Fabrication and characterization of ConA-conjugated curcumin-loaded solid lipid nanoparticles for theranostic applications in lung cancer treatment
Vinit
Nikwade†
a,
Nisha
Choudhary†
b,
Raghu
Solanki
 c,
Ashish
Patel
c,
Ashish
Patel
 *b,
Virendra Kumar
Yadav
*b,
Virendra Kumar
Yadav
 *d,
Saleh H.
Salmen
e,
Abdullah A.
Alarfaj
e,
Mohammad Javed
Ansari
f and
Vivekanand
Chatap
*a
*d,
Saleh H.
Salmen
e,
Abdullah A.
Alarfaj
e,
Mohammad Javed
Ansari
f and
Vivekanand
Chatap
*a
aDepartment of Pharmaceutics, H. R. Patel Institute of Pharmaceutical Education & Research, Shirpur-425405, Maharashtra, India. E-mail: vinitnikwade66@gmail.com; dr.vkchatap@gmail.com
bDepartment of Life Sciences, Hemchandracharya North Gujarat University, Patan, Gujarat, India. E-mail: nishanaseer03@gmail.com; uni.ashish@gmail.com
cDepartment of Biological Sciences and Engineering, Indian Institute of Technology Gandhinagar, Palaj, Gujarat 382355, India. E-mail: raghu.solanki@iitgn.ac.in
dMarwadi University Research Center, Department of Microbiology, Faculty of Sciences, Marwadi University, Rajkot, 360003, Gujarat, India. E-mail: yadava94@gmail.com
eDepartment of Botany and Microbiology, College of Science, King Saud University, P.O Box 2455, Riyadh-11451, Saudi Arabia. E-mail: ssalmen@ksu.edu.sa; aalarfajj@ksu.edu.sa
fDepartment of Botany, Hindu College Moradabad (Mahatma Jyotiba Phule Rohilkhand University Bareilly), 244001, India. E-mail: mjavedansari@gmail.com
First published on 12th December 2024
Abstract
The main issues with current and traditional cancer therapy delivery systems include a lack of selectivity towards tumors, causing harm to healthy cells, low efficiency in loading drugs, and the inability to visually track the drug's localization after administration. These limitations negatively impact the effectiveness of therapy and result in increased treatment costs. Furthermore, conventional cancer therapies typically target tumor cells through a single mechanism, which eventually leads to the emergence of drug resistance. Concanavalin A, a plant lectin derived from jack beans, has the ability to recognise cells and can be used as an efficient targeting agent in cancer therapy. In the current study, the effectiveness of solid lipid nanoparticles (SLNs) loaded with curcumin (CU) and conjugated with ConA has been examined in the fight against A549 human lung cancer cells, with a focus on their anticancer properties. This novel strategy allows for targeted delivery, sustained release, and specific recognition of cancer cells. To verify the successful bonding of ConA to SLNs, we conducted a comparison of the FTIR spectra between the synthesized Cur-SLNs and ConA-SLNs and their respective precursors. Additionally, we employed various techniques, such as XRD (X-ray diffraction), DSC (differential scanning calorimetry), TGA (thermogravimetric analysis), SEM (scanning electron microscopy), particle size analysis, and other methods, to examine the surface morphology and viability of SLNs. The present in vitro study of drug release revealed a sustained release pattern from the ConA-SLNs. The utilization of targeted nanoparticles resulted in a notable increase in the anticancer effectiveness of curcumin, as demonstrated using an anti-proliferation assay. The positive findings from this research indicate the potential of directing nanomedicines towards carbohydrate structures that are overexpressed through lectin (ConA)-mediated delivery in the treatment of lung cancer.
1. Introduction
One of the most widespread and deadliest forms of cancer on a global scale, lung cancer, kills millions of people each year. Lung cancer led to nearly 1.8 million mortalities in 2020 as per the data released by the World Health Organization (WHO). In terms of classification, lung cancer has been typically categorized as small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC).1,2 Lung cancer makes up about 5.9% of all malignancies and 8.1% of cancer fatalities in India. India has lower age-standardized incidence and mortality rates for lung cancer than its neighbours China and Pakistan, but higher rates than Sri Lanka and Bangladesh. In India, lung cancer is more prevalent in men than in women and in urban areas rather than rural. About 80% of lung cancer patients are smokers.3,4 Traditionally, there has been a strong association between lung cancer and smoking, leading to its classification as a social disease accompanied by societal stigma.5 The development of lung metastases occurs frequently in the latter stages of many other cancer types as well. The majority of currently used conventional treatments (administered through intravenous or oral routes) for treating primary lung cancer are ineffective, and metastases cause serious unfavourable side treatments.6 Significantly, the mortality rate due to lung cancer is highest among all commonly occurring cancers and patients have a distressingly low survival span of less than five years.7Surgical treatment, chemotherapy and radiation therapy are common treatment methods for lung carcinoma, with the specific approach determined by factors such as the stage of cancer, the potential for surgical removal, and the overall health condition of the patient.8 However, advanced-stage cancer patients often do not qualify for surgery and chemotherapy. Despite significant efforts in biomarker development for early diagnosis, the goal remains unachieved.9,10 In many cases, chemotherapy is utilized in conjunction with the other two treatment modalities. However, the conventional administration of anticancer drugs, either through oral ingestion or injections, results in the systemic dissemination of these toxic chemicals across the whole body. As a result, drug concentrations in tumor tissues are often insufficient, while normal tissues are exposed to unwanted drug distribution. This conventional chemotherapy approach is associated with significant side effects and suboptimal therapeutic outcomes, which are typically difficult to avoid.11 Consequently, there is a growing need for targeted or localized drug-delivery approaches that aim to enhance the drug dosage specifically in tumors while reducing drug exposure in normally functioning tissues. A targeted drug-delivery strategy is crucial for effective cancer treatment, especially for lung cancer.12 Pulmonary drug delivery refers to a nonintrusive technique of administering medication by inhaling or nebulizing it through the throat and bronchial tubes. This method can effectively treat lung-related conditions like inflammation and narrowing of lungs (asthma), obstructed airflow in lungs (COPD), and microbial infection in lungs (pneumonia) since it enables direct medicine delivery to the lungs.13
Nanomedicines have exhibited promise in the areas of cancer diagnosis, treatment, and monitoring.14 Targeted drug-delivery systems based on nanotechnology have the capability to specifically transport anticancer drugs to cancer cells. In addition to delivering drugs to the desired site, nanomedicines also enhance the solubility and availability of drugs in the bloodstream, enhance their stability, and enable controlled release. These different factors together lower the amount of dose needed, thereby minimizing side effects and improving patient adherence to treatment.15,16 The most commonly employed method for directing drugs or drug-carrier nano-systems to their intended target sites is through the conjugation of targeting ligands. Various techniques, such as covalent and non-covalent conjugation, have been developed for this purpose. The key aspect is to ensure stable and accessible attachment of the ligand to the drug carrier, allowing the ligand to be properly oriented for binding to target receptors. It is important that the coupling reactions do not compromise the functionality or effectiveness of the binding agent and pose no adverse impact on the configuration of the drug administered through nano-systems. Additionally, these coupling reactions need to be optimized to ensure homogeneous binding between ligands and drug-carrying nano-systems.17
Concanavalin A (Con A), which is a plant lectin known for its specific binding to mannose and glucose, has been proposed as a model carbohydrate receptor and extensively studied for its ability to target tumor vasculature. Notably, Con A, a carbohydrate-binding protein, has demonstrated the capacity to agglutinate leukemic cells and transformed cells (due to several agents like simian virus, polyoma virus, carcinogenic chemicals, and X-ray irradiation).18 It is worth mentioning that under similar conditions, Con A does not agglutinate normal cells.19
In the present study, we formulated solid lipid nanoparticles (SLNs) that are conjugated with Con A to selectively bind lung cancer cells and enhance its delivery of curcumin (drug) through pulmonary delivery using a dry powder inhaler. Solid lipid nanoparticles (SLNs)consist of a solid lipid core matrix, known for its high biocompatibility and biodegradability.15,20 Solid lipid nanoparticles (SLNs) offer advantages that encompass both polymeric nanoparticles and liposomes, addressing specific limitations such as acute and chronic toxicity.21 Therefore, SLNs are good candidates for targeting drugs to the cancerous cells.
2. Materials and methods
Materials
Glycerol mono-stearate (GMS) was obtained from the R&D department at Fine Chem Industries located in Mumbai, India. Concanavalin-A (ConA) was purchased from Sisco Research Laboratories Pvt. Ltd. Curcumin (CU) was supplied by Ajanta Pharmaceuticals Ltd (Mumbai, India). Stearic acid (SA) and N-(3,25-dimethylaminopropyl)-N-ethylcarbodiimide (EDC) were purchased from Loba Chem Pvt. Ltd (Mumbai, India). Tween-80 was purchased from Avantor Performance Materials (Thane, India).Fabrication of curcumin (CU)-loaded solid lipid nanoparticles (Cur-SLNs)
SLNs were formulated using a modified solvent injection method.21 Briefly, the lipid GMS and soy lecithin were dissolved in water miscible solvent ethanol then stearic acid was added and the reaction solution was heated up to 60 °C. Simultaneously, 1% (w/v) solution of Tween-80 in the aqueous phase was melted at a temperature of 60 °C. The organic phase was then inserted into the aqueous phase using a syringe at a flow rate of 5 mL min−1. The lipid dispersion was then subjected to probe sonication for 4–5 min. After sonication, the dispersion was stirred for 2 h to evaporate the ethanol and obtain solid lipid nanoparticles. Curcumin-loaded SLNs were prepared by the same method by dissolving the drug into the organic solvent along with the other ingredients.Curcumin encapsulation efficiency and drug loading
The entrapment efficacy of CU is calculated by an indirect method using a UV-visible spectrophotometer (model no.: UV-1800, Shimadzu, Japan). The supernatant obtained after centrifugation was analysed by collecting UV spectra at a wavelength of 419.5 nm using a pre-determined calibration curve for CU. The entrapment efficiency was calculated using the formula:Conjugation of ConA to solid lipid nanoparticles
The conjugation of ConA to the surface of nanoparticles was achieved using a modified carbodiimide method.22 Briefly, 50 mg of solid lipid nanoparticles was added to 5 mL of PBS at pH 7.4 and the carboxylic groups of the SLNs were activated by adding 1 mL of 0.1 M EDC/NHS and the mixture was left to react for 1 h for activation of the carboxylic groups present on the nanoparticles. After that, 5 mg of ConA in 5 mL of PBS at pH 7.4 was added to the mixture, which was stirred overnight for conjugation. The primary amine groups present on ConA reacted with the activated COOH groups of the SLNs and this resulted in the formation of amide linkages.Characterization of SLNs
The average particle size and zeta potential of different SLN formulations, such as BSLN, Cur-SLN, and ConA-SLN, were measured using a particle-size analyzer (Nanoplus3, particulate system, Micromeritics, USA), utilizing a photon correlation spectroscopy technique. The surface morphology of these SLNs was determined using scanning electron microscopy. XRD studies were carried out on samples using a Bruker D2 Phaser (2nd Generation, Germany), which were scanned from 10° to 80°. X-ray scattering measurements were carried out on the pure drug, GMS, Cur-SLNs, BSLNs, and ConA-SLNs. Differential scanning calorimetry (DSC) was carried out to determine the thermal nature of the drug, melting points and the physicochemical interactions of Cur-SLNs, BSLNs, and ConA-SLNs. Utilizing 3 mg of sample, an aluminium pan was closed with a high-pressure press. Using a differential scanning calorimeter (PerkinElmer 4000, USA), thermal analysis was performed under a nitrogen atmosphere over a temperature range of 20 °C–600 °C at a heating rate of 20 °C min−1. Preliminary confirmation of Cur-SLNs, BSLNs, and ConA-SLNs was accomplished using an ATIR spectrophotometer (Bruker, Alpha-II, Germany). The diffuse reflectance spectrum (DRS) method was used to scan the sample from 4000 to 600 cm−1 and estimate the transmission.In vitro drug release study
The dialysis process was utilized to evaluate the drug-release patterns of ConA-SLNs and Cur-SLNs. The release experiment was performed in phosphate buffer of pH 6.8 and pH 7.4 by dissolving samples in the medium at 150 rpm and 37 °C. Separately, 2 mg equivalents of ConA-SLNs and pure CU were mixed in 5 mL of buffer and this sample was transferred into a dialysis bag, which was pre-soaked for 24 h. The bag was clipped at both ends. The materials were then examined using a UV-visible spectrophotometer at λmax of 430.5 and 424.5 nm, respectively. The absorbance obtained was compared with the calibration curve and the percentage cumulative drug release was plotted versus time to evaluate the drug release pattern from this prepared nano-formulation.23Anti-proliferation assay
The MTT assay protocol was used to test the anti-proliferation effect of the samples.24 The cells were cultured in a medium under conditions of 37 °C temperature and 5% CO2 for 24 h with a density of 1 × 104 cells per mL. Then, cells were transferred to microplates (96 wells, tissue culture grade) with a volume of 100 μL and a density of 104 cells per well. The samples were added to the wells at varying concentrations (10, 40, and 100 μg mL−1) in 100 μL of medium. Control wells having DMSO (0.2% in PBS) and a cell line were also included. Each sample assay was replicated three times. The cell survival and viability of the controls were measured after culture and these cells were kept in an incubator (Thermo Scientific, BB150) for 24 h maintained at 37 °C temperature under 5% CO2. The medium was then discarded and 20 μL of MTT solution (5 mg min−1 PBS) was poured into each well. The cells were again incubated in the CO2 incubator for another 4 h at 37 °C. The formation of formazan crystals by living cells was checked under a microscope. The MTT solution was then replaced by adding DMSO (200 μL) to each well, covering them with aluminum foil and leaving them to rest for 10 min at 37 °C. With the help of a microplate reader (Benesphera E21), the absorbance value of each sample was measured at a wavelength of 550 nm.25Lung model study
The deposition of powder in the lungs was assessed using an Andersen cascade impactor with eight stages. The impactor had eight sealed stages in series, with filters on the plates that collected the particles at each stage. The impactor was placed on a flat surface. A vacuum pump was used to draw air through the stages at 28.3 L min−1, and the powder samples were loaded on the top of the impactor. The powder took 10 seconds to settle on the stages, and then the airflow was stopped. The manufacturer provided the cut-off values of the particle aerodynamic diameters for each stage, and these are as follows: pre-separator (10.00 μm), stage-0 (0–9.00 μm), stage-1 (5.8 μm), stage-2 (4.7 μm), stage-3 (3.3 μm), stage-4 (2.1 μm), stage-5 (1.1 μm), stage-6 (0.7 μm), and stage-7 (0.4 μm). The last phase, stage-8, was supposed to gather any residual particles, but this was difficult to achieve. After impaction, the drug that was deposited in various parts of the impactor, for example, the induction port, pre-separator, each plate, capsule residue, and the inhaler device, was washed using methanol or PBS. From the data obtained on drug deposition, various parameters like fine-particle fraction and mass median aerodynamic diameter were calculated.26Statistical analysis
The data are reported as the mean value accompanied by the standard deviation (SD). The software PRISM (Graph Pad) was used and one-way variance analysis (ANOVA) testing was conducted to perform the statistical analysis. A probability level of p < 0.05 was considered to be statistically significant.3. Results and discussion
Preparation of solid lipid nanoparticles
A modified solvent-injection technique was used to develop SLNs. GMS was chosen as the solid-lipid matrix, whereas, Tween® 80 and soy lecithin were selected as the surfactants. Stearic acid was used to introduce some free carboxylic groups onto the nanoparticle surface. CU was dissolved in the organic phase to prepare Cur-SLNs. The average particle sizes of BSLNs, Cur-SLNs, and ConA-SLNs were 247.5 nm and 287.9 nm, 364.2 nm, respectively; the increase in size of ConA-SLNs was due to the conjugation of ConA with Cur-SLNs. The small polydispersity index (PDI) of the nanoparticles revealed their uniform size distribution, ensuring consistency. Both BSLNs and Cur-SLNs exhibited negative zeta potentials of −20.8 ± 3.57 mV and −17.11 ± 4.21 mV, respectively, indicating good physical stability of the SLNs (Table 1). The slight positive shift of the zeta potential of ConA-SLNs may be due to the conjugation of ConA with Cur-SLNs. Cur-SLNs showed the highest values of encapsulation efficiency (%EE) and drug-loading efficiency (DL) of 88% and 4.43%, respectively. These results indicate successful encapsulation of curcumin within the SLNs. Alternatively, the efficiency of curcumin encapsulation within ConA-SLNs was slightly lower than that of Cur-SLNs with 83.2% EE and 4.15% DL, which could be due to the conjugation of ConA affecting the encapsulation and drug retention properties of the SLNs.| PS (nm) | ZP (mV) | PDI | %EE | %DL | |
|---|---|---|---|---|---|
| BSLNs: blank solid lipid nanoparticles; Cur-SLNs: curcumin-loaded solid lipid nanoparticles; ConA-SLNs: concanavalin A-conjugated SLNs; PS: particle size; ZP: zeta potential; PDI: polydispersity index; EE: entrapment efficiency; DL: drug loading. | |||||
| BSLNs | 247.5 ± 5.61 | −20.8 ± 3.57 | 0.291 ± 0.07 | No drug encapsulation | No drug encapsulation |
| Cur-SLNs | 287.9 ± 3.066 | −17.11 ± 4.21 | 0.287 ± 0.106 | 88 ± 1.285 | 4.43 ± 1.05 |
| ConA-SLNs | 364.2 ± 3.52 | −8.06 ± 3.46 | 0.301 ± 0.087 | 83.2 ± 2.55 | 4.15 ± 2.02 |
Physiochemical characterization
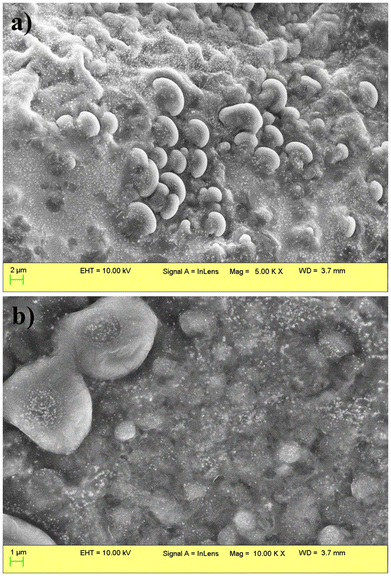
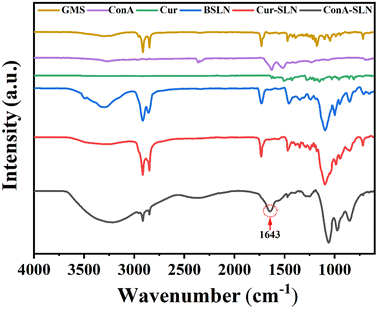
The surface morphology and shape of the nanoparticles were determined using scanning electron microscopy (SEM). The SEM images of ConA-SLNs show that the nanoparticles have a semi-spherical shape and are whitish in appearance, which may be due to the coating of ConA on the surface. The conjugation of ConA slightly alters the surface morphology of the SLNs; hence it was found that the size of ConA-SLNs increased as compared to that of Cur-SLNs. Cur-SLNs show smooth and spherical surface morphology and uniform structure (Fig. 1).The surface conjugation of ConA to SLNs was confirmed through FTIR analysis, as shown in Fig. 2. The IR spectra of Cur-SLNs revealed the characteristic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of the carboxylic group present in stearic acid at 1731 cm−1, which was absent in ConA-conjugated nanoparticles (ConA-SLNs). Furthermore, a new peak at 1643 cm−1 was noticed, indicating the establishment of an amide bond between the amine groups of ConA and the carboxylic groups of the nanoparticles.
O stretching of the carboxylic group present in stearic acid at 1731 cm−1, which was absent in ConA-conjugated nanoparticles (ConA-SLNs). Furthermore, a new peak at 1643 cm−1 was noticed, indicating the establishment of an amide bond between the amine groups of ConA and the carboxylic groups of the nanoparticles.
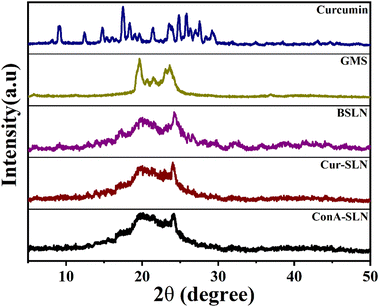
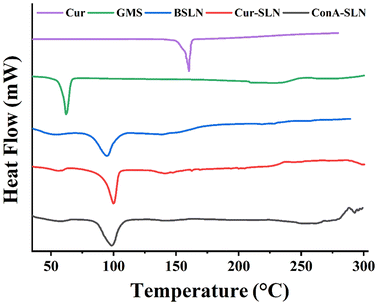
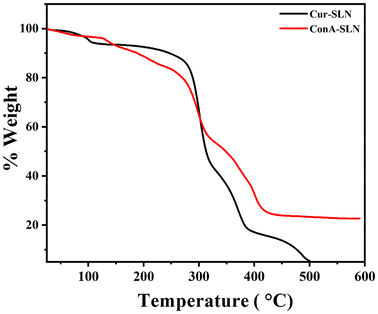
XRD diffractograms of CU, GMS, BSLN, Cur-SLN, and ConA-SLN samples are shown in Fig. 3. The XRD pattern of CU indicates the presence of sharp peaks between 9° and 30°, suggesting its crystalline nature. However, these sharp peaks are not observed in the Cur-SLN sample, indicating that the drug is present either in an amorphous phase or in a dispersed phase within the lipid matrix.27 Glycerol monostearate shows two intense peaks at 19.633° and 23.621°, which are similar to the crystalline nature of lipids. These peaks are also present in the BSLN and Cur-SLN samples, but their intensity is reduced, indicating a decrease in lipid crystallinity.28 The physical state of CU (as a pure compound and after encapsulation) was examined using DSC, as shown in Fig. 4. In the DSC analysis, the endothermic peak of pure CU was observed at 179 °C. However, this peak was absent in the Cur-SLN sample, indicating that the crystalline phase of CU was transformed into an amorphous phase within the nanoparticles. Additionally, a supplementary peak at 99.69 °C was observed in the Cur-SLN thermogram, corresponding to the cryoprotectant (trehalose dihydrate) used during the nanoparticle lyophilization process.29 The DSC thermograph of GMS shows a sharp characteristic peak at 61.99 °C. In the case of the Cur-SLN formulation, the GMS peak exhibited a shifted position from 61 °C to 55.27 °C, accompanied by a broad and shoulder-like shape. It has been noted that lipids display distinct behaviour when present in the bulk form compared to their behaviour in SLN (solid lipid nanoparticle) formulations.30,31 The alteration in the melting point of GMS observed in both Cur-SLN and BSLN formulations can be ascribed to the reduced particle size of the SLNs, leading to an elevated surface energy and the presence of a surfactant.32 Furthermore, lattice defects are formed during the preparation of SLNs, resulting in a reduction in the crystalline structure of lipids used. These disordered or amorphous solids, which possess lower degrees of order, require less energy for melting compared to the original crystalline substances. As a result, they exhibit broader and less-pronounced peaks in the DSC thermogram.33 The TGA curves of Cur-SLNs and ConA-SLNs show the weight loss of the samples over different temperature ranges due to various factors such as evaporation, decomposition and combustion. The conjugation efficiency of ConA on SLNs was calculated by comparing the weight loss of Cur-SLNs and ConA-SLNs and was found to be 21%. The TGA curves also indicate the thermal stability and decomposition behaviour of the lipid matrix and curcumin in both the samples (Fig. 5).
Lung simulation study
It was found that the FPF of formulations is 21.58 ± 0.67% meaning that 21.58 ± 0.67% of the dose delivered from the dry powder inhaler (DPI) has a particle size of less than 5 μm, which is the cut-off size for particles that can reach deep into the lungs and exert a clinical effect. MMAD was found to be 4.55 ± 0.53 μm. Overall, the data obtained from the lung simulation study demonstrate that the formulation exhibits promising aerodynamic properties, with an optimal FPF for deep-lung penetration, a suitable MMAD for deposition in the lower respiratory tract, and a high emitted dose that ensures efficient delivery of the active pharmaceutical ingredient. These characteristics make the formulation a strong candidate for the treatment of respiratory conditions where deep-lung deposition is required for therapeutic action (Table 2).| Group | ED, % | FPF, % | MMAD |
|---|---|---|---|
| ED: emitted dose percentage; FPF: fine particle fraction percentage; MMAD: mass median aerodynamic diameter. | |||
| ConA-SLN | 100.37 ± 0.71 | 21.58 ± 0.67 | 4.55 ± 0.53 |
In vitro drug release study
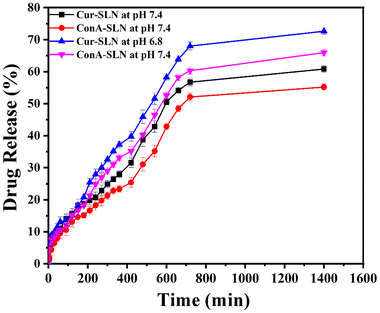
The pattern of release of the drug was investigated by following the dialysis technique in phosphate-buffered saline (PBS) with pH values of 7.4 and 6.8 at a temperature of 37 °C. The Cur-SLN and ConA-SLN formulations exhibit sustained release patterns lasting for 24 h in pH 7.4 PBS, characterized by an initial rapid release of 15% and 13% of both the formulations within 2 h, respectively. This initial burst release may be attributed to the larger surface area of the nanoparticles and the concentration of the drug in the outer shell of the nanoparticles.14,34 Subsequently, the drug-release rate slows down, leading to a cumulative release of 60% and 55% of Cur-SLNs and ConA-SLNs, respectively, over the course of 24 h. This reduction in the release rate could be ascribed to the limited solubility and permeability of CU in the aqueous medium, as well as potential interactions between curcumin and the lipid matrix.14,35 The release mechanism is likely a combination of diffusion and erosion processes.36 The release patterns of Cur-SLNs and ConA-SLNs at pH 6.8 were similar (Fig. 6), with rapid release initially followed by stable release over a period of 24 h. The CPR of Cur-SLNs and ConA-SLNs at pH 6.8 is high compared to that at pH 7.4, which may be due to the higher solubility of CU in acidic pH.In vitro cytotoxicity study – MTT assay
The potential anticancer properties of Cur-SLN and ConA-SLN formulations were examined in the context of A549, a cell line associated with human lung cancer. Cell inhibition is shown in Fig. 7 and 8. The in vitro chemosensitivity of ConA-SLNs was examined across concentrations ranging from 10 to 100 μg mL−1. The positive control, represented by 5-FU, demonstrated greater inhibition at concentrations of both 10 and 100 μg mL−1. However, ConA-SLNs exhibited an enhanced inhibition rate, with the highest growth inhibition observed at the lowest concentration of 33.34 μg mL−1. Similarly, Cur-SLNs demonstrated inhibitory activity against A549 cells, which was comparable to the inhibitory activity of curcumin itself. On the basis of these obtained outcomes, it can be concluded that the synthesized multicomponent nanocomposites, specifically the ConA-conjugated SLNs, have demonstrated enhanced cellular permeation, leading to significant reduction in cell-growth percentage. In the 10–100 μg mL−1 concentration range, steady-state inhibition was observed. This steady-state inhibition can be ascribed to the presence of ConA on the surface of SLNs, which facilitates the recognition of cancer cells. On the other hand, the positive control exhibited a non-linear pattern of inhibition. The anticancer effects of Cur-SLNs and ConA-SLNs were evaluated on an A549 cell line, a type of lung cancer cell. The findings indicate that 5-FU, the reference compound, exhibited the strongest cell-growth suppression at both 10 and 100 μg mL−1 dosages. Nevertheless, ConA-SLNs also exhibited potent inhibition, especially at a concentration of 100 μg mL−1, resulting in a significant decrease in cancer-cell proliferation. ConA-SLNs showed 34.46% inhibition at 10 μg mL−1 and 47.93% at 100 μg mL−1, demonstrating higher efficacy than Cur-SLNs. Although the Cur-SLN formulation also inhibited A549 cells, it did so at a slower rate than ConA-SLNs. The inhibition varied from 25.36% to 42.96% at different concentrations, showing a similar effect to that of curcumin by itself. These findings indicate that ConA-SLNs show improved performance possibly because ConA can target cancer cells more efficiently. Overall, the ConA-SLN formulation demonstrated increased inhibition of cancer cells at concentrations of 10–100 μg mL−1, indicating its potential as a treatment option. The enhanced suppression observed is probably a result of ConA aiding the nanoparticles in identifying and attaching to cancer cells. On the other hand, 5-FU, serving as the positive control, displayed a powerful yet non-linear pattern of inhibition.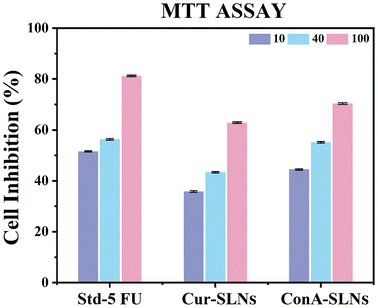 | ||
| Fig. 7 Percentage formulation inhibition of 5 FU (standard), Cur-SLNs and ConA-SLNs on the A549 cell line determined using the MTT assay; values represent mean ± standard deviation (SD) p < 0.05. | ||
 | ||
| Fig. 8 Effect of (A) control, (B) standard 5 FU, (C) Cur-SLNs and (D) ConA-SLNs measured against the A549 lung cancer cell line using the MTT assay. | ||
4. Conclusion
CU-loaded solid lipid nanoparticles were successfully prepared by a simple and scalable modified solvent injection method. The successful conjugation of ConA onto the surface of SLNs has been confirmed through ATR-FTIR analysis. The interaction between the drug and lipid matrix was assessed using DSC, revealing no substantial physical or chemical interactions. XRD results confirmed the encapsulation of the drug within the lipid matrix. SEM analysis further revealed the surface morphology of ConA-SLNs and Cur-SLNs. Particle size measurements confirmed that the formulation was within the nano-size range, while zeta potential analysis indicated the stability of the formulation. In vitro drug release studies revealed the sustained release pattern of the drug. The in vitro study of formulation cytotoxicity toward the A549 lung cancer cell line was carried out using the MTT assay, and the results indicated the anticancer effect of the formulation on the A549 cell line. Furthermore, the aerodynamic properties of the particles in a simulated lung model were evaluated using Anderson's cascade impactor, which shows that the formulation is suitable for inhalation delivery. Overall, this novel ConA-SLN formulation nanocarrier opens up exciting possibilities for targeted drug delivery, treatment monitoring, diagnosis, and sustained drug release.Data availability
Data are available upon request from the authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The project was supported by AICTE under the Research Promotion Scheme (File No. 8-90/FDC/RPS(POLICY-1)/2019-20, dated 14/08/2020).This project was supported by Researchers Supporting Project number (RSP2025R98), King Saud University, Riyadh, Saudi Arabia.
References
- A. Sharma, D. Shambhwani, S. Pandey, J. Singh, H. Lalhlenmawia, M. Kumarasamy, S. K. Singh, D. K. Chellappan, G. Gupta, P. Prasher, K. Dua and D. Kumar, ACS Omega, 2023, 8, 10–41 CrossRef CAS PubMed.
- Q. Wang, Z. H. Gümüş, C. Colarossi, L. Memeo, X. Wang, C. Y. Kong and P. Boffetta, J. Thorac. Oncol., 2023, 18, 31–46 CrossRef CAS PubMed.
- W.-Z. Li, X. Gui, H. Liang, W. Wang, W. Liang and J. He, J. Clin. Oncol., 2024, 42, 11169–11169 CrossRef.
- C. T. Jani, S. A. Kareff, H. Singh, A. S. Zetina and G. Lopes, Cancer Res., 2024, 84, 803–803 CrossRef.
- K. Chansky, M. Rigney and J. C. King, Cancer Med., 2024, 13, e6702 CrossRef.
- J. Kong, F. Xu, J. Qu, Y. Wang, M. Gao, H. Yu and B. Qian, Oncotarget, 2015, 6, 2573–2582 CrossRef PubMed.
- C. Li, S. Lei, L. Ding, Y. Xu, X. Wu, H. Wang, Z. Zhang, T. Gao, Y. Zhang and L. Li, Chin. Med. J. (Engl.), 2023, 136, 1583–1590 Search PubMed.
- B. Liu, H. Zhou, L. Tan, K. T. H. Siu and X.-Y. Guan, Signal Transduction Targeted Ther., 2024, 9, 175 CrossRef.
- V. Mittal, T. El Rayes, N. Narula, T. E. McGraw, N. K. Altorki and M. H. Barcellos-Hoff, 2016, pp. 75–110.
- H. Lemjabbar-Alaoui, O. U. Hassan, Y.-W. Yang and P. Buchanan, Biochim. Biophys. Acta, Rev. Cancer, 2015, 1856, 189–210 CrossRef CAS.
- J. Jin, K. Hu, Y. Zhou and W. Li, PLoS One, 2017, 12, e0184412 CrossRef PubMed.
- H. Shen, L. Wang, W. Chen, K. Menard, Y. Hong, Y. Tian, S. J. Bonacorsi, W. G. Humphreys, F. Y. Lee and J. Gan, Acta Pharm. Sin. B, 2016, 6, 460–467 CrossRef PubMed.
- T. Zhang, Y. Chen, Y. Ge, Y. Hu, M. Li and Y. Jin, Acta Pharm. Sin. B, 2018, 8, 440–448 CrossRef PubMed.
- W. Wang, T. Chen, H. Xu, B. Ren, X. Cheng, R. Qi, H. Liu, Y. Wang, L. Yan, S. Chen, Q. Yang and C. Chen, Molecules, 2018, 23, 1578 CrossRef.
- A. Sultana, M. Zare, V. Thomas, T. S. S. Kumar and S. Ramakrishna, Med. Drug Discovery, 2022, 15, 100134 CrossRef CAS.
- N. Rashidi, M. Davidson, V. Apostolopoulos and K. Nurgali, J. Drug Delivery Sci. Technol., 2024, 95, 105599 CrossRef CAS.
- C. Y. Hsu, A. M. Rheima, M. M. Kadhim, N. N. Ahmed, S. H. Mohammed, F. H. Abbas, Z. T. Abed, Z. M. Mahdi, Z. S. Abbas, S. K. Hachim, F. K. Ali, Z. H. Mahmoud and E. Kianfar, S. Afr. J. Chem. Eng., 2023, 46, 233–270 Search PubMed.
- R. Pop, D. Hodor, C. Cătoi, T. Mocan, L. Mocan and A.-F. Tăbăran, Targets, 2024, 2, 52–63 CrossRef.
- D. Rosenblum, N. Joshi, W. Tao, J. M. Karp and D. Peer, Nat. Commun., 2018, 9, 1410 CrossRef PubMed.
- Y. Duan, A. Dhar, C. Patel, M. Khimani, S. Neogi, P. Sharma, N. Siva Kumar and R. L. Vekariya, RSC Adv., 2020, 10, 26777–26791 RSC.
- V.-A. Duong, T.-T.-L. Nguyen and H.-J. Maeng, Molecules, 2020, 25, 4781 CrossRef CAS.
- D. H. Rich and J. Singh, in Major Methods of Peptide Bond Formation, Elsevier, 1979, pp. 241–261 Search PubMed.
- R. S. Tade, M. P. More, V. K. Chatap, P. O. Patil and P. K. Deshmukh, Mater. Res. Express, 2018, 5, 116102 CrossRef.
- S. B. Wakshe, S. R. Patil, A. D. Patil, P. B. Choudhari, S. B. Patil, P. V. Anbhule, D. Sohn and G. B. Kolekar, J. Mol. Struct., 2020, 1201, 127156 CrossRef CAS.
- V. D. Ragole, S. V. Gayakwad and D. S. Wankhede, J. Iran. Chem. Soc., 2022, 19, 1993–2004 CrossRef CAS.
- S. Chennakesavulu, A. Mishra, A. Sudheer, C. Sowmya, C. Suryaprakash Reddy and E. Bhargav, Asian J. Pharm. Sci., 2018, 13, 91–100 CrossRef CAS PubMed.
- J. Youshia, A. O. Kamel, A. El Shamy and S. Mansour, Eur. J. Pharm. Sci., 2021, 163, 105887 CrossRef CAS PubMed.
- N. Dudhipala and K. Veerabrahma, Drug Delivery, 2016, 23, 395–404 CrossRef CAS.
- D. Pooja, H. Kulhari, M. Kuncha, S. S. Rachamalla, D. J. Adams, V. Bansal and R. Sistla, Mol. Pharm., 2016, 13, 3903–3912 CrossRef CAS PubMed.
- M. Jana, U. K. Biswas, C. S. Patra, B. Debnath, S. Sharma and S. Naskar, Pharm. Nanotechnol., 2024 DOI:10.2174/0122117385312175240502100018.
- A. H. Pagar and A. Y. Pawar, Int. J. Drug Delivery Technol., 2023, 13, 1611–1622 CrossRef.
- M. C. Fontana, J. V. Laureano, B. Forgearini, J. dos Santos, A. R. Pohlmann, S. S. Guterres, B. V. de Araujo and R. C. R. Beck, Int. J. Pharm., 2020, 585, 119429 CrossRef CAS.
- T. Gupta, J. Singh, S. Kaur, S. Sandhu, G. Singh and I. P. Kaur, Front. Bioeng. Biotechnol., 2020, 8, 879 CrossRef.
- D. Sivadasan, K. Ramakrishnan, J. Mahendran, H. Ranganathan, A. Karuppaiah and H. Rahman, Int. J. Mol. Sci., 2023, 24, 6199 CrossRef CAS PubMed.
- S. Doktorovova, E. B. Souto and A. M. Silva, Pharm. Dev. Technol., 2018, 23, 96–105 CrossRef CAS PubMed.
- E. S. Behbahani, M. Ghaedi, M. Abbaspour and K. Rostamizadeh, Ultrason. Sonochem., 2017, 38, 271–280 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally to this work and share the first author position. |
| This journal is © The Royal Society of Chemistry 2025 |