Achieving high performance ultra-broadband near-infrared emission through a multi-site occupancy and energy transfer strategy for NIR LED applications†
Mingkai
Wei
a,
Zixi
Chen
a,
Yongying
Chen
a,
Xinxiang
Liang
a,
Na
Li
a,
Xuejie
Zhang
 *a,
Wei
Li
a,
Haoran
Zhang
*a,
Wei
Li
a,
Haoran
Zhang
 a,
Maxim S.
Molokeev
b and
Bingfu
Lei
a,
Maxim S.
Molokeev
b and
Bingfu
Lei
 *a
*a
aKey Laboratory for Biobased Materials and Energy of Ministry of Education, College of Materials and Energy, South China Agricultural University, Guangzhou 510642, Guangdong, P. R. China. E-mail: tleibf@scau.edu.cn
bLaboratory of Crystal Physics, Kirensky Institute of Physics, Federal Research Center KSC SB RAS, Krasnoyarsk 660036, Russia
First published on 18th March 2025
Abstract
Broadband near-infrared (NIR) phosphor-converted light-emitting diodes (pc-LEDs) are considered to be at the forefront of the development of next-generation NIR light sources. However, the performance of NIR pc-LEDs is severely limited due to the narrow band emission, low quantum efficiency, and thermal quenching of NIR-emitting materials. Herein, an efficient and thermally stable broadband NIR LaMgGa11O19:Cr3+,Yb3+ (LMG:Cr3+,Yb3+) phosphor has been successfully designed by [Cr3+–Yb3+] co-doping. The broadband emission phenomenon of LaMgGa11O19:Cr3+ was confirmed to be due to selective lattice occupancy of Cr3+ ions based on the analysis of crystal structures, crystal field calculations, and fluorescence lifetimes. The NIR emission spectra in the range of 1000–1200 nm were enriched by using the highly efficient energy transfer of Cr3+ → Yb3+ ions and the energy transfer mechanism is discussed in detail. The prepared LMG:Cr3+,Yb3+ phosphors exhibit highly efficient ultra-broadband NIR emission from 650 to 1200 nm under 440 nm excitation with high internal and external quantum efficiencies of 94.2%/40.5% and excellent luminescence thermal stability of 89.3%@373 K. A NIR pc-LED prototype was fabricated by combining the optimized phosphor with a commercial 440 nm blue LED chip, providing 84.5 mW NIR output power at a 350 mA driving current. Finally, the potential applications of the phosphor in night vision lighting and non-destructive testing were demonstrated. The results show that this work is expected to provide a new strategy for efficient ultra-broadband NIR phosphor design.
Introduction
Near-infrared (NIR) pc-LEDs have the advantages of small size, low cost, and spectral tunability, and show great potential for applications in night vision, anti-counterfeiting, non-destructive testing and medical imaging.1–8 As the core component of NIR pc-LED devices, the NIR phosphor directly determines the peak emission, bandwidth, luminous efficacy, and long-term stability of the device.9,10 Generally speaking, the wider the emission band of the NIR phosphor, the more the spectral information that can be obtained from the NIR pc-LED.11–13 However, the absence of suitable NIR phosphors has resulted in the spectral characteristics and light output power of NIR pc-LEDs remaining inadequate for practical applications. Therefore, it is of great practical significance to develop broadband NIR phosphors with high efficiency and thermostability so that the NIR LEDs can meet the requirements of convenient equipment applications.Cr3+ ions are considered as a favorable luminescence center for NIR-emitting phosphors due to their tunable emission bands from deep red to NIR, achieved by adjusting the strength of the crystal field in which they are located.14,15 A variety of Cr3+-doped solid solutions have been successfully developed, and these materials have been widely used in pc-LEDs because they exhibit highly efficient quantum yields and excellent thermal stability in the short wavelength region from 700 to 900 nm.16–19 Nevertheless, there is an increasing demand for phosphor materials capable of achieving intense emission in the long wavelength range in cutting-edge fields such as medicine, imaging and remote sensing detection.20,21 To broaden the emission bandwidth of Cr3+-activated NIR phosphors, researchers have proposed a multi-grid occupation strategy. For example, Jiang et al. reported a broadband emission AlTaO4:Cr3+ NIR phosphor induced by the simultaneous occupation of AlO6 and TaO6 lattices by Cr3+ ions.22 Sun et al. developed a new broadband NIR K2SrGe8O18:Cr3+ phosphor using two Cr3+ lattices, resulting in a full width at half-maximum (FWHM) up to 214 nm.23 Shi et al. prepared La3SnGa5O14:Cr3+ phosphors by the crystalline site-occupation method, whose emission spectrum spans a broad 650–1300 nm range and which show strong emission in the NIR II region (>1000 nm).24 These works provide a reference for further research on tunable NIR luminescent phosphor materials.
Furthermore, researchers have put forth a proposal to expand the luminescence spectrum of Cr3+ ion-activated NIR phosphors through the design of energy transfer mechanisms. A great deal of research has been conducted in this field, resulting in significant advancements. Miao et al. co-doped Cr3+/Ni2+ ions in the LiMgPO4 matrix, which resulted in the generation of broadband NIR emission in the 1100–1600 nm range.25 He et al. co-doped Cr3+ and Nd3+ in Ca2LuZr2Al3O12, resulting in the creation of a highly efficient broadband NIR phosphor with a FWHM of 350 nm; the phosphor demonstrated excellent thermal stability.26 Shi et al. undertook a co-doping process with Yb3+ ions on the InBO3:Cr3+ phosphor, resulting in broader emission spectra and higher internal quantum efficiency (IQE).27 Unfortunately, it is regrettable that these studies continue to exhibit deficiencies in terms of external quantum efficiency (EQE) and thermal stability. Therefore, it is a challenging work to develop new and efficient broadband NIR phosphors to meet the demand for NIR pc-LEDs.
In this study, the successful preparation of novel LaMgGa11O19(LMG):Cr3+,Yb3+ ultra-broadband NIR phosphors via [Cr3+–Yb3+] cation co-doping was achieved. The luminescence properties and energy transfer mechanisms of this material have been studied in depth, which has revealed that the significant red-shift and broadening of the Cr3+ spectrum is attributable to the lattice occupation tendency. Furthermore, the energy transfer process from Cr3+ to Yb3+ has been determined to be a dipole–quadrupole interaction, exhibiting a maximum transfer efficiency of 71.6%. Finally, NIR pc-LED devices were prepared by combining the above phosphors with commercial 440 nm blue LED chips, and their potential applications in night vision imaging and NDT were demonstrated. The results show that this new LMG:Cr3+,Yb3+ phosphor will be promising for future NIR pc-LED applications.
Material and methods
Materials and preparation
A series of LMG phosphor samples were synthesized using a conventional high-temperature solid-phase method. The raw materials were La2O3 (99.99%, Aladdin), MgO (99.99%, Aladdin), Ga2O3 (99.99%, Aladdin), Cr2O3 (99.95%, Aladdin), Yb2O3 (99.99%, Aladdin), and H3BO3 (99.99%, Aladdin). The above ingredients were weighed accurately according to the stoichiometric ratios of the samples, and an amount of boric acid equivalent to 3% of the total mass was incorporated as a flux. All the raw materials were put into an agate mortar and mixed with ethanol and fully ground for 40 min. Then they were put into a corundum crucible and placed in a tube furnace and calcined at 1500 °C for 6 h and then cooled to room temperature. The cooled sample was taken out and ground again for 20 min to obtain the final sample.Characterization
The crystal structure of the samples was confirmed using a powder X-ray diffractometer (XRD) (Ultima IV). Rietveld refinement used TOPAS 4.2. The micro-morphology and elemental distribution of the samples were tested and analyzed using a field emission scanning electron microscope (Verios 460) equipped with an energy dispersive spectroscopy (EDS) analyzer. Diffuse reflectance spectra were recorded using a UV-visible spectrophotometer (PE Lambda950s) and Ba2SO4 was used as a reference sample. X-ray photoelectron spectroscopy (XPS) measurements were executed using a Thermo Scientific K-Alpha system. Excitation spectra, emission spectra, variable temperature spectra and fluorescence lifetimes of the samples were obtained using an FLS1000 spectrometer (Edinburgh). The IQE and absorption efficiency (AE) of the samples were obtained using a UV-NIR absolute quantum yield meter (Quantaurus-QY Plus C13534-12, Hamamatsu) and Ba2SO4 was used as a reference sample.NIR pc-LED fabrication and performance measurement
The NIR pc-LED device was made by mixing LMG:0.2Cr3+,0.05Yb3+ phosphors with AB glue at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 powder–glue ratio, and then coating on a 440 nm blue LED chip followed by encapsulation. The optical properties of the device, such as electroluminescence (EL) spectra, NIR output power, and photoelectric conversion efficiency, were then characterized using an LED test rig (HP8000) equipped with an integrating sphere and an HAAS2000 photoelectric measurement system. The operating temperatures of the LED were recorded using a thermal imaging camera (FLIR E40).
1 powder–glue ratio, and then coating on a 440 nm blue LED chip followed by encapsulation. The optical properties of the device, such as electroluminescence (EL) spectra, NIR output power, and photoelectric conversion efficiency, were then characterized using an LED test rig (HP8000) equipped with an integrating sphere and an HAAS2000 photoelectric measurement system. The operating temperatures of the LED were recorded using a thermal imaging camera (FLIR E40).
Results and discussion
Crystal structure
The crystal structure of LMG is shown in Fig. 1a, which belongs to the structure of magnetoplumbite, a P63/mmc space group with five unique M cation sites [M = Mg2+, Ga3+]. Among them, GaI, GaIV and GaV are 6-coordinated, and GaII and GaIII are 5- and 4-coordinated, respectively. Here [GaIO6] is a regular octahedron and [GaIVO6] and [GaVO6] are relatively irregular octahedra. Mg2+ selectively occupies the GaI and GaIII sites.28 Considering the valence and ionic radius matching rules, the effective ionic radii of Ga3+ and Cr3+ are 0.0620 Å and 0.0615 Å under six-coordination, respectively, with the same valence and similar ionic radii, so substitution is more likely to occur.29 As for the trivalent rare earth ion Yb3+, it is more likely to occupy the La3+ lattice.30The synthesized LMG series phosphors were subjected to XRD tests to determine the phase purity of the samples. Fig. 1b shows the XRD patterns of LMG, LMG:0.2Cr3+, LMG:0.05Yb3+, and LMG:0.2Cr3+,0.05Yb3+ phosphors. The XRD diffraction peaks of all these samples are in agreement with the standard card (PDF#84-0889); no appearance of new peaks was observed, suggesting that the introduction of dopant ions did not alter the crystal structure or induce impurity. Fig. S1(a) and (b)† show the XRD patterns of LMG:xCr3+ (x = 0.01–0.5) and LMG:0.2Cr3+,yYb3+ (y = 0–0.1) phosphors, respectively (insets are partial enlargements of the highest diffraction peaks). The angle of the highest diffraction peak gradually becomes larger with the increase of Cr3+ doping, which may be due to the substitution of the larger Ga3+ ions by the smaller Cr3+ ions. Meanwhile, for the LMG:0.2Cr3+,yYb3+ series phosphors, the angle of the highest diffraction peak is shifted to a large angle with the increase of Yb3+ doping, which leads to cell shrinkage, thus decreasing the crystal plane spacing and shifting the diffraction peak to a higher angle.31 This is consistent with the designed material substitution model. To obtain more detailed crystal data, we performed a Rietveld structure refinement of the samples using LMG as the initial model, and the Rietveld structure refinement of the representative samples is shown in Fig. 1c. No impurity phase was observed, further supporting the conclusion that the samples were pure phases. The residual factor is relatively small, indicating that the results are acceptable. The detailed refinement results are presented in Tables S1 and S2.† The Ga–O bond lengths and average bond lengths of [GaIO6], [GaIVO6] and [GaVO6] are given in Table S3.† The Cr3+ ions occupying the GaI, GaIV and GaV lattice sites are labeled as CrI, CrIV and CrV. The trends of the bond lengths of GaI, GaIV and GaV are shown in Fig. S2d,† where the Ga–O bond lengths decrease with the increase of Cr3+ ion doping, and the changes of GaIV and GaV are more pronounced with respect to those of GaI, which indicates that Cr3+ tends to occupy the GaIV and GaV lattice sites more. Fig. 1d shows the SEM and EDS elemental distribution of the sample LMG:0.2Cr3+,0.05Yb3+ phosphor. It can be observed that the phosphor samples exhibit a uniform dispersion of irregular shapes, with particle sizes ranging from approximately 5 to 20 μm. Further EDS analysis showed that La, Mg, Ga, Cr and Yb elements were uniformly distributed in the sample particles, and the elemental ratio of La, Mg and Ga was about 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.37
1.37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11, which was basically consistent with the stoichiometric ratio of the matrix.
11, which was basically consistent with the stoichiometric ratio of the matrix.
Luminescence properties and energy transfer
Fig. 2a shows the diffuse reflectance spectra of LMG and LMG:0.2Cr3+ phosphors, and the inset shows the body color of LMG and LMG:0.2Cr3+ under daylight. The LMG sample displays a pronounced ultraviolet absorption band, whereas no intrinsic absorption is discernible in the visible and far-red regions. In contrast, the LMG:0.2Cr3+ sample exhibited two additional absorption peaks at 436 nm and 600 nm, in addition to the pronounced UV absorption of the substrate, which was attributed to the 4A2 → 4T1(4F) and 4A2 → 4T2(4F) absorption bands of the Cr3+ ion.32 These phenomena correspond to the LMG matrix base color showing white and the LMG:0.2Cr3+ base color showing gray-green. The optical band gap of the LMG matrix can be calculated using the Kubelka–Munk equation:33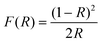 | (1) |
 | (2) |
Fig. 2b illustrates the photoluminescence (PL) and photoluminescence excitation (PLE) spectra of the LMG:0.2Cr3+ sample, which demonstrate that the two excitation peaks are attributed to the energy levels 4A2 → 4T1(4F) and 4A2 → 4T2(4F) of Cr3+, and that the emission at 770 nm is due to the transitions from 2E → 4A2 and 4T2(4F) → 4A2. The PL spectra of the LMG:xCr3+ (x = 0.05–0.5) samples are shown in Fig. 2c. The fluorescence intensity of the samples under blue light excitation at 440 nm shows an increase and then a decrease with the increase of the Cr3+ doping concentration, and reaches a maximum at x = 0.2. This phenomenon can be attributed to the concentration quenching that occurs as a result of the increasing concentration, which involves a non-radiative transition process.34Fig. 2d demonstrates the trend of luminescence intensity and FWHM of Cr3+ doped samples with different concentrations. The fluorescence decay curves of different concentrations of LMG:xCr3+ (x = 0.05–0.5) samples are shown in Fig. 2e, and the fluorescence lifetime of each sample can be fitted by eqn (3):35
 | (3) |
Fig. 2f depicts the Gaussian spectral results of the PL spectrum of the LMG:0.2Cr3+ sample, wherein the emission peaks are accurately represented by three Gaussian peaks. The reliability of the fitted data is indicated by an R2 value of 0.997. In general, it is useful to monitor the excitation spectra at different emission wavelengths to distinguish multiple Cr3+ sites. The excitation spectra monitored at different emission wavelengths (730, 805 and 900 nm) are shown in Fig. 2g. The three excitation spectra are very different, showing a red-shift and broadening, and the calculated crystal field parameters Dq/B are 2.37, 2.11, and 1.93 respectively, suggesting that the three peaks are contributed by three different luminescence centers. The three Cr3+ ion luminescence centers correspond to CrI, CrV and CrIV, and their octahedron coordination Tanabe–Sugano energy levels are shown in Fig. 2h. Among them, CrI is in the crystal field where it is located in the strongest of the three sites, corresponding to the [GaIO6] octahedron with the shortest bond length, emitting NIR light centered at 730 nm, while CrIV and CrV are in the weak crystal-field environment corresponding to the [GaIVO6] and [GaVO6] octahedra, emitting NIR light of long wavelengths centered at 900 nm and 805 nm. Additionally, the fluorescence decay curves of the three aforementioned peaks were monitored and are illustrated in Fig. S5.† The fluorescence lifetimes of peaks I, II and III were 214.29 μs, 143.37 μs and 132.94 μs, respectively, exhibiting a significant discrepancy, which further substantiates the presence of three distinct luminescence centers.
Notably, the PL spectra exhibited a large red-shift (110 nm) and the FWHM of the spectra unfolded dramatically with the doping concentration, which may be related to the tendency of Cr3+ ions to occupy different luminescence centers. To further investigate this phenomenon, Gaussian peak splitting was performed on the PL spectra of LMG:xCr3+ (x = 0.05–0.5) solid solutions (shown in Fig. S6†). The PL spectra of all samples can be well fitted by the three Gaussian peaks. The relationship between the luminous intensity of CrI, CrIV and CrV and the concentration of Cr3+ is shown in Fig. 2i. The three luminescence centers do not contribute equally to the PL spectrum at different concentrations (Fig. S6e†). When the doping concentration is less than 0.2 mol, CrI contributes more to PL than CrIV and CrV, while when the doping concentration is greater than or equal to 0.2 mol, CrIV and CrV contribute more to PL than CrI, which implies that Cr3+ occupies different lattice positions in a selective manner as the doping concentration increases. At higher doping concentrations, Cr3+ ions tend to occupy the [GaIVO6] and [GaVO6] sites of the weak crystal field, exhibiting red-shift and broadening in the spectra. This phenomenon can be attributed to the fact that the various octahedron structures present in the matrix exhibit varying degrees of stability.36,37 The superposition of the three luminescent centers shows the best luminescence when x = 0.2 mol, which is consistent with the phenomenon observed by PL spectroscopy.
Enhancement of LMG:Cr3+ solid solutions in the short-wave NIR is important for their NIR spectroscopic applications. Inspired by the previous work, the absorption of Yb3+ and the emission of Cr3+ partially overlap in the NIR region, and the emission band can be broadened by constructing Cr3+ → Yb3+ energy transfer to improve the luminescence performance of the phosphor.38–42 Therefore, Yb3+ ions were co-doped into LMG:0.2Cr3+ phosphors and their luminescence properties and mechanisms were investigated.
The diffuse reflectance spectra of the LMG:0.2Cr3+ and LMG:0.2Cr3+, 0.05Yb3+ samples and the emission spectrum of the LMG:0.2Cr3+ sample are depicted in Fig. 3a. The diffuse reflectance spectra of LMG:0.2Cr3+,0.05Yb3+ samples show absorption bands in the range of 900–1000 nm, which belong to the characteristic absorption of Yb3+ ions, and are attributed to the 2F5/2 → 2F7/2 transition; no characteristic absorption of Cr4+ ions, which seriously impairs the phosphor performance, is observed in the diffuse reflectance spectra. In addition, the absorption of Yb3+ has a large overlap with the emission spectrum of the LMG:0.2Cr3+ sample, suggesting that energy transfer may be constructed in LMG:0.2Cr3+.
Fig. 3b and c show the XPS fine spectra as well as the XPS full-spectrum profiles of the LMG:0.2Cr3+,0.05Yb3+ samples at the Cr 2p level, respectively. The results show that the peak in the fine spectrum appears at 576.08 eV, corresponding to the 2p3/2 orbital of the Cr3+ ion. No Cr4+ characteristic peak appeared, which further confirmed the absence of Cr4+ in the LMG:0.2Cr3+,0.05Yb3+ phosphor. In addition, the characteristic peaks of La, Mg, Ga, Cr, and Yb elements appeared in the XPS full-spectrum mapping, which further proved the successful synthesis of phosphor samples with LMG as the matrix and Cr3+/Yb3+ as the luminescence center, in line with the result of EDS mapping. Fig. 3d demonstrates the excitation and emission spectra of the LMG:0.2Cr3+ sample and the LMG:0.2Cr3+,0.05Yb3+ sample. Following the introduction of Yb3+, LMG:0.2Cr3+,0.05Yb3+ exhibited additional strong emission peaks in the 1000–1200 nm range in comparison with the LMG:0.2Cr3+ sample, in addition to the emission characteristics of the Cr3+ ion. On monitoring the emission wavelengths of the LMG:0.2Cr3+,0.05Yb3+ samples at 770 nm and 1004 nm, respectively, it was found that the excitation spectra obtained are basically consistent with those of the single-doped samples with Cr3+ ions, which proves that an effective energy transfer from the Cr3+ ions to the Yb3+ ions has occurred.
Fig. 3e shows the PL spectra of the LMG:0.2Cr3+,yYb3+ phosphor with different Yb3+ concentrations under 440 nm excitation, and Fig. 3f demonstrates the trend of the luminescence intensity of the LMG:Cr3+,yYb3+ phosphor at 770 nm and 1004 nm. When Yb3+ ions were introduced, additional emission bands in the range of 1000–1200 nm were observed from the emission spectra in addition to the characteristic emission of Cr3+. With the increase of the Yb3+ doping concentration, the positions of the two characteristic emission peaks did not change significantly, and the emission intensity of the Yb3+ characteristic peaks gradually increased, accompanied by a decrease in the emission intensity of Cr3+, proving that there might be an energy transfer between Cr3+ → Yb3+. Fig. S7a† demonstrates the emission spectra of LMG:0.05Yb3+,xCr3+ phosphors with different Cr3+ concentrations under 440 nm excitation. No characteristic emission was observed from the emission spectra when the concentration of Cr3+ ions was 0, which indicated that LMG:0.05Yb3+ was not able to produce characteristic emission under 440 nm blue light excitation. With the introduction of Cr3+ ions, the characteristic emission of Yb3+ ions appeared in addition to the characteristic peaks of Cr3+ ions, and the emission intensity of the characteristic peaks of Yb3+ gradually increased with the increase of the doping concentration of Cr3+, which also indicated that an effective energy transfer from Cr3+ ions to Yb3+ ions occurred. The quantum efficiency of the solid solution was tested using a BaSO4 reference sample due to the high stability and reflectivity of BaSO4 and the absence of fluorescence interference. Fig. 3g illustrates the PL quantum yield of the LMG:0.2Cr3+,0.05Yb3+ phosphor, which exhibits excellent performance, with an IQE of 94.2% and an EQE of 40.5%. This phenomenon can be attributed to the high EQE observed in the singly doped samples (IQE/EQE = 93.8%/43.0%, shown in Fig. S8†), coupled with the high energy transfer efficiency of Cr3+ → Yb3+. This efficiency is much better than those of many recently reported related phosphors such as SrLaGa3O7:Cr3+,Yb3+ (IQE = 60.5%),43 SrGe4O9:Cr3+ (IQE = 66.9%),44 RbAl3P6O20:Cr3+ (IQE = 66.%),45etc. In addition, the emission bandwidth of the co-doped Yb3+ sample was further broadened compared with that of the single-doped LMG:0.2Cr3+ phosphor. This proves that the phosphor is expected to have potential applications in fields such as nondestructive testing.
To further investigate the energy transfer relationship between Cr3+ and Yb3+ ions, the fluorescence decay curves of all co-doped Yb3+ phosphors were tested. The fluorescence decay curves of LMG:0.2Cr3+,yYb3+ phosphors at 770 nm were monitored under 440 nm excitation, as shown in Fig. 3h, and the fluorescence lifetime of each sample can be fitted by eqn (3). As the concentration of Yb3+ ions increased, the fluorescence lifetime values gradually shortened, indicating a significant energy transfer from Cr3+ → Yb3+, which is the main reason for the decrease in Cr3+ luminescence.
In general, the energy transfer efficiency can be calculated based on the changes in Cr3+ emission intensity or fluorescence lifetime with increasing Yb3+ doping concentration.46 The specific calculation formula is as follows:
 | (4) |
 | (5) |
To further investigate the energy transfer mechanism, based on the Dexter energy transfer formula for multipolar interactions, eqn (6) is obtained:51
 | (6) |
 | (7) |
 | ||
| Fig. 4 (a) Dependence of I0/I of Cr3+ on C6/3, C8/3, and C10/3. (b) Simplified diagram of the Cr3+ → Yb3+ energy transfer process. | ||
Fig. 4b demonstrates the schematic energy transfer between Cr3+ → Yb3+ ions. Under the excitation of 440 nm blue light, the electrons in the ground state of the Cr3+ ion are excited to jump to the excited state, and the electrons in the high excited state will first release energy to jump back to the lowest excited state 4T2. Finally, these electrons may release energy through two pathways. One is that the electrons directly return to the ground state 4A2 and emit NIR light, and the other is that the electrons are transferred from the excited state 4T2 of Cr3+ to the 2F5/2 energy level of Yb3+, which then emits NIR light of a longer wavelength through the 2F5/2 → 2F7/2 energy level leap, resulting in an energy transfer between Cr3+ → Yb3+.
Temperature-dependent properties
Due to the accumulation of heat within the device, the pc-LED operates at temperatures exceeding 100 °C, which results in a loss of luminescence due to thermal quenching. It is therefore essential to evaluate the PL thermal stability of the target NIR phosphor. The temperature-dependent emission spectra of the LMG:0.2Cr3+,0.05Yb3+ phosphor in the range of 293–450 K were tested as an example. The temperature-dependent PL spectra as well as the contour plots of its temperature-dependent PL spectra in the temperature range of 293–450 K are given in Fig. 5a and b. The variation curves of the integral intensity of the emission spectra of LMG:0.2Cr3+,0.05Yb3+ phosphors with temperature are shown in Fig. 5c. The luminescence intensity of the phosphor decreases with increasing temperature due to thermal quenching. The fluorescence emitted by Cr3+ and Yb3+ was integrated separately, and it was found that the luminescence of Yb3+ was consistently better than that of Cr3+, indicating that Yb3+ has better thermal stability. This is important for long wavelength applications of pc-LED devices.55 The thermal stability of LMG:0.2Cr3+,0.05Yb3+ was further evaluated by calculating the activation energy Ea through eqn (8):56 | (8) |
Upon increasing the temperature to 373 K, the luminous intensity of LMG:0.2Cr3+,0.05Yb3+ remains at 89.3% of its initial intensity. Upon increasing the temperature to 423 K, the luminous intensity of the LMG:0.2Cr3+,0.05Yb3+ phosphor remains at 72.7% of the initial intensity, demonstrating excellent thermal stability. Table S4† presents the IQE, EQE, thermal stability and photovoltaic efficiency data for a selection of Cr3+–Yb3+ co-doped NIR-emitting phosphors that have been previously reported in the literature. The results demonstrate that the prepared LMG:0.2Cr3+,0.05Yb3+ solid solution exhibits significant advantages in terms of quantum efficiency and thermal stability properties.
Applications of the NIR pc-LED
The optimized LMG:0.2Cr3+,0.05Yb3+ NIR phosphor was packaged with a commercial 440 nm blue chip for device packaging. Fig. 6a shows the EL curves of the NIR pc-LED device at different driving currents. The images of the NIR pc-LED device before and after energization are shown in the insets of Fig. 6a, respectively. The results demonstrate that the LMG:0.2Cr3+,0.05Yb3+ phosphor is capable of producing efficacious long-wavelength emitted light within the wavelength range of 1000–1200 nm, which is of considerable importance for practical spectral non-destructive testing. With the increase of the driving current from 20 mA to 350 mA, the luminous intensity of the NIR pc-LED device increased continuously and there was no light saturation phenomenon, with 9.9% photoelectric conversion efficiency at 100 mA, and the NIR output power was as high as 84.5 mW at 350 mA (Fig. 6b), which indicated that the LMG:0.2Cr3+,0.05Yb3+ phosphors in the high-throughput NIR pc-LED devices have good potential for application. Table S5† lists the detailed data of the pc-LED output power and efficiency under different current drives.The potential applications of the NIR pc-LED in night vision lighting and non-destructive testing were demonstrated. A pc-LED encapsulated with LMG:0.02Cr3+,0.05Yb3+ phosphors was used as a NIR light source, and a NIR camera was used to take images of plants and boxes with text under daylight and in dark environments with or without NIR light. Under NIR light, succulents can be clearly photographed and imaged, and text and graphics obscured by filters are clearly visible (Fig. 6c). The freshness of the leaves was evaluated through the utilization of the NIR pc-LED and thermal imaging instrumentation. Leaves #1, #2 and #3 were the same leaves that had been dried at room temperature for 1, 2 and 3 days, respectively, following harvesting. It was observed that the temperature of the leaves increased gradually with increasing irradiation time. The temperature at the apex of #2 and #3 was markedly lower than that of the mid-region of the leaves, due to the effects of drying and dehydration, in comparison with #1 (Fig. 6d). This discrepancy may be attributed to the variability in water and sugar contents among leaves at varying degrees of freshness. This can be attributed to the differing rates of absorption of NIR light by different functional groups.57 It is noteworthy that NIR pc-LEDs do not significantly elevate the temperature of the leaves, and the resulting temperature remains harmless. This phenomenon was also observed in grape berries (Fig. S10a†). These findings have significant implications for the integration of NIR spectroscopy into mobile phones or smart wearable devices. Fig. S10b† shows the operating temperature of the fabricated pc-LED versus the driving current and the thermograms after 5 min of operation driven by a 50 mA–300 mA operating current, respectively. When the operating current was increased from 50 mA to 300 mA, the pc-LED device emitted brighter light, while the operating temperature rose from 33.5 °C to 76.1 °C, but the temperature increase is within acceptable limits and the device still operated stably. These results demonstrate that the synthesized LMG:0.02Cr3+,0.05Yb3+ phosphors have a wide range of applications in the field of nondestructive testing as well as imaging techniques.
Conclusion
In summary, the synthesis of the LMG:Cr3+,Yb3+ broadband NIR phosphor with excellent comprehensive performance was achieved by constructing an efficient energy transfer between Cr3+ and Yb3+. Under 440 nm excitation, the LMG:Cr3+,Yb3+ phosphor exhibits a broad emission spectrum from 650 to 1200 nm, shows strong emission near 1000 nm, and exhibits high internal/external quantum efficiencies of 94.8%/40.5%. In addition, it has excellent thermal stability (89.3%@373 K and 72.7%@423 K). Finally, a NIR pc-LED device was fabricated by combining a 440 nm blue LED chip with the LMG:Cr3+,Yb3+ phosphor, with an output power of 84.5 mW at 350 mA and a photovoltaic conversion efficiency of 9.9% at 100 mA, which was successfully applied to non-destructive testing and bioimaging. These results indicate that this novel compound has great potential for future NIR pc-LED applications. This work provides a reference for the development of new efficient broadband NIR phosphors.Data availability
Some of the data that support the findings of this study are available in the ESI† of this article. The remaining data are available from the corresponding author upon reasonable request.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
The work was supported by the National Natural Science Foundation of China (No. 12274144, 22361132525, and 52472161), the Guangdong Provincial Science and Technology Project (No. 2022A1515010229), the Russian Science Foundation (grant 24-43-00006), and the Undergraduate Innovation and Entrepreneurship Training Program grant for Zixi Chen.References
- K. Elzbieciak-Piecka, M. Suta and L. Marciniak, Structurally induced tuning of the relative sensitivity of LaScO3:Cr3+ luminescent thermometers by co-doping lanthanide ions, Chem. Eng. J., 2021, 421, 129757 CAS.
- S. Liu, H. Cai, S. Zhang, Z. Song, Z. Xia and Q. Liu, Site engineering strategy toward enhanced luminescence thermostability of a Cr-doped broadband NIR phosphor and its application, Mater. Chem. Front., 2021, 5, 3841–3849 CAS.
- X. Wu, Q. Lin, Y. Li, J. Peng, W. You, D. Huang and X. Ye, Phase transition and Nd3+/Pr3+ co-doping induced enhancement of NIR emission in ScP3O9: Cr3+ phosphor, Mater. Res. Bull., 2024, 173, 112676 CAS.
- K. Gu and H. Zhong, A general methodology to measure the light-to-heat conversion efficiency of solid materials, Light: Sci. Appl., 2023, 12, 120 CAS.
- C. Li and J. Zhong, Highly Efficient Broadband Near-Infrared Luminescence with Zero-Thermal-Quenching in Garnet Y3In2Ga3O12:Cr3+ phosphors, Chem. Mater., 2022, 34, 8418–8426 CAS.
- A. Balhara, S. K. Gupta, B. Modak, A. K. Yadav, B. S. Naidu and K. Sudarshan, Broadband Shortwave Infrared-Emitting Cr3+- and Ni2+-Codoped Y3Al2Ga3O12Phosphor with Excellent Thermal Stability for Multifunctional Applications, ACS Appl. Mater. Interfaces, 2025, 17, 1509–1521 CAS.
- B. B. Srivastava, S. K. Gupta, S. Mohan and Y. Mao, Molten-Salt-Assisted Annealing for Making Colloidal ZnGa2O4:Cr Nanocrystals with High Persistent Luminescence, Chem. – Eur. J., 2021, 27, 11398–11405 CAS.
- B. B. Srivastava, S. K. Gupta and Y. Mao, Remarkable enhancement of photoluminescence and persistent luminescence of NIR emitting ZnGa2O4:Cr3+ nanoparticles, CrystEngComm, 2020, 22, 2491–2501 CAS.
- D. Liu, G. Li, P. Dang, Q. Zhang, Y. Wei, L. Qiu, H. Lian, M. Shang and J. Lin, Valence conversion and site reconstruction in near-infrared-emitting chromium-activated garnet for simultaneous enhancement of quantum efficiency and thermal stability, Light: Sci. Appl., 2023, 12, 248 CAS.
- F. Zhu, Y. Gao, C. Zhao, J. Pi and J. Qiu, Achieving Broadband NIR-I to NIR-II Emission in an All-Inorganic Halide Double-Perovskite Cs2NaYCl6:Cr3+Phosphor for Night Vision Imaging, ACS Appl. Mater. Interfaces, 2023, 15, 39550–39558 CrossRef CAS PubMed.
- T. Lang, Q. Zhao, X. Jing, G. Guan, S. Fang, Q. Qiang, L. Peng, T. Han, A. N. Yakovlev and B. Liu, Highly efficient near-infrared solid solution phosphors with excellent thermal stability and tunable spectra for pc-LED light sources toward NIR spectroscopy applications, Phys. Chem. Chem. Phys., 2023, 25, 25985–25992 CAS.
- Z. Jia, C. Yuan, Y. Liu, X.-J. Wang, P. Sun, L. Wang, H. Jiang and J. Jiang, Strategies to approach high performance in Cr3+-doped phosphors for high-power NIR-LED light sources, Light: Sci. Appl., 2020, 9, 86 CrossRef CAS PubMed.
- L. Fang, L. Zhang, H. Wu, H. Wu, G. Pan, Z. Hao, F. Liu and J. Zhang, Efficient Broadband Near-Infrared CaMgGe2O6:Cr3+Phosphor for pc-LED, Inorg. Chem., 2022, 61, 8815–8822 CrossRef CAS PubMed.
- Z. Wu, J. Xiang, C. Chen, Z. Li, X. Zhou, Y. Jin and C. Guo, Multi-site occupancy high-efficient Mg2LaTaO6:Cr3+ phosphor for application in broadband NIR pc-LEDs, Ceram. Int., 2024, 50, 5242–5249 CrossRef CAS.
- S. K. Gupta, K. Sudarshan, A. Balhara, S. K. Shaw, J. Bahadur and N. K. Prasad, Highly uniform microspheres of broadband near-infrared-emitting Ba(Hf1(-xCrx)O3 perovskite phosphor, Solid State Commun., 2024, 380, 115443 CrossRef CAS.
- S. Liu, Y. Zheng, D. Peng, J. Zhao, Z. Song and Q. Liu, Near-Infrared Mechanoluminescence of Cr3+Doped Gallate Spinel and Magnetoplumbite Smart Materials, Adv. Funct. Mater., 2023, 33, 2209275 CAS.
- S. Wu, B. Xiao, Y. Xiao, P. Shao, Y. Wang and P. Xiong, Cr3+-activated broadband near-infrared mechanoluminescence in garnet compound, Nano Energy, 2023, 116, 108811 CAS.
- C. Yang, D. Zheng, X. Zou, X. Dai, B. Tang, M. S. Molokeev, X. Zhang, H. Zhang, Y. Liu and B. Lei, Highly-Efficiency Far-Red Emission in Cr3+Activated Ca1.8Mg1.2Al2Ge3O12 toward Plant Precise Lighting, Adv. Opt. Mater., 2024, 12, 2303235 CAS.
- H. Zhu, Y. Li, Y. Xi, C. Xin, C. Zhou, Z. Yang, L. Ruan, Y. Li, Y. Peng, M. S. Molokeev, A. Zolotov, J. Wang, Z. Zhou and M. Xia, Abnormal Lattice Shrinkage, Site Occupation, and Luminescent Properties of Cr3+-Activated β-Al2O3 Structure Phosphors, Laser Photonics Rev., 2025, 19, 2401089 CAS.
- D. Wang, L. Yan, G. Zhu, S. Ma, N. Zhou, S. Li, Z. Li, Y. Cong, X. Bai, X. Han and B. Dong, Achieving Flat Ultra-Broadband NIR Emission in Cr3+Doped Garnet-Type Phosphors Enabled by Structural Regulation Toward Multi-Functional Spectroscopy Applications, Laser Photonics Rev., 2025, 19, 2401256 CAS.
- C. Zhong, Y. Xu, X. Wu, S. Yin, X. Zhang, L. Zhou and H. You, High Output Power and High Quantum Efficiency in Novel NIR Phosphor MgAlGa0.7B0.3O4:Cr3+with Profound FWHM Variation, Adv. Mater., 2024, 36, 2309500 CAS.
- L. Jiang, L. Zhang, X. Jiang, G. Lv and Y. Su, Multiple lattice sites occupied AlTaO4:Cr3+phosphor for luminescence ratiometric thermometry and NIR light source, J. Alloys Compd., 2024, 970, 172544 CAS.
- Y. Sun, M. Shang, Y. Wang, Y. Zhu, X. Xing, P. Dang and J. Lin, The ultra-wideband near-infrared luminescence properties and applications of K2SrGe8O18:Cr3+phosphor, Ceram. Int., 2023, 49, 32619–32627 CAS.
- Y. Shi, Z. Wang, J. Peng, Y. Wang, S. He, J. Li, R. Li, G. Wei, Y. Yang and P. Li, Achieving the ultra-broadband near-infrared La3SnGa5O14:Cr3+phosphor via multiple lattice sites occupation for biological nondestructive detection and night-vision technology, Mater. Today Adv., 2022, 16, 100305 CrossRef CAS.
- S. Miao, Y. Liang, Y. Zhang, D. Chen and X. Wang, Blue LED-Pumped Broadband Short-Wave Infrared Emitter Based on LiMgPO4:Cr3+,Ni2+Phosphor, Adv. Mater. Technol., 2022, 7, 2200320 CrossRef CAS.
- S. He, L. Zhang, J. Zhang, Z. Hao, H. Wu, H. Wu, Y. Luo, G. Pan, F. Liu and J. Zhang, Cr3+and Nd3+co-activated garnet phosphor for NIR super broadband pc-LED application, Mater. Res. Bull., 2022, 151, 111797 CrossRef CAS.
- M. Shi, Q. Shao, L. Yao, S. Yu, Y. Dong and J. Jiang, Molten Salt Synthesis of Broad-Band Near-Infrared InBO3:Cr3+Submicron Phosphor and Its Luminescent Enhancement by Lanthanide Ion Codoping, Inorg. Chem., 2022, 61, 12275–12283 CrossRef CAS PubMed.
- Q. Zhang, D. Liu, Z. Wang, P. Dang, H. Lian, G. Li and J. Lin, LaMgGa11O19:Cr3+,Ni2+as Blue-Light Excitable Near-Infrared Luminescent Materials with Ultra-Wide Emission and High External Quantum Efficiency, Adv. Opt. Mater., 2023, 11, 2202478 CrossRef CAS.
- J. Wang, C. Gong, S. Yang, Q. Zhu, X. Wang and J.-G. Li, Cr3+activated Na3RESi3O9(RE = Y, Lu, Sc) silicate broadband near-infrared phosphors for luminescence towards NIR-II region via a multi-site occupancy strategy, J. Rare Earths, 2024, 42, 1447–1457 CrossRef CAS.
- C. Wang, X. Zhang, C. Zhong, X. Wu, Y. Xu, S. Yin, Q. Yang, L. Zhou and H. You, Enhanced quantum efficiency and thermal stability by crystal-field engineering in a Y(Ga,Al)3(BO3)4:Cr3+,Yb3+phosphor for diverse short-wave infrared applications, J. Mater. Chem. C, 2024, 12, 3515–3525 CAS.
- G. Zheng, C. Lou, Z. Yuan, W. Xiao, L. Shang, J. Zhong, M. Tang and J. Qiu, Rare-Metal-Free Ultrabroadband Near-Infrared Phosphors, Adv. Mater., 2025, 37, 2415791 CAS.
- A. Balhara, S. K. Gupta, B. Modak, M. Abraham, A. K. Yadav, H. V. Annadata, S. Das, N. S. Rawat and K. Sudarshan, Synergy between structural rigidity and cluster defects in a bright near-infrared Cr3+-based phosphor for excellent thermal stability and long afterglow, J. Mater. Chem. C, 2024, 12, 9716–9732 CAS.
- H. Zhang, J. Zhong, F. Du, L. Chen, X. Zhang, Z. Mu and W. Zhao, Efficient and Thermally Stable Broad-Band Near-Infrared Emission in a KAlP2O7:Cr3+ Phosphor for Nondestructive Examination, ACS Appl. Mater. Interfaces, 2022, 14, 11663–11671 CrossRef CAS PubMed.
- W. Zhang, C. Wang, X. Qiao and X. Fan, Simultaneous enhancement of red luminescence and thermal stability of a novel red phosphor: A Li+-Eu3+co-occupied site strategy in BaAl2Ge2O8lattices, J. Rare Earths, 2024, 42, 1846–1854 CrossRef CAS.
- W. Chen, W. Li, X. Zhang, C. Ma, Z. Xia and B. Lei, Carbon dots embedded in lead-free luminescent metal halide crystals toward single-component white emitters, Sci. China Mater., 2022, 65, 2802–2808 CrossRef CAS.
- S. Liu, J. Du, Z. Song, C. Ma and Q. Liu, Intervalence charge transfer of Cr3+-Cr3+aggregation for NIR-II luminescence, Light: Sci. Appl., 2023, 12, 181 CrossRef CAS PubMed.
- C. Zhong, Y. Xu, X. Wu, S. Yin, X. Zhang, L. Zhou and H. You, High Output Power and High Quantum Efficiency in Novel NIR Phosphor MgAlGa0.7B0.3O4:Cr3+with Profound FWHM Variation, Adv. Mater., 2024, 36, 2309500 CrossRef CAS PubMed.
- L. Yao, Q. Shao, S. Han, C. Liang, J. He and J. Jiang, Enhancing Near-Infrared Photoluminescence Intensity and Spectral Properties in Yb3+Codoped LiScP2O7:Cr3+, Chem. Mater., 2020, 32, 2430–2439 CrossRef CAS.
- Y. Zhang, S. Miao, Y. Liang, C. Liang, D. Chen, X. Shan, K. Sun and X.-J. Wang, Blue LED-pumped intense short-wave infrared luminescence based on Cr3+-Yb3+-co-doped phosphors, Light: Sci. Appl., 2022, 11, 136 CrossRef CAS PubMed.
- M. U. Dumesso, W. Xiao, G. Zheng, E. T. Basore, M. Tang, X. Liu and J. Qiu, Efficient, Stable, and Ultra-Broadband Near-Infrared Garnet Phosphors for Miniaturized Optical Applications, Adv. Opt. Mater., 2022, 10, 2200676 CAS.
- D. Xu, Q. Zhang, X. Wu, W. Li and J. Meng, Synthesis, luminescence properties and energy transfer of Ca2MgWO6:Cr3+,Yb3+phosphors, Mater. Res. Bull., 2019, 110, 135–140 CAS.
- Z. Lu, S. Chen, Y. Liu, C. Yuan, R. Li, P. Sun, Z. Luo, Z. Liu and J. Jiang, LiGaP2O7:Cr3+, Yb3+phosphors for broadband NIR LEDs toward multiple applications, J. Alloys Compd., 2023, 956, 170311 CAS.
- Y. Yang, W. Lü, X. Kang, Z. Zhu, Q. Pan and Q. Zeng, Broadening near-infrared emission and enhancing thermal stability of Cr3+-activated SrLaGa3O7 phosphors via Yb3+co-doping, J. Lumin., 2025, 280, 121098 CAS.
- H. Zheng, Z. Shao, F. Ling, L. Li, G. Xiang, Y. Wang and X. Zhou, Broadband near-infrared phosphor SrGe4O9:Cr3+ for NIR-pc-LED applications, Ceram. Int., 2025 Search PubMed , S0272884225006959.
- X. Wu, D. Huang, Q. Lin, L. Han, W. You, J. Zhu and X. Ye, Thermally Stable Broadband Cr3+-Doped RbAl3P6O20 Phosphor for Near-Infrared Spectroscopy Applications, Inorg. Chem., 2025, 64, 3476–3484 CAS.
- S. Wang, Z. Lyu, D. Sun, S. Shen and H. You, Tailoring the Cr3+Emission via Combinatorial Management of Crystal-Field and Energy Transfer for Multiple NIR LEDs, Adv. Opt. Mater., 2024, 12, 2303140 CAS.
- A. D. Sontakke, A. J. Van Bunningen, F. T. Rabouw, S. Meijers and A. Meijerink, Unraveling the Eu2+→ Mn2+Energy Transfer Mechanism in w-LED Phosphors, J. Phys. Chem. C, 2020, 124, 13902–13911 CAS.
- Y. Xiao, Z. Hao, L. Zhang, W. Xiao, D. Wu, X. Zhang, G.-H. Pan, Y. Luo and J. Zhang, Highly Efficient Green-Emitting Phosphors Ba2Y5B5O17with Low Thermal Quenching Due to Fast Energy Transfer from Ce3+to Tb3+, Inorg. Chem., 2017, 56, 4538–4544 Search PubMed.
- M. U. Dumesso, W. Xiao, G. Zheng, E. T. Basore, M. Tang, X. Liu and J. Qiu, Efficient, Stable, and Ultra-Broadband Near-Infrared Garnet Phosphors for Miniaturized Optical Applications, Adv. Opt. Mater., 2022, 10, 2200676 CrossRef CAS.
- Y. Liu, S. He, D. Wu, X. Dong and W. Zhou, Broadband NIR Garnet Phosphors with Improved Thermal Stability via Energy Transfer, ACS Appl. Electron. Mater., 2022, 4, 643–650 CAS.
- S. Miao, Y. Liang, R. Shi, W. Wang, Y. Li and X.-J. Wang, Broadband Short-Wave Infrared-Emitting MgGa2O4:Cr3+, Ni2+Phosphor with Near-Unity Internal Quantum Efficiency and High Thermal Stability for Light-Emitting Diode Applications, ACS Appl. Mater. Interfaces, 2023, 15, 32580–32588 CAS.
- A. A. Melentsova, O. A. Lipina, A. Yu. Chufarov, A. P. Tyutyunnik and V. G. Zubkov, Energy transfer mechanism in infrared-emitting NaYGeO4:Tm3+, Ho3+phosphors, Ceram. Int., 2024, 50, 49545–49551 CAS.
- S. Wang, Z. Lyu, D. Sun, S. Shen and H. You, Tailoring the Cr3+Emission via Combinatorial Management of Crystal-Field and Energy Transfer for Multiple NIR LEDs, Adv. Opt. Mater., 2024, 12, 2303140 CAS.
- S. Zhao, Z. Mu, L. Lou, S. Yuan, M. Liao, Q. Lin, D. Zhu and F. Wu, Broadening and enhancing emission of Cr3+simultaneously by co-doping Yb3+in Ga1.4In0.6SnO5, J. Rare Earths, 2023, 41, 1895–1903 CAS.
- C. Jin, R. Li, Y. Liu, L. Zhang, J. Zhang, P. Sun, Z. Luo and J. Jiang, Efficient and Stable Gd3Ga5O12:Cr3+Phosphors for High-Performance NIR LEDs, Adv. Opt. Mater., 2023, 11, 2300772 CAS.
- Y. Zhang, Z. Gao, Y. Li, S. Chen, M. Han, J. Li, Q. Zhang, Y. Shen, D. Deng and S. Xu, Broadband La2LiSbO6: Cr3+Phosphors with Double Luminescent Centers for NIR pc-LEDs, Inorg. Chem., 2023, 62, 17371–17381 CAS.
- D. Huang, H. Zhu, Z. Deng, H. Yang, J. Hu, S. Liang, D. Chen, E. Ma and W. Guo, A highly efficient and thermally stable broadband Cr3+-activated double borate phosphor for near-infrared light-emitting diodes, J. Mater. Chem. C, 2021, 9, 164–172 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5qi00215j |
| This journal is © the Partner Organisations 2025 |





