Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Oriented catalysis through chaos: high-entropy spinels in heterogeneous reactions
Yalan
Mo
 a,
Xiaohong
Guan
a,
Xiaohong
Guan
 b,
Shaobin
Wang
b,
Shaobin
Wang
 *a and
Xiaoguang
Duan
*a and
Xiaoguang
Duan
 *a
*a
aSchool of Chemical Engineering, The University of Adelaide, Adelaide, SA 5005, Australia. E-mail: shaobin.wang@adelaide.edu.au; xiaoguang.duan@adelaide.edu.au
bSchool of Ecological and Environmental Science, East China Normal University, Shanghai, 200241, China
First published on 27th December 2024
Abstract
High-entropy spinel (HES) compounds, as a typical class of high-entropy materials (HEMs), represent a novel frontier in the search for next-generation catalysts. Their unique blend of high entropy, compositional diversity, and structural complexity offers unprecedented opportunities to tailor catalyst properties for enhanced performance (i.e., activity, selectivity, and stability) in heterogeneous reactions. However, there is a gap in a critical review of the catalytic applications of HESs, especially focusing on an in-depth discussion of the structure–property–performance relationships. Therefore, this review aims to provide a comprehensive overview of the development of HESs in catalysis, including definition, structural features, synthesis, characterization, and catalytic regimes. The relationships between the unique structure, favorable properties, and improved performance of HES-driven catalysis are highlighted. Finally, an outlook is presented which provides guidance for unveiling the complexities of HESs and advancing the field toward the rational design of efficient energy and environmental materials.
1. Introduction
Heterogeneous catalysis is the cornerstone of the modern chemical industry, playing a pivotal role in a wide range of industrial processes, including energy conversion, environmental remediation, and fine chemical production.1 Traditional catalysts, typically composed of noble metals (e.g., Pt, Ir, and Ru) or transition metal oxides (e.g., Co3O4, MnO2, and Fe3O4), have been widely used due to their exceptional catalytic properties.2,3 However, these materials face limitations such as high cost, scarcity, and deactivation under harsh conditions.4–6 Thus, it is necessary to design and develop cost-effective and high-performance catalysts to satisfy the growing demands for practical applications.High-entropy materials (HEMs) are new favorite catalysts featuring multielement composition and complex atomic configurations in the field of catalysis. From high-entropy alloys (HEAs) as the first-reported HEMs, a broader range of HEMs, including high-entropy oxides (HEOs) and other high-entropy ceramics (HECs), have been explored.7–13 Among them, high-entropy spinels (HESs) have attracted significant attention due to their notable catalytic properties stemming from the transition metal components, high electrical conductivity, structural robustness, and stability.14–16 Unlike the rock-salt structure, the spinel structure offers unique opportunities for multicomponent systems due to its large and complex unit cell, consisting of 32 anion sites surrounded by 24 cations arranged in both octahedral and tetrahedral cages.17–19 Such a spinel structure endows HESs with remarkable compositional versatility, enabling fine-tuning of their structural, electronic, and surface properties. This flexibility makes HESs highly versatile and efficient catalysts.
Recent studies have demonstrated the excellent performance of HESs in various catalytic processes, such as the oxygen evolution reaction (OER) and oxygen reduction reaction (ORR) and in Li-ion batteries (LIBs), highlighting their potential as next-generation catalysts.20–25 However, the full potential of HESs in catalytic applications has largely remained untapped due to limited knowledge of the relationships between the structure, properties, and catalytic performance of HESs. The complexity of HESs, arising from the vast compositional space and intricate atomic arrangements, poses significant challenges in elucidating the mechanisms underlying their catalytic behaviour. It is essential to employ advanced experimental and computational methods to systematically study the intricate structure–property–activity relationships of HESs in catalysis. In addition, understanding the unique structural attributes of HESs, such as multiple active metal sites, defect structures, and high configurational entropy to their catalytic activity, can inform the rational design of more efficient and selective catalysts.
This review aims to provide a comprehensive overview of the current research on HESs in heterogeneous catalysis, highlighting key advancements and identifying critical knowledge gaps. Firstly, the fundamentals of HESs are introduced, including a historical overview, definition and features, synthesis methods, and characterisation techniques. Secondly, the research progress of HESs in thermocatalysis, electrocatalysis, and photocatalysis is reviewed. More importantly, the structure–property–performance relationships of HESs and catalytic reaction mechanisms are highlighted and discussed. At the end, challenges and opportunities are discussed to outline potential future directions in developing next-generation HESs for sustainable heterogeneous catalysis.
2. High-entropy spinels
2.1 A historic overview
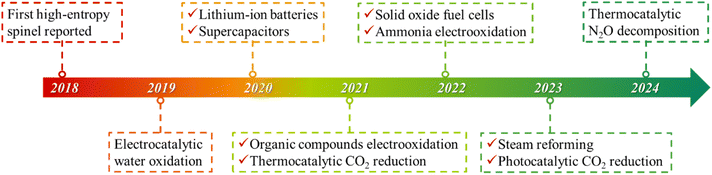
HESs originated from the exploration of an alloy, a metallic substance containing two or more metal or non-metal elements.26 Traditional alloys typically consist of a primary element melted with one or two additional elements in minor quantities, limiting their elemental diversity and broader applications.27 In 2004, Cantor et al. and Yeh et al. introduced “high-entropy alloys”, featuring multiple principal elements in nearly equal atomic proportions, to enhance the diversity and properties of metal alloys.7,28 Inspired by the research on HEAs, Rost et al. extended the high-entropy concept to five-component oxides in 2015, preparing the first HEOs with a rock-salt structure.8 Compared with the simple face-centered cubic (FCC),29 body-centered cubic (BCC),30 and hexagonal close-packed (HECP) structures in HEAs,31 various crystal structures of HEOs have been demonstrated, including rock-salt,32 perovskite,33 spinel,34 fluorite,35 bixbyite,36 pyrochlore,37 and layered O3-type structures.38Rock-salt HEOs which contain only one Wyckoff site for cations have been mostly studied due to their easy formation. With the further development of HEOs, spinel- and perovskite-structured HEOs with multiple Wyckoff sites have attracted increasing attention, because they have more diversified atomic arrangements and thus possess greater flexibility in composition and property tuning.39–42 In 2018, Dąbrowa et al., for the first time, synthesized the single-phase spinel (Co, Cr, Fe, Mn, Ni)3O4 HEO via a solid-state reaction method.43 Since then, numerous HESs have been reported and studied in a wide range of catalytic applications, including electrochemical water splitting,44 anode materials for LIBs,45 supercapacitor electrode materials,46 electrooxidation of organic compounds,47 thermocatalytic CO2 reduction,48 cathode materials for solid oxide fuel cells,49 steam reforming,50etc.16,22,51 A timeline of major developments in HESs and some key applications is illustrated in Fig. 1.
2.2 Definition and features
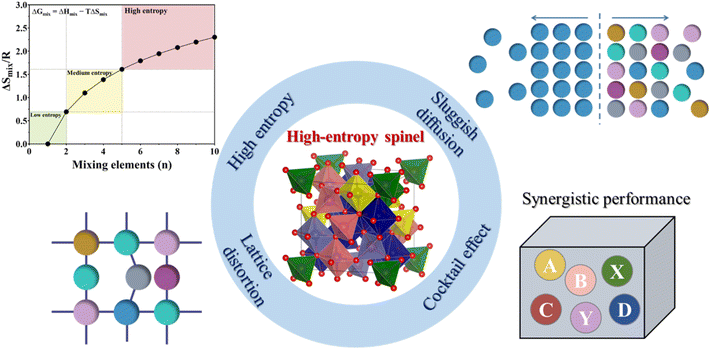
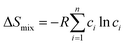 | (1) |
ΔSmix = −R![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n n | (2) |
In this definition, a HEA is a material in which the ideal configurational entropy equals or exceeds the value of five elements in equiatomic proportions, specifically ΔSmix ≥ 1.61R; materials with ΔSmix ≤0.69R are considered “low entropy”, and those with 1.61R > ΔSmix > 0.69R are “medium entropy”.52,53 Other studies considered 1.5R to be the boundary between medium entropy and high entropy and ≤ 1.0R to be the low entropy cutoff.54,55
As HESs contain both cations and anions, the ΔSmix was further extended to56
 | (3) |
It should be noted that the terms “high entropy”, “multicomponent”, and “compositionally complex” are not synonymous. A material being “multicomponent” or “compositionally complex” does not automatically indicate high entropy, as configurational entropy depends on not only the number of components but also their proportions. In addition, although configurational entropy is typically considered the main contributor to the total entropy of a solid, nonconfigurational contributions (such as vibrational, electronic, or magnetic entropy) may dominate the thermodynamics in some cases and should be considered.57
2.2.2.1 High-entropy effect. The high-entropy effect is for the thermodynamic feasibility of the formation of solid solution phases. The phase stability in a multicomponent system is generally described by the Gibbs free energy, and its equation is as follows:
| ΔGmix = ΔHmix − TΔSmix | (4) |
2.2.2.2 Lattice distortion effect. The lattice distortion effect arises from variations in atomic sizes and unsymmetrical bonding among the constituent elements. The disparity in atomic sizes creates a state of thermodynamic nonequilibrium, which may theoretically lower the energy barrier for the adsorption, activation, and transformation of molecules involved in catalysis.64 This effect induces severe lattice strain in HESs, which impedes the dislocation movement and thus hinders the deformation of the crystal lattice under external forces. Tensile lattice strain can also cause an upwards shift of the d-band centre, resulting in stronger adsorption of reactant species on HES surfaces in catalytic reactions.65,66
2.2.2.3 Sluggish diffusion effect. The sluggish diffusion effect is generated by variable diffusion rates, changes in chemical bonds, and divergence of potential energy, which is closely associated with the lattice distortion effect. A severe lattice distortion in HESs is expected to increase the diffusion activation energy of atoms within the lattice, thereby resulting in inhibited atomic diffusion, phase transformation and grain growth.64 The sluggish diffusion effect contributes to the formation of nanoscale HESs, allowing the HESs to maintain structural robustness even under extreme conditions.53
2.2.2.4 Cocktail effect. The cocktail effect signifies a multifaceted synergistic impact resulting from the interaction among various element components in HESs. Many studies indicate that the performance of HESs is not merely a straightforward combination of the properties of each element but is instead unpredictable.65 The cocktail effect is affected not just by the average characteristics of constituent elements, but also by the other three effects discussed above.64 This effect is extensively reflected in various properties of HESs, encompassing their mechanical strength, corrosion resistance, and oxidation resistance.
In summary, HESs exhibit unique features arising from their complex composition and structure. The definition of HESs builds upon the principles of HEAs, incorporating both compositional and entropy-based criteria. In HESs, multiple cations and anions contribute to the increased configurational entropy, which plays a critical role in their stability and catalytic properties.57 The structural features of HESs, including the normal and inverse spinel arrangements, make them highly versatile in tuning their functional properties. The four core effects, including the high-entropy effect in thermodynamics, lattice distortion effect in structures, sluggish diffusion effect in dynamics, and cocktail effect in performance, collectively distinguish HESs from traditional spinels, making them promising candidates for advanced catalytic applications.62 For example, in electrocatalysis, the high-entropy and sluggish diffusion effects enhance the structural robustness of HESs, while lattice distortion optimizes the coordination environment of surface atoms and the adsorption energy of reactants and intermediates.66
2.3 Synthesis
The formation of HESs requires synthesis techniques capable of incorporating more than four elements into a single-phase spinel structure, ensuring homogeneity and phase stability. In addition, synthesized HESs with a high specific surface area, abundant active sites, and robust structural stability are more favorable in catalytic reactions. Therefore, a variety of methods have been used to fabricate HES materials for different catalytic purposes, including conventional synthesis methods (i.e., solid-state and wet-chemical methods) and several emerging methods (e.g., carbothermal shock synthesis).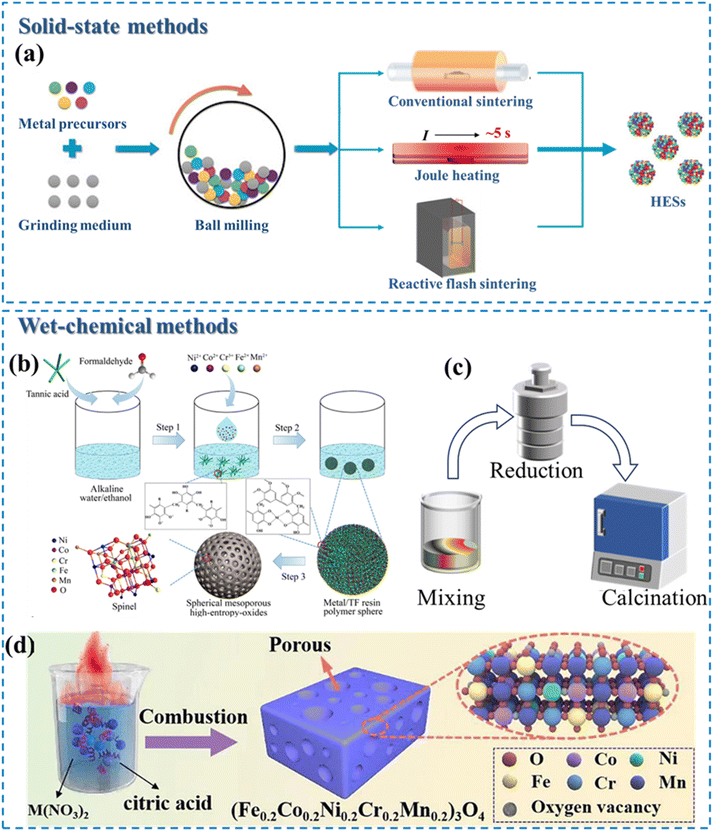 | ||
| Fig. 3 Conventional methods for HES synthesis. (a) Synthetic procedures of different solid-state methods. Reproduced with permission.41 Copyright 2022, John Wiley and Sons. Wet-chemical methods: (b) sol–gel method. Reproduced with permission.67 Copyright 2020, American Chemical Society. (c) Hydro-/solvothermal methods. (d) Solution combustion synthesis. Reproduced with permission.68 Copyright 2023, Elsevier. | ||
Solid-state methods are straightforward and can be easily scaled up for industrial production, making them suitable for large-scale applications. However, achieving a uniform distribution of multiple elements can be challenging due to limited diffusion rates under solid-state conditions.
2.3.2.1 Sol–gel method. This method has been commonly used for preparing nanostructured HESs with high surface areas, which are beneficial for catalytic applications. This method offers precise control over the chemical composition and homogeneity at the molecular level, leading to high-purity and uniformly mixed HESs. A typical sol–gel process involves the hydrolysis and condensation of metal alkoxides or salts to form a gel mixture, followed by drying and calcination to produce HESs. In addition, the sol–gel method allows for the incorporation of dopants or additives to tailor the morphology and structure of HES products. For example, Wang et al. reported the synthesis of spherical mesoporous HESs (Ni–Co–Cr–Fe–Mn oxide) by a sol–gel strategy using a plant polyphenol, formaldehyde, and metal salts as precursors (Fig. 3b).67 The HESs exhibited a high specific surface area (42–143 m2 g−1), large pore size (5.5–8.3 nm), and a unique spherical morphology (∼55 nm).
2.3.2.2 Hydro-/solvothermal methods. This synthesis is a simple, energy-efficient, and well-controlled approach to producing HES nanomaterials.44,50 During the hydro-/solvothermal synthesis process, metal salts are mixed in water or an appropriate solvent and reduced at high temperatures and pressures in a sealed autoclave (Fig. 3c). By adjusting reaction parameters such as temperature, pressure, time, pH value, and precursor and additive concentrations, these methods enable the manipulation of material properties, including crystallinity, morphology, and size.75 Xiao et al. successfully synthesized a defect-rich (CoCrFeMnNi)3O4 HEO catalyst with a single-phase spinel structure via a hydrothermal method.76 Owing to the unique spinel structure and abundant oxygen vacancies, the catalyst displayed a high discharge capacity, superior rate capability, and long-term stability for vanadium redox flow batteries.
2.3.2.3 Solution combustion synthesis (SCS). The SCS is a rapid and self-sustained process to produce HESs.17,77–79 It involves dissolving metal nitrates (acting as oxidizers) and organic fuels (e.g., urea, glycine, or citric acid) in a suitable solvent to form a homogeneous solution. Upon heating, an exothermic redox reaction occurs, generating intense heat and large amounts of gaseous byproducts, resulting in the formation of porous and finely dispersed solid products. He et al. first prepared nano-porous (Fe0.2Co0.2Ni0.2Cr0.2Mn0.2)3O4 with high grain boundary density by a low-temperature SCS method using metal nitrate as an oxidant and citric acid as a complexing agent (Fig. 3d).68 Due to the nano-porous structure and abundant grain boundaries, this catalyst exhibited excellent OER performance.
Carbothermal shock (CTS) synthesis has already shown great promise in HEAs since 2018, offering unique advantages in terms of rapid production, controlled composition, and fine-tuning of properties.83 In recent years, this technique has also been used to synthesize HESs. It involves the rapid heating and cooling of a mixture of metal salt precursors and a carbon source, typically achieved within milliseconds to seconds at extremely high temperatures (1000–1700 °C).80,84 At elevated temperatures, metal salts initially undergo thermal reduction and then turn into liquid metal droplets, resting on a carbon-rich substrate. After rapid cooling to room temperature, the mixture forms a homogeneous HEA, which subsequently oxidizes to spinel oxides under an ambient atmosphere (Fig. 4a). This short reaction time is advantageous as it prevents excessive grain growth, thereby producing nanoscale materials. The resulting HESs are supported on carbon materials, which provide additional benefits such as enhanced electrical conductivity, mechanical strength, and surface area. The carbon support can also play a role in stabilizing the high-entropy spinel structure by preventing agglomeration and facilitating the dispersion of nanoparticles. These features are particularly beneficial for catalytic applications.
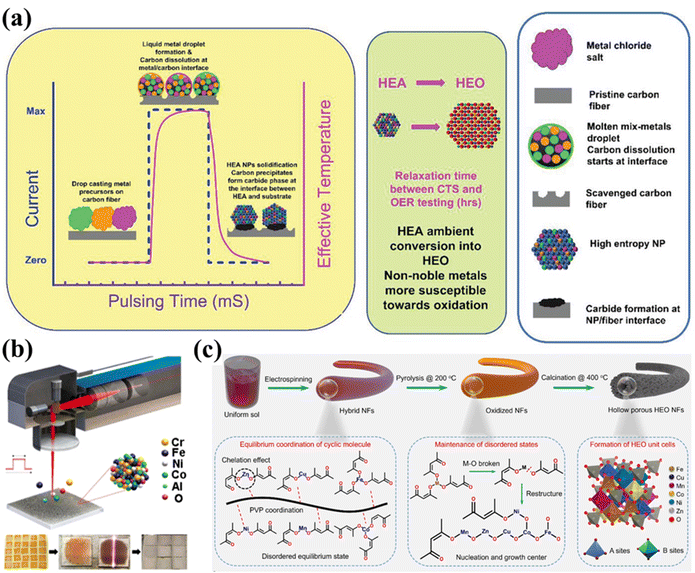 | ||
| Fig. 4 Emerging methods for HES synthesis. (a) Schematic illustration of the carbothermal shock (CTS) synthesis process within mS pulse intervals.80 (b) Laser-based shock methods. Reproduced with permission.81 Copyright 2021, American Chemical Society. (c) Electrospinning. Reproduced with permission.82 Copyright 2024, American Chemical Society. | ||
Abdelhafiz et al. reported that ternary to senary (FeNiCoCrMnV) HES nanoparticles on carbon fibers synthesized by CTS (rapid Joule heating and quenching) showed higher activity towards catalyzing the OER compared to an IrO2 catalyst with two orders of magnitude higher stability than IrO2, due to the stronger interaction with the substrate by metal–carbide bond activated during the OER process.80 In addition to thermal shock, laser shock has also been used to synthesize HESs. For example, Zhu et al. used a scaled-up (up to 51% mass ratio) laser-based shock method to fabricate HES nanoparticles on the surface of conductive carbon black at high speed.85 The carbon black substrate not only served as the conductive network within the HESs as an electrode material in LIBs but also provided optimal conditions for rapid heating and cooling processes used in nanoparticle synthesis. Yang et al. successfully used a pulsed laser to manufacture highly efficient electrocatalytic CoCrFeNiAl HES electrodes for the OER on various substrates (e.g., indium tin oxide, Ni foam, and carbon cloth), as shown in Fig. 4b.81 This pulsed laser enables rapid synthesis with precise control over reaction conditions by adjusting parameters such as pulse energy, pulse duration, and scanning velocity.81,86
Similar to “shock”-based methods, several other techniques have been developed to synthesize HESs, including electrospinning, microwave-assisted synthesis, and spray pyrolysis. Electrospinning can produce nanofibers with high surface areas (Fig. 4c);82,87–89 microwave-assisted synthesis provides fast and uniform heating, leading to shorter reaction times and improved homogeneity of the final products.24,90,91 Spray pyrolysis, on the other hand, is highly versatile and scalable, making it suitable for large-scale production of finely dispersed HES powders.92–94
The structure and functionality of HESs are closely tied to the synthesis method employed, with significant variations observed at the microstructural level.95 Solid-state synthesis, while straightforward and scalable, often struggles with achieving uniform elemental distribution and reproducibility. Wet-chemical methods allow better control over the morphology, composition, and homogeneity, bringing HESs closer to the ideal definition and making them suitable for studies aimed at understanding the fundamental properties of HESs.96 Emerging “shock”-based synthesis techniques offer more efficient, scalable, and tailored production of HESs for enhanced catalytic performance, though they require specialized equipment and conditions. Continuous development and optimization of these synthesis techniques are essential for advancing high-entropy materials and unlocking their full potential in catalysis and other applications.
2.4 Characterization
The characterization of HESs mainly focuses on confirming their single-phase spinel structure and uniform distribution of elements. A series of techniques, including diffraction, microscopy, and spectroscopy, have been employed to characterize the local lattice structure and atomic distribution.43,70,97–99 More importantly, accurate characterization is crucial to identify and quantify active sites, investigate the effect of local structures (e.g., oxygen vacancies and strain) on the catalytic performance, and finally reveal the underlying catalytic mechanisms.X-ray diffraction (XRD) is a powerful tool to determine the crystal structure, phase purity, and lattice parameters of HES materials.43,73 High-resolution electron microscopy techniques, such as scanning electron microscopy (SEM) and transmission electron microscopy (TEM), are standard imaging methods. SEM can visualize the surface morphology, particle size, and elemental distribution of HESs. TEM especially high-resolution TEM (HR-TEM) can reveal the internal structure, crystallography, and defects within HESs and is crucial for studying the nanoscale features of HESs, including grain boundaries, dislocations, and the distribution of different elements within the spinel lattice.21,100 More precisely, high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) can achieve atomic-scale resolution, enabling the visualization of individual atoms and their arrangements within the spinel structure. These methods are often combined with energy-dispersive spectroscopy (EDS or EDX) or electron energy loss spectroscopy (EELS) for elemental mapping. As shown in Fig. 5a–e, the nanoparticle morphology of a HES ((CrMnFeNiZn)3O4) was confirmed using the TEM image (Fig. 5a); lattice spacings of 0.49 and 0.29 nm shown by HR-TEM images (Fig. 5b and c) corresponded to the (111) and (220) planes of the cubic spinel oxide, respectively; the selected area electron diffraction (SAED) pattern (Fig. 5d) was well-indexed to a single-phase spinel structure; the even distribution of Cr, Mn, Fe, Ni, Zn, and O elements was verified by STEM-EDS elemental mapping (Fig. 5e).101
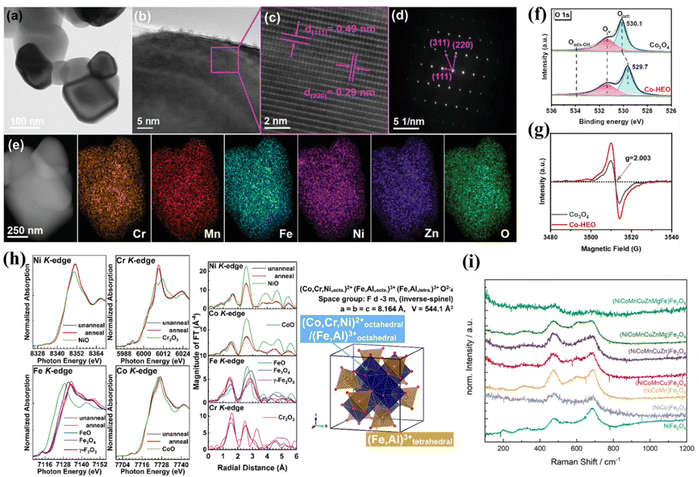 | ||
| Fig. 5 Characterization techniques of HESs. (a) TEM image, (b and c) HR-TEM images, (d) SAED pattern, and (e) STEM-EDS mapping of (CrMnFeNiZn)3O4. Reproduced with permission.101 Copyright 2023, John Wiley and Sons. (f) O 1s XPS spectra and (g) EPR spectra of Co3O4 and (CuMgNiZn)1Co2O4 for oxygen vacancy detection. Reproduced with permission.51 Copyright 2024, American Chemical Society. (h) Ni K-, Co K-, Fe K-, and Cr K-edge XANES and EXAFS spectra of NiCoFeCrAlO powder and schematic illustration of the inverse-spinel structure in this HES. Reproduced with permission.81 Copyright 2021, American Chemical Society. (i) Raman spectra of ferrites with increasingly more cations.24 | ||
In contrast to EDS and EELS, which only examine a small sample area, spectroscopic techniques investigate the bulk and surface structures along with their associated chemical states. X-ray photoelectron spectroscopy (XPS) is frequently used to study the surface composition and chemical states of HESs, which are critical for understanding their catalytic behaviour since only surface atoms directly participate in heterogeneous reactions.16,102 Most studies on HESs also used XPS to identify the oxygen vacancies (Fig. 5f),51 but it is not reliable to take XPS as the only evidence for oxygen vacancy formation since many species hold similar binding energy.103 Electron paramagnetic resonance spectroscopy (EPR) has been a supplementary technique to precisely detect the oxygen vacancies in HESs (Fig. 5g).51 X-ray absorption spectroscopy (XAS) techniques, including extended X-ray absorption spectroscopy fine structure (EXAFS) and X-ray absorption near-edge structure (XANES), are valuable for further understanding chemical states of the constituent elements and their distribution over the octahedral (Oh) and tetrahedral (Td) co-ordination environment in HESs.97,104 XANES examines the oxidation states and coordination chemistry of the tested elements, while EXAFS provides detailed information on the distances between the adsorbing atom and its neighboring atoms, the coordination number, and the types of neighboring atoms. Fig. 5h shows the Ni K-, Co K-, Fe K-, and Cr K-edge XANES and EXAFS spectra of a HES (NiCoFeCrAlO) and a modelled inverse-spinel structure of this HES based on the results.81
Other techniques such as Raman spectroscopy, Fourier-transform infrared (FTIR) spectroscopy, and Mössbauer spectroscopy are also used to characterize HESs.24,99,105 These methods provide detailed insights into the vibrational properties, chemical bonding, surface chemistry, oxidation states, and magnetic properties, complementing other analytical techniques for a comprehensive understanding of these complex materials. For example, as shown in Fig. 5i, Raman spectra of ferrites with increasingly more cations nearly all exhibited a band between 450 and 500 cm−1, which could correspond to the T2g bands arising from asymmetric stretching and bending M–O vibrations at octahedral sites in an inverted binary spinel. In addition, the bands became significantly broader as the number of cations increased, indicating the increased cation disorder also on the octahedral sites.24
Conventionally, the structure of HESs is characterized and compared before and after a reaction via ex situ characterization techniques described above to identify structural changes, surface modifications, and the nature of active sites, thus gaining insights into the catalytic mechanisms.44,45,106 However, in many cases, the structure of a catalyst often differs during the catalytic reaction compared to its structure before or after the reaction, which may lead to a misunderstanding of mechanisms.107 In addition, the process of preparing, handling, and transferring samples for ex situ measurements possibly causes changes in the reacted samples (e.g., contamination and relaxation). In situ/operando characterization techniques enable the real-time monitoring and characterization of the local structure/property/performance, providing a deeper understanding of reaction processes and mechanisms. Many in situ/operando characterization techniques, including XRD, XPS, XAS, Raman, and DRIFTS (diffuse reflectance infrared Fourier transform spectroscopy), have been applied for HES characterization to capture the average atomic or molecular information under real-working conditions.75,108–112 For example, Chang et al. used operando quick-scanning XAS to investigate the lithiation/delithiation mechanism of the (CrMnFeNiCu)3O4 HES.113 The regime was revealed via examining valence/coordination state variations, transition steps, redox sequences, reversibility, and redox overpotential of multiple electroactive centers in the (CrMnFeNiCu)3O4 electrode. Wu et al. applied in situ TEM to monitor the microstructural evolution of (Cr, Mn, Fe, Co, and Ni)3O4 during calcination, offering valuable insights for optimizing HEO synthesis via solid-state methods.114
To assess the properties of HESs, various characterization methods are required to examine their elemental compositions, crystallographic features, microstructure and morphologies, and chemical states. Techniques such as XRD, SEM, TEM, XPS, XAS, EPR, Raman, and FTIR provide detailed insights into the structural and compositional information of HESs, as well as identifying active sites and elucidating catalytic mechanisms. While conventional ex situ techniques are crucial for analyzing structural changes, in situ/operando methods offer real-time insights into catalytic reactions under working conditions. Additionally, advanced characterization techniques, such as three-dimensional atomic electron tomography (3D-AET) and four-dimensional STEM (4D-STEM), are also being explored within the high-entropy community to achieve atomic-level insights into HEMs.115,116 The combination of these characterization methods allows for a comprehensive understanding of structure–property relationships of HESs, which is critical for on-demand HES catalyst design and advancing their catalytic applications.
3. Catalytic applications
As discussed in Section 2.2.2, the marriage of high-entropy design and spinel structure makes HESs exhibit remarkable physicochemical properties with great potential and special advantages in catalytic applications. This section summarizes the recent advances in HESs in thermocatalysis, electrocatalysis, and photocatalysis. Most importantly, the structure–property–performance relationships of HESs and catalytic reaction mechanisms are highlighted.3.1 Thermocatalysis
In the realm of thermocatalysis, HESs have shown promising performance in reactions such as hydrocarbon reforming, oxidation, and hydrogenation reactions. Their high thermal stability and multiple active sites allow them to withstand harsh reaction conditions and provide enhanced catalytic performance.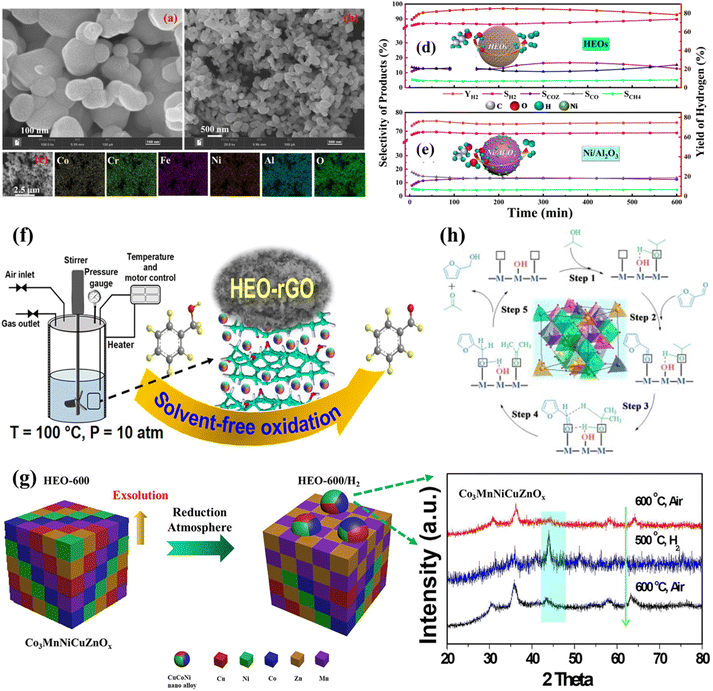 | ||
| Fig. 6 HESs for thermocatalysis. (a and b) The SEM images of the HES (CoCrFeNiAl)3O4, (c) the EDS mapping of the HESs, and the product selectivity and H2 yield of (d) HESs and (e) Ni/Al2O3 after 10 h of ethanol steam reforming.50 (f) (CoFeMnCuNiCr)3O4 on reduced graphene oxide (rGO) for solvent-free aerobic oxidation of benzyl alcohol. Reproduced with permission.105 Copyright 2024, American Chemical Society. (g) Schematic illustration of in situ exsolution of CuCoNi nanoalloys from HES (Co3MnNiCuZnOx) during CO2 hydrogenation, with the XRD patterns of HES, HES/H2, and the re-oxidized sample. Reproduced with permission.48 Copyright 2021, American Chemical Society. (h) Proposed mechanism for the transfer hydrogenation (CTH) reaction of furfural with 2-PrOH on holey lamellar HES nanocrystals with frustrated Lewis pairs. Reproduced with permission.118 Copyright 2023, Elsevier. | ||
In a recent study, a HES ferrite (Co0.2Ni0.2Cu0.2Zn0.2Mg0.2)Fe2O4 catalyst was prepared for H2 production via steam reforming of co-pyrolysis volatiles of polypropylene and waste cooking oil.120 The HES catalyst produced 58.43 mmol g−1 of H2 with a composition of 65.17% at 750 °C, 22.3 times higher than that of 10%Fe/Al2O3 and 10%Ni/Al2O3, and exhibited an extended service life of 9 h without compromising H2 selectivity with a H2 yield of 510.2 mmol gcat.−1 s−1. The improved reductivity originated from the strong C–C breaking ability of reactive oxygen species caused by oxygen vacancies (OVs) and Fe species and the synergistic C–H bond cleavage by Fe, Ni, Co, Cu, and Zn species. Specifically, the elevated temperature caused the reduction of metals, mainly Fe3+ to Fe2+ and Fe2+ to Fe0. To ensure charge balance during the transition of Fe species, a larger number of OVs were formed on the surface, which can activate gaseous oxygen and produce unstable oxygen species to facilitate catalytic reactions. Compared to single-metal-loaded Fe and Ni catalysts, the HESs exhibited better catalytic effects, indicating the catalytic synergy among different metals.
HESs have also been used in the chemical looping process for efficient and stable syngas and H2 coproduction. Zhong et al. utilized (Ni0.2Co0.2Ca0.2Cu0.2Mg0.2)Fe2O4 in chemical looping reforming coupled with water splitting (CLR-WS) for highly efficient and stable hydrogen production using CH4 as a fuel.121 Among the spinel catalysts with different configuration entropies, only HESs exhibited a high H2 yield of 12.66 mmol per gram of oxygen carriers with a purity of 99.1% at 700 °C and maintained 94.76% of their theoretical oxygen transfer capacity (OTC) during 100 cycles, further validating the benefits of multi-element synergy in HESs.
In addition, HESs have also been explored to catalyze CO oxidation,123 CH4 oxidation,124 propane oxidation,125 soot combustion,126etc., highlighting the important role of multicomponents, oxygen vacancies and entropy stabilization in HESs for high-temperature oxidation reactions.
Ma et al. synthesized HES nanocrystals (CrMnFeCoNiO) with abundant frustrated Lewis pairs (FLPs) for hydrogenation of unsaturated compounds, displaying superior catalytic activity and cycling performance under mild conditions.118 The FLPs on the HESs were composed of oxygen vacancies as Lewis acid sites and proximal surface hydroxyls or surface lattice oxygen as Lewis base sites. Attenuated total reflectance-infrared spectroscopy (ATR-IR) analysis and DFT calculations revealed that active regions between FLPs provide a stronger driving force for dissociating alcohols and activating the carbonyl groups of substrates, which enhanced catalytic activity (Fig. 6h).
3.2 Electrocatalysis
Electrocatalysis drives sustainable and clean energy conversion and storage technologies, including water electrolyzers, fuel cells, batteries, and supercapacitors. The diversity in composition and structures of HESs allows for the rational regulation of catalyst surface chemistry, enhancing their efficiency and selectivity in electrochemical transformations, especially in oxygen catalysis and Li-ion batteries.Wang et al. reported the application of HESs ((Co, Cu, Fe, Mn, Ni)3O4) in the OER, which is also the first time that a HEO has served as an efficient catalyst in electrocatalysis. Using hydrophilic multi-walled carbon nanotubes (MWCNTs) to support HES nanoparticles, the resultant HES achieved an overpotential of 1.58 V at a current density of 10 mA cm−2 in 1 M KOH electrolyte and maintained its activity after 12 h of the stability test.44 The better electrocatalytic activity of HESs than that of mixed metal oxides with fewer elements is attributed to the diverse valence states and chemical components of the oxides/hydroxides in the HES layers, which provide numerous intermediates and thus can overcome the kinetic barriers more easily. Operando electrochemical impedance spectroscopy (EIS) analysis revealed that M3+ (M = Co3+, Fe3+, Mn3+ and Ni3+)Oh in the octahedral site is responsible for surface electric double-layer capacitance, while the M2+ (M = Co2+, Fe2+, Mn2+ and Ni2+)Td species in the tetrahedral site were responsible for water oxidation. Zhang et al. prepared entropy-stabilized (Co0.2Mn0.2Ni0.2Fe0.2Zn0.2)Fe2O4via high-energy ball milling with a single-phase spinel structure (Fig. 7a–d).131 The material exhibited both high activity (336 mV at 10 mA cm−2 in 1 M KOH) and stability (maintaining an 89.4% current density after 10 h) in the OER (Fig. 7e and f). XPS analysis revealed that the enhanced OER performance of HESs, compared to two lower-entropy spinels, was attributed to significant lattice distortion and increased configurational entropy resulting from the disordered occupation of multivalent cations. The distorted lattice promoted the formation of high-density oxygen vacancies on the HES surface (Fig. 7g), which can serve as the active sites for H2O absorption. Higher configurational entropy prevented rapid degradation of the lattice structure, which helped maintain oxygen vacancies and thus preserved the active sites during extended OER testing.
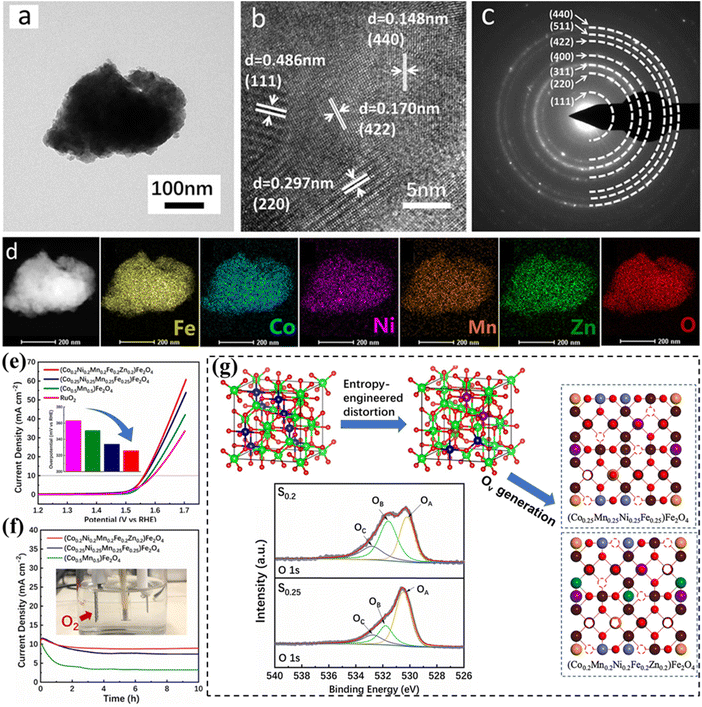 | ||
| Fig. 7 HESs for the OER. (a) TEM image, (b) HRTEM image, (c) SAED pattern, (d) HAADF-STEM image and elemental mapping of (Co0.2Mn0.2Ni0.2Fe0.2Zn0.2)Fe2O4, (e) LSV curves of different spinels and commercial RuO2 (the inset figure shows the overpotential at 10 mA cm−2), (f) chronoamperometry curves, and (g) schematic illustration of the formation of oxygen vacancies on the (400) surfaces of (Co0.25Mn0.25Ni0.25Fe0.25)Fe2O4 (S0.25) and (Co0.2Mn0.2Ni0.2Fe0.2Zn0.2)Fe2O4 (S0.2) caused by the lattice distortion in the O-neighbored octahedral and tetrahedral sublattices with their XRD patterns. Reproduced with permission.131 Copyright 2020, American Chemical Society. | ||
Due to the structural complexity, the regime behind the enhanced performance of HESs still lacks solid experimental evidence and molecular-level understanding. Baek et al. combined theoretical and experimental approaches to examine the OER activity and stability of HESs including Co, Fe, Ni, Cr, and Mn.132 They computationally developed a feasible HES impurity model (Fig. 8a) and used it to calculate the mixing enthalpies (Fig. 8b), demonstrating the thermodynamic stability of the HES system. Guided by the theoretical studies, they synthesized a HES with homogeneous mixing of each element (Co, Fe, Ni, Cr, Mn, and O) shown in Fig. 8c, which exhibited superior OER activity (∼307 mV at 10 mA cm−2 in 1 M KOH) and durability (∼12% increment of the overpotential over 168 h), outperforming lower-entropy oxides. The intermediate (O* and OH*) binding energies for three different active sites (Cr, Co, and Fe) were further calculated based on this model, showing that the overall activity was primarily contributed by Co, followed by Cr and Fe as well as oxyhydroxide (Fig. 8d–f). The high OER activity in HESs originated from the wider intermediate adsorption energy distributions due to the local strain in the active metal site–oxygen bonds.
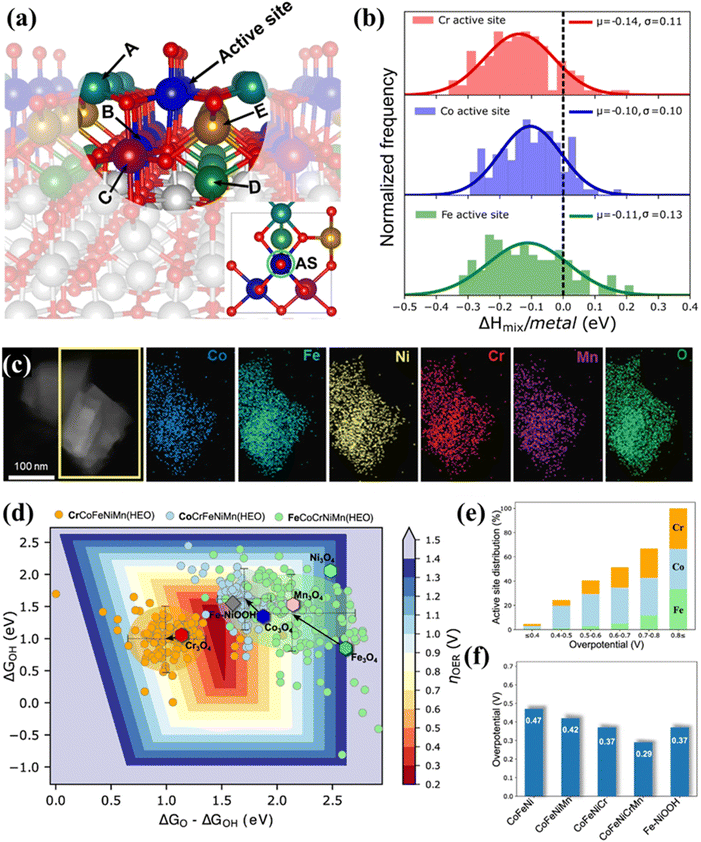 | ||
| Fig. 8 The combination of theoretical and experimental approaches to examine the OER activity and stability of HESs. (a) The HES impurity model containing the OER active site in the centre with adsorbed O* (red) surrounded by 5 metal neighbors (A to E for Cr, Co, Mn, Ni, and Fe) (the inset figure shows the top view of HES containing active sites (ASs)), (b) relative mixing enthalpy per mixing metals for different active sites on the HES surface as referred to their bulk systems, (c) EDS maps of synthesized HES (Co, Fe, Ni, Cr, and Mn), (d) OER activity volcano plot as a 2D heat map of overpotentials based on binding energy calculation of O* and OH*, and scaled OOH* values for HES systems, grouped by the active site along with the pure spinel systems, (e) active site (Cr, Co, and Fe) distribution as a function of OER overpotential for HEO systems and (f) predicted activity trends for different spinel oxide systems with explicitly calculated OOH*.132 | ||
For example, Xu et al. used Fe0.6Mn0.6Co0.6Ni0.6Cr0.6O4 for the first time as the cathode for proton-conducting solid oxide fuel cells (H-SOFCs), which possessed a much better ORR activity (a polarization resistance of 0.057 Ω cm2 at 700 °C shown in Fig. 9a) than a traditional Mn1.6Cu1.4O4 spinel cathode, benefiting the cathode performance (a peak power density of 1052 mW cm−2 shown in Fig. 9b).49 The first-principles calculation showed that the HES had lower O2 adsorption energy than individual oxides (Fe3O4, Mn3O4, Co3O4, NiO, and Cr2O3), indicating more thermodynamically favorable adsorption of O2 on HESs (Fig. 9c). Compared to Mn1.6Cu1.4O4, the HES cathode exhibited higher protonation ability and had a closer O p-band centre to the Fermi level, thereby enhancing the catalytic ORR activity of the cathode.
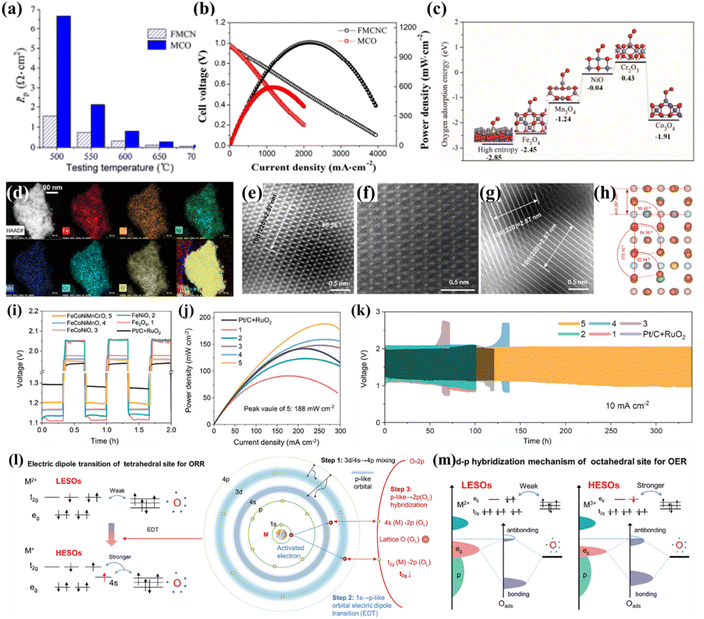 | ||
| Fig. 9 HESs for the ORR. (a) The polarization resistance (Rp) values of the cell using Fe0.6Mn0.6Co0.6Ni0.6Cr0.6O4 (FMCNC) and Mn1.6Cu1.4O4 (MCO) cathodes tested at different temperatures, (b) the fuel cell performance, and (c) O2 adsorption energy on the surface of HES, Fe3O4, Mn3O4, Co3O4, NiO, and Cr2O3.49 (d) STEM image and EDS mapping, (e–g) HAADF-STEM images and (h) the corresponding simulated model of HESs (FeCoNiMnCrO), (i) charge–discharge curves of ZABs at initial three cycles at 10 mA cm−2 using different spinels and commercial catalysts (Pt/C and RuO2) electrodes, (j) powder density, (k) long-term cycling curves at 10 mA cm−2, (l) high-entropy induced robust electric dipole transition in tetrahedral sites for the ORR, and (m) high-entropy induced strong d–p hybridization of octahedral sites for the OER. Reproduced with permission.137 Copyright 2024, John Wiley and Sons. | ||
Recently, Zhang et al. demonstrated that a high-entropy strategy was able to activate and stabilize the tetrahedral sites (potential active sites for the ORR) and enhance the activity of octahedral sites (potential active sites for the OER) in spinel oxides, thereby effectively decoupling the ORR/OER in zinc–air batteries (ZABs).137 The designed HESs displayed the uniform distribution of Fe, Co, Ni, Mn, Cr, and O within the particles (Fig. 9d) and had significant surface reconstruction on the (220) exposure facet due to severe lattice-distortion effects (Fig. 9e–h). The HESs exhibited comparable catalytic activity but superior stability to Pt/C for the ORR as well as superior performance in ZABs (a narrow charge–discharge voltage gap of 0.74 V, a peak power density of 188 mW cm−2, and outstanding long-term cycling durability, as shown in Fig. 9i–k). Combining theoretical and experimental investigations, the superior electrocatalytic performance was attributed to the electron structure redistribution induced by the high-entropy effect. Specifically, the significant lattice distortion in the HESs triggered an intense 1s → 4s electric dipole transition and strong t2g(Co)/eg(Ni)–2p(OL) hybridization, resulting in low-valence Co tetrahedral sites (Coth) and high-valence Ni octahedral sites (Nioh), as shown in Fig. 9l and m. This created dipolar dual-active sites capable of efficiently decoupling the OER/ORR. This study also first used unpaired t2g occupancy as an electronic descriptor for ORR activity for tetrahedral sites and demonstrated that the entropy engineering effectively regulated the electrocatalytic activity descriptors (t2g and eg) of the metal sites for the ORR and OER in HESs.
A HES ((Fe0.2Zn0.2Co0.2Ni0.2Cu0.2)Fe2O4) has also been evaluated as a 2e− ORR electrocatalyst for H2O2 production and exhibited a high H2O2 selectivity ranging from 69.48% to 85.78% under a potential window of 0.2–0.65 V with durability up to 24 h, which were attributed to the high concentration of oxygen vacancies and the synergistic interaction of multiple components.138
Chen et al. synthesized (Ni0.2Co0.2Mn0.2Fe0.2Ti0.2)3O4 as an anode material for LIBs, exhibiting a capacity of ∼560 mA h g−1 at 100 mA g−1 with an excellent capacity retention of 100% after 100 cycles.109 To investigate the lithium storage mechanisms, a series of characterization methods including in operando XANES, in operando XRD, in operando TXM (transition X-ray microscopy), ex situ XPS, and ex situ TEM were conducted to understand the redox reactions and structural changes of the HES during lithiation and delithiation. Combining these results, a mechanism model was proposed (Fig. 10a). During the lithiation process of the HES anode, Ni, Co, Mn, and Fe in the HES were all reduced to the metallic state, and Li ions formed Li2O by a reaction with the metal oxide, while Ti ions formed spinel LiTi2O4, which helped maintain the spinel structure and facilitated the reduced cations to re-occupy the original sites. During the delithiation process, most of the metal nanograins were oxidized back to their original spinel structure. As a result, no significant volume expansion of the HES particles during the lithiation and delithiation processes was observed due to the high-entropy stabilization of the lattice. Patra et al. prepared a series of Co-free HESs (V/Mg/Cu was added to quaternary medium-entropy (CrNiMnFe)3O4, 4M) for LIBs and demonstrated that chemical composition of high-entropy oxides was crucial for achieving phase purity and optimal charge–discharge performance.108 Among the three HESs, only 4MCu had a single-phase spinel structure verified by XRD (Fig. 10b–e) and HRTEM (Fig. 10f–i), which also exhibited the highest rate capacity and cyclability (480 mA h g−1 at 2 A g−1 and almost no capacity decay after 400 cycles as shown in Fig. 10j and k). The high phase purity can maximize the entropy stabilization effects, which helped maintain the crystalline framework of 4MCu during the lithiation/delithiation process and improve its electrode reversibility. In addition, the highest oxygen vacancy concentration of 4MCu among the three HESs was believed to be the decisive factor in its high capability and reversibility, which can modulate the electronic structure and promotes Li+ transport.
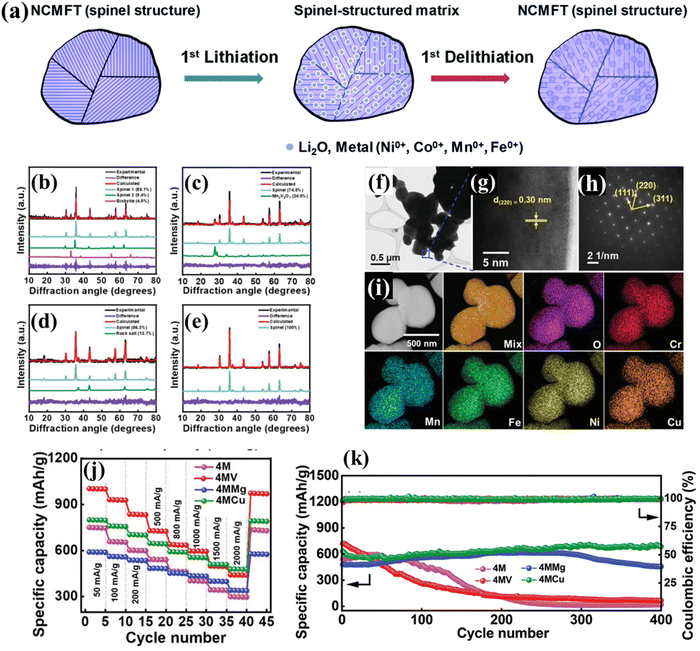 | ||
| Fig. 10 HESs for LIBs. (a) Charge storage mechanism during initial lithiation and delithiation processes.109 XRD patterns of (b) (CrNiMnFe)3O4(4M), (c) (CrNiMnFeV)3O4(4MV), (d) (CrNiMnFeMg)3O4(4MMg), and (e) (CrNiMnFeCu)3O4(4MCu); (f) TEM image, (g) high-resolution lattice image, (h) SAED pattern, and (i) HAADF image and EDS mapping of 4MCu; (j) comparative rate capability of various electrodes, and (k) cycling stability of various electrodes at 500 mA g−1 for 400 cycles. Reproduced with permission.108 Copyright 2022, John Wiley and Sons. | ||
To compare different HESs for LIBs conveniently, Table 1 presents the element composition and electrochemical performance of the current HES anode materials. In addition, HESs have also been reported for the application of electrode materials in sodium ion batteries,92 lithium–sulfur batteries,88 and supercapacitors.46,149,150 HESs offer significant potential for electrochemical energy storage due to their unique multi-cation compositions, which provide enhanced structural stability, high electronic conductivity, and improved ion transport properties. Meanwhile, the ability to fine-tune the composition and defect structures of HESs allows for the optimization of their electrochemical performance.
| Composition | Synthesis method | Initial capacities (mA h g−1)@current density | Cycle retention (cycles)@current density | Rate capability (mA h g−1)@current density | Ref. |
|---|---|---|---|---|---|
| (MgTiZnCuFe)3O4 | Solid-state | 634@100 mA g−1 | 79% (300)@0.1 A g−1 | 272@2 A g−1 | 34 |
| (NiCoMnFeTi)3O4 | Solid-state | 560@100 mA g−1 | 100% (100)@0.1 A g−1 | 343@2.5 A g−1 | 109 |
| (FeCoNiCrMn)3O4 | Hydrothermal | 1235@20 mA g−1 | 90% (200)@0.5 A g−1 | 500@2 A g−1 | 143 |
| (FeCoNiCrMn)3O4 | Solid-state | 680@100 mA g−1 | 60% (300)@0.5 A g−1 | 182@2 A g−1 | 45 |
| (FeCoNiCrMnZnLi)3O4 | Solid-state | 1050@50 mA g−1 | 50% (100)@0.5 A g−1 | 200@2 A g−1 | 144 |
| (Al0.2FeCoNiCrMn)3O4 | Solid-state | 1400@200 mA g−1 | 40% (500)@0.2 A g−1 | 634@3 A g−1 | 77 |
| (CoCrFeMnNi)3O4 | SCS | 1133@100 mA g−1 | 84% (200)@1 A g−1 | 48@10 A g−1 | 78 |
| Sn0.8(Co0.2Mg0.2Mn0.2Ni0.2Zn0.2)2.2O4 | Solid-state | 600@50 mA g−1 | 100% (500)@0.2 A g−1 | 182@1 A g−1 | 145 |
| (CrNiMnFeCu)3O4 | Hydrothermal | 800@50 mA g−1 | 100% (400)@0.5 A g−1 | 480@2 A g−1 | 108 |
| (CrMnFeNiCu)3O4 | Hydrothermal | 750@50 mA g−1 | 100% (150)@0.5 A g−1 | 340@2 A g−1 | 113 |
| (CrMnFeNiCu)3O4 | Hydrothermal | 755@50 mA g−1 | 99% (250)@0.5 A g−1 | 451@2 A g−1 | 75 |
| (FeCoNiCrMn)3O4 | Solid-state | 1645@100 mA g−1 | 86% (1200)@2 A g−1 | 596@2 A g−1 | 141 |
| Li1.8(FeCoZnCrMn)3Ox | Sol–gel | 733@20 mA g−1 | 81% (300)@0.5 A g−1 | 146@1 A g−1 | 87 |
| (CrMnFeNiZn)3O4 (C + T) | Solvothermal | 865@50 mA g−1 | 90% (200)@0.5 A g−1 | 560@3 A g−1 | 101 |
| (ZnCoMnFeAlMg)3O4 | Coprecipitation | 950@1000 mA g−1 | 81% (5000)@2 A g−1 | 300@2 A g−1 | 146 |
| (MnFeCoNiZn)3O4 | Electrospinning | 1284@20 mA g−1 | 100 (550)@0.5 A g−1 | 58@2 A g−1 | 89 |
| (FeCoNiCrZn)3O4 | Sol–gel | 1152@200 mA g−1 | 99% (1000)@1 A g−1 | 220@30 A g−1 | 147 |
| (FeCoNiCuZnMn)3O4 | Solid-state | 740@100 mA g−1 | 86% (100)@0.13 A g−1 | 300@1.6 A g−1 | 84 |
| (CoMnZnNiMg)2CrO4 | SCS | 1483@200 mA g−1 | 95% (200)@0.2 A g−1 | 371@2 A g−1 | 79 |
| (FeCoCrMnZn)3O4 | Solid-state | 477@200 mA g−1 | 95% (2000)@0.2 A g−1 | 829@2 A g−1 | 142 |
| (CrFeMnNiCo3)3O4 | SCS | 506@200 mA g−1 | 100% (800)@0.2 A g−1 | 147@2 A g−1 | 148 |
| (MgCoNiCuZn)Fe2O4 | Solid-state | 812@50 mA g−1 | 80% (650)@1 A g−1 | 87@10 A g−1 | 71 |
| (LiFeNiMnCuZn)3O4 | Laser-based | 866@500 A g−1 | 100% (800)@0.5 A g−1 | 585@2 A g−1 | 85 |
Qi et al. designed ultrathin high-entropy Fe-based spinel oxide (Co0.2Ni0.2Zn0.2Mg0.2Cu0.2)Fe2O4 (A5Fe2O4) nanosheets for the NO3−RR to NH3.22 A5Fe2O4 had a thickness of only 4.3 nm (Fig. 11a) investigated by atomic force microscopy (AFM) with a spinel structure (Fig. 11b and c) and abundant mesoporous structures between two interconnected nanoparticles (Fig. 11d). These A5Fe2O4 nanosheets showed excellent performance for the NO3−RR with an NH3 yield rate of ≈2.1 mmol h−1 cm−2 at −0.5 V versus the reversible hydrogen electrode, outperforming other binary Fe-based spinels (AFe2O4), as shown in Fig. 11e. DFT calculations suggested that introducing multicomponents could significantly narrow the bandgap and increase states around the Fermi level, achieving the transformation of spinel oxides from semiconductors into metalloids (Fig. 11f and g). The doped transition metals (Co, Ni, Zn, and Cu) with lower crystal field splitting and higher electronegativity could influence the bandgap by lowering the energy of the unoccupied t2g states of B metals and introduce a new state within the bandgap of A metals, which made all metals lose their own electronic properties and become degenerated (Fig. 11j–l). The narrower bandgap of A5Fe2O4 was also confirmed by diffuse reflectance spectra (DRS) measurements, which endowed A5Fe2O4 with a high electron conductivity, more than an order of magnitude higher than that of AFe2O4 (Fig. 11m). In addition, the density of states (DOS) of A5Fe2O4 near the Fermi level was mainly contributed by d-orbitals of Fe (Fig. 11h and i), indicating that the electrocatalytic activity of the HES mainly originated from Fe sites. The lower surface work function (WF) of A5Fe2O4 than that of AFe2O4 indicated that electrons could more easily transfer from the catalyst surface to the adsorbed reaction intermediates, thereby promoting catalytic reaction kinetics. Mechanistic investigations by experimental and computational methods revealed that constructing high-entropy A5Fe2O4 could adjust the adsorption strength of NO3− and other reaction intermediates for boosting the NO3−RR (Fig. 11n and o).
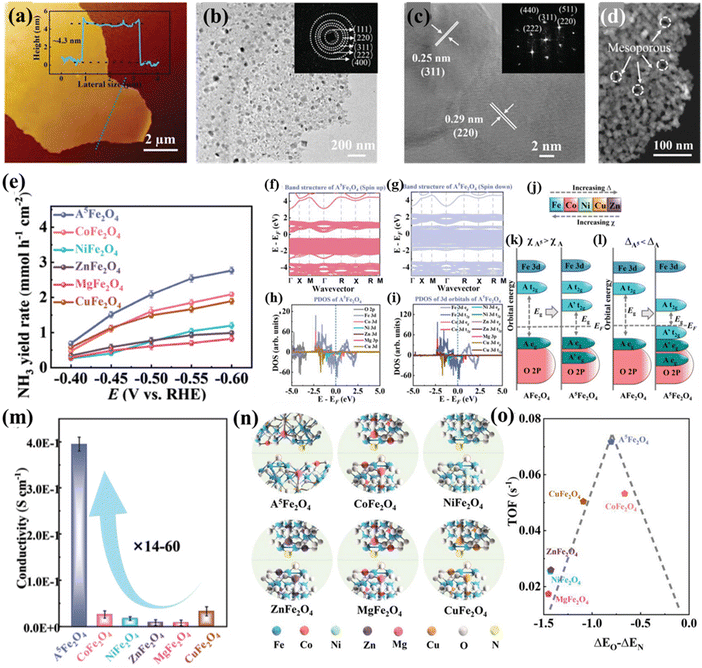 | ||
| Fig. 11 HESs for the electrochemical NO3−RR. (a) Thickness measurement of (Co0.2Ni0.2Zn0.2Mg0.2Cu0.2)Fe2O4 (A5Fe2O4) nanosheets by AFM, (b) TEM (the inset shows the SAED patterns), (c) HRTEM, and (d) HAADF-STEM image of A5Fe2O4, (e) NH3 yield rate of A5Fe2O4 and binary spinel oxides at different potentials, the calculated band structure of A5Fe2O4 (f) (spin up) and (g) (spin down), (h and i) PDOS for A5Fe2O4, (j) trends in crystal field splitting Δ and electronegativity χ for the five metals of the HES, schematic illustration that (k) adding a transition metal with greater electronegativity reduced the bandgap energy by introducing states between the eg and t2g states of the original transition metal and (l) adding a transition metal of lower crystal field splitting reduced the bandgap energy both by introducing new occupied t2g states above the eg states of the original transition metal and by lowering its unoccupied t2g states, (m) electron conductivity of all spinel oxides, (n) optimized structural model of adsorbate nitrogenous compounds (*NO, *N, *NH, *NH2, and *NH3) and oxycompounds (*NO3, *NO2, and *OH) on A5Fe2O4 and other binary spinels, and (o) a volcano-type relationship between the binding difference of O and N (ΔEO − ΔEN) and TOF values of different spinel catalysts. Reproduced with permission.22 Copyright 2022, John Wiley and Sons. | ||
3.3 Photocatalysis and advanced oxidation
Despite being less studied in the area of photocatalysis, HESs have the ability to arrange various components and tune properties to achieve a photoresponse, which has attracted increasing attention in photocatalysis in recent years. For example, Zhang et al. explored the photocatalytic potential of porous HES nanofibers (NFs) of (NiCuMnCoZnFe)3O4 for CO2 reduction.82 A series of characterization studies showed that the as-prepared HES NFs had a high surface area of 66.48 m2 g−1, abundant lattice strains and defects, a dual bandgap, and semi-metallic properties. The dual-bandgap structure and half-metallicity endowed them with remarkable light-absorbing capacities (Fig. 12a). In situ FTIR results show that the incorporation of multiple elements created a wide range of synergistic catalytic sites capturing intermediates and protons, and oxygen vacancies can serve as active sites for electron–hole separation and CO2 adsorption and help break C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds to produce CO (Fig. 12b). All of these made the HES NFs excellent photocatalysts to efficiently convert CO2 into CH4 and CO with high yields of 8.03 and 15.89 μmol g−1 h−1, respectively, without using photosensitizers or sacrificial agents.
O bonds to produce CO (Fig. 12b). All of these made the HES NFs excellent photocatalysts to efficiently convert CO2 into CH4 and CO with high yields of 8.03 and 15.89 μmol g−1 h−1, respectively, without using photosensitizers or sacrificial agents.
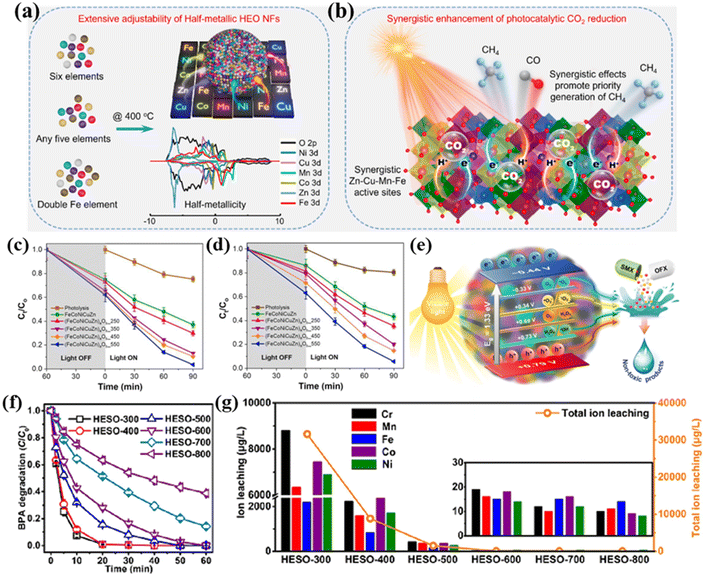 | ||
| Fig. 12 HESs for photocatalysis and AOPs. (a) Structurally adjustable HES nanofibers with half metallicity, and (b) schematic illustration of the synergistic activation and conversion of CO2 into CH4 of the HES nanofibers. Reproduced with permission.82 Copyright 2024, John Wiley and Sons. Visible light-induced photocatalytic degradation of (c) SMX and (d) OFX by FeCoNiCuZn and (FeCoNiCuZn)aOb, and (e) schematic illustration of the photocatalytic degradation mechanism of (FeCoNiCuZn)aOb under visible light irradiation.152 (f) BPA degradation efficiency and (g) the concentration of total leached metal ions in PMS-AOPs systems with (Cr0.2Mn0.2Fe0.2Co0.2Ni0.2)3O4 (HESO) calcined at different temperatures as catalysts. Reproduced with permission.153 Copyright 2023, Elsevier. | ||
Das et al. applied HESs (FeCoNiCuZn)aOb for photocatalytic degradation of organic pollutants in water and wastewater treatment processes.152 As shown in Fig. 12c and d, the (FeCoNiCuZn)aOb nanoparticles exhibited a good photocatalytic degradation efficiency of above 95% for two model antibiotics (i.e., sulfamethoxazole and ofloxacin) under visible light irradiation for 90 min without any significant metal leaching. This outstanding photocatalytic performance can be mainly attributed to the narrow bandgaps, reduced electron–hole recombination and thus extended carrier-lifetime, and enhanced electronic conductivity (Fig. 12e). In addition, the even distribution of metal cations with different oxidation states in HESs promoted the activation of lattice oxygen in the form of surface-confined oxygen vacancies and the formation of reactive species for oxidation reactions.
In addition to the catalytic applications above, HESs have also been explored as catalysts in advanced oxidation processes (AOPs) for organic pollutant degradation. Wang et al. demonstrated that spherical mesoporous HESs (e.g., Ni–Co–Cr–Fe–Mn spinel oxide) could effectively activate PMS for degradation of methylene blue (MB).67 Nearly 95% of MB was degraded after 60 min with a total organic carbon removal of 71.7%. Radical scavenging experiments indicated that both SO4˙− and HO˙ were involved in the oxidation and SO4˙− played a dominant role. After the reaction, the concentrations of leached metal ions (Ni, Co, Cr, Fe, and Mn) in the treated solution were very low, indicating the structural robustness of HESs in oxidation reactions. Zhang et al. successfully prepared an entropy-stabilized HES ((Cr0.2Mn0.2Fe0.2Co0.2Ni0.2)3O4) as a PMS catalyst for bisphenol A (BPA) degradation.153 As shown in Fig. 12f and g, the HES calcined at 600 °C achieved complete degradation of BPA within 60 min and also exhibited good structural and chemical stability, which had a minimal total metal ion leaching (82 μg L−1) and maintained an efficiency of over 90% even after 5 cycles. XPS analysis before and after the reaction indicated that Co(II) was the dominant active site for PMS activation to generate radicals (HO˙ and SO4˙−) for rapid BPA degradation. The presence of oxygen vacancies could contribute to the generation of reactive oxygen species and enhance the catalytic performance of catalysts. DFT calculations suggested that the adsorption energy of PMS molecules on the Co sites in the HES was more negative than that of a binary spinel ((Co0.2Fe0.8)3O4), indicating that the electronic structures of Co in HES were optimized by its disorder arrangement of metal sites and defect structure, thus promoting electron transfer between the Co sites and PMS. Due to the acidic nature of PMS, it is crucial to develop stable HESs without severe metal leaching during reactions to avoid secondary pollution.
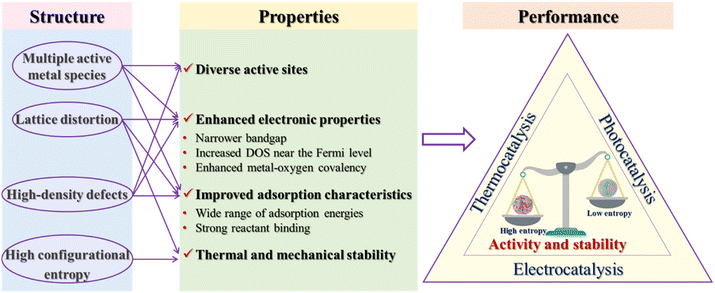
4. Discussion
The diverse catalytic applications of HESs, as summarized above, highlight their potential in thermocatalysis, electrocatalysis, photocatalysis and AOPs. While catalytic mechanisms of HESs have also been investigated for each reaction, variations across studies have been observed. As research on HESs is currently in its nascent stages, further efforts are still required to reveal the complex structure–property–performance relationships of HESs in various catalytic processes. The compositional and structural diversity of HESs makes it extremely challenging to reveal the mechanisms underlying their enhanced catalytic performance compared with low-entropy spinels or other catalysts. Here, we summarize the strategies for fine-tuning the properties of HESs arising from the flexible high-entropy structure to benefit the catalytic performance of HESs as shown in Fig. 13. Key structural characteristics of HESs including multiple active metal species, lattice distortion, high-density defects, and high configurational entropy lead to the optimization of critical catalytic properties such as active site diversity, electronic properties, adsorption characteristics, and stability. These properties collectively enhance the catalytic performance of HESs in comparison to lower entropy materials.In heterogeneous catalysis, active sites are responsible for reactant adsorption, bond breaking and forming, interfacial charge transfer, intermediate stabilization, and product production and desorption. For metal-based catalysis, multiple active metal cations with various valence states in HESs can provide more active sites than traditional spinels.127 Rather than merely reflecting the average activity of each metal site, these elements demonstrate significantly higher activity due to the collaborative and synergistic effects resulting from their homogeneous distribution and inter-element interactions.40,106 As a result, the multiple metal cations in HES can provide favorable adsorption sites for different reactants/intermediates and pathways, thus facilitating the overall catalytic performances.154 Several studies suggested that there is no simple linear trend between the molar ratio of each element and the related catalytic activity, confirming the synergy between multiple metal cations in HESs.48,155
In addition, high-density defects in HESs can also act as active sites, contributing to the excellent catalytic performance of HESs. In HES phases, the diverse bonding configurations between various metal ions and oxygen atoms generate a disordered electronic environment around the oxygen atoms, which will induce the detachment of these ions to form defects.156 These defect structures include point defects (e.g., vacancies and interstitials), line defects (e.g., dislocations), and interfaces (e.g., grain boundaries and surfaces).10,68,157 In particular, oxygen vacancies have been demonstrated to influence the catalytic activity by providing additional catalytic sites, modifying electronic properties of surrounding metals, and affecting the adsorption configuration of reaction intermediates, which have attracted the most interest in the high entropy community to improve the catalytic performance of HESs.21,78,108,122,158–163
The electronic structure of catalysts is considered a critical factor in regulating electron transfer processes and determining the binding strength between catalysts and adsorbates.164 An explicit electronic structure analysis could provide important clues for understanding the enhanced catalytic performance of HESs. In transition metal oxides, the d-electrons of transition metals play a major role in shaping their electronic structures. The presence of various metal elements in HESs leads to an extremely complex electronic landscape with new electronic states that may facilitate catalytic reactions.15,23,49,165,166 More importantly, high entropy configuration induces significant lattice distortion and defects in spinels, fundamentally altering the electronic properties of spinels, such as the band structure, DOS, the distribution of electronic states, and eg orbital occupancy.16,22,45,82,137,163,167,168 For example, Katzbaer et al. demonstrated band gap narrowing in a high-entropy aluminate spinel oxide (Fe0.2Co0.2Ni0.2Cu0.2Zn0.2)Al2O4 with a 0.9 eV band gap, significantly narrower than the band gaps of its single-metal end members (ranging from 1.6 to 4.2 eV).14 First-principles calculations showed that the narrower band gap originated from the wider energy distribution of the 3d states due to the different electronegativities and crystal field splitting energies between 3d transition metals. Tian et al. found that the Co–O octahedron in spinel undergoes asymmetric distortion in a high-entropy atomic environment, resulting in the redistribution of internal charges in the Co–O octahedron.23 Near the Fermi level, the DOS of HESs ((Co0.2Mn0.2Ni0.2Fe0.2Cr0.2)3O4) was obviously higher than that of Co3O4 and (Co0.25Ni0.25Fe0.25Mn0.25)3O4, which means the highest conductivity of HESs among the three materials. The difference between the O p band center and the Co d band center in HESs was also smaller, indicating stronger Co–O covalency. More importantly, the overall d band center of HES had an upward shift closer to the Fermi level, which is favorable for the strong adsorption of reaction intermediate on the HES surface. Adsorption energy of reactive intermediates on the catalyst surface is one of the most important descriptors for catalytic reactions, which successfully bridge the gap between a catalyst's structure and activity through the Sabatier principle and the d-band theory.169 DFT calculations in many studies have indicated that HESs can provide access to a broad range of adsorption energies which could be optimum for the intermediates due to their diverse components and configurations.21,132,170
One of the primary benefits of high-entropy engineering for spinels is structural stabilization. The spinel structure provides not one but two cation sublattices: a tetrahedrally coordinated A site forming a diamond lattice and an octahedrally coordinated B site forming a pyrochlore lattice.18 A significant increase in configurational entropy can be anticipated when both sublattices are occupied by a mixture of cations.57,82,97,171 Due to the high-entropy effect, HESs obtain lower formation energy of the spinel structure and increase its thermodynamic stability, especially at high temperatures. Kinetically, high-entropy mixing may also enhance structural stability due to lattice distortion, which can create significant diffusion barriers that help prevent phase segregation, particularly at low temperatures. This stability is crucial for maintaining the integrity of the catalysts under harsh reaction conditions, as evidenced by their consistent performance in high-temperature and electrochemical catalytic reactions.143,172 For example, Han et al. reported that the high-entropy effect stabilized the spinel structure of (Fe0.2Mg0.2Mn0.1Al0.3Cr0.2)3O4 at 900 °C even with a large amount of oxygen converted and thus promoted the exsolution and stabilization of substantial Fe0, which resulted in extensive metal–oxide interfaces with metal–oxygen vacancy pairs responsible for the efficient and stable solar thermochemical water splitting.110
5. Summary and outlook
HESs have emerged as a typical representative of HEMs, offering more possibilities for future energy and environmental materials. In this review, the recent advances in applying HESs for heterogeneous catalysis are presented, including the unique features, synthesis, characterization, and catalytic applications. From the wide range of studies, the underlying relationships among the special structure, favorable catalytic properties, and performance improvement of HESs in catalysis are summarized and discussed. Although significant progress has been made in the study of HESs, further efforts are required in several areas, including precise synthesis, advanced characterization, and expanded applications, to fully unlock the potential of HESs in catalysis. Notably, despite efforts made to elucidate the structure–property–performance relationships of HESs, a comprehensive and deep understanding is still lacking due to the compositional and structural complexity of HESs. Many studies commonly attributed the excellent performance of HESs in catalytic reactions to the presence of oxygen vacancies and/or a synergistic effect, but it remains unclear how oxygen vacancies specifically impact the performance of HESs in a catalytic reaction and what the exact nature of the synergistic effect is. More importantly, accurate identification of active sites is crucial for the design, synthesis, modification, and enhancement of metal-based catalysts. For HESs, the vast number of potential surface atom arrangements makes it challenging to identify the actual active sites by studying only one or a few models. This requires an integrated approach combining experimental and computational studies to provide insights into the structure and properties directly related to catalytic performance of HESs and help elucidate the catalytic reaction mechanisms, optimizing their performance for various catalytic applications.The development of advanced in situ/operando characterization techniques will offer valuable insight into the fundamental understanding of active sites and reaction pathways in HESs for catalysis. For instance, in situ TEM allows real-time tracking of phase transformations induced by catalytic reactions and morphological changes of individual HES nanoparticles.173 By analyzing in situ electron diffraction (ED) data along with the spatiotemporal changes in valence states of metal species, the formation sequences of distinct phases can be clearly identified. This provides direct insights into the conversion and reaction kinetics, thereby enhancing our understanding of the dynamic catalytic mechanisms. Operando XAS is another powerful tool for revealing the interactions among elements within a high-entropy phase and the dynamic interplay between active sites and reactants, intermediates, or products during a reaction.174 However, for complex systems such as HESs, “trial and error” experiments are low-efficiency and time-consuming, while conventional theoretical simulations involve high computational costs and limited accuracy. With the fast development of computational chemistry, artificial intelligence (AI) and machine learning (ML) have revolutionized the field of materials science by enabling the prediction of properties, optimization of compositions, and discovery of new materials. For example, ML regression can be trained on experimental data to predict properties based on compositional and structural features and optimize the composition of catalysts to achieve desired properties by exploring a vast compositional space efficiently. In addition, high-throughput experimental methods, enabled by the advancement in instrumentation and electronics, will speed up the generation of extensive results and promote the identification of desired products, thereby enriching knowledge in computational design. It can be envisioned that these data-driven methodologies will unveil the complexities of HESs and advance the field toward the rational design of robust HESs with enhanced properties for catalytic applications.
While this review has focused on HESs, the underlying concepts can be applicable to other HEMs as well. We anticipate that the exploration of HESs in catalysis will innovate traditional paradigms of material design, promoting a multidisciplinary approach that integrates materials science, chemical engineering, sustainable technology, and computational chemistry. Advancements in this field could revolutionise the development of new and complex catalysts, moving beyond the limitations of conventional transition metals and oxides to achieve greater efficiency, stability, and environmental compatibility. This progress could provide more effective solutions for tackling global challenges in energy and sustainability.
Data availability
No primary research results, software or code have been included and no new data were generated or analyzed as part of this review.Author contributions
The manuscript was written by Y. L. Mo. X. G. Duan and S. B. Wang supervised the work and contributed to revising and refining the manuscript. X. H. Guan provided valuable suggestions for the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the financial support from the Australian Research Council (Grant No. DP230102406 and FL230100178).References
- R. Schlogl, Angew Chem. Int. Ed. Engl., 2015, 54, 3465–3520 CrossRef PubMed.
- Y. J. Li, Y. J. Sun, Y. N. Qin, W. Y. Zhang, L. Wang, M. C. Luo, H. Yang and S. J. Guo, Adv. Energy Mater., 2020, 10, 1903120 CrossRef.
- S. Yuan, X. Duan, J. Q. Liu, Y. Ye, F. Lv, T. Liu, Q. Wang and X. B. Zhang, Energy Storage Mater., 2021, 42, 317–369 CrossRef.
- L. Cui, X. Zhao, H. Xie and Z. Zhang, ACS Catal., 2022, 12, 13334–13348 CrossRef.
- M. Huang, Y. S. Li, C. Q. Zhang, C. Cui, Q. Q. Huang, M. Li, Z. Qiang, T. Zhou, X. Wu and H. Q. Yu, Proc. Natl. Acad. Sci. U. S. A., 2022, 119, e2202682119 CrossRef PubMed.
- Y. Li, Y. Sun, Y. Qin, W. Zhang, L. Wang, M. Luo, H. Yang and S. Guo, Adv. Energy Mater., 2020, 10, 1903120 CrossRef.
- J. W. Yeh, S. K. Chen, S. J. Lin, J. Y. Gan, T. S. Chin, T. T. Shun, C. H. Tsau and S. Y. Chang, Adv. Eng. Mater., 2004, 6, 299–303 CrossRef.
- C. M. Rost, E. Sachet, T. Borman, A. Moballegh, E. C. Dickey, D. Hou, J. L. Jones, S. Curtarolo and J. P. Maria, Nat. Commun., 2015, 6, 8485 CrossRef CAS PubMed.
- C. Oses, C. Toher and S. Curtarolo, Nat. Rev. Mater., 2020, 5, 295–309 CrossRef CAS.
- S. Schweidler, M. Botros, F. Strauss, Q. S. Wang, Y. J. Ma, L. Velasco, G. C. Marques, A. Sarkar, C. Kübel, H. Hahn, J. Aghassi-Hagmann, T. Brezesinski and B. Breitung, Nat. Rev. Mater., 2024, 9, 266–281 CrossRef.
- X. M. Xu, Z. P. Shao and S. P. Jiang, Energy Technol., 2022, 10, 2200573 CrossRef CAS.
- B. Wang, X. Zhu, X. Pei, W. Liu, Y. Leng, X. Yu, C. Wang, L. Hu, Q. Su, C. Wu, Y. Yao, Z. Lin and Z. Zou, J. Am. Chem. Soc., 2023, 145, 13788–13795 CrossRef CAS.
- D. Liu, P. F. Guo, H. G. Pan and R. B. Wu, Prog. Mater. Sci., 2024, 145, 101300 CrossRef CAS.
- R. R. Katzbaer, F. M. Dos Santos Vieira, I. Dabo, Z. Mao and R. E. Schaak, J. Am. Chem. Soc., 2023, 145, 6753–6761 CrossRef CAS PubMed.
- X. Yang, S. Liping, L. Qiang, H. Lihua and Z. Hui, J. Mater. Chem. A, 2022, 10, 17633–17641 RSC.
- W. Hooch Antink, S. Lee, H. S. Lee, H. Shin, T. Y. Yoo, W. Ko, J. Shim, G. Na, Y. E. Sung and T. Hyeon, Adv. Funct. Mater., 2023, 34, 2309438 CrossRef.
- K. H. Nam, Z. Wang, J. Luo, C. Huang, M. F. Millares, A. Pace, L. Wang, S. T. King, L. Ma, S. Ehrlich, J. Bai, E. S. Takeuchi, A. C. Marschilok, S. Yan, K. J. Takeuchi and M. M. Doeff, Chem. Mater., 2024, 36, 4481–4494 CrossRef CAS PubMed.
- G. H. J. Johnstone, M. U. Gonzalez-Rivas, K. M. Taddei, R. Sutarto, G. A. Sawatzky, R. J. Green, M. Oudah and A. M. Hallas, J. Am. Chem. Soc., 2022, 144, 20590–20600 CrossRef CAS PubMed.
- L. Min, J. P. Barber, Y. Wang, S. V. Gayathri Ayyagari, G. E. Niculescu, E. Krysko, G. R. Bejger, L. Miao, S. H. Lee, Q. Zhang, N. Alem, C. M. Rost and Z. Mao, J. Am. Chem. Soc., 2024, 146, 24320–24329 CrossRef CAS.
- C. R. Feng, Y. F. Zhou, M. Chen, L. N. Zou, X. M. Li, X. W. An, Q. Zhao, P. Xiaokaiti, A. Abudula, K. Yan and G. Q. Guan, Appl. Catal., B, 2024, 349, 123875 CrossRef.
- Y. F. Liu, C. C. Ye, L. Chen, J. B. Fan, C. Liu, L. Xue, J. W. Sun, W. Q. Zhang, X. Wang, P. Xiong and J. W. Zhu, Adv. Funct. Mater., 2024, 34, 2314820 CrossRef.
- S. Qi, Z. Lei, Q. Huo, J. Zhao, T. Huang, N. Meng, J. Liao, J. Yi, C. Shang, X. Zhang, H. Yang, Q. Hu and C. He, Adv. Mater., 2024, 36, e2403958 CrossRef PubMed.
- G. Tian, H. Xu, X. Wang, X. Wen, P. Liu, S. Liu, T. Zeng, F. Fan, S. Wang, C. Wang, C. Zeng and C. Shu, ACS Nano, 2024, 18, 11849–11862 CrossRef.
- J. Zander, J. P. Wölfel, M. Weiss, Y. Jiang, N. Cheng, S. Zhang and R. Marschall, Adv. Funct. Mater., 2023, 34, 2310179 CrossRef.
- B. Ran, H. Li, R. Cheng, Z. Yang, Y. Zhong, Y. Qin, C. Yang and C. Fu, Adv. Sci., 2024, 11, e2401034 CrossRef PubMed.
- Y. B. Zhou, X. W. Shen, T. Qian, C. L. Yan and J. M. Lu, Nano Res., 2023, 16, 7874–7905 CrossRef.
- E. P. George, D. Raabe and R. O. Ritchie, Nat. Rev. Mater., 2019, 4, 515–534 CrossRef.
- B. Cantor, I. T. H. Chang, P. Knight and A. J. B. Vincent, Mater. Sci. Eng., A, 2004, 375, 213–218 CrossRef.
- Z. Z. Li, S. T. Zhao, R. O. Ritchie and M. A. Meyers, Prog. Mater. Sci., 2019, 102, 296–345 CrossRef.
- B. Chen, S. Li, H. Zong, X. Ding, J. Sun and E. Ma, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 16199–16206 CrossRef.
- Y. J. Zhao, J. W. Qiao, S. G. Ma, M. C. Gao, H. J. Yang, M. W. Chen and Y. Zhang, Mater. Des., 2016, 96, 10–15 CrossRef.
- D. Bérardan, S. Franger, A. Meena and N. Dragoe, J. Mater. Chem. A, 2016, 4, 9536–9541 RSC.
- S. C. Jiang, T. Hu, J. Gild, N. X. Zhou, J. Y. Nie, M. D. Qin, T. Harrington, K. Vecchio and J. Luo, Scr. Mater., 2018, 142, 116–120 CrossRef.
- H. Chen, N. Qiu, B. Wu, Z. Yang, S. Sun and Y. Wang, RSC Adv., 2020, 10, 9736–9744 RSC.
- J. Gild, M. Samiee, J. L. Braun, T. Harrington, H. Vega, P. E. Hopkins, K. Vecchio and J. Luo, J. Eur. Ceram. Soc., 2018, 38, 3578–3584 CrossRef.
- X. Y. Ping, B. Meng, X. H. Yu, Z. Y. Ma, X. Y. Pan and W. Lin, Chem. Eng. J., 2023, 464, 142649 CrossRef.
- L. A. Pressley, A. Torrejon, W. A. Phelan and T. M. McQueen, Inorg. Chem., 2020, 59, 17251–17258 CrossRef.
- C. Zhao, F. Ding, Y. Lu, L. Chen and Y. S. Hu, Angew Chem. Int. Ed. Engl., 2020, 59, 264–269 CrossRef PubMed.
- A. Sarkar, Q. Wang, A. Schiele, M. R. Chellali, S. S. Bhattacharya, D. Wang, T. Brezesinski, H. Hahn, L. Velasco and B. Breitung, Adv. Mater., 2019, 31, e1806236 CrossRef PubMed.
- Z. Sun, Y. J. Zhao, C. Sun, Q. Ni, C. Z. Wang and H. B. Jin, Chem. Eng. J., 2022, 431, 133448 CrossRef.
- G. M. Tomboc, X. D. Zhang, S. Choi, D. Kim, L. Y. S. Lee and K. Lee, Adv. Funct. Mater., 2022, 32, 2205142 CrossRef.
- T. X. Nguyen, Y. C. Liao, C. C. Lin, Y. H. Su and J. M. Ting, Adv. Funct. Mater., 2021, 31, 2101632 CrossRef.
- J. Dąbrowa, M. Stygar, A. Mikuła, A. Knapik, K. Mroczka, W. Tejchman, M. Danielewski and M. Martin, Mater. Lett., 2018, 216, 32–36 CrossRef.
- D. D. Wang, Z. J. Liu, S. Q. Du, Y. Q. Zhang, H. Li, Z. H. Xiao, W. Chen, R. Chen, Y. Y. Wang, Y. Q. Zou and S. Y. Wang, J. Mater. Chem. A, 2019, 7, 24211–24216 RSC.
- D. Wang, S. D. Jiang, C. Q. Duan, J. Mao, Y. Dong, K. Z. Dong, Z. Y. Wang, S. H. Luo, Y. G. Liu and X. W. Qi, J. Alloys Compd., 2020, 844, 156158 CrossRef.
- B. Liang, Y. Ai, Y. Wang, C. Liu, S. Ouyang and M. Liu, Materials, 2020, 13, 5798 CrossRef PubMed.
- K. Gu, D. Wang, C. Xie, T. Wang, G. Huang, Y. Liu, Y. Zou, L. Tao and S. Wang, Angew Chem. Int. Ed. Engl., 2021, 60, 20253–20258 CrossRef.
- J. H. Zhao, J. F. Bao, S. Z. Yang, Q. Niu, R. Y. Xie, Q. Y. Zhang, M. S. Chen, P. F. Zhang and S. Dai, ACS Catal., 2021, 11, 12247–12257 CrossRef CAS.
- Y. S. Xu, X. Xu and L. Bi, J. Adv. Ceram., 2022, 11, 794–804 CrossRef.
- C. Wang, W. Liu, M. Liao, J. Weng, J. Shen, Y. Chen and Y. Du, Nanoscale, 2023, 15, 8619–8632 RSC.
- B. Li, X. Duan, T. Zhao, B. Niu, G. Li, Z. Zhao, Z. Yang, D. Liu, F. Zhang, J. Cheng and Z. Hao, Environ. Sci. Technol., 2024, 58, 2153–2161 CrossRef PubMed.
- Y. Jien-Wei, Ann. Chim.: Sci. Mater., 2006, 31, 633–648 CrossRef.
- Y. Sun and S. Dai, Sci. Adv., 2021, 7, eabg1600 CrossRef.
- B. S. Murty, J.-W. Yeh, S. Ranganathan and P. P. Bhattacharjee, High-Entropy Alloys, Elsevier, 2019 Search PubMed.
- J. Wu, H. Wang, N. Liu, B. Jia and J. Zheng, Small, 2024, e2403162 CrossRef PubMed.
- A. Sarkar, B. Breitung and H. Hahn, Scr. Mater., 2020, 187, 43–48 CrossRef.
- S. S. Aamlid, M. Oudah, J. Rottler and A. M. Hallas, J. Am. Chem. Soc., 2023, 145, 5991–6006 CrossRef PubMed.
- R. J. Hill, J. R. Craig and G. V. Gibbs, Phys. Chem. Miner., 1979, 4, 317–339 CrossRef.
- K. E. Sickafus, J. M. Wills and N. W. Grimes, J. Am. Ceram. Soc., 1999, 82, 3279–3292 CrossRef.
- Q. Zhao, Z. Yan, C. Chen and J. Chen, Chem. Rev., 2017, 117, 10121–10211 CrossRef PubMed.
- J. W. Yeh, JOM, 2013, 65, 1759–1771 CrossRef.
- W. L. Hsu, C. W. Tsai, A. C. Yeh and J. W. Yeh, Nat. Rev. Chem, 2024, 8, 471–485 CrossRef PubMed.
- S. A. Lee, J. W. Bu, J. W. Lee and H. W. Jang, Small Sci., 2023, 3, 2200109 CrossRef CAS.
- M. H. Tsai and J. W. Yeh, Mater. Res. Lett., 2014, 2, 107–123 CrossRef.
- Y. Y. Zhai, X. R. Ren, B. L. Wang and S. Z. Liu, Adv. Funct. Mater., 2022, 32, 2207536 CrossRef CAS.
- Y. Zhang, D. Wang and S. Wang, Small, 2022, 18, e2104339 CrossRef.
- G. Wang, J. Qin, Y. Feng, B. Feng, S. Yang, Z. Wang, Y. Zhao and J. Wei, ACS Appl. Mater. Interfaces, 2020, 12, 45155–45164 CrossRef CAS.
- L. He, H. J. Kang, G. Y. Hou, X. S. Qiao, X. Jia, W. Qin and X. H. Wu, Chem. Eng. J., 2023, 460, 141675 CrossRef CAS.
- B. Musicó, Q. Wright, T. Z. Ward, A. Grutter, E. Arenholz, D. Gilbert, D. Mandrus and V. Keppens, Phys. Rev. Mater., 2019, 3, 104416 CrossRef.
- B. L. Liang, Y. L. Ai, Y. L. Wang, C. H. Liu, S. Ouyang and M. J. Liu, Materials, 2020, 13, 5798 CrossRef CAS PubMed.
- R. Q. Ren, D. X. Wu, J. Y. Zhang, X. Y. You, Z. K. Xu, J. Y. Yang, H. Ren, G. Y. Zhu, Y. Z. Zhang and S. Y. Dong, J. Mater. Chem. A, 2024, 12, 3251–3257 RSC.
- P. K. Tyagi and S. K. Jha, Materialia, 2024, 33, 101967 CrossRef CAS.
- A. Q. Mao, F. Quan, H. Z. Xiang, Z. G. Zhang, K. Kuramoto and A. L. Xia, J. Mol. Struct., 2019, 1194, 11–18 CrossRef CAS.
- R. Z. Zhang and M. J. Reece, J. Mater. Chem. A, 2019, 7, 22148–22162 RSC.
- T. X. Nguyen, C.-C. Tsai, J. Patra, O. Clemens, J.-K. Chang and J.-M. Ting, Chem. Eng. J., 2022, 430, 132658 CrossRef.
- X. H. Xiao, D. M. Kabtamu, A. M. Demeku, G. C. Chen, Y. T. Ou, Z. J. Huang, N. Y. Hsu, H. H. Ku, Y. M. Wang and C. H. Wang, J. Power Sources, 2024, 597, 234178 CrossRef.
- H. Z. Xiang, H. X. Xie, Y. X. Chen, H. Zhang, A. Q. Mao and C. H. Zheng, J. Mater. Sci., 2021, 56, 8127–8142 CrossRef.
- J. Zhao, X. Yang, Y. Huang, F. Du and Y. Zeng, ACS Appl. Mater. Interfaces, 2021, 13, 58674–58681 CrossRef.
- Q. B. An, S. Li, J. J. Zhou, S. J. Ji, Z. S. Wen and J. C. Sun, Adv. Eng. Mater., 2023, 25, 2300585 CrossRef.
- A. Abdelhafiz, B. M. Wang, A. R. Harutyunyan and J. Li, Adv. Energy Mater., 2022, 12, 2200742 CrossRef.
- J. X. Yang, B. H. Dai, C. Y. Chiang, I. C. Chiu, C. W. Pao, S. Y. Lu, I. Y. Tsao, S. T. Lin, C. T. Chiu, J. W. Yeh, P. C. Chang and W. H. Hung, ACS Nano, 2021, 15, 12324–12333 CrossRef PubMed.
- L. Zhang, S. Xia, X. Zhang, Y. Yao, Y. Zhang, S. Chen, Y. Chen and J. Yan, ACS Nano, 2024, 18, 5322–5334 Search PubMed.
- Y. Yao, Z. Huang, P. Xie, S. D. Lacey, R. J. Jacob, H. Xie, F. Chen, A. Nie, T. Pu, M. Rehwoldt, D. Yu, M. R. Zachariah, C. Wang, R. Shahbazian-Yassar, J. Li and L. Hu, Science, 2018, 359, 1489–1494 CrossRef PubMed.
- Q. Dong, M. Hong, J. Gao, T. Li, M. Cui, S. Li, H. Qiao, A. H. Brozena, Y. Yao, X. Wang, G. Chen, J. Luo and L. Hu, Small, 2022, 18, e2104761 Search PubMed.
- S. Y. Zhu, W. Nong, L. J. J. Nicholas, X. Cao, P. L. Zhang, Y. Lu, M. Z. Xiu, K. Huang, G. Wu, S. W. Yang, J. S. Wu, Z. Liu, M. Srinivasan, K. Hippalgaonkar and Y. Z. Huang, J. Mater. Chem. A, 2024, 12, 11473–11486 Search PubMed.
- H. K. Beere, N. S. Reddy, P. Kulkarni, K. Samanta, H. Y. Jung and D. Ghosh, J. Energy Storage, 2024, 80, 110325 CrossRef.
- B. Petrovičovà, W. Xu, M. G. Musolino, F. Pantò, S. Patanè, N. Pinna, S. Santangelo and C. Triolo, Appl. Sci., 2022, 12, 5965 CrossRef.
- L. Tian, Z. Zhang, S. Liu, G. Li and X. Gao, Energy Environ. Mater., 2021, 5, 645–654 CrossRef.
- C. Triolo, M. Maisuradze, M. Li, Y. Liu, A. Ponti, G. Pagot, V. Di Noto, G. Aquilanti, N. Pinna, M. Giorgetti and S. Santangelo, Small, 2023, 19, e2304585 CrossRef.
- D. Wang, C. Duan, H. He, Z. Wang, R. Zheng, H. Sun, Y. Liu and C. Liu, J. Colloid Interface Sci., 2023, 646, 89–97 CrossRef CAS.
- H. He, P. Kou, Z. Zhang, D. Wang, R. Zheng, H. Sun, Y. Liu and Z. Wang, J. Colloid Interface Sci., 2024, 653, 179–188 CrossRef CAS.
- W. S. Bian, H. J. Li, Z. X. Zhao, H. L. Dou, X. Q. Cheng and X. M. Wang, Electrochim. Acta, 2023, 447, 142157 CrossRef CAS.
- T. G. Brandt, A. R. Tuokkola, M. J. Yu and R. M. Laine, Chem. Eng. J., 2023, 474, 145495 CrossRef CAS.
- D. Stenzel, B. Zhou, C. Okafor, M. V. Kante, L. Lin, G. Melinte, T. Bergfeldt, M. Botros, H. Hahn, B. Breitung and S. Schweidler, Front. Energy Res., 2022, 10, 942314 CrossRef.
- M. U. González-Rivas, S. S. Aamlid, M. R. Rutherford, J. Freese, R. Sutarto, N. Chen, E. E. Villalobos-Portillo, H. Castillo-Michel, M. Kim, H. Takagi, R. J. Green and A. M. Hallas, J. Am. Chem. Soc., 2024, 146, 26048–26059 CrossRef.
- M. Brahlek, M. Gazda, V. Keppens, A. R. Mazza, S. J. McCormack, A. Mielewczyk-Gryń, B. Musico, K. Page, C. M. Rost and S. B. Sinnott, APL Mater., 2022, 10, 11 Search PubMed.
- M. Fracchia, M. Manzoli, U. Anselmi-Tamburini and P. Ghigna, Scr. Mater., 2020, 188, 26–31 CrossRef CAS.
- M. Einert, A. Waheed, S. Lauterbach, M. Mellin, M. Rohnke, L. Q. Wagner, J. Gallenberger, C. Tian, B. M. Smarsly, W. Jaegermann, F. Hess, H. Schlaad and J. P. Hofmann, Small, 2023, 19, e2205412 CrossRef.
- A. Sarkar, B. Eggert, R. Witte, J. Lill, L. Velasco, Q. S. Wang, J. Sonar, K. Ollefs, S. S. Bhattacharya, R. A. Brand, H. Wende, F. M. F. de Groot, O. Clemens, H. Hahn and R. Kruk, Acta Mater., 2022, 226, 117581 CrossRef CAS.
- H. Minouei, M. Kheradmandfard, M. Saboktakin Rizi, M. Jalaly, D.-E. Kim and S. I. Hong, Appl. Surf. Sci., 2022, 576, 151719 CrossRef CAS.
- T. X. Nguyen, J. Patra, C. C. Tsai, W. Y. Xuan, H. Y. T. Chen, M. S. Dyer, O. Clemens, J. Li, S. B. Majumder, J. K. Chang and J. M. Ting, Adv. Funct. Mater., 2023, 33, 2300509 CrossRef.
- N. J. Usharani, H. Sanghavi and S. S. Bhattacharya, J. Alloys Compd., 2021, 888, 161390 CrossRef.
- Z. Wang, R. Lin, Y. Huo, H. Li and L. Wang, Adv. Funct. Mater., 2021, 32, 2109503 CrossRef.
- K. Iwase and I. Honma, ACS Appl. Energy Mater., 2022, 5, 9292–9296 CrossRef.
- S. Mehrabi-Kalajahi, A. O. Moghaddam, F. Hadavimoghaddam, R. Salari, M. A. Varfolomeev, A. L. Zinnatullin, K. R. Minnebaev, D. A. Emelianov, D. A. Uchaev, R. Fereidonnejad, O. Zaitseva, G. R. Khasanova, E. A. Trofimov, A. Rozhenko, A. Cabot and F. G. Vagizov, ACS Appl. Nano Mater., 2024, 7, 5513–5524 CrossRef.
- C. Q. Duan, X. L. Li, D. Wang, Z. Y. Wang, H. Y. Sun, R. G. Zheng and Y. G. Liu, Sustainable Energy Fuels, 2022, 6, 1479–1488 RSC.
- J. Dou, Z. Sun, A. A. Opalade, N. Wang, W. Fu and F. F. Tao, Chem. Soc. Rev., 2017, 46, 2001–2027 RSC.
- J. Patra, T. X. Nguyen, C. C. Tsai, O. Clemens, J. Li, P. Pal, W. K. Chan, C. H. Lee, H. Y. T. Chen, J. M. Ting and J. K. Chang, Adv. Funct. Mater., 2022, 32, 2110992 CrossRef.
- T. Y. Chen, S. Y. Wang, C. H. Kuo, S. C. Huang, M. H. Lin, C. H. Li, H. Y. T. Chen, C. C. Wang, Y. F. Liao, C. C. Lin, Y. M. Chang, J. W. Yeh, S. J. Lin, T. Y. Chen and H. Y. Chen, J. Mater. Chem. A, 2020, 8, 21756–21770 RSC.
- Y. J. Han, M. Tian, C. J. Wang, T. Zong and X. D. Wang, Appl. Catal., B, 2023, 339, 123096 CrossRef.
- Y. Sun, T. Tang, L. Xiao, J. Han, X. Bai, M. Shi, S. Chen, J. Sun, Y. Ma and J. Guan, Chemistry, 2024, 30, e202303779 Search PubMed.
- M. Y. Zhang, X. Y. Lu, K. L. Luo, J. Ye, J. L. Dong, N. N. Lu, X. P. Wang, Q. Niu, P. F. Zhang and S. Dai, Appl. Catal., B, 2024, 349, 123845 CrossRef.
- X. F. Luo, J. Patra, W. T. Chuang, T. X. Nguyen, J. M. Ting, J. Li, C. W. Pao and J. K. Chang, Adv. Sci., 2022, 9, e2201219 CrossRef PubMed.
- Y. T. Yeh, C. W. Huang, A. Y. Hou, C. Y. Huang, Y. D. Lin and W. W. Wu, Small, 2024, 20, e2307284 CrossRef PubMed.
- H. Liu, Y. Zhang, L. Zhang, X. Mu, L. Zhang, S. Zhu, K. Wang, B. Yu, Y. Jiang, J. Zhou and F. Yang, J. Am. Chem. Soc., 2024, 146, 20193–20204 CrossRef CAS.
- S. Huang, Z. Qiu, J. Zhong, S. Wu, X. Han, W. Hu, Z. Han, W. N. Cheng, Y. Luo, Y. Meng, Z. Hu, X. Zhou, S. Guo, J. Zhu, X. Zhao and C. C. Li, Adv. Mater., 2024, 36, 2405170 CrossRef CAS.
- W. Li, G. Zhao, J. Zhong and J. Xie, Fuel, 2024, 358, 130155 CrossRef CAS.
- M. W. Ma, L. P. Li, G. Tian, Z. B. Geng, X. Zhang, X. Zhao and G. S. Li, Chem. Eng. J., 2023, 470, 144291 CrossRef CAS.
- S. H. Albedwawi, A. AlJaberi, G. N. Haidemenopoulos and K. Polychronopoulou, Mater. Des., 2021, 202, 109534 CrossRef CAS.
- L. J. Xu, L. D. Wang, Y. Li, Q. B. Song, Z. P. Tian, C. Wang, M. Zhao and N. B. Gao, Chem. Eng. J., 2024, 488, 150767 CrossRef CAS.
- M. Zhong, T. Xu, C. Wang, Y. Teng, Y. Cai, Z. Zhang, B. Xiao and X. Wang, Chem. Eng. J., 2024, 487, 150521 CrossRef CAS.
- L. Yuan, C. Xu, S. Zhang, M. Yu, X. Wang, Y. Chen and L. Dai, J. Colloid Interface Sci., 2023, 640, 359–371 CrossRef CAS PubMed.
- S. Y. Du and P. F. Zhang, AIChE J., 2024, 70, e18470 CrossRef CAS.
- S. Y. Nie, L. Wu, L. C. Zhao, X. Zheng, S. Z. Yang and P. F. Zhang, Chem Catal., 2021, 1, 648–662 CrossRef CAS.
- Y. Ma, Y. Zhang, Y. Jian, Z. Jiang, S. Chai, L. Li, L. Xia and C. He, Fuel, 2023, 353, 129171 Search PubMed.
- X. L. Duan, X. P. Wang, L. Xu, T. T. Ma, Y. Shu, S. T. Hou, Q. Niu and P. F. Zhang, J. Mater. Chem. A, 2023, 11, 19696–19706 RSC.
- A. Taniguchi, T. Fujita and K. Kobiro, Dalton Trans., 2024, 53, 8124–8134 RSC.
- L. An, C. Wei, M. Lu, H. Liu, Y. Chen, G. G. Scherer, A. C. Fisher, P. Xi, Z. J. Xu and C. H. Yan, Adv. Mater., 2021, 33, e2006328 CrossRef.
- C. C. McCrory, S. Jung, J. C. Peters and T. F. Jaramillo, J. Am. Chem. Soc., 2013, 135, 16977–16987 CrossRef.
- C. Triolo, K. Moulaee, A. Ponti, G. Pagot, V. Di Noto, N. Pinna, G. Neri and S. Santangelo, Adv. Funct. Mater., 2023, 34, 2306375 CrossRef.
- Y. Zhang, T. Lu, Y. Ye, W. Dai, Y. Zhu and Y. Pan, ACS Appl. Mater. Interfaces, 2020, 12, 32548–32555 CrossRef PubMed.
- J. Baek, M. D. Hossain, P. Mukherjee, J. Lee, K. T. Winther, J. Leem, Y. Jiang, W. C. Chueh, M. Bajdich and X. Zheng, Nat. Commun., 2023, 14, 5936 CrossRef PubMed.
- Z. Lin, B. Ma, Z. H. Chen and Y. K. Zhou, Ceram. Int., 2023, 49, 23057–23067 CrossRef.
- V. Prabhahari, R. Praveena and K. S. Babu, J. Alloys Compd., 2024, 986, 174152 CrossRef.
- Z. Jin, J. Lyu, K. Hu, Z. Chen, G. Xie, X. Liu, X. Lin and H. J. Qiu, Small, 2022, 18, e2107207 Search PubMed.
- C. Ozgur, T. Erdil, U. Geyikci, C. Okuyucu, E. Lokcu, Y. E. Kalay and C. Toparli, Glob Chall., 2024, 8, 2300199 CrossRef.
- Q. Zhang, Z. Zheng, R. Gao, X. Xiao, M. Jiao, B. Wang, G. Zhou and H. M. Cheng, Adv. Mater., 2024, 36, e2401018 Search PubMed.
- D. Y. Li, L. P. Sun, Q. Li, T. Xia, L. H. Huo and H. Zhao, J. Alloys Compd., 2022, 913, 165148 CrossRef CAS.
- X. Zou, Y. R. Zhang, Z. P. Huang, K. Yue and Z. H. Guo, Chem. Commun., 2023, 59, 13535–13550 RSC.
- R. Guo, Y. Yang, C. Zhao, F. Huo, J. Xue, J. He, B. Sun, Z. Sun, H. K. Liu and S. X. Dou, Adv. Funct. Mater., 2023, 34, 2313168 CrossRef.
- B. Xiao, G. Wu, T. D. Wang, Z. G. Wei, Y. W. Sui, B. L. Shen, J. Q. Qi, F. X. Wei and J. C. Zheng, Nano Energy, 2022, 95, 106962 CrossRef.
- B. Xiao, G. Wu, T. Wang, Z. Wei, Z. Xie, Y. Sui, J. Qi, F. Wei, X. Zhang, L. B. Tang and J. C. Zheng, ACS Appl. Mater. Interfaces, 2023, 15, 2792–2803 CrossRef.
- T. X. Nguyen, J. Patra, J. K. Chang and J. M. Ting, J. Mater. Chem. A, 2020, 8, 18963–18973 RSC.
- C. Duan, K. Tian, X. Li, D. Wang, H. Sun, R. Zheng, Z. Wang and Y. Liu, Ceram. Int., 2021, 47, 32025–32032 CrossRef.
- M. Mozdzierz, K. Swierczek, J. Dabrowa, M. Gajewska, A. Hanc, Z. Feng, J. Cieslak, M. Kadziolka-Gawel, J. Plotek, M. Marzec and A. Kulka, ACS Appl. Mater. Interfaces, 2022, 14, 42057–42070 CrossRef.
- S. Li, Z. J. Peng and X. L. Fu, J. Adv. Ceram., 2023, 12, 59–71 CrossRef.
- X. B. Yang, H. Q. Wang, Y. Y. Song, K. T. Liu, T. T. Huang, X. Y. Wang, C. F. Zhang and J. Li, ACS Appl. Mater. Interfaces, 2022, 14, 26873–26881 CrossRef PubMed.
- C. Liu, J. Bi, L. Xie, X. Gao and J. Rong, J. Energy Storage, 2023, 71, 108211 CrossRef.
- B. Talluri, M. L. Aparna, N. Sreenivasulu, S. S. Bhattacharya and T. Thomas, J. Energy Storage, 2021, 42, 103004 CrossRef.
- Y. Yin, W. B. Zhang, X. L. Zhang, M. M. Theint, J. L. Yang, Z. Q. Yang, J. J. Li, S. Liang and X. J. Ma, Dalton Trans., 2023, 52, 9005–9016 RSC.
- S. He, V. Somayaji, M. Wang, S.-H. Lee, Z. Geng, S. Zhu, P. Novello, C. V. Varanasi and J. Liu, Nano Res., 2021, 15, 4785–4791 CrossRef.
- S. Das, S. Kumar, S. Sarkar, D. Pradhan, C. S. Tiwary and S. Chowdhury, J. Mater. Chem. A, 2024, 12, 16815–16830 RSC.
- L. J. Zhang, N. Wang, F. Y. Wang, P. Xu, X. J. Han and Y. C. Du, Chem. Eng. J., 2024, 488, 150826 Search PubMed.
- H. D. Li, J. P. Lai, Z. J. Li and L. Wang, Adv. Funct. Mater., 2021, 31, 2106715 CrossRef.
- V. Strotkotter, O. A. Krysiak, J. Zhang, X. Wang, E. Suhr, W. Schuhmann and A. Ludwig, Chem. Mater., 2022, 34, 10291–10303 CrossRef PubMed.
- B. Zhao, Y. Du, Z. Yan, L. Rao, G. Chen, M. Yuan, L. Yang, J. Zhang and R. Che, Adv. Funct. Mater., 2022, 33, 2209924 CrossRef.
- J. Y. Wang, J. H. Zhang, H. F. Yu, L. Chen, H. Jiang and C. Z. Li, ACS Mater. Lett., 2024, 6, 1739–1745 CrossRef.
- K. Y. Zhu, F. Shi, X. F. Zhu and W. S. Yang, Nano Energy, 2020, 73, 104761 CrossRef.
- D. Yan, Y. Li, J. Huo, R. Chen, L. Dai and S. Wang, Adv. Mater., 2017, 29, 1606459 CrossRef PubMed.
- H. B. Tao, L. Fang, J. Chen, H. B. Yang, J. Gao, J. Miao, S. Chen and B. Liu, J. Am. Chem. Soc., 2016, 138, 9978–9985 CrossRef.
- S. L. Fereja, Z. Zhang, Z. Fang, J. Guo, X. Zhang, K. Liu, Z. Li and W. Chen, ACS Appl. Mater. Interfaces, 2022, 14, 38727–38738 CrossRef PubMed.
- N. Ci, Y. Hu, Q. Li, J. Cheng, H. Zhang, D. Li, K. Li, K. M. Reddy, L. Ci, G. Xie, X. Liu and H. J. Qiu, Small Methods, 2023, e2301322 Search PubMed.
- X. M. He, Z. Q. Zhang, T. Qiao, H. Liu, X. W. Jiang, T. F. Xing and S. L. Wang, ACS Appl. Nano Mater., 2023, 6, 19573–19580 CrossRef.
- L. Guo, J. Zhou, F. Liu, X. Meng, Y. Ma, F. Hao, Y. Xiong and Z. Fan, ACS Nano, 2024, 18, 9823–9851 CrossRef PubMed.
- H. Wu, Q. Lu, Y. Li, J. Wang, Y. Li, R. Jiang, J. Zhang, X. Zheng, X. Han, N. Zhao, J. Li, Y. Deng and W. Hu, Nano Lett., 2022, 22, 6492–6500 CrossRef.
- M. Einert, M. Mellin, N. Bahadorani, C. Dietz, S. Lauterbach and J. P. Hofmann, ACS Appl. Energy Mater., 2022, 5, 717–730 CrossRef.
- Q. Zhang, Y. Hu, H. Wu, X. Zhao, M. Wang, S. Wang, R. Feng, Q. Chen, F. Song, M. Chen and P. Liu, ACS Nano, 2023, 17, 1485–1494 CrossRef.
- Y. X. Lao, X. Q. Huang, L. P. Liu, X. L. Mo, J. L. Huang, Y. T. Qin, Q. Q. Mo, X. F. Hui, Z. Yang and W. Jiang, Chem. Eng. J., 2024, 481, 148428 CrossRef.
- J. Zhang, H. B. Yang, D. Zhou and B. Liu, Chem. Rev., 2022, 122, 17028–17072 CrossRef PubMed.
- T. Loffler, A. Savan, H. Meyer, M. Meischein, V. Strotkotter, A. Ludwig and W. Schuhmann, Angew Chem. Int. Ed. Engl., 2020, 59, 5844–5850 CrossRef.
- M. Fracchia, M. Coduri, P. Ghigna and U. Anselmi-Tamburini, J. Eur. Ceram. Soc., 2024, 44, 585–594 CrossRef CAS.
- C. Y. Huang, C. W. Huang, M. C. Wu, J. Patra, T. X. Nguyen, M. T. Chang, O. Clemens, J. M. Ting, J. Li, J. K. Chang and W. W. Wu, Chem. Eng. J., 2021, 420, 129838 CrossRef CAS.
- L. Su, J. Ren, T. Lu, K. Chen, J. Ouyang, Y. Zhang, X. Zhu, L. Wang, H. Min, W. Luo, Z. Sun, Q. Zhang, Y. Wu, L. Sun, L. Mai and F. Xu, Adv. Mater., 2023, 35, 2205751 CrossRef PubMed.
- C.-Y. Wu, Y.-C. Hsiao, Y. Chen, K.-H. Lin, T.-J. Lee, C.-C. Chi, J.-T. Lin, L.-C. Hsu, H.-J. Tsai, J.-Q. Gao, C.-W. Chang, I.-T. Kao, C.-Y. Wu, Y.-R. Lu, C.-W. Pao, S.-F. Hung, M.-Y. Lu, S. Zhou and T.-H. Yang, Sci. Adv., 2024, 10, eadl3693 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2025 |