxide (CuO) Nanoparticles Biosynthesized by Bacillus sp. FU4: Optimization of Experiment Design, Pharm. Sci., 2017, 23, 198–206 CrossRef.
- N. Norouzi, Y. Ong, V. G. Damle, M. B. Habibi Najafi and R. Schirhagl, Effect of medium and aggregation on antibacterial activity of nanodiamonds, Mater. Sci. Eng., C, 2020, 112, 110930 CrossRef CAS PubMed.
- A. Azam, A. S. Ahmed, M. Oves, M. S. Khan, S. S. Habib and A. Memic, Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: a comparative study, Int. J. Nanomed., 2012, 7, 6003–6009 CrossRef CAS PubMed.
- S. Marín, A. J. Ramos and V. Sanchis, Comparison of methods for the assessment of growth of food spoilage moulds in solid substrates, Int. J. Food Microbiol., 2005, 99, 329–341 CrossRef PubMed.
- S. W. Kim, J. H. Jung, K. Lamsal, Y. S. Kim, J. S. Min and Y. S. Lee, Antifungal Effects of Silver Nanoparticles (AgNPs) against Various Plant Pathogenic Fungi, Mycobiology, 2012, 40, 53–58 CrossRef CAS PubMed.
- E. Ibarra-Laclette, J. Blaz, C.-A. Pérez-Torres, E. Villafán, A. Lamelas, G. Rosas-Saito, L. A. Ibarra-Juárez, C. D. J. García-Ávila, A. I. Martínez-Enriquez and N. Pariona, Antifungal Effect of Copper Nanoparticles against Fusarium kuroshium, an Obligate Symbiont of Euwallacea kuroshio Ambrosia Beetle, J. Fungi, 2022, 8, 347 CrossRef CAS PubMed.
- J. Robertson, C. McGoverin, F. Vanholsbeeck and S. Swift, Optimisation of the Protocol for the LIVE/DEAD® BacLightTM Bacterial Viability Kit for Rapid Determination of Bacterial Load, Front. Microbiol., 2019, 10, 801 CrossRef PubMed.
- C. Bankier, Y. Cheong, S. Mahalingam, M. Edirisinghe, G. Ren, E. Cloutman-Green and L. Ciric, A comparison of methods to assess the antimicrobial activity of nanoparticle combinations on bacterial cells, PLoS One, 2018, 13, e0192093 CrossRef PubMed.
- D. V. Francis, M. N. Jayakumar, H. Ahmad and T. Gokhale, Antimicrobial Activity of Biogenic Metal Oxide Nanoparticles and Their Synergistic Effect on Clinical Pathogens, Int. J. Mol. Sci., 2023, 24, 9998 CrossRef CAS PubMed.
- M. Himly, D. N. Foster, I. Bottoli, J. S. Iacovoni and P. K. Vogt, The DF-1 Chicken Fibroblast Cell Line: Transformation Induced by Diverse Oncogenes and Cell Death Resulting from Infection by Avian Leukosis Viruses, Virology, 1998, 248, 295–304 CrossRef CAS PubMed.
- A. Baer and K. Kehn-Hall, Viral Concentration Determination Through Plaque Assays: Using Traditional and Novel Overlay Systems, J. Visualized Exp., 2014, 93, e52065 Search PubMed.
- S. Park, H. H. Park, S. Y. Kim, S. J. Kim, K. Woo and G. Ko, Antiviral Properties of Silver Nanoparticles on a Magnetic Hybrid Colloid, Appl. Environ. Microbiol., 2014, 80, 2343–2350 CrossRef PubMed.
- P. Merkl, S. Long, G. M. McInerney and G. A. Sotiriou, Antiviral Activity of Silver, Copper Oxide and Zinc Oxide Nanoparticle Coatings against SARS-CoV-2, Nanomaterials, 2021, 11, 1312 CrossRef CAS PubMed.
- DIN Media, Antibacterial products - Test for antibacterial activity and efficacy, 2010, JIS Z 2801:2010-12-20, https://www.dinmedia.de/de/norm/jis-z-2801/149158779, (accessed 12/02, 2024).
- ISO 22196, Measurement of antibacterial activity on plastics and other non-porous surfaces, International Standard, 2011, p. 2 Search PubMed.
- S. M. Imani, L. Ladouceur, T. Marshall, R. Maclachlan, L. Soleymani and T. F. Didar, Antimicrobial Nanomaterials and Coatings: Current Mechanisms and Future Perspectives to Control the Spread of Viruses Including SARS-CoV-2, ACS Nano, 2020, 14, 12341–12369 CrossRef CAS PubMed.
- G. Martínez, M. Merinero, M. Pérez-Aranda, E. Pérez-Soriano, T. Ortiz, E. Villamor, B. Begines and A. Alcudia, Environmental Impact of Nanoparticles' Application as an Emerging Technology: A Review, Materials, 2020, 14, 166 CrossRef PubMed.
- Y. Huang, X. Guo, Y. Wu, X. Chen, L. Feng, N. Xie and G. Shen, Nanotechnology's frontier in combatting infectious and inflammatory diseases: prevention and treatment, Signal Transduction Targeted Ther., 2024, 9, 34 CrossRef PubMed.
- G. E. Yılmaz, I. Göktürk, M. Ovezova, F. Yılmaz, S. Kılıç and A. Denizli, Antimicrobial Nanomaterials: A Review, Hygiene, 2023, 3, 269–290 CrossRef.
- A. Karnwal, G. Kumar, G. Pant, K. Hossain, A. Ahmad and M. B. Alshammari, Perspectives on Usage of Functional Nanomaterials in Antimicrobial Therapy for Antibiotic-Resistant Bacterial Infections, ACS Omega, 2023, 8, 13492–13508 CrossRef CAS PubMed.
- M. Himly, M. Geppert, S. Hofer, N. Hofstätter, J. Horejs-Höck and A. Duschl, When Would Immunologists Consider a Nanomaterial to be Safe? Recommendations for Planning Studies on Nanosafety, Small, 2020, 16, 1907483 CrossRef CAS PubMed.
- M. Bundschuh, J. Filser, S. Lüderwald, M. S. McKee, G. Metreveli, G. E. Schaumann, R. Schulz and S. Wagner, Nanoparticles in the environment: where do we come from, where do we go to?, Environ. Sci. Eur., 2018, 30, 6 CrossRef PubMed.
- N. G. Bastús, J. Comenge and V. Puntes, Kinetically Controlled Seeded Growth Synthesis of Citrate-Stabilized Gold Nanoparticles of up to 200 nm: Size Focusing versus Ostwald Ripening, Langmuir, 2011, 27, 11098–11105 CrossRef PubMed.
- J. Singh, T. Dutta, K.-H. Kim, M. Rawat, P. Samddar and P. Kumar, ‘Green’ synthesis of metals and their oxide nanoparticles: applications for environmental remediation, J. Nanobiotechnol., 2018, 16, 84 CrossRef CAS PubMed.
- M. Mishra, M. Sharma, R. Dubey, P. Kumari, V. Ranjan and J. Pandey, Green synthesis interventions of pharmaceutical industries for sustainable development, Curr. Res. Green Sustainable Chem., 2021, 4, 100174 CrossRef CAS.
- J. S. Moodley, S. B. N. Krishna, K. Pillay and P. Govender, Green Synthesis of Metal Nanoparticles for Antimicrobial Activity, IntechOpen, 2020 Search PubMed.
- S. Ahmed, M. Ahmad, B. L. Swami and S. Ikram, A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise, J. Adv. Res., 2016, 7, 17–28 CrossRef CAS PubMed.
- E. Turunc, R. Binzet, I. Gumus, G. Binzet and H. Arslan, Green synthesis of silver and palladium nanoparticles using Lithodora hispidula (Sm.) Griseb.(Boraginaceae) and application to the electrocatalytic reduction of hydrogen peroxide, Mater. Chem. Phys., 2017, 202, 310–319 CrossRef CAS.
- S. S. Sana and L. K. Dogiparthi, Green synthesis of silver nanoparticles using Givotia moluccana leaf extract and evaluation of their antimicrobial activity, Mater. Lett., 2018, 226, 47–51 CrossRef CAS.
- V. Dhand, L. Soumya, S. Bharadwaj, S. Chakra, D. Bhatt and B. Sreedhar, Green synthesis of silver nanoparticles using Coffea arabica seed extract and its antibacterial activity, Mater. Sci. Eng., C, 2016, 58, 36–43 CrossRef CAS PubMed.
- A. Chahardoli, N. Karimi and A. Fattahi, Nigella arvensis leaf extract mediated green synthesis of silver nanoparticles: Their characteristic properties and biological efficacy, Adv. Powder Technol., 2018, 29, 202–210 CrossRef CAS.
- B. Turakhia, P. Turakhia and S. Shah, Green synthesis of zero valent iron nanoparticles from Spinacia oleracea (spinach) and its application in waste water treatment, J. Adv. Res. Appl. Sci., 2018, 5, 46–51 Search PubMed.
- S. C. Guadarrama-Reyes, R. A. Morales-Luckie, V. Sánchez-Mendieta, M. G. González-Pedroza, E. Lara-Carrillo, U. Velazquez-Enriquez, V. Toral-Rizo and R. Scougall-Vilchis, Green Synthesis of Silver Nanoparticles Using Heterotheca inuloides and Its Antimicrobial Activity in Catgut Suture Threads, IntechOpen, 2019 Search PubMed.
- N. S. Alsaiari, F. M. Alzahrani, A. Amari, H. Osman, H. N. Harharah, N. Elboughdiri and M. A. Tahoon, Plant and Microbial Approaches as Green Methods for the Synthesis of Nanomaterials: Synthesis, Applications, and Future Perspectives, Molecules, 2023, 28, 463 CrossRef CAS PubMed.
- R. Gaur, Environmental impact and life cycle analysis of green nanomaterials, Elsevier, 2022, pp. 513–539 Search PubMed.
- S. Ying, Z. Guan, P. C. Ofoegbu, P. Clubb, C. Rico, F. He and J. Hong, Green synthesis of nanoparticles: Current developments and limitations, Environ. Technol. Innovation, 2022, 26, 102336 Search PubMed.
- B. Salieri, D. A. Turner, B. Nowack and R. Hischier, Life cycle assessment of manufactured nanomaterials: Where are we?, NanoImpact, 2018, 10, 108–120 CrossRef.
- E. Parliament, Circular economy: definition, importance and benefits, https://www.europarl.europa.eu/topics/en/article/20151201STO05603/circular-economy-definition-importance-and-benefits, (accessed 08/27, 2024).
- European-Commission, Safe and sustainable by design, https://research-and-innovation.ec.europa.eu/research-area/industrial-research-and-innovation/chemicals-and-advanced-materials/safe-and-sustainable-design_en, (accessed 08/27, 2024).
- European-Commission, 2050 long-term strategy, https://climate.ec.europa.eu/eu-action/climate-strategies-targets/2050-long-term-strategy_en, (accessed 08/24, 2024).
- M. Kryuchkov, J. Adamcik and V. L. Katanaev, Bactericidal and Antiviral Bionic Metalized Nanocoatings, Nanomaterials, 2022, 12, 1868 CrossRef CAS PubMed.
- F. Eshboev, N. Mamadalieva, P. Nazarov, H. Hussain, V. Katanaev, D. Egamberdieva and S. Azimova, Antimicrobial Action Mechanisms of Natural Compounds Isolated from Endophytic Microorganisms, Antibiotics, 2024, 13, 271 CrossRef CAS PubMed.
- T. Joseph, D. Kar Mahapatra, A. Esmaeili, Ł. Piszczyk, M. Hasanin, M. Kattali, J. Haponiuk and S. Thomas, Nanoparticles: Taking a Unique Position in Medicine, Nanomaterials, 2023, 13, 574 CrossRef CAS PubMed.
- V. F. McNeill, Addressing the Global Air Pollution Crisis: Chemistry's Role, Trends Chem., 2019, 1, 5–8 CrossRef CAS.
- European-Commission, European Missions - Restore our Ocean and Waters by 2030, EU Search PubMed.
- OECD, Test No. 202: Daphnia sp. Acute Immobilisation Test, OECD Guidelines for the Testing of Chemicals, Section 2, 2004, DOI:10.1787/9789264069947-en.
- A. Mackevica, L. M. Skjolding, A. Gergs, A. Palmqvist and A. Baun, Chronic toxicity of silver nanoparticles to Daphnia magna under different feeding conditions, Aquat. Toxicol., 2015, 161, 10–16 CrossRef CAS PubMed.
- J. Hou, Y. Zhou, C. Wang, S. Li and X. Wang, Toxic Effects and Molecular Mechanism of Different Types of Silver Nanoparticles to the Aquatic Crustacean Daphnia magna, Environ. Sci. Technol., 2017, 51, 12868–12878 CrossRef CAS PubMed.
- Z. Liu, C. R. Malinowski and M. S. Sepúlveda, Emerging trends in nanoparticle toxicity and the significance of using Daphnia as a model organism, Chemosphere, 2022, 291, 132941 CrossRef CAS PubMed.
- P. A. Holden, F. Klaessig, R. F. Turco, J. H. Priester, C. M. Rico, H. Avila-Arias, M. Mortimer, K. Pacpaco and J. L. Gardea-Torresdey, Evaluation of Exposure Concentrations Used in Assessing Manufactured Nanomaterial Environmental Hazards: Are They Relevant?, Environ. Sci. Technol., 2014, 48, 10541–10551 CrossRef CAS PubMed.
- OECD, Test No. 222: Earthworm Reproduction Test (Eisenia fetida/Eisenia andrei), OECD Guidelines for the Testing of Chemicals, Section 2, 2016, DOI:10.1787/9789264264496-en.
- S. V. A. C. Samarasinghe, K. Krishnan, R. J. Aitken, R. Naidu and M. Megharaj, Chronic effects of TiO2 and ZnO nanoparticles to earthworm Eisenia fetida, Environ. Chem. Ecotoxicol., 2023, 5, 129–134 CrossRef CAS.
- S. Peixoto, R. G. Morgado, M. Prodana, D. N. Cardoso, C. Malheiro, J. Neves, C. Santos, Z. Khodaparast, M. D. Pavlaki, S. Rodrigues, S. M. Rodrigues, I. Henriques and S. Loureiro, Responses of soil microbiome to copper-based materials (nano and bulk) for agricultural applications: An indoor-mesocosm experiment, NanoImpact, 2024, 34, 100506 CrossRef CAS PubMed.
- W. A. Shoults-Wilson, O. I. Zhurbich, D. H. McNear, O. V. Tsyusko, P. M. Bertsch and J. M. Unrine, Evidence for avoidance of Ag nanoparticles by earthworms (Eisenia fetida), Ecotoxicology, 2011, 20, 385–396 CrossRef CAS PubMed.
- AMI2030, Advanced Materials 2030 Initiative, https://www.ami2030.eu/, (accessed 08/27, 2024).
- O. A. Rugaie, A. A. H. Abdellatif, M. A. El-Mokhtar, M. A. Sabet, A. Abdelfattah, M. Alsharidah, M. Aldubaib, H. Barakat, S. M. Abudoleh, K. A. Al-Regaiey and H. M. Tawfeek, Retardation of Bacterial Biofilm Formation by Coating Urinary Catheters with Metal Nanoparticle-Stabilized Polymers, Microorganisms, 2022, 10, 1297 CrossRef CAS PubMed.
- K. E. Rudd, S. C. Johnson, K. M. Agesa, K. A. Shackelford, D. Tsoi, D. R. Kievlan, D. V. Colombara, K. S. Ikuta, N. Kissoon, S. Finfer, C. Fleischmann-Struzek, F. R. Machado, K. K. Reinhart, K. Rowan, C. W. Seymour, R. S. Watson, T. E. West, F. Marinho, S. I. Hay, R. Lozano, A. D. Lopez, D. C. Angus, C. J. L. Murray and M. Naghavi, Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study, Lancet, 2020, 395, 200–211 CrossRef PubMed.
- J. Nandhini, E. Karthikeyan and S. Rajeshkumar, Nanomaterials for wound healing: Current status and futuristic frontier, Bio/Technology, 2024, 6, 26–45 CAS.
- G. A. Seisenbaeva, K. Fromell, V. V. Vinogradov, A. N. Terekhov, A. V. Pakhomov, B. Nilsson, K. N. Ekdahl, V. V. Vinogradov and V. G. Kessler, Dispersion of TiO2 nanoparticles improves burn wound healing and tissue regeneration through specific interaction with blood serum proteins, Sci. Rep., 2017, 7, 15448 CrossRef PubMed.
- Q. Qiu, S. Chen, Y. Li, Y. Yang, H. Zhang, Z. Quan, X. Qin, R. Wang and J. Yu, Functional nanofibers embedded into textiles for durable antibacterial properties, Chem. Eng. J., 2020, 384, 123241 CrossRef CAS.
- H. Rabiei, S. Farhang Dehghan, M. Montazer, S. S. Khaloo and A. G. Koozekonan, UV protection properties of workwear fabrics coated with TiO2 nanoparticles, Front. Public Health, 2022, 10, 929095 CrossRef PubMed.
- S. Barage, J. Lakkakula, A. Sharma, A. Roy, S. Alghamdi, M. Almehmadi, M. J. Hossain, M. Allahyani and O. Abdulaziz, Nanomaterial in Food Packaging: A Comprehensive Review, J. Nanomater., 2022, 2022, 1–12 Search PubMed.
- I. S. Arvanitoyannis and L. A. Bosnea, Recycling of Polymeric Materials Used for Food Packaging: Current Status and Perspectives, Food Rev. Int., 2001, 17, 291–346 CrossRef CAS.
- V. Venture, Voyager Venture - Smart Labels, https://www.voyagerventure.com/, (accessed 08/27, 2024).
- U. Nations, 2024 Revision of World Population Prospects, https://population.un.org/wpp/, (accessed 08/27, 2024).
- Y. Kang, S. Khan and X. Ma, Climate change impacts on crop yield, crop water productivity and food security – A review, Prog. Nat. Sci., 2009, 19, 1665–1674 CrossRef.
- C. Rathbone and S. Ullah, Ammonia emissions from nitrogen fertilised agricultural soils: controlling factors and solutions for emission reduction, Environ. Chem., 2023, 21, EN23010 CrossRef.
- K. Richardson, W. Steffen, W. Lucht, J. Bendtsen, S. E. Cornell, J. F. Donges, M. Drüke, I. Fetzer, G. Bala, W. Von Bloh, G. Feulner, S. Fiedler, D. Gerten, T. Gleeson, M. Hofmann, W. Huiskamp, M. Kummu, C. Mohan, D. Nogués-Bravo, S. Petri, M. Porkka, S. Rahmstorf, S. Schaphoff, K. Thonicke, A. Tobian, V. Virkki, L. Wang-Erlandsson, L. Weber and J. Rockström, Earth beyond six of nine planetary boundaries, Sci. Adv., 2023, 9(37), 1–16 Search PubMed.
- P. Zhang, Z. Guo, S. Ullah, G. Melagraki, A. Afantitis and I. Lynch, Nanotechnology and artificial intelligence to enable sustainable and precision agriculture, Nat. Plants, 2021, 7, 864–876 CrossRef PubMed.
- M. Abbasi Khalaki, M. Moameri, B. Asgari Lajayer and T. Astatkie, Influence of nano-priming on seed germination and plant growth of forage and medicinal plants, Plant Growth Regul., 2021, 93, 13–28 CrossRef CAS.
- A. Homez-Jara, H. Cardenas-Roa, M. Montealegre, L.-T. Lim, M. G. Corradini, H. A. Váquiro-Herrera and A. Sandoval-Aldana, Postharvest Treatments of Hass Avocado (Persea americana Mill.) and Estimation of Its Quality Using Hyperspectral Imaging (HSI), ACS Food Sci. Technol., 2023, 3, 932–944 CrossRef CAS.
- F. R. Cassee, E. A. J. Bleeker, C. Durand, T. Exner, A. Falk, S. Friedrichs, E. Heunisch, M. Himly, S. Hofer, N. Hofstätter, D. Hristozov, P. Nymark, A. Pohl, L. G. Soeteman-Hernández, B. Suarez-Merino, E. Valsami-Jones and M. Groenewold, Roadmap towards safe and sustainable advanced and innovative materials. (Outlook for 2024-2030), Comput. Struct. Biotechnol. J., 2024, 25, 105–126 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2025 |