VRBE and HRBE schemes of lanthanides: design of dual-luminescence-center long persistent luminescence phosphors with Pr3+ or/and Tb3+ doping in Mg3Y2Ge3O12 garnet with high storage capacity for anti-counterfeiting, information storage, and X-ray imaging†
Yuanying
Lin
a,
Chengzhuo
Ming
a,
Ruonan
Xuan
b and
Weisheng
Liu
 *a
*a
aCollege of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou 730000, PR China. E-mail: liuws@lzu.edu.cn; Tel: +86-18993168326
bSchool of Chemistry and Chemical Engineering, Qinghai Normal University, Xining 810008, PR China
First published on 20th March 2025
Abstract
The development of X-ray charged long persistent luminescence (LPL) phosphors with excellent storage capacity remains a significant challenge. These phosphors have multifunctional applications in white light-emitting diodes (w-LEDs), multi-mode anti-counterfeiting, information storage, and X-ray imaging. Herein, by combining the refined chemical shift model, optical spectroscopy, and thermoluminescence (TL) curves, the host-referred and vacuum-referred binding energy (HRBE and VRBE) schemes about Mg3Y2Ge3O12:Ln3+(MYGO:Ln, Ln = Pr or/and Tb) phosphors are constructed and validated. MYGO:Pr,Tb avoids the re-absorption issue in the blue light region and agrees well with blue phosphors for w-LED applications. MYGO:Pr,Tb Charged with X-rays (Xc), the TL intensity ratios to commercial SrAl2O4:Eu2+,Dy3+/BaFBr(I):Eu2+ are 1.517 and 1.435, respectively, and 50% of the carriers remain for over 1000 h. Finally, the MYGO series phosphors are successfully applied in multi-mode anti-counterfeiting, information storage, and X-ray imaging.
1. Introduction
Since the discovery of X-rays, they have played a central role in various aspects of human life. In particular, X-ray indirect detection has found widespread applications in medical imaging, industrial machinery nondestructive testing, civil aviation security inspections, and nuclear physics engineering.1–11 The key to X-ray indirect detection lies in the ability of imaging materials to absorb X-rays and convert them into visible luminescence signals.11–14 Halide chalcogenide scintillators, as conventional X-ray imaging materials, offer high light yield but are often complex to synthesize, produce significant pollutants, and suffer from poor stability due to decomposition caused by water, air, or ionizing radiation.15–21 These limitations have hindered their broader commercial applications. Long persistent luminescence (LPL) storage materials have emerged as a promising alternative, offering advantages such as ease of synthesis, environmental friendliness, and high stability. These materials overcome the drawbacks of traditional halide chalcogenide scintillators and are being increasingly used to meet societal demands for efficient information storage.22–29 Additionally, LPL materials play an important role in multi-mode anti-counterfeiting, battery-free radiation dose measurement, X-ray imaging, and high-capacity storage.23,25,26,30–34Luminescence centers are a key component of phosphors, determining their emission range.23,25,26 To date, green LPL phosphors such as SrAl2O4:Eu2+,Dy3+ and BaFBr(I):Eu2+, where a single lanthanide ion serves as the luminescence center, remain the benchmark for LPL and storage materials. However, red and yellow phosphors are relatively scarce.25,26,35 Moreover, conventional yellow phosphors suffer from re-absorption in the blue light region and lack red spectral components, resulting in low color rendering and uneven white light tones in novel lighting applications such as w-LEDs.36 Dual-luminescence-center yellow LPL materials can overcome these issues, enabling proportional chemical sensing, multi-color displays, and imaging, thereby advancing complex anti-counterfeiting technologies. These characteristics make the development of dual-luminescence-center LPL materials a reasonable strategy for encryption of military information and multi-mode anti-counterfeiting applications.
Traps are another crucial component of phosphors, as their concentration and depth significantly influence LPL and storage properties.21,23,37 While the LPL mechanism remains insufficiently understood, accurate methods for predicting trap depths are in high demand.23 The host-referred and vacuum-referred binding energy (HRBE and VRBE) schemes, based on Dorenbos’ theory, enable the determination of energy levels for divalent and trivalent lanthanide ions and the prediction of trap depths.38–40 Efficient LPL and storage phosphor design can be achieved by adjusting the type and concentration of lanthanide ions to regulate the trap depth and carrier distribution.23 However, HRBE and VRBE schemes have thus far been applied to only a limited number of materials.25
This study focuses on the development of dual-luminescence-center yellow LPL materials for X-ray imaging and anti-counterfeiting applications. In 2020, Jiang et al. first reported a Mg3Y2Ge3O12:Ce3+ (MYGO:Ce) garnet phosphor and achieved red-shifted Ce3+ emission by substituting Ge4+ with Si4+ to enlarge the host bandgap.41 Subsequent studies by Liao et al., Hou et al., and Meng et al. introduced various dopants such as Eu3+, Ce3+, and Bi3+, enhancing properties like thermal stability, tunable luminescence, and extended LPL durations.42–44 Recently, Krieke et al. reported MYGO:Tb3+, a garnet LPL phosphor with bright blue-green tunable luminescence.45 These findings suggest that the MYGO host has the potential for achieving dual-luminescence-center LPL materials with multifunctional applications. In this work, we successfully constructed the HRBE and VRBE schemes for MYGO, predicting trap depths of 1.68 eV and 1.86 eV for Pr3+ and Tb3+, respectively. These depths meet the requirements for high-capacity information storage, which typically requires trap depths greater than 1.00 eV. This study provides valuable insights for advancing the development of multi-mode optical anti-counterfeiting materials and information encryption technologies. Regarding the LPL performance of MYGO:Ln (MYGO:Pr, MYGO:Tb, and MYGO:Pr,Tb), the τp, τt, and τc values under 254 nm ultraviolet (UV) lamp charging (Uc) were approximately 405, 179, and 48 min, respectively. Additionally, the initial LPL intensities for all three types exceeded 100 mcd m−2. MYGO:Pr (τp = 405 min) demonstrated a significant advantage among lanthanide-activated red LPL materials. As a dual-luminescence-center system, Pr3+ and Tb3+ produced distinct emission responses, enabling MYGO:Pr,Tb to exhibit yellow LPL under Uc. This exceptional performance highlights its potential for multi-level anti-counterfeiting applications. Regarding the storage performance of MYGO:Ln, the experimental trap depths of Pr3+ and Tb3+, derived from the thermoluminescence (TL) curves of MYGO:Pr,Tb under X-ray charging (Xc), were measured as 1.684 and 1.867 eV, respectively. These values align well with the predictions from the HRBE and VRBE schemes. Additionally, the Xc TL curves indicated that 49% of the carriers stored in MYGO:Pr,Tb remained intact after 1000 h. When compared to commercial phosphors SrAl2O4:Eu2+,Dy3+/BaFBr(I):Eu2+, the TL intensity ratios (r1/r4, r2/r5, and r3/r6) for MYGO:Pr, MYGO:Tb, and MYGO:Pr,Tb were higher, with values of 1.298/1.228, 0.504/0.477, and 1.517/1.435, respectively. These results further emphasize the superior storage performance of MYGO:Ln phosphors. The LPL mechanism model elucidated the storage and release processes of carriers, identified defect types and trap depths, and detailed the energy level transitions and emission processes of the dual-luminescence centers, Pr3+ and Tb3+. Through comprehensive analyses of photoluminescence (PL), LPL, TL, and thermally stimulated luminescence (TSL) properties of MYGO:Pr, MYGO:Tb, and MYGO:Pr,Tb (MYGO:Ln), the multifunctional applications of deep-trap LPL phosphors in multi-mode anti-counterfeiting, information storage, and X-ray imaging have been realized. This study provides a robust theoretical foundation and experimental framework for further prediction and validation of energy levels in lanthanide-doped garnets. It also offers a theoretical perspective for the rational design of novel LPL and storage phosphors.
2. Results and discussion
XRD and DRS of MYGO
Mg3Y2Ge3O12 (MYGO) is a unique garnet due to its multiple cationic lattice sites and structural properties that allow multiple cations to occupy the same lattice site. This characteristic facilitates the formation of a distorted lattice environment and complex traps.45,46 For A3B2C3O12 garnets, there are three distinct cationic sites: the dodecahedral A site with a coordination number (CN) of 8, the octahedral B site (CN = 6), and the tetrahedral C site (CN = 4).47 The ionic distribution in these sites is primarily determined by the relative size of the ions: larger cations occupy the A site, medium-sized cations are located in the B site, and smaller cations reside in the C site. However, MYGO belongs to a group called inverse garnets, analogous to inverse spinels.46 The XRD patterns of MYGO:Ln are shown in Fig. 1(a) and Fig. S1.† The recorded patterns align well with the standard reference card for MYGO (no. AMCSD#0016886), confirming that all MYGO:Ln samples are single phase.46,47. As shown in Fig. 1(a), the slightly leftward shift of the XRD peaks (2θ values) with increasing concentrations of Pr3+ and/or Tb3+ is attributed to their larger ionic radii (Pr3+: 1.126 Å, Tb3+: 1.040 Å) compared to Y3+ (1.019 Å) for CN = 8. This shift indicates lattice expansion in the garnet structure.24–26,45 The structural model of MYGO is presented in Fig. 1(b). In the MYGO structure, Mg2+ (0.89 Å) shares the dodecahedral A position with Y3+ despite their significant differences in ionic radii. Consequently, the chemical formula can be expressed as (Y2Mg)Mg2Ge3O12.45 This substitution distorts the local environment, promoting the formation of cationic and anionic vacancies, which enhance luminescence properties. Considering the similarity in ionic radii and coordination numbers, it is reasonable for lanthanide ions (Ln) to occupy the Y3+ site (Y/MgO8 dodecahedron) as luminescence centers.45 To further investigate the crystal structure, XRD patterns were analyzed using Rietveld refinement, as shown in Fig. 1(c and d), Fig. S2a, b, and Table S2.† The refinement results show good convergence, with χ2 values of 2.26 (host), 2.08 (Pr), 2.17 (Tb), and 1.93 (Pr,Tb), respectively. These results confirm that the crystal system of MYGO:Ln is an inverse-garnet-type cubic structure, based on the Ia3d (230) space group.To investigate the absorption properties of the host, the ultraviolet-visible diffuse reflectance spectra (UVV-DRS) at room temperature (RT) are presented in the inset of Fig. 1(e). According to the Mott and Davis theory, the fundamental absorption threshold (Efa) values of the samples were calculated as 5.626 (host) 5.888 (Pr), 5.849 (Tb) and 5.855 (Pr and Tb) eV, as shown in Fig. 1(e, f) and Fig. S2c, d.†23,48,49 Due to the relatively coarse DRS data for the host, the Efa value of 5.626 eV calculated by extrapolation from eqn (S1) and (2)† may not be entirely accurate. This is corroborated by the smoother DRS curves of MYGO:Ln, which provide more reliable results. The UVV-DRS and absorption curves (inset of Fig. S2c†) reveal that an absorption band with a peak at 217 nm (5.714 eV) is present in both MYGO:Ln and the host. This band is attributed to the fundamental absorption threshold of the host. Based on this common feature, the Efa value of the host was corrected to 5.714 eV, providing a more reasonable and accurate estimate. Notably, the absorption edge was found to be blue-shifted, and the detailed calculations and underlying reasons for this blue shift are explained in Fig. S2† in the discussion section. The SEM images of MYGO:Ln (Fig. S3a–d†) show that the samples consist of spherical particles with sizes ranging from approximately 0.3 μm to 5 μm. The EDS analysis (Fig. S3e–h†) confirms that Ln3+ ions are effectively doped into the MYGO lattice.
HRBE and VRBE schemes in MYGO
To analyze the trapping and releasing processes of charge carriers, the VRBE and HRBE schemes in MYGO have been constructed and exploited. A unique feature of the VRBE and HRBE schemes is their ability to compare the binding energy of electrons in the conduction band (CB), valence band (VB), or 4f ground states (GS) of lanthanides across various compounds using a common energy reference.23–26 The Efa of MYGO is 5.714 eV. The band gap, influenced by electron–hole binding energy and temperature, is approximately calculated using Formula (1) as a rule of thumb:39,50| Eex = Efa + 0.008(Eex)2 | (1) |
U(6, A) = 5.44 + 2.834![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−εc/2.2) exp(−εc/2.2) | (2) |
 | (3) |
PL and RL in MYGO:Ln
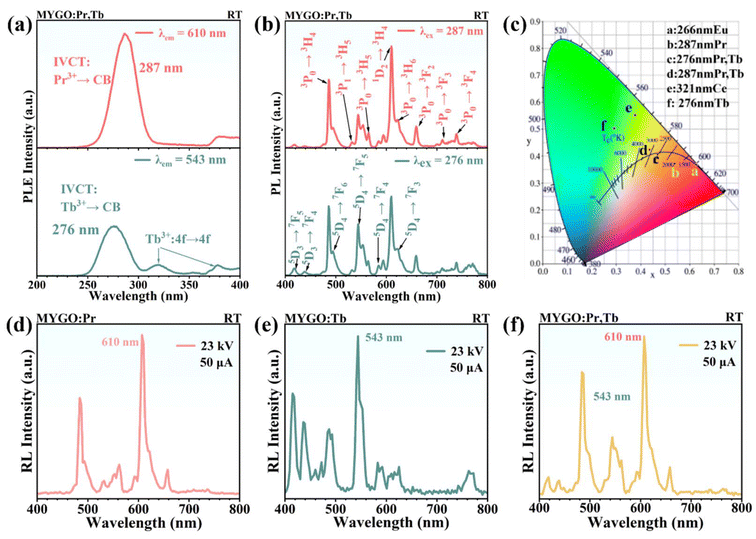
As shown in Fig. 3(a and b), the PLE and PL spectra of MYGO:Pr,Tb were recorded at RT to analyze the optical transition processes of the ions.33 In Fig. 3(a), the PLE spectrum monitored at 610 nm shows an excitation band at ≈287 nm attributed to IVCT: Pr3+ → CB, while the 370–400 nm band is hypothesized to originate from host intrinsic defects. The PLE spectrum monitored at 544 nm shows two excitation bands at ≈276 nm and 320 nm, along with weaker bands in the 350–390 nm range. The strongest excitation band at 276 nm is attributed to IVCT: Tb3+ → CB. The most intense excitation band peaked at 276 nm is associated with IVCT: Tb3+ → CB. This assignment (IVCT: Pr3+/Tb3+ → CB) agrees well with the HRBE and VRBE schemes in Fig. 2(e). The excitation band at 320 nm in the investigated host remains ambiguous, potentially arising from the f–d transitions of Tb3+ in a distorted environment or point defects in the material. Excitation bands in the 350–390 nm range are attributed to the spin-forbidden f–f transitions of Tb3+. In Fig. 3(b), excitation at 287 nm (Pr3+ emissions) reveals several Pr3+ 4f–4f transition peaks at 487, 532, 564, 610, 622, 660, 711, and 739 nm, corresponding to transitions 3P0 → 3H4, 3P1 → 3H5, 3P0 → 3H5, 1D2 → 3H4, 3P0 → 3H6, 3P0 → 3F2, 3P0 → 3F3, and 3P0 → 3F4, respectively. For excitation at 276 nm (Tb3+ emissions), the bands correspond to 5D3 → 7F5 (417 nm), 5D3 → 7F4 (438 nm), 5D4 → 7F6 (488 nm), 5D4 → 7F5 (544 nm), 5D4 → 7F4 (584 nm), and 5D4 → 7F3 (619 nm). Fig. 3(b) shows that partial overlap of excitation bands results in PL spectra of co-doped MYGO:Pr,Tb displaying characteristic emission peaks of both Pr3+ and Tb3+. This indicates that co-doping does not cause fluorescence quenching and achieves dual-luminescence-center emissions. To better evaluate luminescence properties, the PL quantum yields (PLQYs) of MYGO:Ln were measured. Fig. S5† shows that the PLQYs of MYGO:Pr, MYGO:Tb, and MYGO:Pr,Tb are 68.2%, 88.8%, and 71.1%, respectively, indicating that the PL properties of MYGO:Ln are influenced by the type of doping ion. The highest PLQY of 90.4% is achieved with Tb3+ doping, confirming the excellent PL properties of MYGO:Tb. Fig. 3(a and b) and Fig. S5† show that the yellow MYGO:Pr,Tb phosphors have PL peaks between 450 nm and 800 nm, with an excitation range below 450 nm, effectively restricting blue light re-absorption. MYGO:Pr,Tb is well matched to blue phosphors excitable by near-UV chips and can be integrated into w-LEDs. Fig. 3(c) presents the CIE color coordinates of the MYGO series at (0.63, 0.37), (0.54, 0.37), (0.47, 0.41), (0.44, 0.42), (0.38, 0.55), and (0.29, 0.50), respectively. This clearly indicates that different PL colors arise from distinct luminescence centers. To assess the feasibility of applying the MYGO series to X-ray multifunctional fields, X-ray RL spectra were recorded. As shown in Fig. 3(d–f), the radioluminescence (RL) spectra of MYGO:Pr (d), MYGO:Tb (e), and MYGO:Pr,Tb (f) closely resemble their respective PL spectra (Fig. S4†). These results demonstrate that MYGO:Ln can be excited by X-rays, with the RL performance closely linked to the luminescence center ions.LPL processes in MYGO:Ln
To identify the recombination centers and clarify the LPL decay processes, RT LPL decay spectra were recorded after Uc.22Fig. 4(a–c) show 2D contour plots of MYGO:Pr (a), MYGO:Tb (b), and MYGO:Pr,Tb (c) for durations of 120, 60, and 15 min, respectively. The LPL spectra of MYGO:Pr and MYGO:Tb, ranging from 400 to 800 nm, exhibit similar trends, with the LPL intensity decreasing over time. For MYGO:Pr,Tb, the LPL decay spectra, resembling the PL spectra, primarily consist of Pr3+: 3P0 → 3H4, 3H6 and Tb3+: 5D3, 5D4 → 7Fj transitions. In Fig. 4(a), the LPL decay curves for Pr3+: 3P0 → 3H4, 3H6 emissions at 487 and 610 nm exhibit a rapid initial decay within 20 min, followed by a slower decrease. In Fig. 4(b), the LPL decay curves for Tb3+: 5D4 → 7F5 emission (λem = 543 nm) show low initial intensity and rapid decay. In Fig. 4(c), the decay curves at 487, 543, and 610 nm for MYGO:Pr,Tb indicate that Pr3+ has a higher LPL intensity than Tb3+, and both ions serve as LPL and recombination centers. As shown in Fig. 3(d), the initial intensity after Uc exceeded 100 mcd m−2, and the τp, τt, and τc values were approximately 405, 179, and 48 min, respectively, demonstrating excellent luminescence performance for MYGO:Ln. To study electron and hole trapping and release processes, RT isothermal LPLE spectra of MYGO:Pr,Tb (monitored at 487, 543, and 610 nm) were recorded after 200–400 nm photon charging, as shown in Fig. 4(e–g). In Fig. 4(h), the LPLE plots of MYGO:Pr,Tb are similar to the PLE spectra but exhibit a blue shift. This indicates that the LPL of MYGO:Pr,Tb phosphors is driven by IVCT: Pr3+/Tb3+ → CB. Notably, no intrinsic defect bands appear in the LPLE spectra, indicating that intrinsic defect generation is not involved in the LPL processes.PSL processes in MYGO:Ln
Photo-stimulated luminescence (PSL) is a phenomenon where low-energy light stimulates materials excited by high-energy light to emit luminescence.22 It is widely applied in advanced optical storage technologies. Some charge carriers trapped in the material cannot be activated at RT. Stored carriers can be released through low-energy photo-stimulation. Deep traps in PSL materials prevent information loss. In other words, PSL phenomena can qualitatively predict information storage properties. Therefore, MYGO:Pr,Tb was investigated as a PSL research object to explore the possibility of containing independent deep traps, as shown in Fig. 5(a–d). Real-time PSL curves were recorded after Uc. The 808 nm and 980 nm NIR lasers were alternately turned “on” and “off” every 30 s for 30 min, causing alternating jumps and drops in PSL intensity. This indicates that electrons in deep traps cannot be fully cleared by thermal perturbation at RT but are effectively excited by NIR stimulation. For MYGO:Pr,Tb, the 808 nm and 980 nm NIR PSL (PSL-808, PSL-980) enable partially responsive optical information readout with a high response rate. These results suggest that MYGO:Pr,Tb can enable multi-mode anti-counterfeiting applications, including PL, LPL, and NIR PSL. As shown in Fig. 5(e), the X-ray storage performance of MYGO was quantified by comparing the TL curves of MYGO:Ln with commercial phosphors SrAl2O4:Eu2+,Dy3+ and BaFBr(I):Eu2+ after Xc. For MYGO:Ln, the peak temperature of the TL band (Tm) shifted to higher values, with TL intensity ratios of 1.298/1.228 (r1/r4), 0.504/0.477 (r2/r5), and 1.517/1.435 (r3/r6), respectively. To explore anti-counterfeiting applications, TL curves (Xc) of MYGO:Pr,Tb with varying durations were analyzed. As shown in Fig. 5(f), the TL intensity increased with Xc time, and TL peaks shifted to the right. The inset of Fig. 5(f) shows that the integrated TL intensity fits the equation TL × 10−8 = 2.002 × t − 0.422 (R2 = 0.9996), where t represents the X-ray charging time. This suggests that MYGO:Pr,Tb storage phosphors are promising candidates for X-ray detection dosimeters.TL curves and trap depths of MYGO:Ln
To determine the trap depths of MYGO:Ln and validate the VRBE and HRBE models, we systematically studied and recorded their TL curves. Fig. 6(a–c) show the TL curves of MYGO:Ln (310–650 K) after Xc at heating rates (β) ranging from 0.3 to 1.5 K s−1. As β increased, the TL intensity (ITL) of MYGO:Ln strengthened, and Tm shifted to higher temperatures. To analyze the recombination and luminescence centers during TL decay, TL curves of MYGO:Ln at β = 1 K s−1 in Fig. 6(d) were Gaussian fitted and compared. The fitting parameters for TL curves are listed in Tables S5–7.† In Fig. 6(d1) and (d3), Tm for MYGO:Tb (Tm-Tb) is higher than that for MYGO:Pr (Tm-Pr). In Fig. 6(d3), ITL for MYGO:Pr (ITL-Pr) is approximately 2.5 times that of MYGO:Tb (ITL-Tb). These bands are speculated to originate from unknown intrinsic defects, impurities, or Ln3+. To better understand the type and depth of traps, Gaussian-fitted curves of MYGO:Ln in Fig. 6(d) and Fig. S6–8† were analyzed. TL curves of MYGO:Pr [Fig. 6(d3), Fig. S6†] and MYGO:Tb [Fig. 6(d1), Fig. S7†] each contain four fitted TL peaks. Fitted TL bands 1–3 of MYGO:Pr and MYGO:Tb share similar shapes and Tm values (≈380, 420, and 490 K). Therefore, The TL fitted curves of MYGO:Pr and MYGO:Tb are divided into two parts: low-temperature bands 1–3 (bands H) and high-temperature bands 4 (band P, Tm = 549 K in MYGO:Pr) and 5 (band T, Tm = 569 K in MYGO:Tb). Bands H, P and T correspond to host-induced traps (intrinsic defects), Pr3+-induced defects, and Tb3+-induced defects, respectively. For MYGO:Pr,Tb, five TL fitted peaks are shown in Fig. 6(d2) and Fig. S8.† The low-temperature bands 1–3 (Tm ≈ 382 K, 427 K, 489 K) are similar to the bands H of MYGO:Pr and MYGO:Tb, while the high-temperature bands 4 (Tm ≈ 543 K) and 5 (Tm ≈ 581 K) resemble band P (Tm ≈ 549 K) of MYGO:Pr and band T (Tm ≈ 569 K) of MYGO:Tb, respectively. Thus, the Gaussian-fitted TL curves of MYGO:Pr,Tb can be divided into three parts: bands H (1–3), band P (4), and band T (5). Therefore, the TL curves of MYGO:Ln comprise bands H, P, and T, corresponding to host intrinsic defects, Pr3+ traps, and Tb3+ traps, respectively. Fig. 6(e–h) display TL bands P for MYGO:Pr, bands T for MYGO:Tb, and bands P and T (Pc, Tc) for MYGO:Pr,Tb after Xc. Source data are provided in Fig. S6–8.† Finally, trap depths were estimated using formula (4) and the variable β rate profiles of TL bands P, T, Pc and Tc in Fig. 6(e–h):| βE/(kBTm2) = s×exp(−E/kBTm) | (4) |
Here, β (K s−1) is the heating rate, E (eV) is the trap depth, kB is the Boltzmann constant, Tm (K) is the TL peak temperature, and s (s−1) is the frequency factor. By plotting ln (Tm2) against 1/(kB × Tm), straight lines representing trap depths E (slopes) and frequency factors s are shown in Fig. 6(j) and Table S9.† In Fig. 6(j), fitted lines from the same traps are parallel and exhibit consistent trap depths E. Trap depths E for bands P, T, Pc and Tc are calculated as 1.668, 1.684, 1.867, and 1.798 eV, respectively. Based on Fig. 6, Tb3+ co-doping generates new hole traps and increases trap depths, while Pr3+ co-doping enhances the TL intensity via Coulomb interactions. Altogether, Pr3+ and Tb3+ co-doping improves the luminescence performance through higher TL intensity and greater trap depths. Notably, the trap depths agree well with the energy differences between 4f-Pr3+/Tb3+ and the VB top in the VRBE and HRBE schemes. Consequently, we identified a trap depth engineering strategy for MYGO and successfully developed novel deep-trap LPL and X-ray storage materials.
Charge carrier trapping and release processes in MYGO garnet
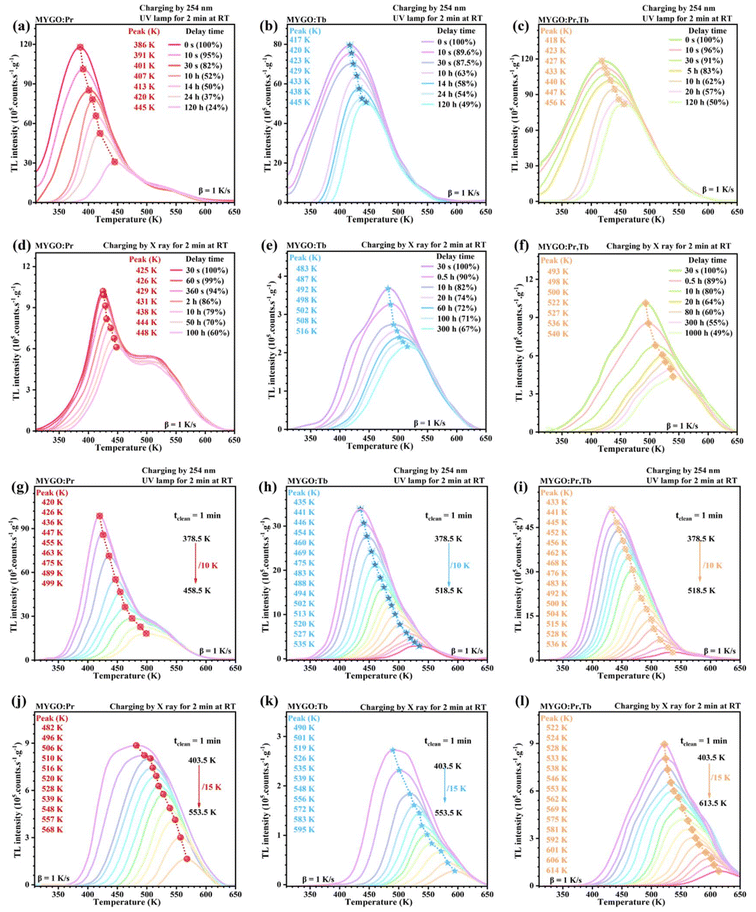
Higher initial storage capacity and lower release rates are critical for superior storage material performance. The integral area percentage (ITL) and Tm of TL curves represent carrier storage capacity and release rates. TL curves of MYGO:Ln (310–650 K) were investigated after Uc or Xc for 2 min to quantify the storage capacity in the temperature-cleaning (tclean) or decay time (td) dimensions. Fig. 7 shows that ITL gradually decreases and Tm shifts to higher temperatures with increasing tclean or td. In the td dimension, Fig. 7(a and b) illustrate TL curve trends for MYGO:Pr (a) and MYGO:Tb (b) as td increases (0 s–120 h) after Uc. The ITL ratio of MYGO:Pr to MYGO:Tb at 0 s is 1.5, indicating that MYGO:Pr has higher initial storage capacity. Compared to Tm-Pr (≈386 K), Tm-Tb (≈417 K) indicates deeper trap depths in MYGO:Tb. In Fig. 7(a and b), MYGO:Tb retains 49% of carriers at td = 120 h, demonstrating a lower release rate and reduced RT thermal perturbation. Fig. 7(c) shows that MYGO:Pr,Tb retains high initial ITL, Tm (≈418 K), and 50% of carriers at td = 120 h, combining the high storage capacity of MYGO:Pr and low release rate of MYGO:Tb. The type of charging energy significantly affects the storage capacity. In Fig. 7(d–f), ITL after Xc decreases by an order of magnitude compared to Uc, but Tm increases to 425 K (d), 483 K (e), and 493 K (f), indicating that carriers shift to deeper traps with lower release rates. For MYGO:Pr,Tb, half of the carriers are released after 100 h (Xc) or 1000 h (Uc).Therefore, Fig. 7(a–f) illustrate that Pr3+ and Tb3+ co-doping in MYGO enhances the storage performance compared to single doping in the td dimension. And X-rays are more suitable for long-term storage applications, while 254 nm UV is better for large-capacity storage. In the tclean dimension, storage performance was evaluated by uniformly increasing tclean. Fig. 7(g–l) show that Tm shifted to higher temperatures after Xc (g–i) or Uc (j–l) for MYGO:Ln. The TL curves for tclean and td yielded similar results, showing that MYGO:Pr (g and j), MYGO:Tb (h and k), and MYGO:Pr,Tb (i and l) exhibit higher initial storage capacity, lower release rates, or both. Fig. 7(g–i) show that after Uc and increasing tclean, carrier release is the fastest in MYGO:Pr (j) but slower and more gradual in MYGO:Tb (h) and MYGO:Pr,Tb (i). The initial tclean (Xc) was raised to 403 K. In Fig. 7(j and k), as tclean increases from 403.5 K to 553.5 K, stored carriers are slowly released, and Tm shifts to 482 K (MYGO:Pr) or 495 K (MYGO:Tb). For MYGO:Pr,Tb (i and l), major carrier release requires Tm to exceed 518.5 K (Uc) or 613.5 K (Xc). Fig. 7 confirms that MYGO:Ln, especially MYGO:Pr,Tb, is a novel deep-trap X-ray storage material with excellent storage performance.
Applications
Mechanism of LPL
Above all, the possible LPL mechanism in MYGO:Ln is proposed and illustrated in Fig. 10. Under 254 nm UV or X-ray charging, electrons in the VB are excited to the CB, leaving behind numerous holes in the VB (1). Some electrons immediately return to the ground state, producing direct PL from Pr3+ or Tb3+. Simultaneously, holes in the VB or electrons in the CB are trapped by hole traps (Ln3+: Pr3+ and/or Tb3+) or electron traps (MYGO host), respectively (2). After the excitation ceases, trapped electrons and holes are released into the CB and VB, respectively, under thermal interference at RT (LPL) or additional energy stimulation, e.g. TSL or PSL (3). Ln3+ (case 1 or 2) first attracts an electron or a hole (4) and subsequently attracts a hole or an electron, forming an exciton under Coulomb force. Alternatively, released electrons and holes directly recombine to form excitons, which migrate through the lattice and are eventually captured by Ln3+ (5). In all cases, Ln3+ binds to excitons, acquires their recombination energy, and transitions to the excited state (6). After nonradiative relaxation, LPL arises from the 4f → 4f transition of Ln3+. | ||
| Fig. 10 The schematic illustration of the LPL mechanism of the MYGO:Pr,Tb phosphor depending on the VRBE and HRBE models. | ||
3. Conclusions
In this work, Mg3Y2Ge3O12 (MYGO) series LPL materials were synthesized using a high-temperature solid-phase method. Combining the refined chemical shift model, optical spectroscopy, and TL curves, the HRBE and VRBE schemes for MYGO materials were constructed and validated, predicting trap depths of Pr3+ and Tb3+ as 1.68 eV and 1.86 eV, respectively. Experimental trap depths calculated from Xc TL curves matched the predictions. Lanthanide ions (Pr3+ and/or Tb3+) act as deep hole traps, while host intrinsic defects serve as electron traps. LPL decay curves demonstrate excellent performance of the MYGO series, with initial intensities exceeding 100 mcd m−2 and τp, τt, and τc measured as 405, 179, and 48 min, respectively. The red LPL phosphor MYGO:Pr offers a significant advantage, as most lanthanide-activated LPL materials last less than 6 h. As a novel yellow LPL phosphor, MYGO:Pr,Tb emits across 400–800 nm with an excitation range below 400 nm, addressing issues like blue light re-absorption and insufficient red components, which affect color rendering and white light uniformity. Regarding storage ability, 49% of carriers in MYGO:Pr,Tb can be retained over 1000 h.TL intensity ratios for MYGO:Pr, MYGO:Tb, and MYGO:Pr,Tb compared to commercial SrAl2O4:Eu2+,Dy3+/BaFBr(I):Eu2+ are 1.298/1.228 (r1/r4), 0.504/0.477 (r2/r5), and 1.517/1.435 (r3/r6), respectively. Based on the LPL mechanism model, the processes of energy level transitions at the luminescence center, as well as the storage and release of carriers, have been elucidated. Finally, the MYGO series demonstrates multifunctional applications in anti-counterfeiting, information storage, and X-ray imaging through PL, LPL, TL, and TSL. Above all, MYGO:Pr,Tb has the prospects of being matched well with blue phosphors and used for w-LED applications, and the MYGO series can be an excellent candidate for multifunctional applications in LPL, anti-counterfeiting, advanced displays, high-capacity storage and X-ray imaging. This study offers a theoretical framework and experimental methodology for designing novel LPL and storage phosphors.
4. Experimental section
Materials and synthesis
According to the stoichiometric ratio, the raw materials MgO (99.99%), Y2O3 (99.99%), GeO2 (99.99%), Pr7O11 (99.99%), Tb2O3 (99.99%), CeO2 (99.99%), and Eu2O3 (99.99%) were added to an agate mortar. Then, an appropriate amount of absolute ethanol was poured in and the mixture was ground for 0.5 h. The detailed compositions are listed in Table S1.† The mixture was calcined in a high-temperature tube furnace at 1623 K for 6 h in a muffle furnace under air, finally cooled to room temperature (RT), and the temperature rate of rise and fall obtained was 5 K min−1.Device preparation
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 21871122 and 21431002) and the Fundamental Research Funds for the Central Universities (Grant No. lzujbky-2021-kb17).References
- S. Cho, S. Kim and J. Kim,
et al., Hybridisation of perovskite nanocrystals with organic molecules for highly efficient liquid scintillators, Light: Sci. Appl., 2020, 9, 159 CrossRef PubMed
.
- J. Zhao, L. Zhao and Y. Deng,
et al., Perovskite-filled membranes for flexible and large-area direct-conversion X-ray detector arrays, Nat. Photonics, 2020, 14, 612–617 CrossRef
.
- W. Zhu, W. Ma and Y. Su,
et al., Low-dose real-time X-ray imaging with nontoxic double perovskite scintillators, Light: Sci. Appl., 2020, 9, 112 CrossRef CAS PubMed
.
- Z. Li, G. Peng and Z. Li,
et al., Hydrogen Bonds Strengthened Metal-Free Perovskite for Degradable X-ray Detector with Enhanced Stability, Flexibility and Sensitivity, Angew. Chem., Int. Ed., 2023, 62, 10 Search PubMed
.
- J. Liu, B. Shabbir and C. Wang,
et al., Flexible, Printable Soft-X-Ray Detectors Based on All-Inorganic Perovskite Quantum Dots, Adv. Mater., 2019, 31, 30 Search PubMed
.
- W. Pan, B. Yang and G. Niu,
et al., Hot-Pressed CsPbBr3 Quasi-Monocrystalline Film for Sensitive Direct X-ray Detection, Adv. Mater., 2019, 31, 44 Search PubMed
.
- F. Zhang, Y. Zhou and Z. Chen,
et al., Thermally Activated Delayed Fluorescence Zirconium-Based Perovskites for Large-Area and Ultraflexible X-ray Scintillator Screens, Adv. Mater., 2022, 34, 43 Search PubMed
.
- F. Zhou, Z. Li and W. Lan,
et al., Halide Perovskite, a Potential Scintillator for X-Ray Detection, Small Methods, 2020, 4, 10 Search PubMed
.
- F. Qiu, G. Peng and Y. Xu,
et al., Sequential Vacuum Evaporated Copper Metal Halides for Scalable, Flexible, and Dynamic X-Ray Detection, Adv. Funct. Mater., 2023, 33, 36 Search PubMed
.
- X. Zhou, K. Han and Y. Wang,
et al., Energy-Trapping Management in X-Ray Storage Phosphors for Flexible 3D Imaging, Adv. Mater., 2023, 35, 16 Search PubMed
.
- A. Jana, S. Cho and S. A. Patil,
et al., Perovskite: Scintillators, direct detectors, and X-ray imagers, Mater. Today, 2022, 55, 110–136 CrossRef CAS
.
- Z. Yang, T. Wang and X. Xu,
et al., Fiber Optic Plate Coupled Pb-Free Perovskite X-ray Camera Featuring Low-Dose-Rate Imaging toward Dental Diagnosis, J. Phys. Chem. Lett., 2023, 14, 326–333 CrossRef CAS PubMed
.
- Y. Wu, J. Feng and Z. Yang,
et al., Halide Perovskite: A Promising Candidate for Next-Generation X-Ray Detectors, Adv. Sci., 2022, 10, 1 Search PubMed
.
- X. He, M. Xia and H. Wu,
et al., Quasi-2D Perovskite Thick Film for X-Ray Detection with Low Detection Limit, Adv. Funct. Mater., 2021, 32, 7 Search PubMed
.
- H. Li, J. Song and W. Pan,
et al., Sensitive and Stable 2D Perovskite Single Crystal X-ray Detectors Enabled by a Supramolecular Anchor, Adv. Mater., 2020, 32, 40 Search PubMed
.
- W. Ma, T. Jiang and Z. Yang,
et al., Highly Resolved and Robust Dynamic X-Ray Imaging Using Perovskite Glass Ceramic Scintillator with Reduced Light Scattering, Adv. Sci., 2021, 8, 15 Search PubMed
.
- W. Pan, H. Wu and J. Luo,
et al., Cs2AgBiBr6 single-crystal X-ray detectors with a low detection limit, Nat. Photonics, 2017, 11, 726–732 CrossRef CAS
.
- H. Wei, Y. Fang and P. Mulligan,
et al., Sensitive X-ray detectors made of methylammonium lead tribromide perovskite single crystals, Nat. Photonics, 2016, 10, 333–339 CrossRef CAS
.
- M. Xia, J. H. Yuan and G. Niu,
et al., Unveiling the Structural Descriptor of A3B2X9 Perovskite Derivatives toward X-Ray Detectors with Low Detection Limit and High Stability, Adv. Funct. Mater., 2020, 30, 24 Search PubMed
.
- B. Yang, L. Yin and G. Niu,
et al., Lead-Free Halide Rb2CuBr3 as Sensitive X-Ray Scintillator, Adv. Mater., 2019, 31, 44 Search PubMed
.
- H. Zhang, Z. Yang and M. Zhou,
et al., Reproducible X-ray Imaging with a Perovskite Nanocrystal Scintillator Embedded in a Transparent Amorphous Network Structure, Adv. Mater., 2021, 33, 40 Search PubMed
.
- S. Ding, H. Guo and P. Feng,
et al., A New Near-Infrared Long Persistent Luminescence Material with Its Outstanding Persistent Luminescence Performance and Promising Multifunctional Application Prospects, Adv. Opt. Mater., 2020, 8, 18 Search PubMed
.
- Y. Lin, C. Ming and Z. Wang,
et al., Co-doped long persistent luminescence materials LiSr3SiO4Cl3:Eu2+,Ln3+ (Ln = Dy, Ho, Er): construction and verification of VRBE and HRBE scheme and their multifunctional applications, Inorg. Chem. Front., 2023, 10, 5071–5081 RSC
.
- T. Lyu and P. Dorenbos, Towards information storage by designing both electron and hole detrapping processes in bismuth and lanthanide-doped LiRE(Si,Ge)O4 (RE = Y, Lu) with high charge carrier storage capacity, Chem. Eng. J., 2020, 400, 124776 CrossRef CAS
.
- T. Lyu and P. Dorenbos, Vacuum-Referred Binding Energies of Bismuth and Lanthanide Levels in LiTaO3 Perovskite: Toward Designing Energy Storage Phosphor for Anti-Counterfeiting, X-Ray Imaging, and Mechanoluminescence, Laser Photonics Rev., 2022, 16, 10 Search PubMed
.
- T. Lyu, P. Dorenbos and Z. Wei, Designing LiTaO3:Ln3+,Eu3+ (Ln = Tb or Pr) perovskite dosimeter with excellent charge carrier storage capacity and stability for anti-counterfeiting and flexible X-ray imaging, Chem. Eng. J., 2023, 461, 141685 CrossRef CAS
.
- Y. Yang, P. Zhang and W. Xie,
et al., Time-dependent multicolor evolution of photoluminescence and afterglow in lanthanide-doped gallate, Chem. Eng. J., 2023, 476, 146487 CrossRef CAS
.
- B. Yu, Y. J. Wang and Y. Y. Lin,
et al., HKUST-1 nano metal–organic frameworks combined with ZnGa2O4:Cr3+ near-infrared persistent luminescence nanoparticles for in vivo imaging and tumor chemodynamic and photothermal synergic therapy, Nanoscale, 2022, 14, 8978–8985 RSC
.
- S. Zhao, Z. Wang and Y. Lin,
et al., Multi-level information security realized in ortho-stannic acid magnesium with a single activator of Tb3+, Inorg. Chem. Front., 2021, 8, 3522–3531 RSC
.
- D. Gao, Q. Kuang and F. Gao,
et al., Achieving opto-responsive multimode luminescence in Zn1+XGa2–2XGO4:Mn persistent phosphors for advanced anti-counterfeiting and information encryption, Mater. Today Phys., 2022, 27, 100765 CrossRef CAS
.
- C. Jia, J. Yu and Y. Hu,
et al., Deep-trap persistent materials for future rewriteable optical information storage, Phys. Chem. Chem. Phys., 2024, 26, 19591–19605 RSC
.
- D. Heggen, R. Zilenaite and E. Ezerskyte,
et al., A Standalone, Battery-Free Light Dosimeter for Ultraviolet to Infrared Light, Adv. Funct. Mater., 2021, 32, 14 Search PubMed
.
- P. Zhang, W. Xie and Z. Wang,
et al., Time-dependent dynamic multicolor afterglow of simple LiGa5O8:Eu3+/Tb3+particles for advanced anticounterfeiting and encryption, Inorg. Chem. Front., 2022, 9, 4022–4029 RSC
.
- Z.-H. Zuo, Y. Y. Peng and J. Li,
et al., Thermal-responsive dynamic color-tunable persistent luminescence from green to deep red for advanced anti-counterfeiting, Chem. Eng. J., 2022, 446, 16976 CrossRef
.
- P. Huang, Z. Wen and Y. Yu,
et al., High charge carrier storage capacity and wide range X-rays to infrared photon sensing in LiLuGeO4:Bi3+,Ln3+ (Ln = Pr, Tb, or Dy) for anti-counterfeiting and information storage applica-tions, Mater. Chem. Front., 2023, 7, 168–182 RSC
.
- G. Liu, Y. Wang and T. Seto, A Giant Stokes Shift in Wide-Band Red Phosphor [Ca0.33(Sr1−xBax)0.67]7(SiO3)6Cl2: Eu2+, Adv. Opt. Mater., 2024, 12, 16 Search PubMed
.
- L. Li, T. Li and Y. Hu,
et al., Mechanism of the trivalent lanthanides’ persistent luminescence in wide bandgap materials, Light: Sci. Appl., 2022, 11, 5071–5081 Search PubMed
.
- P. Dorenbos, Ce3+ 5d-centroid shift and vacuum referred 4f-electron binding energies of all lanthanide impurities in 150 different compounds, J. Lumin., 2002, 99, 283 CrossRef CAS
.
- R. Zhou, L. Lin and C. Liu,
et al., Insight into Eu redox and Pr3+ 5d emission in KSrPO4 by VRBE scheme construction, Dalton Trans., 2018, 47, 306–313 RSC
.
- Y. Zhuang, Y. Lv and L. Wang,
et al., Trap Depth Engineering of SrSi2O2N2:Ln2+,Ln3+ (Ln2+ = Yb, Eu; Ln3+ = Dy, Ho, Er) Persistent Luminescence Materials for Information Storage Applications, ACS Appl. Mater. Interfaces, 2018, 10, 1854–1864 CrossRef CAS PubMed
.
- Z. Q. Jiang, Y. H. Wang and L. S. Wang, Enhanced Yellow-to-Orange Emission of Si-Doped Mg3Y2Ge3O12: Ce3+ Garnet Phosphors for Warm White Light-Emitting Diodes, J. Electrochem. Soc., 2010, 157(5), J155–J158 CrossRef CAS
.
- M. Liao, Z. Mu and S. Zhang,
et al., A red phosphor Mg3Y2Ge3O12: Bi3+, Eu3+ with high brightness and excellent thermal stability of luminescence for white light-emitting diodes, J. Lumin., 2019, 210, 202–209 CrossRef CAS
.
- D. Hou, R. Huang and J.-Y. Li,
et al., Expanded visible-near-infrared temperature sensing properties in view of ultra-broadband tunable luminescence in Mg3Y2Ge3O12:Ce3+, Cr3+ phosphors with advanced applications, J. Lumin., 2022, 251, 119204 CrossRef CAS
.
- X. Meng, Z. Wang and S. Wu,
et al., Improving the luminescence and afterglow properties of Mg3Y2Ge3O12:Cr3+ by co-doping Bi3+, Ceram. Int., 2020, 46, 18903–18910 CrossRef CAS
.
- G. Krieke, G. Doke and A. Antuzevics,
et al., Tuneable persistent luminescence of novel Mg3Y2Ge3O12 garnet, J. Alloys Compd., 2022, 922, 166312 CrossRef CAS
.
- C. Ji, Z. Huang and X. Tian,
et al., Novel red emitting phosphors Mg3Y2Ge3O12:Sm3+ with high color purity and excellent thermal stability used in W-LEDs, J. Alloys Compd., 2020, 825, 154176 CrossRef CAS
.
- H. Lin, J. Xu and Q. Huang,
et al., Bandgap Tailoring via Si Doping in Inverse-Garnet Mg3Y2Ge3O12:Ce3+ Persistent Phosphor Potentially Applicable in AC-LED, ACS Appl. Mater. Interfaces, 2015, 7, 21835–21843 CrossRef CAS PubMed
.
- A. Mao, Z. Zhao and J. Wang,
et al., Crystal structure and photo-luminescence of Gd3Ga2(Al3(-xSix)(O12(-yNy):Ce3+ phosphors for AC-warm LEDS, Chem. Eng. J., 2019, 368, 924–932 CrossRef CAS
.
- F. Xie, J. Li and D. Xu,
et al., Layer-structure-suppressed concentration quenching of Dy3+ luminescence and the realization of a single phase white light-emitting phosphor cooperated with Tm3+, Inorg. Chem. Front., 2022, 9, 3797–3807 RSC
.
- P. Dorenbos, Charge transfer bands in optical materials and related defect level location, Opt. Mater., 2017, 69, 8–22 CrossRef CAS
.
- P. Dorenbos, Ce3+ 5d-centroid shift and vacuum referred 4f-electron binding energies of all lanthanide impurities in 150 different compounds, J. Lumin., 2013, 135, 93–104 CrossRef CAS
.
- P. Dorenbos, Determining binding energies of valence-band electrons in insulators and semiconductors via lanthanide spectroscopy, Phys. Rev. B:Condens. Matter Mater. Phys., 2013, 87, 3 CrossRef
.
- J. Ueda, R. Maki and S. Tanabe, Vacuum Referred Binding Energy (VRBE)-Guided Design of Orange Persistent Ca3Si2O7:Eu2+ Phosphors, Inorg. Chem., 2017, 56, 10353–10360 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4qi02823f |
| This journal is © the Partner Organisations 2025 |