 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A perspective on the potential impact of microplastics and nanoplastics on the human central nervous system
Kimia
Moiniafshari
 a,
Alessandra
Zanut
a,
Alessandra
Zanut
 a,
Andrea
Tapparo
a,
Andrea
Tapparo
 a,
Paolo
Pastore
a,
Paolo
Pastore
 a,
Sara
Bogialli
a,
Sara
Bogialli
 a and
Fazel
Abdolahpur Monikh
a and
Fazel
Abdolahpur Monikh
 *ab
*ab
aDepartment of Chemical Sciences, University of Padova, Padua, Italy. E-mail: fazel.monikh@unipd.it
bInstitute for Nanomaterials, Advanced Technologies, and Innovation, Technical University of Liberec, Bendlova 1409/7, 460 01, Liberec, Czech Republic
First published on 29th January 2025
Abstract
Humans are constantly exposed to microplastics and nanoplastics (MNPs). Although significant gaps remain in our understanding of their adverse effects on human health, it is increasingly evident that MNPs can penetrate physiological barriers and accumulate in various locations within the human body. Analytical limitations in tracking and measuring nanoplastics in physiological media may persist for several years before we can accurately detect these particles in the human body and establish a clear link between exposure to them and associated hazards. In addition to the few studies that have emerged recently, our knowledge of chemicals with properties similar to those of MNPs, as well as other types of nanomaterials, suggests that MNPs may cross the blood–brain barrier (BBB) and potentially induce damage to the human central nervous system. Here, we provide an overview of the limited number of studies available on this topic and present a perspective on the potential pathways through which MNPs may penetrate the BBB. We also discuss the main mechanisms by which MNPs could potentially impact the central nervous system (CNS), with a focus on neurodegenerative diseases such as Alzheimer's disease (AD), Parkinson's disease (PD), multiple sclerosis (MS), and amyotrophic lateral sclerosis (ALS). This information could contribute to the development of tailored studies exploring the negative effects of MNPs on the CNS.
Environmental significanceThe pervasive presence of microplastics and nanoplastics (MNPs) in the environment poses an emerging threat to both ecosystems and human health. While significant research has been conducted on MNP contamination in aquatic environments, their impact on the central nervous system (CNS) and their role in exacerbating neurodegenerative diseases such as Alzheimer's and Parkinson's remains underexplored. This perspective highlights the potential pathways through which MNPs may cross the blood–brain barrier (BBB), inducing oxidative stress and neuroinflammation, that can lead to neurodegenerative disease. By addressing these previously overlooked environmental pollutants, this work aims to inspire targeted studies that can inform better regulation of plastic waste, ultimately protecting human neurological health in an increasingly plastic-laden world. Understanding the mechanistic links between environmental exposure and CNS disorders could lead to transformative policy changes and preventive measures to safeguard future generations. |
1. Introduction
The increase in plastic products has led to a global environmental challenge with far-reaching consequences. Plastic degradation (by physical, chemical, or biological processes) in the environment releases a staggering amount of micro- and nanoplastics (MNPs).1 Microplastics are plastic particles and fragments with a size smaller than 5 mm,2 while nanoplastics are generally defined as particles with a size smaller than 1 μm.3 Note that some definitions consider nanoplastics to be particles smaller than 100 nm. This broad definition itself may contribute to the challenges associated with investigating and handling nanoplastics.4 The most common plastic debris in the environment is reported to be polyethene, propylene, polystyrene, and polyvinyl chloride,5,6 although that differs from location to location, which are found mainly accumulating in aquatic ecosystems. In recent decades, the exposure of humans and other organisms to MNPs has increased significantly due to industrial production, application, and mismanagement of plastic waste.7Organisms are exposed to MNPs mainly through inhalation8 and ingestion9 (Fig. 1). Air-borne exposure of humans can occur in both indoor and outdoor environments, although the concentration of MNPs and the flow are different.10 The outdoor air typically has more flow and circulation, resulting in a lower MNP concentration. In contrast, indoor environments, where infants and children spend more time, have a higher risk of exposure due to the expected higher concentrations of MNP.8,9 Water-born exposure is another significant pathway for MNs. Humans can be exposed to MNPs by drinking tap water, consuming beverages from single-use plastic bottles, or even by drinking tea10,11 soft drinks12 or beer. Furthermore, several food sources including seafood,13 sugar,14 honey, and salt,10 are reported to be sources for intake of MNPs. The increase in exposure routes for MNPs is particularly concerning for children, as their developing bodies are more vulnerable to the potential health impacts of MNPs.15
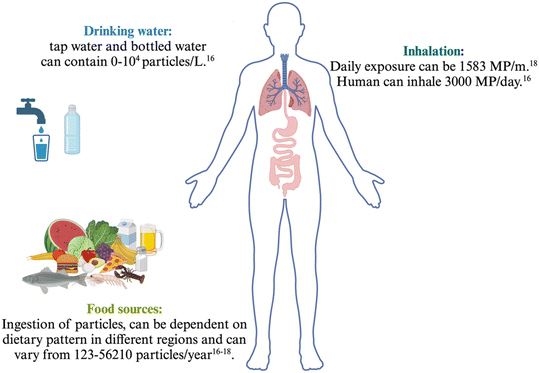 | ||
| Fig. 1 Exposure to MNPs through the common routes. According to the WHO and EFSA report, human can be exposed to different amounts of MNPs in their daily life through various routes, particularly by ingestion and inhalation.16–18 | ||
The effects of MNPs on humans are not well understood due to limitations in human tissue sampling and analytical techniques for in situ characterization of these particles.19 In the human body, the nervous system is a complex network containing a large number of neurons that regulate physiological activities.20 Although studies on the effect of exposure to MNPs on the nervous system are limited, MNPs are potentially able to cross physiological barriers, e.g. the blood–brain barrier (BBB).21 Translocation and accumulation of MNPs in the brain are expected to cause various forms of damage, mainly through induction of oxidative stress22 leading to an increase in vulnerability to neurodegenerative disease. Although not yet reported, there appears to be a potential link between increased exposure to MNPs and the rising number of reports on neurodegenerative disorders. This may be important as the data reported by the World Health Organisation (WHO) indicated an increase of up to 18% in the prevalence of neurodegenerative disorders which are known as the seventh cause of death23,24 (Fig. 2).
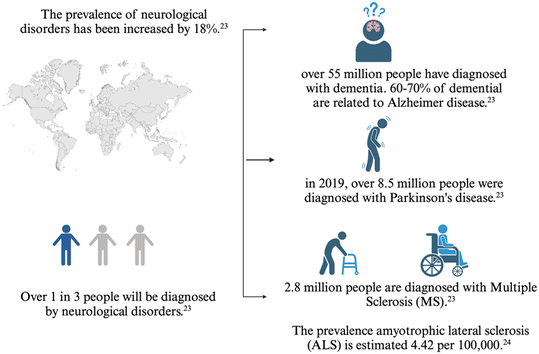 | ||
| Fig. 2 Schematic describing the incidence of neurodegenerative diseases in the population. According to WHO, the prevalence of neurological disorders has risen within the past years. Among the diagnosed neurodegenerative disorders, Alzheimer's disease is known as the most prevalent disease, Parkinson's disease, multiple sclerosis and amyotrophic lateral sclerosis are the other prevalent diseases, repectively.23,24 | ||
From the available body of evidence on the toxicity of MNPs, it is apparent that, like other nanomaterials, the effects of MNPs are largely driven by their oxidative stress-inducing properties. The increase in oxidative stress can affect the structure and function of neural cells, potentially contributing to the development of neurodegenerative diseases25,26 and the disruption of neurotransmitters.27 This disruption is particularly concerning regarding acetylcholine (ACh) and dopamine28 which are the leading causes of neurodegenerative diseases such as Alzheimer's disease (AD) and Parkinson's disease (PD).29 For example, Gou et al.30 demonstrated that exposure to MNPs can increase the aggregation of amyloid-β leading to Alzheimer's. Liang et al.,31 reported that MNPs can increase PD by increasing inflammation and neural cell dysfunction. Moreover, Cao et al.,32 demonstrated that MNPs can affect neurodevelopment, behavioral abnormalities, and motor neuron function by increasing oxidative stress.
Linking exposure to hazards from MNPs remains a challenge at this stage of scientific progress.
After internalisation, the biological fate of MNPs in the human body, and consequently, their effects are dictated by the physicochemical characteristics of the particle such as particle size, charge, shape, and chemical composition.33 However, to date, there has been no straightforward and systematic investigation showing that the presence of MNPs can drive the hazard in the brain, depending on their characteristics.
This perspective aims to provide an overview of the potential entry of MNPs into the brain and link the exposure and the particle physicochemical properties to neurodegenerative diseases such as AD, PD, and other neurodegenerative diseases. Additionally, it offers insights into the mechanisms of MNPs' effects on the aforementioned diseases. Finally, the perspective highlights the research needed to understand the connection between MNP exposure and the development of these diseases. This perspective excludes biodegradable polymeric materials such as polyvinyl alcohol, polyethene glycol, polyvinylpyrrolidone, and polyphenylene sulfide, and focuses primarily on the most common polymer types (Table 1).
| Polymer type | Particle size | Mechanism of toxicity | Neurodegenerative diseases | Ref. |
|---|---|---|---|---|
| Polystyrene | 30–50 nm | Microglia activation, neural suppression, and decrease in neural development | Memory loss, cognitive decline | 34, 35 |
| 25, 50 nm | Dopaminergic neuron degeneration, α-synuclein aggregation | PD | 36 | |
| 30 nm | Oxidative stress, TDP-43 aggregation | ALS | 37 | |
| 25, 50 nm | Decrease in myeline protein, decrease myeline thickness, increase cell apoptosis | MS | 38 | |
| 80, 100 nm | Increase in amyloid-β aggregation | AD | 39, 40 | |
| Polyethylene | <20–50 μm | Oxidative stress, amyloid plaque aggregation | Cognitive decline, AD | 41, 42 |
| Polypropylene | 10–30 nm | Oxidative stress, AChE activity reduction, α-synuclein aggregation | Cognitive decline, memory deficit, AD, PD | 43, 44 |
| Polyvinyl chloride (PVC) | 100 nm | Increase in α-synuclein aggregation | PD | 45 |
2. Mechanisms of MNP entry into the brain
The mechanism through which MNPs enter the brain primarily involves their penetration through the BBB46 (Fig. 3). More research is still needed to fully understand these pathways for MNPs.47,48 In general, internalization of MNPs can occur via two main mechanisms: passive penetration and active endocytosis,49 which includes both pinocytosis and phagocytosis.49 Endocytosis can occur through clathrin-mediated pathways (typically for small nanoplastics) and caveolin-mediated pathways (usually for larger particles).50 The mechanisms of MNP penetration are influenced by their physicochemical properties, such as particle size, shape, chemical composition, and surface charge.51,52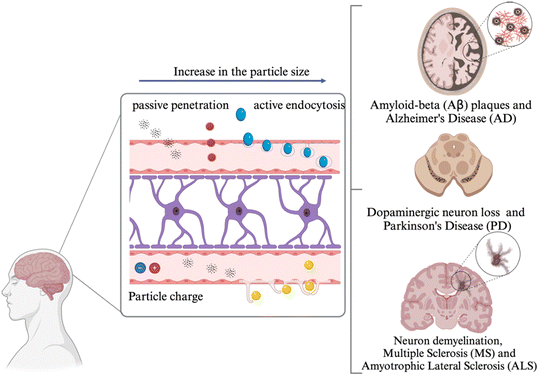 | ||
| Fig. 3 Consequence of MNP penetration through BBB. Particles with smaller size, can pass the BBB more likely through passive penetration. As the particle size increases, particles are more likely to penetrate through active endocytosis. Particle charge can also affect their penetration through tight junction impairment and damage to cells due to their ability have electrostatic interaction with BBB membrane along with impairment in tight junctions.53–55 | ||
To date, very few studies have investigated how the size of MNPs influences their penetration through the BBB. This is due to the complexity of conducting such experiments, which require MNPs of different sizes that are detectable in the complex matrices of biological media.56 Zhou et al.,53 showed that a higher number of smaller-size PS (100 nm) could enter the brain of zebrafish embryos compared to their larger counterparts (500 and 1000 nm), consequently, causing more neurotoxicity. This study used fluorescent-labelled PS particles, utilizing fluorescence as a tracer to facilitate the detection of their penetration into the brain. However, it is worth mentioning that the use of fluorescent-labelled particles to represent nanoplastics has fallen out of favour due to the potential release of dyes from the particles,56 which can lead to inaccurate results and conclusions regarding particle uptake. Thus, strong evidence for the penetration of MNPs through the BBB based on particle size remains questionable. Yin et al.57 used adverse effect assessments to confirm that smaller nanoplastics can penetrate the brain more readily than microplastics. Their findings suggested that the smaller particles appeared to be more toxic.
Nevertheless, medical and environmental studies have already used different types of metallic nanomaterials to demonstrate that particle size plays a crucial role in the ability of nanomaterials to cross the BBB.52 For example, the study by Liu et al.58 compared different particle sizes of silica nanomaterials for their ability to pass through cerebral endothelial cells in mice. The results indicated that smaller particles (25 nm) were taken up by the brain more efficiently than the 50 nm and 100 nm particles. It has been suggested that large-size nanomaterials are more likely to penetrate through active routes, including pinocytosis (micropinocytosis) and phagocytosis.50,59 Although the transferability of this knowledge to MNPs is questionable due to differences in chemistry and particle density, factors that significantly influence cellular penetration, these studies provide a basic understanding and a starting point for conducting similar experiments with MNPs.
The shape of MNPs can also influence their penetration through the BBB into the brain as it can modulate their binding to the cell. Da Silva-Candal et al.60 investigated the toxicity of spherical and rod-shaped PS nanoplastics in mice. They demonstrated that rod-shaped nanoplastics can have greater binding capacity compared to the spherical shape PS MNPs. This finding is further supported by Kolhar et al.,61 who compared nano-rods and nano-spheres and showed a higher accumulation of rod-shaped particles. Much more evidence on the influence of particle shape on their penetration in BBB is available for other nanomaterials. For example, Salatin et al.62 reported that rod-shaped gold (Au) nanomaterials have higher affinity and efficacy for endothelial cells, resulting in greater uptake. Sharp-shaped Au nanomaterials instead, can penetrate the membrane more easily compared to the other shapes, resulting in particle localization. The study by Niaz et al.63 on Au nanomaterials demonstrated that the sharp edges of particles enable them to penetrate and cause damage to the membrane more easily than other shapes, which is also reported for the nano-stars and nano-spike-shaped particles.
An important factor that can influence the penetration of MNPs through the BBB, and that also distinguishes different nanomaterials from one another, is the chemical composition of the particles. A recent study by Abdolahpur Monikh et al.21 showed that nanoplastics composed of PS and PVC can cross the BBB, albeit at low concentrations. The study further highlighted that the presence of a biological corona (proteins and metabolites) significantly modulates the penetration efficiency of nanoplastics across the BBB.21 The formation of the corona on the nanoplastics was dictated by the chemical composition of the particles. It was also reported that PS, PE, PP, and PVC were able to enter the CNS, and PP and PE caused more inflammation.33 The study by Abdolahpur Monikh et al.64 demonstrated that different chemical compositions can have different effects on the behaviour of zebrafish embryos, even at the same particle size. It is worth mentioning that, so far, PS MNPs have been more extensively investigated than other plastic particles. This creates a challenge in understanding how chemical composition may contribute to the biological fate and toxicity of the particles.65
The particle surface charge is another key indicator of the ability of MNPs to penetrate the BBB.27 Negatively charged particles are unlikely to pass easily through the membrane due to electrostatic interaction. Although Ikeda et al.66 showed that ionic imbalance in the cell membrane can lead to direct penetration of negative particles. The study by Chen et al.67 demonstrated that 20 nm negatively charged silica nanomaterials can penetrate the BBB in the zebrafish model. However, the increase in the particle size led to a decrease in penetration. On the other hand, Chirio et al.54 showed that positively charged nanoplastics have a greater ability to penetrate the BBB and localize in cells. Shan et al.55 reported that negatively charged PS (50 nm) could penetrate through BBB due to their ability to affect the tight junctions. It is also confirmed by Grodzicki et al.,68 that negatively charged PS nanoplastics are more likely to accumulate in rodent brains compared to positively charged PS nanoplastics with the same particle size of 50 nm. Another mechanism through which positively charged MNPs can penetrate the BBB is by inducing damage to the cell membrane as a result of impairment of the membrane structure.69 The study by Zhang et al.70 demonstrated that positively charged PS nanoplastics (20–100 nm size) can increase the permeability of the BBB. Although there is some evidence that nanoplastics can pass through the BBB and cause neurodegeneration, there remains a lack of definitive data.71
3. Consequence of MNPs passage into the brain
The consequences of MNPs' presence in the brain are not yet fully understood, as these non-biodegradable materials can undergo biotransformation—such as biological corona formation, polymer swelling, and surface functionalization56 which alters their characteristics and potentially influences their effects. Subsequently, MNPs might damage the structure and function of the brain.72 Studies on brain cells in mice revealed that exposure to MNPs can induce neuron and astrocyte apoptosis while reducing cell viability.73 Moreover, a study on zebrafish embryos showed that MNPs can penetrate and accumulate mainly in lipid-rich regions such as the brain in a dose-dependent manner.74 It is reported that MNP decrease the number of Purkinje cells and mitochondria function in the brain, mainly through an increase in oxidative stress.75–77 This can irreducibly damage neurons and accelerate neurodegenerative processes due to neuron loss and disruption in the balance of neurotransmitters, especially acetylcholine and dopamine, leading to neurodegenerative disease.78 The question to raise is what consequences the presence of MNPs and the associated brain damage might have on the nervous system and what brain diseases could be linked to MNPs. Although it may be challenging to answer or draw definitive conclusions, in this perspective, we review the published results to identify whether any patterns might emerge.3.1. Alzheimer's disease
Alzheimer's disease is a progressive neurodegenerative disease accompanied by memory loss, known as the most common type of dementia.79 AD is characterized by the accumulation of amyloid-beta (Aβ) plaques in the brain and misfolded protein inside neurons80 MNPs have recently been studied for their potential role in exacerbating neurodegenerative conditions such as AD.34 Exposure to nanomaterials has been linked to AD by increasing oxidative stress, inflammation, protein aggregation, and synaptic dysfunction.41,42,81 It has been reported that MNPs accelerate Aβ aggregation52 by changing the secondary structure of the protein30 mainly through oxidative stress, inflammation, and alterations in cellular metabolism.80 Gou et al.30 demonstrated that exposure to PS nanoplastics size 70–150 nm could cause trigger the progression of AD, specifically by inducing Aβ nucleation and aggregation, along with cell membrane damage, increased ROS and Ca2+. Sun et al.39 evaluated the effect of 80 nm PS nanoplastics on mice brains and found that exposure to PS could increase their accumulation in the brain and lead to Aβ aggregation and AD. The study by Paing et al.35 indicated that PS nanoplastics (30–50 nm), can internalize into the microglial cells over astrocytes in vitro and the mice brains, resulting in increased inflammation and cognitive decline. Moreover, Bai et al.,40 confirmed that exposure to 100 nm-sized amino-modified PS nanoplastics in mice can cause brain damage and show AD-like characteristics.Despite the emerging evidence, significant gaps remain in understanding the specific molecular mechanisms through which MNPs contribute to AD.50,80 Given the similarities between MNPs and other nanomaterials, it is reasonable to hypothesize that MNPs may follow pathological pathways similar to those of other metallic nanomaterials which are reported to destabilize protein secondary structures, leading to protein misfolding and amyloid aggregation. One may argue that most of the observed adverse effects associated with metallic nanomaterials are linked to their dissolution and the release of ions, whereas MNPs do not undergo such dissolution. However, this may not be entirely accurate, as there remains a significant gap in knowledge and analytical methods to clearly distinguish between the effects of ionic and particulate forms of metallic nanomaterials. Furthermore, most protocols used to detect the effects of nanomaterials, in general, are based on those developed for chemicals and metallic ions rather than particles. These protocols are predominantly focused on oxidative stress mechanisms, creating the impression that toxicological studies are designed with an “I see what I expect to see” approach, rather than aiming to identify new endpoints specifically associated with the particulate form of nanomaterials. Finally, exposure to MNPs has been linked to increased protein denaturation, fibrillation, and amyloid aggregation, all of which are key contributors to AD progression.
3.2. Parkinson's disease
Parkinson's disease (PD), is another progressive neurodegenerative disorder. This is primarily characterized by the loss of dopaminergic neurons in the “Substantia nigra”, leading to motor impairment and cognitive loss.82 The pathological pathway of PD, includes the accumulation of α-synuclein,43–45,83,84 protein misfolding, and formation of Lewy bodies within neurons, contributing to the degeneration of dopaminergic neurons.85 Similar to AD, nanomaterials can contribute to PD by inducing increased oxidative stress, neuroinflammation, and the aggregation of α-synuclein protein.86 As indicated in the study of Huang et al.87 the exposure of SH-SY5Y cells to 50–100 nm sized PS nanoplastics, resulted in mitochondrial dysfunction and dopaminergic neuron damage. Liang et al.88 showed that exposure of mice to PS and PE microplastics with a particle size of 5 μm caused dysfunction of mitochondrial energy, leading to restriction of the energy reservoir, especially in excitatory neurons, and PD-like disorder. Another way MNPs can cause PD is by increasing dopaminergic neuron degradation and α-synuclein protein aggregation.83 For example, C. elegans was exposed to PS (25 nm) and showed degradation of dopaminergic neurons and α-synuclein protein aggregation.36Despite some studies reporting the neurotoxicity of MNPs, primarily due to oxidative stress, the underlying mechanisms remain unclear. We believe that to better understand the toxicity of MNPs, it is essential to develop new toxicology guidelines, such as those by the OECD. These guidelines should incorporate endpoints beyond those used for conventional chemicals, enabling not only the linkage of exposure to hazard but also a deeper understanding of the mechanisms by which MNPs induce PD and other neurological disorders. It also remains to be investigated whether alterations in gene expression and apoptosis contribute to increased neural cell death, or if changes in neurotransmission pathways play a more significant role in the development of PD.
3.3. Other neurodegenerative diseases
Amyotrophic lateral sclerosis (ALS) is characterized by progressive neuronal degeneration that causes motor dysfunction and cognitive loss.89 The mechanism recognized so far for the development of ALS following exposure to MNPs is an increase in oxidative stress, which may activate inflammatory responses.90 Exposure to MNP can increase the possibility of ALS from the level of DNA and gene expression91 and increased oxidative stress.92 Sun et al.37 exposed TDP-43 cells to PS (20 nm) and reported damage in motor neurons. A similar result was shown by Chen et al.,93 where rodent exposure to PS with a particle size of 50 nm could increase neural injury and cognitive decline that could be linked to ALS.Multiple sclerosis is an autoimmune and inflammatory disease characterized by neural demyelination and sensory, motor, and cognitive decline.94 Exposure to MNPs can increase the risk of MS, in a similar way to other neurodegenerative diseases.95 The results from de Oliveira et al.96 demonstrated that patients diagnosed with MS, had higher serum metallic nanomaterials in their blood serum, suggesting that the increase in nanomaterials can increase the risk of developing MS. Zhang et al.38 evaluated the effect of exposure to PS (25 and 50 nm) in rats and the results indicated that the exposure reduced myelin basic proteins, myelin thickness, and myelin formation in the fetal brain which are the leading cause for MS. Similarly, the study on zebrafish conducted by Aliakbarzadeh et al.97 indicated that exposure to PS with a particle size of 57.7 nm caused neural cell damage in the brain and a decrease in the number of myelinated axons.
Research on other types of nanomaterials has also revealed similar neurotoxic effects that might be transferable to MNPs. For example, studies involving silver nanomaterials have shown that they can induce inflammation in neuronal cells, contributing to neurodegeneration.98,99 Similarly, titanium dioxide nanomaterials have demonstrated the ability to penetrate the BBB and trigger neuroinflammatory responses, leading to neuronal damage.100,101 These findings from different nanomaterials studies suggest commonalities in the mechanisms by which various particles can lead to different neurodegenerative diseases. This reinforces the idea that there are potential risks posed by MNPs, given their similar physicochemical properties and biological interactions.
One important factor in inducing neurodegenerative diseases as a result of exposure to MNPs, as well as other nanomaterials is the duration of exposure. This factor must be carefully reviewed and incorporated into the new guidelines being developed for MNP toxicity testing. Table 2 summarizes the key findings from recent animal studies investigating the effects of MNPs on neural tissues and neurodegenerative diseases, highlighting that the duration of exposure plays a critical role in the neurotoxic effects of MNPs. Studies with longer exposure periods, such as those by Bai et al.40 (15 weeks), Liang et al.31 (28 days), and Aliakbarzadeh et al.97 (45 days), revealed more pronounced neurotoxic outcomes, including brain damage, mitochondrial dysfunction, and neurodegeneration. Shorter exposure periods, such as the 7-day exposure in Sun et al.39 and the 5-day exposure in Zhou et al.53 still demonstrated significant neurotoxic effects, but with varying degrees of impact depending on the particle size and administration route. Overall, these findings suggest that extended exposure to MNPs increases the likelihood and severity of neurodegenerative effects, highlighting the importance of considering exposure duration in the development of new toxicology guidelines for MNPs.
| MNP type and size | Exposure duration and pathways | Mechanisms of toxicity | Ref. |
|---|---|---|---|
| PS nanoplastic (100 nm, 500 nm, 1000 nm) | 120 h (5 days) exposure to10 mg L−1 PS nanoplastic | The smaller sized PS nanoplastic could penetrate the BBB in zebrafish larvae easier and cause more neurotoxicity | 53 |
| PS nanoplastics (200 nm) | 100 μg per animal weight, administered through the jugular vein | Rod-shaped nanoplastics demonstrated greater binding capacity in the mice brain compared to spherical-shaped PS nanoplastics | 60 |
| PS nanoplastic (50 nm) | 28 days oral exposure to PS 0.25–250 mg kg−1 body weight (BW) PS nanoplastics | The exposure outcome was mitochondrial dysfunction, energy restriction in excitatory neurons, and PD like disorders in mice | 31 |
| PS microplastics (5 μm) | 6 weeks exposure to 1, 10 and 100 mg L−1 | The chiken exposure to PS microplastics impaired tight junction proteins expression and reduced number of Purkinje cells, resulting in neurotoxicity | 76 |
| PS nanoplastics (80 nm) | 7 days 50 mg per bodyweight intranasal administration (INA), gastric feeding (GF) and intratracheal instillation (ITI) exposure to PS nanoplastics | Exposure increased particle accumulation in the mice brain and led to Aβ aggregation, a cause for AD | 39 |
| Amino-modified PS nanoplastics (100 nm) | 15 weeks exposure to oral 40 mg kg−1 body weight of PS nanoplastics | Mice exposure caused brain damage and characteristics of AD | 40 |
| PS nanoplastics (20–80 nm) | 45 days of PS nanoplastics 0.1, 1, 10, and 100 μg L−1 | The exposure caused neural cell damage and a decrease in myelinated axons in zebrafish | 97 |
Perspective and future directions
The threat MNPs pose to neurodegenerative diseases highlights the deep interconnectedness between human health and environmental well-being. The consequences of persistent plastic pollution extend far beyond its visible impacts on the environment; they may be quietly undermining brain health, with long-lasting and devastating repercussions. Most of the investigated effects of MNPs are based on oxidative stress, which is attributed to two main factors: 1) the research protocols commonly used to study the adverse effects of MNPs are primarily centred around oxidative stress, even though the aims and mechanisms of the studies may differ; and 2) it is well-established that the effects of MNPs, like other nanomaterials, are largely driven by their oxidative stress-inducing properties. Exposure to MNPs can affect the CNS both structurally and functionally, which may serve as a predictor of neurodegenerative diseases and neurodevelopmental disorders at all stages of life. Available evidence confirms that MNPs impact development, growth, and behaviour.There are several mechanisms by which MNPs can adversely affect neural health, mainly dependent on the dose and duration of exposure. By crossing biological barriers and accumulating in tissue, MNPs increase oxidative stress and trigger immune response. This leads to higher levels of inflammatory mediators and increased gene expression of inhibitory proteins, while the expression and function of regulatory proteins decrease. These effects are associated with increased lipid peroxidation resulting in DNA damage, the accumulation of misfolded proteins, activation of apoptosis pathways and neuroinflammation. Furthermore, MNPs may reduce the expression of neurotransmitter-related genes and decrease the activity of antioxidant enzymes, even if the expression of proteins involved in oxidative stress increases in different regions of the brain and neural cells. This can lead to significant disruption in the expression of proteins crucial for neural development, which can regulate the structure and plasticity of the CNS. Considering the function and potential effects on neurodegenerative disease and neurodevelopmental disorders, MNPs can decrease important genes involved in cognition, which is the leading cause of cognitive decline.
The mechanisms by which MNPs induce oxidative stress and inflammatory responses, and impair myelin formation align closely with those observed for other nanomaterials like Ag and TiO2. This convergence of evidence underscores the urgent need for comprehensive investigations into the neurotoxic effects of MNPs. Establishing causative links between exposure and disease progression will be vital for developing effective preventive strategies and interventions to mitigate the health risks posed by MNPs in our increasingly plastic-laden environment. More studies are needed to clarify dose–response relationships, the long-term consequences of chronic exposure, and the differential effects of various plastic types and additives. Understanding precisely how MNPs harm the brain will be crucial in developing effective interventions.
While MNPs have been hypothesized to affect the structure and function of the neurons, there is a lack of extensive studies confirming this effect. Understanding how MNPs may disrupt this complex brain network is crucial, as it could have far-reaching health implications. Evidence for the uptake of spherical MNPs by cells currently relies on indirect assumptions rather than definitive visual or experimental proof. Developing methods to directly track and visualize the uptake of spherical MNPs is necessary to fully understand their cellular interactions and potential toxicity. Research is needed to determine if specific antioxidants can protect against MNP-induced neurotoxicity and the optimal antioxidant strategies for mitigating these risks. Additionally, exploring how different plastic compositions and particle shapes affect their ability to cross the BBB will provide a more comprehensive understanding of the potential health risks associated with MNPs.
Data availability
The data supporting this article has been included in the article.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work received financial support the Project DISC_ASSEGNIBIRD2222_01 and FSE-REACT-EU, PON Ricerca e Innovazione 2014–2020 DM 1062/202, Cod: 19-G-12549-3.References
- L. G. Barboza, A. D. Vethaak, B. R. Lavorante, A. K. Lundebye and L. Guilhermino, Marine microplastic debris: An emerging issue for food security, food safety and human health, Mar. Pollut. Bull., 2018, 133, 336–348 CrossRef CAS PubMed.
- B. Hou, F. Wang, T. Liu and Z. Wang, Reproductive toxicity of polystyrene microplastics: In vivo experimental study on testicular toxicity in mice, J. Hazard. Mater., 2021, 405, 124028 CrossRef CAS PubMed.
- B. Nguyen, D. Claveau-Mallet, L. M. Hernandez, E. G. Xu, J. M. Farner and N. Tufenkji, Separation and analysis of microplastics and nanoplastics in complex environmental samples, Acc. Chem. Res., 2019, 52(4), 858–866 CrossRef CAS PubMed.
- J. Gigault, H. El Hadri, B. Nguyen, B. Grassl, L. Rowenczyk, N. Tufenkji, S. Feng and M. Wiesner, Nanoplastics are neither microplastics nor engineered nanoparticles, Nat. Nanotechnol., 2021, 16(5), 501–517 CrossRef CAS PubMed.
- V. V. Annenkov, E. N. Danilovtseva, S. N. Zelinskiy and V. A. Pal'shin, Submicro-and nanoplastics: How much can be expected in water bodies?, Environ. Pollut., 2021, 278, 116910 CrossRef CAS PubMed.
- S. Rist and N. B. Hartmann, Aquatic ecotoxicity of microplastics and nanoplastics: lessons learned from engineered nanomaterials, Freshwater microplastics: Emerging environmental contaminants?, 2018, pp. 25–49 Search PubMed.
- J. Lojk, L. Babič, P. Sušjan, V. B. Bregar, M. Pavlin, I. Hafner-Bratkovič and P. Veranič, Analysis of the direct and indirect effects of nanoparticle exposure on microglial and neuronal cells in vitro, Int. J. Mol. Sci., 2020, 21(19), 7030 CrossRef CAS PubMed.
- A. Facciolà, G. Visalli, M. Pruiti Ciarello and A. Di Pietro, Newly emerging airborne pollutants: current knowledge of health impact of micro and nanoplastics, Int. J. Environ. Res. Public Health, 2021, 18(6), 2997 CrossRef PubMed.
- M. B. Paul, V. Stock, J. Cara-Carmona, E. Lisicki, S. Shopova, V. Fessard, A. Braeuning, H. Sieg and L. Böhmert, Micro-and nanoplastics–current state of knowledge with the focus on oral uptake and toxicity, Nanoscale Adv., 2020, 2(10), 4350–4367 RSC.
- M. Kosuth, S. A. Mason and E. V. Wattenberg, Anthropogenic contamination of tap water, beer, and sea salt, PLoS One, 2018, 13(4), e0194970 CrossRef PubMed.
- E. Dube and G. E. Okuthe, Plastics and Micro/Nano-Plastics (Mnps) in the environment: occurrence, impact, and toxicity, Int. J. Environ. Res. Public Health, 2023, 20(17), 6667 CrossRef CAS PubMed.
- M. F. Diaz-Basantes, J. A. Conesa and A. Fullana, Microplastics in honey, beer, milk and refreshments in Ecuador as emerging contaminants, Sustainability, 2020, 12(14), 5514 CrossRef.
- U. Anand, S. Dey, E. Bontempi, S. Ducoli, A. D. Vethaak, A. Dey and S. Federici, Biotechnological methods to remove microplastics: a review, Environ. Chem. Lett., 2023, 21(3), 1787–1810 CrossRef CAS PubMed.
- S. Afrin, M. M. Rahman, M. N. Hossain, M. K. Uddin and G. Malafaia, Are there plastic particles in my sugar? A pioneering study on the characterization of microplastics in commercial sugars and risk assessment, Sci. Total Environ., 2022, 837, 155849 CrossRef CAS PubMed.
- H. K. Ageel, S. Harrad and M. A. Abdallah, Occurrence, human exposure, and risk of microplastics in the indoor environment, Environ. Sci.: Processes Impacts, 2022, 24(1), 17–31 RSC.
- https://www.who.int/publications/i/item/9789240054608 .
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), Statement on the presence of microplastics and nanoplastics in food, with particular focus on seafood, EFSA J., 2016, 14(6), 4501 Search PubMed.
- A. Lusher, P. Hollman and J. Mendoza-Hill, Microplastics in fisheries and aquaculture: status of knowledge on their occurrence and implications for aquatic organisms and food safety, FAO, 2017 Search PubMed.
- Y. Feng, C. Tu, R. Li, D. Wu, J. Yang, Y. Xia, W. J. Peijnenburg and Y. Luo, A systematic review of the impacts of exposure to micro-and nano-plastics on human tissue accumulation and health, Eco-Environ. Health, 2023, 2(4), 195–207 CrossRef.
- A. M. Sousa, K. A. Meyer, G. Santpere, F. O. Gulden and N. Sestan, Evolution of the human nervous system function, structure, and development, Cell, 2017, 170(2), 226–247 CrossRef CAS PubMed.
- F. A. Monikh, Š. Lehtonen, J. Kekäläinen, I. Karkossa, S. Auriola, K. Schubert, A. Zanut, S. Peltonen, J. Niskanen, M. Bandekar and M. von Bergen, Biotransformation of nanoplastics in human plasma and their permeation through a model in vitro blood-brain barrier: An in-depth quantitative analysis, Nano Today, 2024, 59, 102466 CrossRef.
- M. Panizzolo, V. H. Martins, F. Ghelli, G. Squillacioti, V. Bellisario, G. Garzaro, D. Bosio, N. Colombi, R. Bono and E. Bergamaschi, Biomarkers of oxidative stress, inflammation, and genotoxicity to assess exposure to micro-and nanoplastics. A literature review, Ecotoxicol. Environ. Saf., 2023, 267, 115645 CrossRef CAS PubMed.
- https://www.who.int/news/item/14-03-2024-over-1-in-3-people-affected-by-neurological-conditions--the-leading-cause-of-illness-and-disability-worldwide .
- K. A. Ravichandran and M. T. Heneka, Inflammasome activation in neurodegenerative diseases, Essays Biochem., 2021, 65(7), 885–904 CrossRef CAS.
- R. Martin-Folgar, M. C. González-Caballero, M. Torres-Ruiz, A. I. Cañas-Portilla, M. de Alba González, I. Liste and M. Morales, Molecular effects of polystyrene nanoplastics on human neural stem cells, PLoS One, 2024, 19(1), e0295816 CrossRef CAS PubMed.
- K. S. Hwang, Y. Son, S. S. Kim, D. S. Shin, S. H. Lim, J. Y. Yang, H. N. Jeong, B. H. Lee and M. A. Bae, Size-dependent effects of polystyrene nanoparticles (PS-NPs) on behaviors and endogenous neurochemicals in zebrafish larvae, Int. J. Mol. Sci., 2022, 23(18), 10682 CrossRef CAS.
- A. J. Gan, K. F. Chia, C. L. Lim, B. K. Tan, S. F. Wong, S. M. Chye, C. O. Leong and R. Y. Koh, Neurotoxicity of nanoplastics: A review, F1000Research., 2024, 13, 793 Search PubMed.
- F. Xiong, J. Liu, K. Xu, J. Huang, D. Wang, F. Li and R. Sun, Microplastics induce neurotoxicity in aquatic animals at environmentally realistic concentrations: A meta-analysis, Environ. Pollut., 2023, 318, 120939 CrossRef CAS PubMed.
- H. J. Heusinkveld, T. Wahle, A. Campbell, R. H. Westerink, L. Tran, H. Johnston and R. P. Schins, Neurodegenerative and neurological disorders by small inhaled particles, Neurotoxicology, 2016, 56, 94–106 CrossRef CAS PubMed.
- X. Gou, Y. Fu, J. Li, J. Xiang, M. Yang and Y. Zhang, Impact of nanoplastics on Alzheimer's disease: Enhanced amyloid-β peptide aggregation and augmented neurotoxicity, J. Hazard. Mater., 2024, 465, 133518 CrossRef CAS PubMed.
- B. Liang, Y. Huang, Y. Zhong, Z. Li, R. Ye, B. Wang, B. Zhang, H. Meng, X. Lin, J. Du and M. Hu, Brain single-nucleus transcriptomics highlights that polystyrene nanoplastics potentially induce Parkinson's disease-like neurodegeneration by causing energy metabolism disorders in mice, J. Hazard. Mater., 2022, 430, 128459 CrossRef CAS PubMed.
- X. Cao, W. Xie, M. Feng, J. Chen, J. Zhang, J. Luo and Y. Wang, Nanoplastic Exposure Mediates Neurodevelopmental Toxicity by Activating the Oxidative Stress Response in Zebrafish (Danio rerio), ACS Omega, 2024, 9(14), 16508–16518 CrossRef CAS PubMed.
- C. Urani, R. Barbieri, S. Alloisio and M. Tesauro, From the Environment to Molecular Interactions of Nanoplastics: Unraveling the Neurotoxic Impacts and the Implications in Neurodegenerative Processes, Appl. Sci., 2024, 14(16), 7280 CrossRef CAS.
- J. Windheim, L. Colombo, N. C. Battajni, L. Russo, A. Cagnotto, L. Diomede, P. Bigini, E. Vismara, F. Fiumara, S. Gabbrielli and A. Gautieri, Micro-and nanoplastics' effects on protein folding and amyloidosis, Int. J. Mol. Sci., 2022, 23(18), 10329 CrossRef CAS PubMed.
- Y. M. Paing, Y. Eom, G. B. Song, B. Kim, M. G. Choi, S. Hong and S. H. Lee, Neurotoxic effects of polystyrene nanoplastics on memory and microglial activation: Insights from in vivo and in vitro studies, Sci. Total Environ., 2024, 924, 171681 CrossRef CAS PubMed.
- A. Jeong, S. J. Park, E. J. Lee and K. W. Kim, Nanoplastics exacerbate Parkinson's disease symptoms in C. elegans and human cells, J. Hazard. Mater., 2024, 465, 133289 CrossRef CAS PubMed.
- H. Sun, B. Yang, Q. Li, X. Zhu, E. Song, C. Liu, Y. Song and G. Jiang, Polystyrene nanoparticles trigger aberrant condensation of TDP-43 and amyotrophic lateral sclerosis-like symptoms, Nat. Nanotechnol., 2024, 1–2 Search PubMed.
- Y. Zhang, L. Tian, J. Chen, X. Liu, K. Li, H. Liu, W. Lai, Y. Shi, B. Lin and Z. Xi, Selective bioaccumulation of polystyrene nanoplastics in fetal rat brain and damage to myelin development, Ecotoxicol. Environ. Saf., 2024, 278, 116393 CrossRef CAS PubMed.
- M. Sun, M. Zhang, F. Di, W. Bai, J. Sun, M. Zhang, J. Sun, M. Li and X. Liang, Polystyrene nanoplastics induced learning and memory impairments in mice by damaging the glymphatic system, Ecotoxicol. Environ. Saf., 2024, 284, 116874 CrossRef CAS PubMed.
- H. Bai, Y. Wu, H. Li, Y. Zhu, R. Che, F. Wang and C. Zhang, Cerebral neurotoxicity of amino-modified polystyrene nanoplastics in mice and the protective effects of functional food Camellia pollen, Sci. Total Environ., 2024, 912, 169511 CrossRef CAS PubMed.
- Y. Sincihu, M. F. Lusno, T. M. Mulyasari, S. M. Elias, I. K. Sudiana, K. Kusumastuti, L. Sulistyorini and S. Keman, Wistar rats hippocampal neurons response to blood low-density polyethylene microplastics: a pathway analysis of SOD, CAT, MDA, 8-OHdG expression in hippocampal neurons and blood serum Aβ42 levels, Neuropsychiatr. Dis. Treat., 2023, 73–83 CrossRef CAS PubMed.
- J. Wang, Y. Yang, Y. Shi, L. Wei, L. Gao and M. Liu, Oxidized/unmodified-polyethylene microplastics neurotoxicity in mice: Perspective from microbiota-gut-brain axis, Environ. Int., 2024, 185, 108523 CrossRef CAS PubMed.
- G. Yang, C. Gong, X. Zheng, F. Hu, J. Liu, T. Wang, X. Chen, M. Li, Z. Zhu, L. Zhang and R. Li, Early clues and molecular mechanism involved in neurodegenerative diseases induced in immature mice by combined exposure to polypropylene microplastics and DEHP, Environ. Pollut., 2023, 336, 122406 CrossRef CAS PubMed.
- F. Poncin-Epaillard, C. Mille, D. Debarnot, W. Zorzi, B. El Moualij, A. Coudreuse, G. Legeay, I. Quadrio and A. Perret-Liaudet, Study of the adhesion of neurodegenerative proteins on plasma-modified and coated polypropylene surfaces, J. Biomater. Sci., Polym. Ed., 2012, 23(15), 1879–1893 CrossRef CAS PubMed.
- S. Ghosal, S. Bag and S. Bhowmik, Insights into the Binding Interactions between Microplastics and Human α-Synuclein Protein by Multispectroscopic Investigations and Amyloidogenic Oligomer Formation, J. Phys. Chem. Lett., 2024, 15, 6560–6567 CrossRef CAS PubMed.
- J. Xie, Y. Sun, Y. Ma, D. Wu and Z. Zhang, Blood-brain barrier damage accelerates the accumulation of micro-and nanoplastics in the human central nervous system, J. Hazard. Mater., 2024, 480, 136028 CrossRef CAS PubMed.
- T. G. Iversen, T. Skotland and K. Sandvig, Endocytosis and intracellular transport of nanoparticles: Present knowledge and need for future studies, Nano Today, 2011, 6(2), 176–185 CrossRef CAS.
- P. Foroozandeh and A. A. Aziz, Insight into cellular uptake and intracellular trafficking of nanoparticles, Nanoscale Res. Lett., 2018, 13(1), 339 CrossRef PubMed.
- I. Canton and G. Battaglia, Endocytosis at the nanoscale, Chem. Soc. Rev., 2012, 41(7), 2718–2739 RSC.
- S. Liu, X. Wu, W. Gu, J. Yu and B. Wu, Influence of the digestive process on intestinal toxicity of polystyrene microplastics as determined by in vitro Caco-2 models, Chemosphere, 2020, 256, 127204 CrossRef CAS PubMed.
- T. D. Brown, N. Habibi, D. Wu, J. Lahann and S. Mitragotri, Effect of nanoparticle composition, size, shape, and stiffness on penetration across the blood–brain barrier, ACS Biomater. Sci. Eng., 2020, 6(9), 4916–4928 CrossRef CAS PubMed.
- S. Mazumdar, D. Chitkara and A. Mittal, Exploration and insights into the cellular internalization and intracellular fate of amphiphilic polymeric nanocarriers, Acta Pharm. Sin. B, 2021, 11(4), 903–924 CrossRef CAS PubMed.
- R. Zhou, D. Zhou, S. Yang, Z. Shi, H. Pan, Q. Jin and Z. Ding, Neurotoxicity of polystyrene nanoplastics with different particle sizes at environment-related concentrations on early zebrafish embryos, Sci. Total Environ., 2023, 872, 162096 CrossRef CAS PubMed.
- D. Chirio, M. Gallarate, E. Peira, L. Battaglia, E. Muntoni, C. Riganti, E. Biasibetti, M. T. Capucchio, A. Valazza, P. Panciani and M. Lanotte, Positive-charged solid lipid nanoparticles as paclitaxel drug delivery system in glioblastoma treatment, Eur. J. Pharm. Biopharm., 2014, 88(3), 746–758 CrossRef CAS PubMed.
- S. Shan, Y. Zhang, H. Zhao, T. Zeng and X. Zhao, Polystyrene nanoplastics penetrate across the blood-brain barrier and induce activation of microglia in the brain of mice, Chemosphere, 2022, 298, 134261 CrossRef CAS PubMed.
- F. A. Monikh, M. G. Vijver, R. Kortet, I. Lynch and W. J. Peijnenburg, Emerging investigator series: perspectives on toxicokinetics of nanoscale plastic debris in organisms, Environ. Sci.: Nano, 2022, 9(5), 1566–1577 RSC.
- K. Yin, Y. Wang, H. Zhao, D. Wang, M. Guo, M. Mu, Y. Liu, X. Nie, B. Li, J. Li and M. Xing, A comparative review of microplastics and nanoplastics: Toxicity hazards on digestive, reproductive and nervous system, Sci. Total Environ., 2021, 774, 145758 CrossRef CAS.
- D. Liu, B. Lin, W. Shao, Z. Zhu, T. Ji and C. Yang, In vitro and in vivo studies on the transport of PEGylated silica nanoparticles across the blood–brain barrier, ACS Appl. Mater. Interfaces, 2014, 6(3), 2131–2136 CrossRef CAS PubMed.
- V. Kopatz, K. Wen, T. Kovács, A. S. Keimowitz, V. Pichler, J. Widder, A. D. Vethaak, O. Hollóczki and L. Kenner, Micro-and nanoplastics breach the blood–brain barrier (BBB): Biomolecular corona's role revealed, Nanomaterials, 2023, 13(8), 1404 CrossRef CAS PubMed.
- A. Da Silva-Candal, T. Brown, V. Krishnan, I. Lopez-Loureiro, P. Ávila-Gómez, A. Pusuluri, A. Pérez-Díaz, C. Correa-Paz, P. Hervella, J. Castillo and S. Mitragotri, Shape effect in active targeting of nanoparticles to inflamed cerebral endothelium under static and flow conditions, J. Controlled Release, 2019, 309, 94–105 CrossRef CAS PubMed.
- P. Kolhar, A. C. Anselmo, V. Gupta, K. Pant, B. Prabhakarpandian, E. Ruoslahti and S. Mitragotri, Using shape effects to target antibody-coated nanoparticles to lung and brain endothelium, Proc. Natl. Acad. Sci. U. S. A., 2013, 110(26), 10753–10758 CrossRef CAS PubMed.
- S. Salatin, S. Maleki Dizaj and A. Yari Khosroushahi, Effect of the surface modification, size, and shape on cellular uptake of nanoparticles, Cell Biol. Int., 2015, 39(8), 881–890 CrossRef CAS PubMed.
- S. Niaz, B. Forbes and B. T. Raimi-Abraham, Exploiting endocytosis for non-spherical nanoparticle cellular uptake, Nanomanufacturing, 2022, 2(1), 1–6 CrossRef.
- F. A. Monikh, M. Durão, P. V. Kipriianov, H. Huuskonen, J. Kekäläinen, S. Uusi-Heikkilä, E. Uurasjärvi, J. Akkanen and R. Kortet, Chemical composition and particle size influence the toxicity of nanoscale plastic debris and their co-occurring benzo (α) pyrene in the model aquatic organisms Daphnia magna and Danio rerio, NanoImpact, 2022, 25, 100382 CrossRef CAS PubMed.
- A. Santoro, M. Marino, L. N. Vandenberg, M. A. Szychlinska, E. P. Lamparelli, F. Scalia, N. D. Rocca, R. D'Auria, G. M. Giovanna Pastorino, G. D. Porta and F. F. Operto, PLASTAMINATION: Outcomes on the Central Nervous System and Reproduction, Curr. Neuropharmacol., 2024, 22(11), 1870–1898 CrossRef CAS PubMed.
- Y. Ikeda, H. Nakamura, S. Ohsaki and S. Watano, Direct translocation of a negatively charged nanoparticle across a negatively charged model cell membrane, Phys. Chem. Chem. Phys., 2021, 23(17), 10591–10599 RSC.
- Y. P. Chen, C. M. Chou, T. Y. Chang, H. Ting, J. Dembélé, Y. T. Chu, T. P. Liu, C. A. Changou, C. W. Liu and C. T. Chen, Bridging Size and Charge Effects of Mesoporous Silica Nanoparticles for Crossing the Blood–Brain Barrier, Front. Chem., 2022, 10, 931584 CrossRef CAS PubMed.
- W. Grodzicki, K. Dziendzikowska, J. Gromadzka-Ostrowska and M. Kruszewski, Nanoplastic impact on the gut-brain axis: current knowledge and future directions, Int. J. Mol. Sci., 2021, 22(23), 12795 CrossRef PubMed.
- E. Fröhlich, C. Meindl, E. Roblegg, B. Ebner, M. Absenger and T. R. Pieber, Action of polystyrene nanoparticles of different sizes on lysosomal function and integrity, Part. Fibre Toxicol., 2012, 9, 1–3 CrossRef PubMed.
- L. Zhang, J. Fan, G. Li, Z. Yin and B. M. Fu, Transcellular model for neutral and charged nanoparticles across an in vitro blood–brain barrier, Cardiovasc. Eng. Technol., 2020, 11, 607–620 CrossRef PubMed.
- M. Prüst, J. Meijer and R. H. Westerink, The plastic brain: neurotoxicity of micro-and nanoplastics, Part. Fibre Toxicol., 2020, 17, 1–6 CrossRef PubMed.
- A. Ucar, V. Parlak, F. B. Ozgeris, A. C. Yeltekin, M. E. Arslan, G. Alak, H. Turkez, E. M. Kocaman and M. Atamanalp, Magnetic nanoparticles-induced neurotoxicity and oxidative stress in brain of rainbow trout: Mitigation by ulexite through modulation of antioxidant, anti-inflammatory, and antiapoptotic activities, Sci. Total Environ., 2022, 838, 155718 CrossRef CAS PubMed.
- Y. Liu, J. Li, K. Xu, J. Gu, L. Huang, L. Zhang, N. Liu, J. Kong, M. Xing, L. Zhang and L. Zhang, Characterization of superparamagnetic iron oxide nanoparticle-induced apoptosis in PC12 cells and mouse hippocampus and striatum, Toxicol. Lett., 2018, 292, 151–161 CrossRef CAS PubMed.
- E. G. Schniederjan, Employing spICP-MS to measure uptake, distribution, and toxicity of a heterogenous mixture of nanoplastics in zebrafish Embryo-Larvae, Doctoral dissertation, Texas Tech University, 2024 Search PubMed.
- S. Liu, Y. He, J. Yin, Q. Zhu, C. Liao and G. Jiang, Neurotoxicities induced by micro/nanoplastics: A review focusing on the risks of neurological diseases, J. Hazard. Mater., 2024, 134054 CrossRef CAS PubMed.
- K. Yin, D. Wang, H. Zhao, Y. Wang, Y. Zhang, Y. Liu and M. Xing, Polystyrene microplastics up-regulates liver glutamine and glutamate synthesis and promotes autophagy-dependent ferroptosis and apoptosis in the cerebellum through the liver-brain axis, Environ. Pollut., 2022, 307, 119449 CrossRef CAS PubMed.
- K. Yin, H. Lu, Y. Zhang, L. Hou, X. Meng, J. Li, H. Zhao and M. Xing, Secondary brain injury after polystyrene microplastic-induced intracerebral hemorrhage is associated with inflammation and pyroptosis, Chem.-Biol. Interact., 2022, 367, 110180 CrossRef CAS PubMed.
- L. Shapiro and N. Katchur, Microplastic Exposure and the of Parkinson's Disease: The Effects of microplastics in the body and similarities to the pathogenesis of Parkinson's Disease, J. Stud. Res., 2022, 11(3), 3–11 Search PubMed.
- Z. Breijyeh and R. Karaman, Comprehensive review on Alzheimer's disease: causes and treatment, Molecules, 2020, 25(24), 5789 CrossRef CAS PubMed.
- U. Sehar, P. Rawat, A. P. Reddy, J. Kopel and P. H. Reddy, Amyloid beta in aging and Alzheimer's disease, Int. J. Mol. Sci., 2022, 23(21), 12924 CrossRef CAS PubMed.
- A. Waris, A. Ali, A. U. Khan, M. Asim, D. Zamel, K. Fatima, A. Raziq, M. A. Khan, N. Akbar, A. Baset and M. A. Abourehab, Applications of various types of nanomaterials for the treatment of neurological disorders, Nanomaterials, 2022, 12(13), 2140 CrossRef CAS PubMed.
- D. J. Surmeier, Determinants of dopaminergic neuron loss in Parkinson's disease, FEBS J., 2018, 285(19), 3657–3668 CrossRef CAS PubMed.
- M. X. Henderson, J. Q. Trojanowski and V. M. Lee, α-Synuclein pathology in Parkinson's disease and related α-synucleinopathies, Neurosci. Lett., 2019, 709, 134316 CrossRef CAS PubMed.
- B. Li, W. Song, Y. Cheng, K. Zhang, H. Tian, Z. Du, J. Wang, J. Wang, W. Zhang and L. Zhu, Ecotoxicological effects of different size ranges of industrial-grade polyethylene and polypropylene microplastics on earthworms Eisenia fetida, Sci. Total Environ., 2021, 783, 147007 CrossRef CAS PubMed.
- S. Ramesh and A. S. Arachchige, Depletion of dopamine in Parkinson's disease and relevant therapeutic options: A review of the literature, AIMS Neurosci., 2023, 10(3), 200 Search PubMed.
- A. Mohammadipour, H. Haghir and A. Ebrahimzadeh Bideskan, A link between nanoparticles and Parkinson's disease. Which nanoparticles are most harmful?, Rev. Environ. Health, 2020, 35(4), 545–556 CrossRef CAS PubMed.
- Y. Huang, B. Liang, Z. Li, Y. Zhong, B. Wang, B. Zhang, J. Du, R. Ye, H. Xian, W. Min and X. Yan, Polystyrene nanoplastic exposure induces excessive mitophagy by activating AMPK/ULK1 pathway in differentiated SH-SY5Y cells and dopaminergic neurons in vivo, Part. Fibre Toxicol., 2023, 20(1), 44 CrossRef CAS PubMed.
- B. Liang, Y. Huang, Y. Zhong, Z. Li, R. Ye, B. Wang, B. Zhang, H. Meng, X. Lin, J. Du and M. Hu, Brain single-nucleus transcriptomics highlights that polystyrene nanoplastics potentially induce Parkinson's disease-like neurodegeneration by causing energy metabolism disorders in mice, J. Hazard. Mater., 2022, 430, 128459 CrossRef CAS PubMed.
- K. A. Jellinger, The spectrum of cognitive dysfunction in amyotrophic lateral sclerosis: an update, Int. J. Mol. Sci., 2023, 24(19), 14647 CrossRef CAS PubMed.
- K. D. Patel, Z. Keskin-Erdogan, P. Sawadkar, N. S. Sharifulden, M. R. Shannon, M. Patel, L. B. Silva, R. Patel, D. Y. Chau, J. C. Knowles and A. W. Perriman, Oxidative stress modulating nanomaterials and their biochemical roles in nanomedicine, Nanoscale Horiz., 2024, 9(10), 1630–1682 RSC.
- A. Eisen, E. P. Pioro, S. A. Goutman and M. C. Kiernan, Nanoplastics and Neurodegeneration in ALS, Brain Sci., 2024, 14(5), 471 CrossRef CAS PubMed.
- C. Urani, R. Barbieri, S. Alloisio and M. Tesauro, From the Environment to Molecular Interactions of Nanoplastics: Unraveling the Neurotoxic Impacts and the Implications in Neurodegenerative Processes, Appl. Sci., 2024, 14(16), 7280 CrossRef CAS.
- J. Chen, Y. Zhang, X. Liu, K. Li, H. Liu, W. Lai, Y. Shi, Z. Xi, L. Yan, L. Tian and B. Lin, Effects of exposure to nano-plastic drinking during pregnancy on cognitive related proteins in offspring of SD rats, Environ. Pollut. Bioavailability, 2024, 36(1), 2292104 CrossRef.
- G. Papiri, G. D'Andreamatteo, G. Cacchiò, S. Alia, M. Silvestrini, C. Paci, S. Luzzi and A. Vignini, Multiple sclerosis: inflammatory and neuroglial aspects, Curr. Issues Mol. Biol., 2023, 45(2), 1443–1470 CrossRef CAS PubMed.
- A. Weiss and Y. Ding, The impact of microplastics on neurodegenerative diseases and underlying molecular mechanisms: A narrative review, Environmental Disease, 2024, 9(3), 60–64 CrossRef.
- M. de Oliveira, F. B. Santinelli, P. N. Lisboa-Filho and F. A. Barbieri, The blood concentration of metallic nanoparticles is related to cognitive performance in people with multiple sclerosis: An exploratory analysis, Biomedicines, 2023, 11(7), 1819 CrossRef CAS PubMed.
- F. Aliakbarzadeh, M. Rafiee, F. Khodagholi, M. R. Khorramizadeh, H. Manouchehri, A. Eslami, F. Sayehmiri and A. Mohseni-Bandpei, Adverse effects of polystyrene nanoplastic and its binary mixtures with nonylphenol on zebrafish nervous system: From oxidative stress to impaired neurotransmitter system, Environ. Pollut., 2023, 317, 120587 CrossRef CAS PubMed.
- L. Strużyńska and J. Skalska, Mechanisms underlying neurotoxicity of silver nanoparticles, Cellular and Molecular Toxicology of Nanoparticles, 2018, pp. 227–250 Search PubMed.
- H. Yang, S. Niu, M. Guo and Y. Xue, Molecular mechanisms of silver nanoparticle-induced neurotoxic injury and new perspectives for its neurotoxicity studies: a critical review, Environ. Pollut., 2024, 124934 CrossRef CAS PubMed.
- Y. Lin, C. Hu, A. Chen, X. Feng, H. Liang, S. Yin, G. Zhang and L. Shao, Neurotoxicity of nanoparticles entering the brain via sensory nerve-to-brain pathways: injuries and mechanisms, Arch. Toxicol., 2020, 94, 1479–1495 CrossRef CAS PubMed.
- A. Krawczyńska, K. Dziendzikowska, J. Gromadzka-Ostrowska, A. Lankoff, A. P. Herman, M. Oczkowski, T. Królikowski, J. Wilczak, M. Wojewódzka and M. Kruszewski, Silver and titanium dioxide nanoparticles alter oxidative/inflammatory response and renin–angiotensin system in brain, Food Chem. Toxicol., 2015, 85, 96–105 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2025 |
