 Open Access Article
Open Access ArticlePhosphor-converted light-emitting diodes in the marine environment: current status and future trends
Maofeng
Hua
a,
Shuifu
Liu
ab,
Lei
Zhou
 *a,
Jean-Claude
Bünzli
*a,
Jean-Claude
Bünzli
 *cd and
Mingmei
Wu
*cd and
Mingmei
Wu
 *a
*a
aZhuhai Key Laboratory of Optoelectronic Functional Materials and Membrane Technology, School of Chemical Engineering and Technology, Sun Yat-sen University, Zhuhai, 519082, P. R. China. E-mail: zhoul8@mail.sysu.edu.cn; ceswmm@mail.sysu.edu.cn
bCollege of Materials, Xiamen University, Xiamen 361005, P. R. China
cDepartment of Applied Biology and Chemical Technology, The Hong Kong Polytechnic University, Kowloon, Hong Kong 999077, P. R. China
dInstitute of Chemical Sciences and Engineering, Swiss Federal Institute of Technology Lausanne (EPFL), Lausanne, Switzerland. E-mail: jean-claude.bunzli@epfl.ch
First published on 10th January 2025
Abstract
The exploitation and utilization of resources in marine environments have ignited a demand for advanced illumination and optical communication technologies. Light-emitting diodes (LEDs), heralded as “green lighting sources”, offer a compelling solution to the technical challenges of marine exploration due to their inherent advantages. Among the myriad of LED technologies, phosphor-converted light-emitting diodes (pc-LEDs) have emerged as frontrunners in marine applications. At the heart of pc-LEDs lie phosphor materials, colour-converters that orchestrate the emission of light. In the marine environment, blue-green light with a wavelength of 440–570 nm exhibits the deepest penetration depth, while other wavelengths are rapidly attenuated in the shallow layer. Additionally, specific wavelengths of light are crucial for particular applications. However, the moist marine environment as well as the demand for continuous and stable lighting pose a formidable challenge to the environmental stability of the phosphors. Therefore, developing blue-green phosphors with exceptional colour purity and high thermal and moisture stability is paramount for marine applications. Herein, this review delves into the application of LED and pc-LED technology in underwater optical communication and marine fishery, exploring the development strategies of phosphors tailored for the marine environment. The insights presented serve as a valuable reference for further research on phosphors and pc-LED devices.
1 Introduction
The relentless pursuit of sustainable development has propelled coastal nations towards the rational exploitation and utilization of marine resources, fostering a vibrant marine economy. Solid-state lighting technology has emerged as a transformative force in this endeavor, offering innovative solutions for both underwater and surface marine applications, such as light communications and marine fishery. 1–4 Primary traditional marine lighting devices, relying on incandescent and metal halide (MH) lamps, are plagued by inherent drawbacks, including exorbitant energy consumption, limited lifespan and inefficient performances. More sustainable alternatives, for instance, light-emitting diodes (LEDs), especially the white light-emitting diodes (WLEDs) are now replacing obsolete light sources, establishing themselves as the frontrunners in marine applications due to their exceptional longevity, low energy consumption, minimal heat radiation, and rapid response time. 5–8WLEDs can be fabricated through two primary approaches: either by combining three primary-colour (red/green/blue) LEDs or by employing phosphor-converted LEDs (pc-LEDs) to transform the blue or near-ultraviolet (n-UV) emission of a single LED chip into white light. 6,9 The latter approach offers compelling advantages over the former in terms of cost-effectiveness and manufacturing simplicity. Beyond white light, pc-LEDs fitted with adequate phosphors can also transform the short-wavelength emission from LED chips into a spectrum tailored to specific applications. 10,11 Moreover, pc-LEDs can be tailored with specific phosphors to emit wavelengths suited for various marine applications, enhancing energy efficiency beyond the capabilities of traditional white light sources. In addition, the marine environment, with its pervasive humidity and salinity, poses a formidable challenge to the stability of phosphors. To ensure the longevity and reliability of pc-LEDs in marine applications, phosphor materials must be meticulously engineered. This necessitates the development of anti-thermal-quenching and moisture-resistant phosphors that retain their luminescent properties whatever the environment they are working in.
Significant advances in pc-LED technology have been achieved recently but there is a lack of comprehensive reviews interweaving the application of LED devices with the development of phosphor materials for the marine environment. This paper aims to bridge this gap by providing an overarching perspective on the role of pc-LEDs in the marine field, delving into the specific requirements of marine applications, and reviewing the development of high-performance phosphors for pc-LEDs (Fig. 1). It also intends to serve as a valuable reference for researchers and practitioners alike, paving the way for the transformative application of pc-LEDs in the marine field.
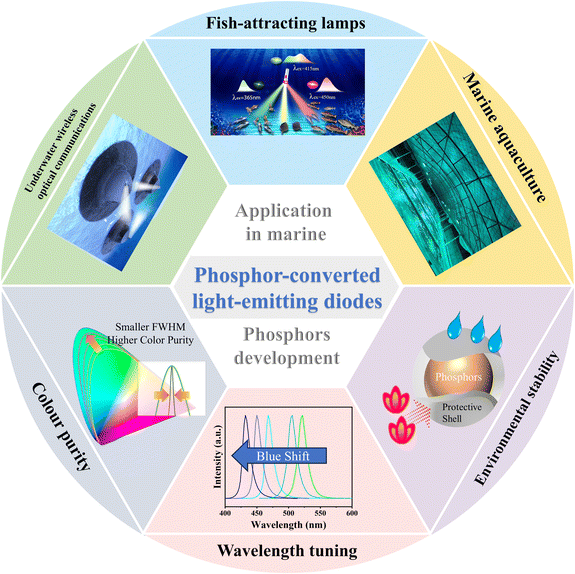 | ||
| Fig. 1 Research content of this review. The figure in the section of colour purity is reprinted with permission from ref. 12. Copyright 2021, Springer Nature. | ||
2 Application of pc-LEDs in marine environments
2.1 Underwater wireless optical communications (UWOC)
Traditional wireless communication modes, such as acoustic communications and radio communications, are often limited in their ability to simultaneously achieve high-speed and long-distance transmission. Visible light communications (VLC) have emerged as a promising alternative for maritime applications due to their adjustable bandwidth and long transmission distance. 13,14 Additionally, the visible-light band, with a spectral range between 380 and 780 nm, is unlicensed, unlike radio frequencies, allowing for utilization without authorization, and it also ensures high privacy and safety since it is not sensitive to electromagnetic interferences. 15 Underwater wireless optical communications (UWOC) have the potential to play a significant role in a wide range of applications, including tactical surveillance, pollution monitoring, oil control and maintenance, offshore explorations, climate change monitoring, and oceanographic research. 16 A typical UWOC system can be divided into three main components: the transmitter unit, the water channel, and the receiver module. Laser diodes (LDs) or LEDs are commonly employed as receivers and transmitters in UWOC systems (Fig. 2a). Moreover, LEDs offer advantages in terms of cost, and lifetime in comparison to LDs. 17 In a LED-based UWOC system, a photoelectric conversion device is incorporated into the receiver to convert the transmitted light signal into electric signal.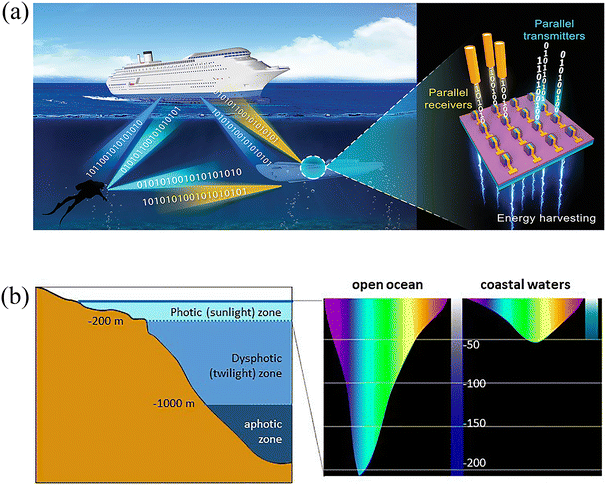 | ||
| Fig. 2 (a) Schematic diagram of a duplex UWOC system based on a LED array. Reproduced with permission from ref. 25. Copyright 2021, WILEY-VCH. (b) Penetration of light into the water column and illustration of the depth at which different colours penetrate ocean waters. Reproduced with permission from ref. 29. Copyright 2020, Frontiers. | ||
Recently, pc-LED devices have gained interest in UWOC applications. However, the quantum-confined stark effect (QCSE) of the InGaN/GaN LED quantum wells and phosphor materials pose a challenge, limiting the modulation bandwidth. 18,19 To address this issue and improve data transmission rates, several solutions have been proposed, which include the use of analogue equalizers at the transmitter and receiver sides, the implementation of a blue-light bandpass filter at the receiver to eliminate the phosphorescent component, and the adoption of different modulation schemes such as quadrature amplitude modulation (QAM) and orthogonal frequency division multiplexing (OFDM). 20,21 Minh et al. successfully increased the bandwidth of a WLED to 50 MHz using an analogue equalizer, achieving a transmission rate of 100 Mbps. 22 Additionally, reducing the active LED area reduces device capacitance and increases the bias current density, thereby widening the modulation bandwidth. 23 This principle has led to the development of Micro-LED arrays. 24 Tian et al. documented a peak modulation bandwidth of 251.3 MHz and a data rate of 660 Mbps using green micro-LEDs in a duplex UWOC system. 25 Xu et al. proposed a LED-to-LED UWOC system based on series of connected micro-LED arrays. Zhang et al. designed a real-time UWOC system with four blue LEDs, achieving a maximum communication range of 25.4 m at 80 Mbps. 26 The normalized detection bandwidth obtained demonstrates that increasing the number of LEDs improves the performance and practicability of LED UWOC systems.
Another critical aspect to consider in UWOC is the influence of water depth on the spectral components and light intensity. Purple light with a wavelength below 360 nm and red light with a wavelength above 600 nm are rapidly absorbed by surface water, while blue-green light tends to penetrate much deeper, reaching up to a level of 200 m in the open ocean (Fig. 2b). 27–29 This penetration may be adequate with respect to the distance requirement of UWOC data transmissions. A shore-to-underwater VLC system has been proposed by Chung et al., taking into account the varying light penetrability in onshore and underwater environments. 30 The signal was transmitted from the lighthouse equipped with WLEDs and received by a beacon that relayed the signal to the undersea receivers comprised of positive-intrinsic-negative photodiodes (PDs). The signal was then fed to a green LED driver and transmitted through an undersea optical channel. This study demonstrated that an underwater data rate transmission of up to 1 Gbps can be achieved over shorter distances, surpassing the performance of WLED-based VLC systems. Therefore, the development of blue or green LED devices specifically tailored for underwater applications holds great promise for advancing UWOC technology.
Nowadays, UWOC has emerged as a unique technology enabling high-speed data and moderate-distance communications in undersea environments. Numerous applications that demand high data transmission rates, such as real-time video transmission and remote-operated vessel control, stand to benefit significantly from UWOC. To date, UWOC has heralded the advent of a 100 meter high-speed connectivity paradigm. Besides, UWOC transmission of 40 m/1 Gbps and 100 m/1 Gbps levels can be achieved using a wide-bandwidth photomultiplier tube (PMT) and a high-bandwidth single-photon counter, respectively. 31,32 Future research direction in UWOC will focus on LED-based transmission, optimal coding, and modulation technologies, aiming to achieve high-speed, long-distance, and real-time communications. In practical applications, the intricacies of the subaquatic milieu, including the presence of marine plankton, hydrodynamic perturbations, and other disruptive elements, exert a clear influence on the integrity and performance of UWOC systems. 33 Specifically, mobile UWOC network systems must account for the architectural parameters of subaquatic optical systems, including the emission divergence angle, the reception field of view, and other pivotal determinants.
2.2 Marine fishery
| Cephalopod | Fish | Crustacean | |
|---|---|---|---|
| Visual structure | Single eye | Single eye | Compound eye |
| Identifiable light colour | Colour blindness | Wide range | Blue-green (440–570 nm), purple-ultraviolet (360–440 nm) |
| Optimum colour or wavelength | Blue, green, and white | Blue-green: cyprinus carpio, carassius cuvieri, amphiprion clarkii | 480–500 nm (deep sea), 510–525 nm (coastal) green light (530 nm), broadband white light |
| Red: verasper moseri perca fluviatilis, silurus meridionalis, anguilla marmorata, wallago attu |
Most of the research in this area has focused on evaluating the influence of pc-LED emitting different colours and intensities on fish-attracting efficiency. For instance, a study revealed that Japanese flying squids exhibit heightened sensitivity to blue and green light, with a peak spectral sensitivity at 482 nm. 41 Additionally, research demonstrated the successful application of combined white LED and blue LED configurations in attracting squids, both in controlled tank settings and open-sea trials. 42 Furthermore, researchers have recognized the variations in phototaxis behaviour across different life stages. Juvenile fish typically exhibit stronger phototaxis responses compared to their adult counterparts. This implies that employing full-spectrum white light or inappropriate light intensities can lead to the undesirable capture of predominantly juvenile fish, potentially jeopardizing the ecosystem and compromising fishing efficiency. 43 Extensive research has sought to unravel the phototactic patterns of various commercially important fish species using LED as fishing light sources. 44–46 Despite advances in understanding phototactic behaviour, the connection between phosphors design in pc-LEDs and fish-attracting lamp efficacy remains elusive and understudied. This gap persists due to the limited understanding of the underlying mechanisms governing light-mediated attraction and repulsion in marine organisms. 36 Besides, the application of commercial fish-attracting lamps is limited by many factors, such as light pollution caused by excessive power of flashlights, mismatch between lighting systems and flashboats, etc. However, recent discoveries offer promising solutions; for instance, Liu et al. successfully synthesized a novel phosphor material with multi-colour tuneable emission covering the spectral sensitivity range of most marine fish species. 47 Further elucidating the fundamental reasons behind variations in photosensitivity holds potential for optimizing fish-attracting lamps. Such progress paves the way for more sustainable and productive fishing practices, characterized by enhanced energy efficiency and reduced ecological impact.
Despite significant progress in understanding the impact of light on aquaculture, further research is needed to elucidate the precise mechanisms governing these responses. Additionally, the design of pc-LED systems for marine ranches faces challenges associated with the long-term lighting requirements and the harsh environment characterized by high humidity and salinity. Continued advancements in pc-LED technology and a deeper understanding of light-mediated responses in various species hold great potential for optimizing the productivity and sustainability of marine ranches.
By leveraging the versatility of pc-LEDs, marine ranches can provide customized lighting environments for diverse aquaculture species. Tailoring light colour, intensity, and photoperiod based on the specific needs of each species offers a promising avenue for enhancing growth, development, and ultimately, profitability. Continued research efforts dedicated to unravelling the intricacies of light-mediated responses in aquatic organisms, coupled with further technological advancements in pc-LEDs, are paving the way for sustainable and efficient aquaculture.
3 Developing phosphors for pc-LEDs in marine environments
Existing pc-LED devices are primarily designed for terrestrial applications and often exhibit limitations such as low energy efficiency and short lifespans under marine temperature and humidity conditions. Additionally, their broad emission bands hinder precise characterization of the impact of specific light on marine organisms. Therefore, pc-LEDs with tailored light colours and enhanced stability are crucial for effective deployment in practical marine applications. This review focuses on the role of phosphors, the key luminescent components in pc-LEDs, in addressing these critical challenges. By exploiting the tunability of rare-earth and transition metal ions, strategies for optimizing phosphor properties can be designed that meet the specific demands of the marine environment.Phosphors dictate the optical properties of LEDs, such as luminous efficiency, colour temperature and colour rendering index (CRI). They are typically composed of host materials and activators; the former often include silicates, phosphates, aluminates, borates, perovskites, and nitrogen oxides, and the latter often include Ce3+, Eu2+, Bi3+, Ln3+ (Ln = Sm, Eu, Tb, Dy, …) or other transition metal ions. A large library of inorganic phosphors with specific emission colours has been developed and implemented in pc-LEDs, demonstrating high quantum efficiency at room temperature (Table 2). Notably, the inherent tunability of doping ions provides a powerful tool for manipulating the photoluminescent (PL) properties of phosphors. Several strategies can be employed to achieve the desired characteristics for marine applications, including colour tuning and stability enhancement.
| Colour | Matrix | Doped activator | Excitation maximum (nm) | Emission maximum (nm) | Ref. |
|---|---|---|---|---|---|
| Red | M2Si5N8 (M = Ca, Sr, Ba) | Eu2+ | 450 | 623 (Ca) | 61 and 62 |
| 640 (Sr) | |||||
| 650 (Ba) | |||||
| SrLiAl3N4 | Eu2+ | 440 | 650 | 63 | |
| K2SiF6 | Mn4+ | 450 | 630 | 64 | |
| CaAlSiN3 | Eu2+ | 450 | 650 | 65 | |
| Yellow | Y3Al5O12 | Ce3+ | 460 | 560 | 66 |
| Sr[Li2.5Al1.5O3N] | Tb3+ | 460 | 578 | 67 | |
| Green | NaK2Li[Li3SiO4]4 | Eu2+ | 450 | 528 | 68 |
| β-SiAlON | Eu2+ | 325 | 540, 529 | 69 | |
| Lu3Al5O12 | Ce3+ | 460 | 530 | 70 | |
| Ba2SiO4 | Eu2+ | 395 | 511 | 71 | |
| Blue | BaMgAl10O17 | Eu2+ | 254 | 450 | 72 |
| Ca4SnGe3O12 | Bi3+ | 383 | 442 | 73 | |
| K2SrBa(PO4)2 | Eu2+ | 330 | 425 | 74 | |
| Ba3Y2B6O15 | Ce3+ | 365 | 446 | 75 |
3.1 Blue-green phosphors with hight colour purity
Marine environment presents a unique and challenging frontier for pc-LED applications. The inherent attenuation of light in water necessitates the use of wavelengths with enhanced penetration, such as blue-green light (440–570 nm). Furthermore, the various photobiological responses of marine organisms highlight the importance of precise light control, achievable through narrow-band emission of phosphors. A series of narrow-band blue or green emitting phosphors have been developed for the backlighting of display, and these phosphors meet the colour requirements of the marine field. 76–81 This section introduces such blue- or green-emitting phosphors, as well as strategies to improve their colour purity.For green-emitting phosphors, β-SiAlON: Eu2+ has dominated commercial applications for the past decade. Xie et al. reported Eu2+-doped β-SiAlON (Si5.6Al0.4O0.4N7.6) phosphors with a strong response to excitation in the ultraviolet (UV) region and blue regions, and a peak emission at 535 nm (Fig. 3a). 82 However, β-SiAlON phosphors show broadband emission in a range from 525 to 540 nm, ultimately failing to meet the best emission range for marine applications. Besides, high-temperature synthesis with nitrogen oxide restricts further development. In contrast, UCr4C4-type compounds, M(A, B)4X4, emerge as more promising candidates for Eu2+-doped commercial narrow-band phosphors (M is an alkali metal ion or alkaline earth metal ion, A and B are coordinated metal ions, X is oxygen or nitrogen ions). The corner-sharing and edge-sharing connections between [AX4] and [BX4] tetrahedra create a highly condensed three-dimensional network achieving narrow-band emission when doped with Eu2+. Xia et al. pioneered this approach with oxide-based RbLi(Li3SiO4)2:Eu2+ (RLSO:Eu2+) and RbNa(Li3SiO4)2:Eu2+ (RNSO:Eu2+). 83,84 Compared to β-SiAlON:Eu2+, these phosphors exhibit narrower emission bands (RLSO:Eu2+-λem = 530 nm, full width at half height FWHM = 42 nm; RNSO: Eu2+-λem = 523 nm, FWHM = 41 nm) and more suitable emission peak positions (Fig. 3b). These phosphors showcase narrow-band emission with high internal quantum efficiency (IQE) and excellent thermal stability. However, the presence of a large amount of alkali metal cations renders them susceptible to hydrolysis, leading to poor moisture resistance. To address this, surface modification or structural regulation can be applied. The detailed strategies will be discussed in Section 3.4.
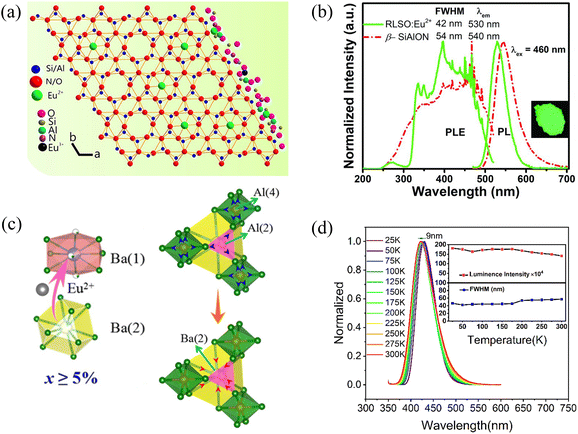 | ||
| Fig. 3 (a) Crystal structure model of β-SiAlON. Reproduced with permission from ref. 82. Copyright 2018, ACS. (b) PLE and PL spectra of RLSO:8%Eu2+ and the commercial β-SiAlON:Eu2+ phosphors. The inset is a digital photograph of green RLSO:8%Eu2+ phosphor under 365 nm UV lamp illumination. Reproduced with permission from ref. 83. Copyright 2018, WILEY-VCH. (c) The proposed mechanism of the blue-shifted emission upon increasing Eu concentration (left) and the preferential site-occupancy of Eu2+ into Ba(1) and Ba(2) sites when x ≥ 5% (right). Reproduced with permission from ref. 100. Copyright 2018, ACS. (d) PL spectra of the LaScO3:Bi3+ sample under 344 nm excitation. The inset shows the luminescence intensity and FWHM from 25 K to 300 K. Reproduced with permission from ref. 107. Copyright 2022, Elsevier. | ||
In addition to the Eu2+-doped phosphors described above, inorganic luminescent materials doped with Tb3+ are promising for their potential to emit narrow-band green light at 544 nm. However, the challenge lies in the weak UV absorption of Tb3+ ions, which is due to the parity-forbidden nature of f–f electric-dipole transitions. A common solution to overcome this is co-doping with Ce3+ as a sensitizer to enhance Tb3+ excitation through energy transfer. Notable examples include the borates Sr2LiScB4O10:Ce3+, Tb3+, Ba2Lu5B5O17:Ce3+, Tb3+, the silicates Gd4.67Si3O13:Ce3+, Tb3+, the aluminates Ca2TbHf2Al3O12:Ce3+, CaYAlO4:Tb3+, Ce3+, and the phosphate Na3Tb(PO4)2:Ce3+. 85–90 However, the 5d orbitals of Ce3+ extend beyond the electron shell, making it highly sensitive to environmental perturbations. This sensitivity leads to a broad absorption band and a wide, tuneable emission spectrum. 91 It is frequently reported that Ce3+-activated phosphors tend to yield broadband emission, their FWHM values being usually larger than those of Eu2+-doped phosphors. Therefore, most Ce3+/Tb3+ co-doped phosphors have non-negligible Ce3+ emission in the UV and blue regions, reducing green colour purity. Besides that, divalent manganese (Mn2+) also emerges as an alternative dopant for phosphors with high colour purity, due to the characteristic narrow-band green emission of Mn2+ in tetrahedral coordinating environments; on the other hand, in octahedral positions, Mn2+ emits in the red. 92,93 Xie et al. reported Mn2+ doped γ-aluminum oxynitride (γ-AION) emitting a green band at 512 nm with FWHM of 32 nm under 445 nm excitation, surpassing β-SiAlON:Eu2+ in emission wavelength, bandwidth, and colour purity. 94 However, similarly to Tb3+, Mn2+-activated blue/green phosphors often suffer from low absorption or/and external quantum efficiency (EQE) due to parity forbidden electric-dipole d–d transitions. 95 Strategies like ion substitution and energy transfer can improve the external quantum efficiency, as seen in γ-AION:Mn2+, Mg2+ and γ-AION:Mn2+, Eu2+, 96,97 but there is still a problem of low internal quantum efficiency. It is noteworthy that the emission of Mn2+ is easily affected by the host crystal. Part of host materials with a strong charge transfer band absorption can be the ideal hosts for Mn2+, and the optimal absorption peak has to match well with common n-UV or blue LED chips. 98 Accordingly, Wang et al. designed a series of gallate phosphors and successfully prepared NaGa11−yAlyO17:Mn2+ (y = 0, 1, 2, 3) narrow-band green phosphor with an excellent external quantum efficiency (56.1%). 99
Substrates for blue-emitting phosphors encompass a range of materials, including silicates, phosphates, aluminates, borates, and nitrogen oxides. Eu2+, Ce3+, or Bi3+ are commonly employed activator ions due to their favorable photoluminescent properties. Among commercially established blue phosphors, BaMgAl10O17:Eu2+ (BAM:Eu2+) stands out for its high luminous efficiency. 72 However, drawbacks such as poor high-temperature stability and a broad emission band necessitate further development. In this regard, Lin et al. reported a promising alternative: the Eu2+-doped BaAl12O19 (BAO) blue-emitting phosphor, exhibiting both high efficiency (IQE 90%) and colour purity (90%). 100 Due to the expansion of the Al polyhedron, the Ba(2) site becomes unsuitable for Eu2+ ions, favoring their preferential occupation of the single Ba(1) site, thereby leading to narrow-band emission and high thermal stability (Fig. 3c). Besides, several narrow-band blue-emitting phosphors have been reported, such as AELi2[Be4O6]:Eu2+(AE = Sr, Ba), RbNa3(Li3SiO4)4:Eu2+, AEP8N14:Eu2+(AE = Ca, Sr, Ba), Sr[B8O11(OH)4]:Eu2+. 101–104 Some, like UCr4C4-type phosphors, suffer from limited moisture stability, and others, like nitride phosphors, require harsh preparation conditions, hindering their commercial application. As the promising alternative, some perovskite-structure phosphors can be the particularly fascinating hosts for Eu2+. Leng et al. reported a zero-thermal-quenching perovskite-like K2BaPO4F:1.5% Eu2+ phosphor with an ultra-narrow-band (FWHM = 25 nm) blue-emission and a high colour purity (99%), whose emission intensity can remain 91% of its pristine value after being exposed to the ambient atmosphere for 4 months. 105 Additionally, bismuth (Bi3+) ions in various phosphor hosts can minimize spectral overlap and emit light across the ultraviolet to orange-red spectrum due to their 6p–6s transition. The ns2-nsp transition of Bi3+ is more sensitive to local structure than 5d–4f electron transition of Eu2+, posing a challenge in developing Bi3+-activated, narrow-band emission phosphors. 73,106–109 To date, only a few such phosphors have been identified. For example, Wang et al. found that LaScO3:Bi3+ perovskite-structure phosphors exhibit a notably narrow blue emission with high colour purity (92.26%) and internal quantum efficiency (98%) (Fig. 3d). 107 Lin et al. developed a Sr10P6O25:Bi3+ phosphor with a narrow-band blue emission and good thermal stability (>82% at 150 °C), attributed to the small Stokes shift and introduction of Bi3+ into highly symmetric lattice sites. 108 Chen et al. reported that garnet-type Ca4Hf(GeSi)3O12(CHGSO):xBi3+ phosphors show a narrow-band blue emission with a high colour purity of ∼95% under the excitation of n-UV LED chips. 109 The large band gap, high lattice rigidity and high symmetry of these phosphate, perovskite, and garnet hosts make them ideal for narrow-band emission of Bi3+ ions.
These studies provide valuable insights into the design criteria for phosphors with narrow-band emission and high colour purity. Generally, a rigid host crystal structure is crucial to minimize electron-phonon coupling, while a highly symmetrical coordination environment around the active ion helps reduce the influence of crystal-field splitting. 110–112 Furthermore, a single lattice site for doping minimizes spectral overlap from multiple luminescence centres. Regarding the activator ions mentioned above, most transition metals and trivalent rare earth ions show weak and narrow absorption in the UV region, while Bi3+, Ce3+/Eu2+ display broadband absorption due to the parity-allowed s–p and 4f–5d transitions, respectively. Furthermore, Ce3+ has a broader emission band than Eu2+, attributed to the spin–orbit and crystal-field splitting of the 2F ground state. Overall, Eu2+ and Bi3+ may be the dominant choices for developing phosphors with high colour purity. The design of these phosphors is a cornerstone of photoluminescence (PL) conversion, and the strategies for controlling the emission peak position are explored in the next section.
3.2 Wavelength tuning of blue-green emitting phosphors for marine pc-LED applications
Achieving high-colour-purity blue or green light in phosphors necessitates not only narrow emission half-peak widths but also precise control over the emission wavelength. This control depends on several factors, including the intricate interplay between the crystal structure and the coordination environment surrounding the luminescent centre. The reasons for the emission shift can be attributed to crystal-field splitting and electron cloud rearrangement effects. This section explores some common methods for manipulating PL through targeted modifications.One such method involves cation or anion substitution within the host lattice. This approach alters crucial parameters like bond lengths between the luminescent centre and its ligands, the coordination polyhedron volume, and consequently, the crystal-field strength around the centre. Such alterations directly influence the phosphor's emission spectrum. For instance, in the Ca9−xSrxLiGd2/3(PO4)7:Eu2+ system, the emission wavelength red-shifts from 485 nm to 535 nm because of Ca2+ ions being replaced by Sr2+ ions, attributed to crystal-field splitting and energy transfer between cation sites (Fig. 4a). 113 Also, an obvious blue shift of the emission bands of Gd3TaO7:Bi3+ (λem = 505 nm) can be detected upon cation substitution, varying to 493 nm for Gd2YTaO7:Bi3+, 484 nm for Gd2LuTaO7:Bi3+ and 471 nm for Gd2LaTaO7:Bi3+.114 Similar effects have been observed for anion substitution, for instance in Sr2Ba(AlO4F)1−x(SiO5)x: Ce3+ phosphors. 115 Beyond single substitutions, a more versatile approach known as chemical unit co-substitution involves the simultaneous replacement of multiple atomic units within the host. This strategy offers increased control over luminescence properties. For example, Xia et al. successfully tuned the emission colour from green to blue in Ca2(Al1−xMgx)(Al1−xSi1+x)O7:Eu2+ phosphors by replacing the [Al3+–Al3+] unit with [Mg2+–Si4+] (Fig. 4b). 116 In another case, Zhang et al. used chemical unit co-substitution in the lutetium aluminium garnet (LuAG) host, replacing LuO8/AlO4 units with SrO8/SiO4, to develop the efficient and thermally stable green phosphor Lu2SrAl4SiO12:Ce3+ (LSAS:Ce3+), which emits a blue-shifted emission peaking at 514 nm. 117 Similarly, Huang et al. observed a noticeable wavelength-tuning from 423 nm to 525 nm in the orthosilicate-orthophosphate structured phosphor K1−x(BaSr)(1+x)/2(SixP1−x)O4:Eu2+ (0 ≤ x ≤ 1) by employing a co-substitution strategy, which also improved the thermal stability due to an expanded bandgap and the introduced defect levels associated with the higher content of the phosphate anion group. 118
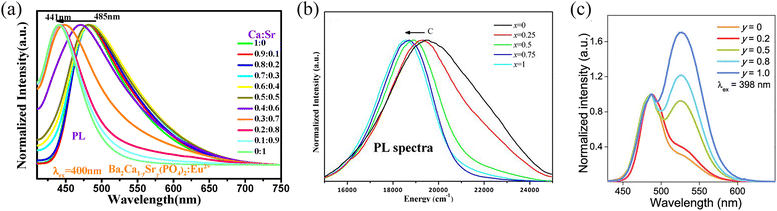 | ||
| Fig. 4 (a) Normalized PL spectra of Ba2Ca1−ySry(PO4)2:Eu2+. Reproduced with permission from ref. 113. Copyright 2020, Elsevier. (b) PL spectra of the phosphors Ca1.98(Al1−xMgx)(Al1−xSi1+x)O7:0.02Eu2+ (x = 0, 0.25, 0.5, 0.75, and 1) as a function of x. Reproduced with permission from ref. 116. Copyright 2015, ACS. (c) Normalized PL spectra of CsKaNa1.98−yLiy(Li3SiO4)4:0.02Eu2+ (0 ≤ y ≤ 1). Reproduced with permission from ref. 76. Copyright 2019, ACS. | ||
Adjusting the doping concentration typically impacts luminescence intensity according to the concentration quenching mechanism. However, in certain phosphors, this adjustment also affects the lattice site occupancy, leading to an emission wavelength shift. 119 For example, in the Ba1−xEuxSi2O2N2 system, increasing Eu2+ concentration red-shifts the emission peak from 490 nm to 500 nm. 120 This phenomenon stems from the shorter bond lengths formed between Eu2+ and its surrounding O/N ligands when it replaces larger Ba2+ ions. The resulting stronger crystal field weakens the energy of the 5d–4f transitions, leading to the observed red shift. Similar effects occur in La3−xCexSi6N11 and Sr1−xEuxSi2O2N2 phosphor systems. 121,122 When the host matrix possesses multiple available cation sites, manipulating the activator ion distribution across these sites, known as crystal-site engineering, offers another avenue for photoluminescence tuning. For example, Liang et al. demonstrated this approach in CsKNa2(Li3SiO4)4:Eu2+ phosphors. Introducing Li+ ions controlled the migration of Eu2+ ions from K+ sites to Na+ sites, achieving spectral tuning from a cyan narrow band to a green narrow band (Fig. 4c). 76 Similarly, in KSrScSi2O7 phosphors, increasing the Eu2+ doping concentration triggers its migration from the Sr2+ sites to the Sc3+ sites through doping concentration-dependent crystal-site engineering, enabling tuneable photoluminescence from blue to cyan. 123 Further exemplifying this principle, Ca3La3(1−x)Ce3x(BO3)5 phosphors exhibit different emission changes with increasing Ce3+ concentration. 124 Concretely, the emission intensity from the Ce(2)3+ site steadily increases, while that from the Ce(1)3+ site decreases. This behaviour arises from the preferential occupation of Ce(2)3+ sites at higher doping concentrations. These examples show the effectiveness of various strategies, including cation/anion substitution, chemical unit co-substitution, and crystal-site engineering, in optimizing phosphor optical performances and achieving specific blue or green emission characteristics.
3.3 Thermal stability of phosphors for pc-LED
In practical applications of pc-LED devices, in addition to the specific requirements for emission colours and colour purity, the stability of luminescence must also be considered, with the most critical aspects being chemical and thermal stabilities. 106,125,126 In marine applications, the primary chemical stability to consider is moisture resistance, which will be detailed in the following section. 127,128 While designing phosphors with high thermal stability is presented in this section. The thermal stability of PL in phosphors is evaluated by the thermal quenching (TQ) behaviour, which can be influenced by the electronic and/or crystal structures, structural rigidity, and chemical composition of the phosphors. 129 Within several years, many efforts have been devoted to designing anti-TQ phosphors, and the design strategies can be summarized as defect engineering, structural modulation, surface coating, and other strategies (e.g., energy transfer, crystallinity enhancement, negative/zero thermal expansion, and glass technology). 112,130–134Defect engineering in phosphor materials refers to the deliberate introduction, manipulation, or control of defects within the structure to enhance or modify the optical properties. For phosphors with high thermal stability, energy levels associated with defects within the bandgap can inhibit TQ through energy transfer (ET) between the defect levels and luminescence centres. For example, the introduced SrBa trap levels in K2Sr1.25Ba0.75(PO4)2:Eu2+ (KS1.25BP:Eu2+) phosphors enhance the thermal stability compared to the isomorphic K2Ba2(PO4)2:Eu2+ and K2Sr2(PO4)2:Eu2+ phosphors (Fig. 5a). 135Fig. 5b illustrates that these intentionally created point defects, mainly SrBa, serve as electron-trapping centres. They store and transfer energy to the excitation band, leading to an emission increase, which counteracts the usual emission losses due to non-radiation (NR) transition at high temperatures. Some other blue or green emitting phosphors such as Sr2SiO4:Ce3+/K+, BaGa2O4:Bi3+, Ba4(CaLa)(PO4)3Cl:Eu2+, Na2MgAl10O17:Eu2+ also present high thermal stability via defect engineering. 136–139 Moreover, ET can occur not only between the defect levels and luminescence centres, but also in multiple luminescence centres through the interaction between two activators or electric multipole interactions. 140 Sensitizers can efficiently absorb UV/n-UV light and transfer energy to other luminescent centres, significantly enhancing the PL thermal stability of activators. In addition, PL thermal stability of luminescence centres can be enhanced by crystallinity improvement through adding flux during synthesis process of phosphors. Zhang et al. added a flux of K2CO3 into K3YSi2O7:Eu phosphors to improve the crystallinity and hinder the NR quenching process, so that integral intensity at 200 °C and quantum yield of the optimal sample can reach up to 200% enhancement compared to phosphors without flux adding. 141 The appropriate use of flux not only regulates the average size and shape of phosphor particles but also suppresses the impurity formation. This results in a pure phase with enhanced crystallinity and reduced surface defects, significantly impeding the non-radiative (NR) quenching process.
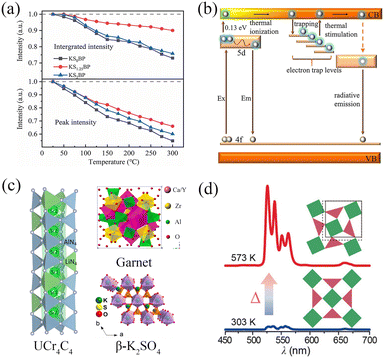 | ||
| Fig. 5 (a) The emission retention of peak intensity and integrated intensity of KSxBP:Eu2+ (x = 0, 1.25, 2) as a function of temperature and (b) a suggested model for describing the mechanism of improved thermal stability. Reproduced with permission from ref. 135. Copyright 2022, Elsevier. (c) UCr4C4 structure, garnet structure, and β-K2SO4 structure model. 142–144 Reproduced with permission from ref. 142–144. Copyright 2023, ACS. Copyright 2023, Elsevier. Copyright 2018, ACS. (d) Thermal enhancement in Er3+-doped orthorhombic Yb2W3O12 by negative thermal expansion. Reproduced with permission from ref. 145. Copyright 2023, WILEY-VCH. | ||
The preceding strategies are mainly based on the relationship between anti-TQ properties and the corresponding electronic structure of phosphor. Another aspect to be considered is the crystal structure. The TQ properties of phosphors can be strongly affected by structural modulations, which influence the local lattice environment around the luminescent ions. Concretely, a rigid structural framework such as β-K2SO4-, garnet-, and UCr4C4-type compound, can significantly reduce emission losses during the thermal processing (Fig. 5c). 142–144 Besides, chemical substitution can lead to enhanced structural rigidity and a concomitant decrease in lattice vibration frequency, thereby increasing the quenching temperature and moisture stability. This method and surface coating method will be introduced in Section 3.4 in more detail. Notably, several negative thermal expansion materials have been used as the matrix to develop anti-TQ phosphors. A 29-fold enhancement of green emission was observed in Yb2W3O12:Er3+ as the temperature rose from 30 to 300 °C, which may result from the increased efficiency in the accumulation of excitation energy due to the contraction and distortion of the host lattice (Fig. 5d). 145
3.4 Moisture stability of phosphors for pc-LED
Considering the applications of pc-LEDs in the humid and salt-fog marine environment, it is necessary to combine structural regulation, surface modification, and other methods to further improve the luminescence stability of narrow-band blue-green phosphors. Fluoride and sulfide phosphors, for instance, exhibit strong hydrophilicity, readily undergoing hydrolysis or oxidation of the luminescent center or the inorganic matrix. 146,147 Besides, silicate- and nitride-based phosphors exhibit low emission efficiency under humid conditions. 63,148 These limitations hinder the application of pc-LED devices reliant on such phosphors in maritime environments. Extensive research has identified various factors contributing to moisture-induced degradation, including structure decomposition, 149 luminescence centre oxidation, 150 and surface layer hydrolysis. 151 Consequently, phosphors for pc-LEDs often fall short of performance requirements in complex, humid atmospheres. To address this challenge, researchers have explored several strategies to enhance moisture resistance, including ionic substitution, surface modification, and the development of glass-ceramics phosphors, phosphor-in-glass (PiG) composites, and single-crystal phosphors.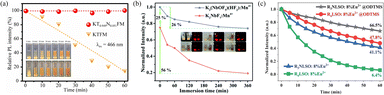 | ||
| Fig. 6 (a) Relative emission intensity (λex = 466 nm) and photographs of K2Ti0.949Nb0.051F6.051:5.0%Mn4+ (the upper illustration) and K2TiF6:5.0%Mn4+ (the inferior illustration) after immersion in deionized water for different times. Reproduced with permission from ref. 153. Copyright 2021, Elsevier. (b) Normalized emission intensity and photographs of K3(NbOF5)(HF2): Mn4+ and K2NbF7:Mn4+ red phosphors at 630 nm after various immersion times. Reproduced with permission from ref. 155. Copyright 2019, the Royal Society of Chemistry. (c) Time-dependent normalized PL intensities of R3NLSO:8%Eu2+@ODTMS, R4LSO:8%Eu2+@ODTMS, R3NLSO:8%Eu2+, and R4LSO:8%Eu2+ immersed in water. Reproduced with permission from ref. 157. Copyright 2021, WILEY-VCH. | ||
3.4.2.1 Protective coating layers. As mentioned previously, alkaline earth silicate and aluminate substrates are popular choices for blue-green phosphors due to their suitable properties for pc-LEDs. However, their instability in water hinders their commercial viability in marine environments. To address this problem, protective homogeneous coating layers using materials like SiO2, Al2O3, and MgO, organo-silicone, resin, polyurethane, oleic acid (OA), and alkyl phosphates can be deposited. 163,164 For instance, Yoo et al. demonstrated plasma coating of SrAl2O4:Eu2+, Dy3+ (SAO) with silane-based material, confirming no detrimental effects on the luminescence performance. This plasma-coated SAO phosphor retained over 50% of its initial luminescence intensity after 48 h in water. Ma et al. found that after being soaked in deionized water, the luminescence intensity of K2SiF6:Mn4+ phosphors using oleylamine (OAm) as an organic coating layer remained 85.2% of its initial value, while that of non-coated KSFM decreased to 15.4%. 165 Xia et al. developed a RLSO:Eu2+ phosphor with superior thermal stability but poor humidity resistance. 83 Heterogeneous protective layers, such as those obtained using atomic layer deposition (ALD), offer advantages like uniformity, densification, and precise control over layer thickness. By ALD strategy, Xia et al. created a dual-core-shell structure in RLSO:Eu2+@Al2O3@ODTMS (ODTMS is octadecyltrimethoxysilane), significantly enhancing water corrosion resistance (Fig. 7a). 166 However, these heterogeneous coatings exhibit long-term detachment issues under high temperatures and humidity. In contrast, the homogeneous core–shell approach offers better stability and reduces non-radiative transitions compared to heterogeneous coatings, and does not easily fall off in a high-humidity environment. Current reports on the moisture resistance of homogeneously coated core–shell phosphors are more common for Mn4+-doped red fluoride phosphors. Chen et al. assembled a KTF:Mn4+@KTF core–shell phosphor via reverse cation exchange. 161 The KTF shell not only protects the [MnF6]2− group inside the phosphor from hydrolysis, but, also, effectively cuts off energy migration to the surface layer, thereby increasing the luminous efficiency, especially for high-concentration phosphors (Fig. 7b).
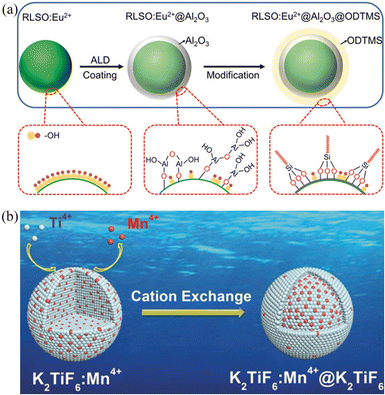 | ||
| Fig. 7 (a) Schematic illustration of surface coating via atomic layer deposition (ALD) for the RLSO: Eu2+ phosphor and subsequent hydrophobic surface modification with ODTMS on RLSO:Eu2+@Al2O3 surface. Reproduced with permission from ref. 166. Copyright 2020, WILEY-VCH. (b) Illustration of the construction of KTF: Mn4+@KTF core–shell phosphor through a reverse cation-exchange strategy. Reproduced with permission from ref. 161. Copyright 2019, WILEY-VCH. | ||
3.4.2.2 Passivation treatment. Weak reducing agents like phosphoric acid, 167 H2O2,168 oxalic acid, 169 and mandelic acid 170 can be used to form a surface passivation shell, reduce surface defects and improve chemical stability. This method offers advantages such as post-processing on existing phosphors or potential reduction of defects affecting ions like Mn4+ and Eu2+. Li et al. demonstrated successful passivation of K2SiF6:Mn4+ with sodium sulfite, the phosphor maintaining 90.8% of its initial intensity after 6 h in water. 171 Ulteriorly, Chang et al. found that K2SiF6:Mn4+ phosphors maintained 95.2% of their emission intensity after immersing in deionized water for 6 h. 172 Reducing agent passivation is an environmentally friendly, cost-effective, and simple approach for enhancing moisture resistance while preserving the high luminous efficiency of pc-LED phosphors.
In summary, surface modification presents a versatile and effective approach for improving the moisture stability of phosphors, paving the way for their successful application in marine environments. Exploring and optimizing coating materials and passivation strategies is needed for further advancements in this field.
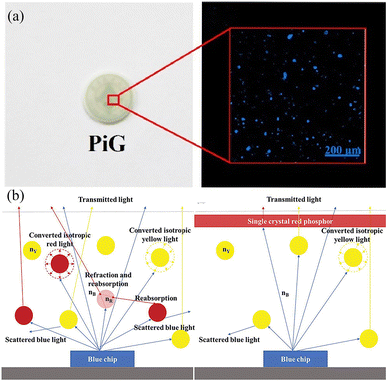 | ||
| Fig. 8 (a) Digital picture and confocal laser scanning microscope image of PiG. Reproduced with permission from ref. 179. Copyright 2020, Elsevier. (b) Luminescence behaviors of powdery (left) and crystal phosphors (right) in WLEDs. Reproduced with permission from ref. 183. Copyright 2020, WILEY-VCH. | ||
However, PiG and transparent ceramics face limitations in luminescence efficiency due to light scattering and reflection losses. 182 Single crystals, in contrast, offer advantages like superior moisture stability, reduced backscattering and minimized self-absorption due to fewer grain boundaries (Fig. 8b). This, along with minimized crystal defects, makes their luminescence less susceptible to ambient moisture. 183 Zhang et al. reported a micrometre-size K2SiF6:Mn4+ (KSFM) single crystal with excellent moisture resistance obtained via saturated crystallization. 184 Wu et al. reported a Cs2TiF6:Mn4+ (CTFM) single crystal with 98.7% internal quantum efficiency and strong moisture stability compared to the corresponding powder. 185 Notably, adding a homogeneous Cs2TiF6 shell using epitaxial growth further enhanced stability. While currently pc-LEDs are focused on red fluoride phosphors, similar materials can be developed for preparing blue-green phosphors, such as those with perovskite structures.
4 Conclusions and outlook
As the field of solid-state pc-LED devices for marine applications continues to grow, innovative strategies to optimize and enhance the luminescence properties of phosphors offer exciting potential for future advancements. This review delves into the current applications of pc-LEDs in marine environments and explores strategies for developing suitable phosphors tailored to the unique demands of these environments. In underwater optical communications systems, pc-LED devices must meet stringent requirements of short response time, high bandwidth, and efficient information transfer. For marine fisheries, pc-LED devices that can precisely control light intensity, light colour, and photoperiod are crucial for optimizing the growth and well-being of specific species. To address these demands, previous research on narrow-band-emitting phosphors, photoluminescence tuning, and environment-resistant phosphors provide valuable foundations for designing acceptable phosphors.But despite the encouraging progress in phosphor discovery, several challenges and opportunities remain unexplored. These include the following aspects.
4.1 Distance and scope of optical beam transmission in UWOC
Water type, absorption, scattering, and other propagation losses significantly impact the range of the optical beam underwater. Blue-green wavelengths from pc-LED correspond to a low attenuation window and facilitate high-bandwidth communication over moderate distances.4.2 Species-specific lighting needs in fisheries
Diverse aquatic species exhibit varying lighting needs and photosensitivity. The optimum light colour for most aquatic organisms lies within the blue-green spectral range. Strengthening research on improving the characteristics of underwater pc-LED light devices and on the photobiological effects of aquatic organisms will aid in developing lighting devices with emission wavelengths tailored to specific species.4.3 Balancing narrow-band emission and environmental stability
While exploring phosphors with highly rigid and ordered structures for narrow-band blue or green emission is essential, achieving high environmental stability remains a challenge. Further research is warranted to develop phosphors that can simultaneously achieve both narrow-band emission and moisture resistance.In conclusion, this review provides a comprehensive overview of the application directions and research progress in phosphor materials suitable for marine environments. By addressing the aforementioned challenges and opportunities, the development of marine pc-LED devices can be further advanced, paving the way for transformative applications in underwater communication, fisheries, and aquaculture.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Author contributions
All the authors contributed to the literature search, writing, and editing of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the support of Zhuhai Key Laboratory of Optoelectronic Functional Materials and Membrane Technology, grants from the National Natural Science Foundation of China (NSFC, no. 52272174), its joint project with Guangdong Province (no. U22A20135, U1801253, U1702254 and U1301242), the bilateral project of China–Serbia for “highly efficient, thermally stable and moisture-resistant red-emitting Mn4+-activated single-crystal phosphors for high-performance warm WLEDs”, and the Oceano company for new phosphors and their applications. We are also grateful to Prof. Zhengye Xiong of Guangdong Ocean University for his support in the literature search.References
- L. Wang, Z. Qi, P. Liu, F. Hu, J. Li and Y. Wang, J. Lightwave Technol., 2023, 41, 5951–5957 CAS.
- A. G. Nasser and M. A. A. Ali, J. Opt. Commun., 2024, 44, s1355–s1363 CrossRef.
- C. Wang, Q. Chen, Z. Xiong, Z. Chen and R. Ye, PLoS One, 2024, 19, e0301434 CrossRef CAS PubMed.
- M.-H. Wang, Y.-J. Hsieh, H.-H. Chang and Y.-S. Wang, Aquaculture, 2024, 578, 740112 CrossRef.
- M. Yu, C. Liu, L. Zhang and Y. Tang, Front. Mar. Sci., 2022, 9, 2439 Search PubMed.
- M.-H. Fang, Z. Bao, W.-T. Huang and R.-S. Liu, Chem. Rev., 2022, 122, 11474–11513 CrossRef CAS.
- N. D. Nhat, T. Van Dan, N. D. Q. Tram, N. Q. Lich, H. D. Phuc and N. N. Phuoc, Aquacult. Fish., 2023, 8, 551–557 Search PubMed.
- S. K. Gupta, J. P. Zuniga, M. Abdou, M. P. Thomas, M. D. A. Goonatilleke, B. S. Guiton and Y. Mao, Chem. Eng. J., 2020, 379, 122314 CrossRef CAS.
- B. Shao, J. Huo and H. You, Adv. Opt. Mater., 2019, 7, 1900319 CrossRef.
- Z. Liu, Z. Li, T. Seto and Y. Wang, Adv. Opt. Mater., 2023, 11, 2300845 CrossRef CAS.
- C. Chen, M. Jin, J. Xiang, J. Zheng, P. Guo and C. Guo, Ceram. Int., 2023, 49, 25232–25239 CrossRef CAS.
- J. M. Ha, S. H. Hur, A. Pathak, J.-E. Jeong and H. Y. Woo, NPG Asia Mater., 2021, 13, 53 CrossRef CAS.
- B. A. Vijayalakshmi, S. Lekashri, M. Gomathi, R. Ashwini, B. Arunsundar and M. Nesasudha, J. Opt., 2024, 2, 1–10 Search PubMed.
- A. Elfikky, A. I. Boghdady, A. G. AbdElkader, E. E. Elsayed, K. W. Palitharathna, Z. Ali, M. Singh, S. A. H. Mohsan, M. Mahmoud and M. H. Aly, Opt. Quantum Electron., 2024, 56, 55 CrossRef.
- L. Perera, P. Ranaweera, S. Kusaladharma, S. Wang and M. Liyanage, IEEE Open J. Commun. Soc., 2024, 5, 1753–1802 Search PubMed.
- G. Schirripa Spagnolo, L. Cozzella and F. Leccese, Sensors, 2020, 20, 2261 CrossRef.
- J. U. Rahman, S. Khan, V. Jain, A. Rajiv, S. Dasi, K. F. Fawy, P. K. Jindal and R. Sivaranjani, Rev. Inorg. Chem., 2024, 5, 44 Search PubMed.
- T. Lu, X. Lin, W. Guo, C.-C. Tu, S. Liu, C.-J. Lin, Z. Chen, H.-C. Kuo and T. Wu, Opto-Electron. Sci., 2022, 1, 220020–220024 CAS.
- R. Wan, L. Wang, J. Huang, X. Yi, H.-C. Kuo and J. Li, Adv. Photonics Res., 2021, 2, 2100093 CrossRef CAS.
- M. Singh, S. A. Abd El-Mottaleb, A. Atieh and M. H. Aly, Opt. Eng., 2024, 63, 078105 Search PubMed.
- K. Bera, R. Parthiban and N. Karmakar, IEEE Access, 2023, 11, 122833–122841 Search PubMed.
- H. Le Minh, D. O'Brien, G. Faulkner, L. Zeng, K. Lee, D. Jung, Y. Oh and E. T. Won, IEEE Photonics Technol. Lett., 2009, 21, 1063–1065 Search PubMed.
- D. Li, S. Liu, Z. Qian, Q. Liu, K. Zhou, D. Liu, S. Sheng, B. Sheng, F. Liu and Z. Chen, Adv. Mater., 2022, 34, 2109765 CrossRef CAS PubMed.
- W. C. Miao, F. H. Hsiao, Y. Sheng, T. Y. Lee, Y. H. Hong, C. W. Tsai, H. L. Chen, Z. Liu, C. L. Lin and R. J. Chung, Adv. Opt. Mater., 2024, 12, 2300112 CrossRef CAS.
- R. Lin, X. Liu, G. Zhou, Z. Qian, X. Cui and P. Tian, Adv. Opt. Mater., 2021, 9, 2002211 CrossRef CAS.
- M. Zhang and H. Zhou, Sensors, 2023, 23, 7649 CrossRef CAS PubMed.
- D. R. Mishra, S. Narumalani, D. Rundquist and M. Lawson, ISPRS J. Photogramm. Remote Sens., 2005, 60, 48–64 CrossRef.
- Z. Lee, C. Hu, S. Shang, K. Du, M. Lewis, R. Arnone and R. Brewin, J. Geophys. Res.:Oceans, 2013, 118, 4241–4255 CrossRef.
- J. Falcón, A. Torriglia, D. Attia, F. Viénot, C. Gronfier, F. Behar-Cohen, C. Martinsons and D. Hicks, Front. Neurosci., 2020, 14, 1183 Search PubMed.
- A. R. Darlis, W. A. Cahyadi and Y.-H. Chung, Wirel. Pers. Commun., 2018, 99, 681–694 CrossRef.
- K. Sun, B. Han, J. Yang, B. Li, B. Zhang, K. Liu and C. Li, Photonics, 2023, 10, 596 CrossRef.
- T. Shen, J. Guo, H. Liang, Y. Li, K. Li, Y. Dai and Y. Ai, Photonics, 2023, 10, 1238 CrossRef.
- J. Fu, K. Zhu, S. A. H. Mohsan and Y. Li, J. Mar. Sci. Eng., 2023, 11, 547 CrossRef.
- G. Kreimer, K.-i. Wakabayashi, P. Hegemann and C. Dieckmann, The Chlamydomonas Sourcebook, 2023, vol. 3, pp. 391–419 Search PubMed.
- H.-J. Kim, S. Umino and G. Satuito, Hydrobiologia, 2024, 2, 1–10 Search PubMed.
- K. Q. Nguyen and P. D. Winger, Reviews in Fisheries Science & Aquaculture, 2019, 27, 106–126 Search PubMed.
- R. Torres, A. M. Campos, J. Goldman, I. Barrote, L. Mata and J. Silva, J. Appl. Phycol., 2024, 36, 627–637 CrossRef.
- C. Huang, X. Nie, J. Wei, Y. Wang, K. Hong, X. Mu, C. Liu, Z. Chu, X. Zhu and L. Yu, Aquacult. Res., 2024, 2024, 8897473 CrossRef.
- N. Yeh, P. Yeh, N. Shih, O. Byadgi and T. C. Cheng, Renewable Sustainable Energy Rev., 2014, 32, 611–618 CrossRef.
- G. B. Nair, H. Swart and S. Dhoble, Prog. Mater. Sci., 2020, 109, 100622 CrossRef.
- H. Matsui, G. Takayama and Y. Sakurai, Fish. Sci., 2016, 82, 303–309 CrossRef.
- H. Jeong, S. Yoo, J. Lee and Y.-I. An, Renewable Energy, 2013, 54, 101–104 CrossRef.
- Y. Ina, Y. Sakakura, Y. Tanaka, T. Yamada, K. Kumon, T. Eba, H. Hashimoto, J. Konishi, T. Takashi and K. Gen, Fish. Sci., 2017, 83, 537–542 CrossRef CAS.
- D. A. Guggiana-Nilo and F. Engert, Front. Behav. Neurosci., 2016, 10, 160 Search PubMed.
- J. D. Karlsen, V. Melli and L. A. Krag, ICES J. Mar. Sci., 2021, 78, 2818–2829 CrossRef.
- Y. Qi, C. Liu, G. Yuan, H. Guo, J. Näslund, Y. Wang, J. Ru, Y. Ou, X. Chai and X. Zhang, Animals, 2024, 14, 1701 CrossRef PubMed.
- S. Liu, D. Wen, R. Du, C. Jiang, J. Chen, J. Li, L. Zhou, M. S. Molokeev and M. Wu, Adv. Opt. Mater., 2023, 11, 2203151 CrossRef CAS.
- X. Zhang, D. Sun, X. Zhang and H. Yang, Aquaculture, 2021, 534, 736339 CrossRef.
- H. S. Shin, J. Lee and C. Y. Choi, Fish. Sci., 2012, 78, 549–556 CrossRef CAS.
- T. Yamanome, K. Mizusawa, E. I. Hasegawa and A. Takahashi, J. Exp. Zool., Part A, 2010, 311A, 73–79 CrossRef.
- S. M. Noureldin, A. M. Diab, A. S. Salah and R. A. Mohamed, Aquaculture, 2021, 538, 736532 CrossRef CAS.
- A. B. Head and J. A. Malison, J. World Maric. Soc., 2010, 31, 73–80 Search PubMed.
- Y.-T. Lin, W.-C. Hung, Y.-F. Yeh, K.-M. Lu, D.-H. Cherng and Y.-S. Han, Zool. Stud., 2023, 62, e28 Search PubMed.
- S. S. Giri, S. K. Sahoo, B. B. Sahu, A. K. Sahu and S. Ayyappan, Aquaculture, 2002, 213, 151–161 CrossRef.
- W. G. Reis, W. Wasielesky Jr, P. C. Abreu, H. Brandão and D. Krummenauer, Aquaculture, 2023, 563, 738924 CrossRef.
- T. Wijgerde, P. Henkemans and R. Osinga, Aquaculture, 2012, 344, 188–193 CrossRef.
- J. Dou, G. Zhang, C. Shi, C. Song, C. Mu, Y. Ye and C. Wang, Aquacult. Eng., 2021, 93, 102158 CrossRef.
- L. Ye, Y. Zhang, J. Zhao, X. Zhao, J. Li and Z. Ye, Trans. ASABE, 2021, 64, 997–1005 CAS.
- H. Ma, P. Wei, X. Li, S. Liu, Y. Tian, Q. Zhang and Y. Liu, Aquaculture, 2021, 542, 736840 CrossRef CAS.
- Y. J. Choi, S. G. N. R. Park, A.-H. Jo and J.-H. Kim, Fishes, 2023, 8, 77 CrossRef.
- P. F. Smet, K. Van den Eeckhout, A. J. J. Bos, E. van der Kolk and P. Dorenbos, J. Lumin., 2012, 132, 682–689 CrossRef CAS.
- C.-W. Yeh, W.-T. Chen, R.-S. Liu, S.-F. Hu, H.-S. Sheu, J.-M. Chen and H. T. Hintzen, J. Am. Chem. Soc., 2012, 134, 14108–14117 CrossRef CAS.
- P. Pust, V. Weiler, C. Hecht, A. Tuecks, A. S. Wochnik, A.-K. Henss, D. Wiechert, C. Scheu, P. J. Schmidt and W. Schnick, Nat. Mater., 2014, 13, 891–896 CrossRef CAS PubMed.
- A. Vanetsev, P. Põdder, M. Oja, N. M. Khaidukov, V. N. Makhov, V. Nagirnyi, I. Romet, S. Vielhauer, H. Mändar and M. Kirm, J. Lumin., 2021, 239, 118389 CrossRef CAS.
- K. Zhu, Z. Chen, H. Liu, X. Yi, Y. Wang, J. Chen, X. Yuan and G. Liu, J. Alloys Compd., 2023, 946, 169353 CrossRef CAS.
- X. Liu, Y. Zhu, Z. Cheng, Y. Wang, F. Tian, Z. Liu, W. Li, G. Zhou, J. Zou and D. Y. Kosyanov, J. Am. Ceram. Soc., 2024, 107, 1061–1069 CrossRef CAS.
- G. J. Hoerder, S. Peschke, K. Wurst, M. Seibald, D. Baumann, I. Stoll and H. Huppertz, Inorg. Chem., 2019, 58, 12146–12151 CrossRef CAS PubMed.
- M.-H. Fang, C. O. M. Mariano, K.-C. Chen, J.-C. Lin, Z. Bao, S. Mahlik, T. Lesniewski, K.-M. Lu, Y.-R. Lu and Y.-J. Wu, Chem. Mater., 2021, 33, 1893–1899 CrossRef CAS.
- Y. Gao, J. Iihama, D. Hamana, R. Iwasaki, S. Honda, T. Asaka, M. Kumari, T. Hayakawa, S. Bernard and P. Thomas, Int. J. Appl. Ceram. Technol., 2023, 20, 768–779 CrossRef CAS.
- A. Markovskyi, V. Gorbenko, T. Yokosawa, J. Will, E. Spiecker, M. Batentschuk, J. Elia, A. Fedorov, M. Pakuła and M. Kaczmarek, J. Alloys Compd., 2022, 929, 167159 CrossRef CAS.
- Y. Wang, P. P. Zhang, L. Luo, X. T. Chen, R. Guo and J. Y. Xu, J. Lumin., 2021, 239, 118317 CrossRef CAS.
- K. B. Kim, Y. I. Kim, H. G. Chun, T. Y. Cho, J. S. Jung and J. G. Kang, Chem. Mater., 2002, 14, 5045–5052 CrossRef CAS.
- W. Xia, F. Du, Q. Zhao, L. Du, Z. Leng and Z. Tang, J. Lumin., 2025, 277, 120896 CrossRef CAS.
- T. Yang, Z. Ma, S. Huang, T. Zhang, K. Zhao, L. Yin, Q. Gu and P. Cao, J. Alloys Compd., 2022, 892, 162066 CrossRef CAS.
- A. C. Duke, S. Hariyani and J. Brgoch, Chem. Mater., 2018, 30, 2668–2675 CrossRef.
- W. Wang, M. Tao, Y. Liu, Y. Wei, G. Xing, P. Dang, J. Lin and G. Li, Chem. Mater., 2019, 31, 9200–9210 CrossRef.
- Y. Zhu, Y. Liang, S. Liu, H. Li and J. Chen, Adv. Opt. Mater., 2019, 7, 1801419 CrossRef.
- Z. Long, M. Liu, X.-g. Wu, K. Gu, G. Yang, Z. Chen, Y. Liu, R. Liu and H. Zhong, Nat. Synth., 2023, 2, 1–9 CrossRef.
- T. Jansen, J. Gorobez, M. Kirm, M. Brik, S. Vielhauer, M. Oja, N. Khaidukov, V. Makhov and T. Jüstel, ECS J. Solid State Sci. Technol., 2017, 7, R3086 CrossRef.
- X. Zhang, M.-H. Fang, Y.-T. Tsai, A. Lazarowska, S. Mahlik, T. Lesniewski, M. Grinberg, W. K. Pang, F. Pan and C. Liang, Chem. Mater., 2017, 29, 6781–6792 CrossRef.
- L. Yang, S. Gai, H. Ding, D. Yang, L. Feng and P. Yang, Adv. Opt. Mater., 2023, 11, 2202382 CrossRef.
- S. Li, L. Wang, D. Tang, Y. Cho, X. Liu, X. Zhou, L. Lu, L. Zhang, T. Takeda and N. Hirosaki, Chem. Mater., 2018, 30, 494–505 CrossRef.
- M. Zhao, H. Liao, L. Ning, Q. Zhang, Q. Liu and Z. Xia, Adv. Mater., 2018, 30, 1802489 CrossRef.
- M. Liao, Z. Mu, Q. Wang, X. Zhang, H. Dong, M. Wen and F. Wu, J. Alloys Compd., 2020, 837, 155084 CrossRef.
- H. Chen and Y. Wang, Inorg. Chem., 2019, 58, 7440–7452 CrossRef PubMed.
- Y. Xiao, Z. Hao, L. Zhang, X. Zhang, G.-H. Pan, H. Wu, H. Wu, Y. Luo and J. Zhang, J. Mater. Chem. C, 2018, 6, 5984–5991 RSC.
- Y. Jin, Q. Wang, H. Zhou, L. Zhang and J. Zhang, Ceram. Int., 2016, 42, 3309–3316 CrossRef CAS.
- L. Zhou, J. Hong, X. Li, J. Shi, P. A. Tanner, K. L. Wong and M. Wu, Adv. Opt. Mater., 2020, 8, 2000523 CrossRef CAS.
- W. Xiao, X. Liu, J. Zhang and J. Qiu, Adv. Opt. Mater., 2019, 7, 1801677 CrossRef.
- S. Huang, L. Yu, K. Peng, Y. Zhao, J. Wang and M. Shang, J. Am. Ceram. Soc., 2021, 104, 5848–5858 CrossRef CAS.
- C. Dou, Z. Song and Q. Liu, J. Mater. Chem. C, 2024, 12, 11209–11241 RSC.
- B. Su, G. Zhou, J. Huang, E. Song, A. Nag and Z. Xia, Laser Photonics Rev., 2021, 15, 2000334 CrossRef CAS.
- R. Zhang, H. Xie, W. Liu, K. Zhan, H. Liu, Z. Tang and C. Yang, ACS Appl. Mater. Interfaces, 2023, 15, 47238–47249 CrossRef CAS PubMed.
- L. Liu, L. Wang, C. Zhang, Y. Cho, B. Dierre, N. Hirosaki, T. Sekiguchi and R.-J. Xie, Inorg. Chem., 2015, 54, 5556–5565 CrossRef CAS PubMed.
- P. Dang, H. Lian and J. Lin, Adv. Opt. Mater., 2022, 11, 2202511 Search PubMed.
- R.-J. Xie, N. Hirosaki, X.-J. Liu, T. Takeda and H.-L. Li, Appl. Phys. Lett., 2008, 92, 201905 CrossRef.
- A. D. Sontakke, A. J. Van Bunningen, F. T. Rabouw, S. Meijers and A. Meijerink, J. Phys. Chem. C, 2020, 124, 13902–13911 CrossRef CAS.
- C. Zhan, H. Zhu, S. Liang, Y. Huang, Z. Wang and M. Hong, Inorg. Chem. Front., 2024, 11, 826–836 RSC.
- G. Lu, Y. Wang, K. Ma, X. Chen, W. Geng, T. Liu, S. Xu, J. Zhang and B. Chen, Ceram. Int., 2024, 50, 16190–16200 CrossRef CAS.
- Y. Wei, L. Cao, L. Lv, G. Li, J. Hao, J. Gao, C. Su, C. C. Lin, H. S. Jang and P. Dang, Chem. Mater., 2018, 30, 2389–2399 CrossRef CAS.
- P. Strobel, C. Maak, V. Weiler, P. J. Schmidt and W. Schnick, Angew. Chem., Int. Ed., 2018, 57, 8739–8743 CrossRef CAS PubMed.
- H. Liao, M. Zhao, M. S. Molokeev, Q. Liu and Z. Xia, Angew. Chem., Int. Ed., 2018, 57, 11728–11731 CrossRef CAS PubMed.
- S. Wendl, L. Eisenburger, P. Strobel, D. Günther, J. P. Wright, P. J. Schmidt, O. Oeckler and W. Schnick, Chem.–Eur. J., 2020, 26, 7292–7298 CrossRef CAS.
- P. Liang, W.-L. Lian and Z.-H. Liu, Chem. Commun., 2021, 57, 3371–3374 RSC.
- Z. Leng, D. Zhang, H. Bai, H. He, Q. Qing, J. Zhao and Z. Tang, J. Mater. Chem. C, 2021, 9, 13722–13732 RSC.
- J. Ding and Q. Wu, Dalton Trans., 2024, 53, 15403–15411 RSC.
- H. Yang, P. Li, X. Fu, Z. Ye, X. Huo, Y. Wang, Q. Wu, H. Suo, L. Li and Z. Wang, Mater. Today Chem., 2022, 26, 101050 CrossRef.
- P. Dang, W. Yang, Q. Zhang, D. Liu, H. Lian, G. Li and J. Lin, Opt. Mater.:X, 2022, 13, 100136 Search PubMed.
- S. Piao, Y. Wang, G. Zhu, J. Zhang, X. Zhang, D. Wu, Y. Cao, X. Li and B. Chen, J. Mater. Chem. C, 2021, 9, 14777–14787 RSC.
- H. Dong, L.-D. Sun and C.-H. Yan, J. Am. Chem. Soc., 2021, 143, 20546–20561 CrossRef PubMed.
- Z. Pan, V. Castaing, L. Yan, L. Zhang, C. Zhang, K. Shao, Y. Zheng, C. Duan, J. Liu and C. Richard, J. Phys. Chem. C, 2020, 124, 8347–8358 CrossRef.
- Q. Liu, P. Dang, G. Zhang, H. Lian, Z. Cheng, G. Li and J. Lin, Chem. Mater., 2024, 36, 1763–1772 CrossRef.
- H. Zhu, Z. Wang, X. Zhang, D. Wang, J. Zhao, Y. Sun, Z. Yang and P. Li, Ceram. Int., 2020, 46, 25556–25567 CrossRef.
- J. Ding, Z. An, Z. Wang, Y. Dai, W. Yu, J. Deng, Y. Lai, X. Huang and Q. Wu, Chem. Eng. J., 2023, 477, 147122 CrossRef.
- K. A. Denault, N. C. George, S. R. Paden, S. Brinkley, A. A. Mikhailovsky, J. Neuefeind, S. P. DenBaars and R. Seshadri, J. Mater. Chem., 2012, 22, 18204–18213 RSC.
- Z. Xia, C. Ma, M. S. Molokeev, Q. Liu, K. Rickert and K. R. Poeppelmeier, J. Am. Chem. Soc., 2015, 137, 12494–12497 CrossRef PubMed.
- Y. Xiao, W. Xiao, L. Zhang, Z. Hao, G.-H. Pan, Y. Yang, X. Zhang and J. Zhang, J. Mater. Chem. C, 2018, 6, 12159–12163 RSC.
- T. Yang, T. Zhang, T. D. Christopher, S. Huang and P. Cao, Ceram. Int., 2024, 50, 13862–13870 CrossRef CAS.
- P. Dai, Q. Wang, M. Xiang, T.-M. Chen, X. Zhang, Y.-W. Chiang, T.-S. Chan and X. Wang, Chem. Eng. J., 2020, 380, 122508 Search PubMed.
- Y. Q. Li, A. Delsing, G. De With and H. Hintzen, Chem. Mater., 2005, 17, 3242–3248 Search PubMed.
- N. Kijima, T. Seto and N. Hirosaki, ECS Trans., 2009, 25, 247 CrossRef.
- V. Bachmann, C. Ronda, O. Oeckler, W. Schnick and A. Meijerink, Chem. Mater., 2009, 21, 316–325 Search PubMed.
- S. Lai, M. Zhao, Y. Zhao, M. S. Molokeev and Z. Xia, ACS Mater. Au, 2022, 2, 374–380 CrossRef CAS.
- C. Liu, H. Liang, X. Kuang, J. Zhong, S. Sun and Y. Tao, Inorg. Chem., 2012, 51, 8802–8809 Search PubMed.
- Y. Wei, Z. Cheng and J. Lin, Chem. Soc. Rev., 2019, 48, 310–350 Search PubMed.
- C. Zhang, T. Uchikoshi, T. Takeda and N. Hirosaki, Phys. Chem. Chem. Phys., 2023, 25, 24214–24233 RSC.
- Y. Zhang, J. Ling, Y. Li, W. Xu, Y. Zhou and M. Hong, J. Lumin., 2022, 247, 118886 CrossRef CAS.
- M.-H. Liu, T.-T. Li and D. L. Zhang, Mater. Res. Bull., 2022, 154, 111944 CrossRef CAS.
- P. Dang, W. Wang, H. Lian, G. Li and J. Lin, Adv. Opt. Mater., 2022, 10, 2102287 Search PubMed.
- P. Dang, G. Li, X. Yun, Q. Zhang, D. Liu, H. Lian, M. Shang and J. Lin, Light:Sci. Appl., 2021, 10, 29 Search PubMed.
- W. Zhou, J. Fan, J. Luo, L. Lin, J. Zhou, J. Zhang, Z. Zhu and X. Zhang, Inorg. Chem., 2022, 61, 8767–8781 Search PubMed.
- T. Zheng, L. Luo, P. Du, S. Lis, U. R. Rodriguez-Mendoza, V. Lavin and M. Runowski, Chem. Eng. J., 2022, 446, 136839 CrossRef CAS.
- L. Cao, W. Li, B. Devakumar, N. Ma, X. Huang and A. F. Lee, ACS Appl. Mater. Interfaces, 2022, 14, 5643–5652 Search PubMed.
- T. Zheng, L. Luo, P. Du, S. Lis, U. R. Rodriguez-Mendoza, V. Lavin, I. R. Martin and M. Runowski, Chem. Eng. J., 2022, 443, 136414 Search PubMed.
- T. Yang, T. Zhang, S. Huang, T. D. Christopher, Q. Gu, Y. Sui and P. Cao, Chem. Eng. J., 2022, 435, 133873 CrossRef CAS.
- K. Zhao, Z. Ma, L. Yin, B. Hui, H. Si, X. Tong, H. Tang, P. Cao and S. Huang, Inorg. Chem. Front., 2023, 10, 2314–2324 Search PubMed.
- Y. Xu, S. Yin, X. Wu, C. Zhong, X. Zhang, L. Zhou and H. You, Ceram. Int., 2024, 50, 1159–1165 Search PubMed.
- Q. Zong, D. Zhao, R. J. Zhang, L. Jia and S.-Y. Zhu, Ceram. Int., 2023, 49, 24794–24801 CrossRef CAS.
- H. Li, J. Cai, R. Pang, G. Liu, S. Zhang, L. Jiang, D. Li, C. Li, J. Feng and H. Zhang, J. Mater. Chem. C, 2019, 7, 13088–13096 RSC.
- X. Lv, R. Xiao, J. Liu, C. Yang, Y. Xin and N. Guo, Inorg. Chem. Front., 2024, 11, 1668–1682 Search PubMed.
- W. Zhou, Z. Sun, J. Luo, X. Zhang, Q. Pang and L. Zhou, J. Alloys Compd., 2021, 854, 157188 CrossRef CAS.
- J. Bouquiaux, S. Poncé, Y. Jia, A. Miglio, M. Mikami and X. Gonze, Chem. Mater., 2023, 35, 5353–5361 Search PubMed.
- M. Qu, Z. Pang, T. Li, Q. Liu, Y. Yang and X. Zhang, Ceram. Int., 2023, 49, 792–800 Search PubMed.
- J. Qiao, L. Ning, M. S. Molokeev, Y.-C. Chuang, Q. Liu and Z. Xia, J. Am. Chem. Soc., 2018, 140, 9730–9736 Search PubMed.
- H. Zou, X. Yang, B. Chen, Y. Du, B. Ren, X. Sun, X. Qiao, Q. Zhang and F. Wang, Angew. Chem., Int. Ed., 2019, 58, 17255–17259 CrossRef CAS PubMed.
- J. Tang, X. Zhang, S. Liao, Y. Zhu, Y. Han, H. Su, Z. Qiu, S. Lian and J. Zhang, Adv. Opt. Mater., 2024, 16, 2401811 CrossRef.
- X. Wang, Z. Qiu, Y. Li, Q. Mi, W. Zhou, S. Ai, J. Xu, Y. Liu and S. Lian, J. Mater. Chem. C, 2019, 7, 5931–5936 RSC.
- H. Yunsheng, H. Jianhua, W. Zhuang, X. Huang and H. Huaqiang, J. Rare Earths, 2011, 29, 911–914 CrossRef.
- X. Zhong, D. Deng, T. Wang, Y. Li, Y. Yu, J. Qiang, S. Liao, Y. Huang and J. Long, J. Lumin., 2022, 243, 118643 CrossRef CAS.
- Y. H. Kim, P. Arunkumar, B. Y. Kim, S. Unithrattil, E. Kim, S.-H. Moon, J. Y. Hyun, K. H. Kim, D. Lee and J.-S. Lee, Nat. Mater., 2017, 16, 543–550 CrossRef CAS.
- B. Guo, M. Wen, H. Tang, S. Lishik, X. Fan, G. Zhang and J. Fan, Laser Photonics Rev., 2024, 18, 2300838 CrossRef CAS.
- T. Lang, T. Han, S. Fang, J. Wang, S. Cao, L. Peng, B. Liu, V. I. Korepanov and A. N. Yakovlev, Chem. Eng. J., 2020, 380, 122429 CrossRef CAS.
- J. Zhou, F. Liu, J. Li, B. Milićević, J. Yan, Z. Wu and M. Wu, Opt. Mater., 2021, 117, 111184 CrossRef CAS.
- X. Zhong, D. Deng, T. Wang, Y. Li, Y. Yu, J. Qiang, S. Liao, Y. Huang and J. Long, Inorg. Chem., 2022, 61, 5484–5494 CrossRef CAS PubMed.
- G. Pang, F. Hong, X. Liu, Y. Zhang, C. Zhang, T. Dong, Z. Fu, G. Liu, J. Wang, D. Li and X. Dong, Dalton Trans., 2021, 50, 17290–17300 RSC.
- W. B. Im, N. George, J. Kurzman, S. Brinkley, A. Mikhailovsky, J. Hu, B. F. Chmelka, S. P. DenBaars and R. Seshadri, Adv. Mater., 2011, 23, 2300–2305 CrossRef.
- M. Liao, Q. Wang, Q. Lin, M. Xiong, X. Zhang, H. Dong, Z. Lin, M. Wen, D. Zhu and Z. Mu, Adv. Opt. Mater., 2021, 9, 2100465 CrossRef.
- S. Li, Z. Shi, F. Zhang, L. Wang, Z. Ma, D. Wu, D. Yang, X. Chen, Y. Tian and Y. Zhang, ACS Appl. Mater. Interfaces, 2020, 12, 46330–46339 CrossRef.
- Y. Jia, Y. Pan, Y. Li, L. Zhang, H. Lian and J. Lin, Inorg. Chem., 2021, 60, 231–238 CrossRef.
- P. Balaz, M. Achimovicova, M. Balaz, P. Billik, Z. Cherkezova-Zheleva, J. Manuel Criado, F. Delogu, E. Dutkova, E. Gaffet, F. Jose Gotor, R. Kumar, I. Mitov, T. Rojac, M. Senna, A. Streletskii and K. Wieczorek-Ciurowa, Chem. Soc. Rev., 2013, 42, 7571–7637 RSC.
- D. Huang, H. Zhu, Z. Deng, Q. Zou, H. Lu, X. Yi, W. Guo, C. Lu and X. Chen, Angew. Chem., Int. Ed., 2019, 58, 3843–3847 CrossRef.
- J. Xiong, N. Liu, X. Hu, Y. Qi, W. Liu, J. Dai, Y. Zhang, Z. Dai, X. Zhang and Y. Huang, Adv. Energy Mater., 2022, 12, 2201787 CrossRef.
- Y. Liu, H. Wang, D. Chen, L. Gao, X. Wang, X. Jian, C. Mu, X. Xu, Y. Zhao and L. Yin, Ceram. Int., 2021, 47, 3244–3251 CrossRef.
- R. Li, X. Fang, J. Ren, B. Chen, X. Yuan, X. Pan, P. Zhang, L. Zhang, D. Tu and Z. Fang, Nanoscale, 2021, 13, 12494–12504 RSC.
- C. Chang, W. Ye, C. Zuo, Y. Li, Z. Wen, L. Guo, Y. Cao and C. Ma, ACS Sustainable Chem. Eng., 2023, 11, 15887–15897 CrossRef.
- M. Zhao, K. Cao, M. Liu, J. Zhang, R. Chen, Q. Zhang and Z. Xia, Angew. Chem., Int. Ed., 2020, 59, 12938–12943 CrossRef PubMed.
- Q. Zhou, L. Dolgov, A. M. Srivastava, L. Zhou, Z. Wang, J. Shi, M. D. Dramićanin, M. G. Brik and M. Wu, J. Mater. Chem. C, 2018, 6, 2652–2671 RSC.
- Y. Zhou, E. Song, T. Deng, Y. Wang, Z. Xia and Q. Zhang, Adv. Mater. Interfaces, 2019, 6, 1802006 CrossRef.
- H. Yu, B. Wang, X. Bu, Y.-g. Liu, J. Chen, Z. Huang and M. Fang, Ceram. Int., 2020, 46, 18281–18286 CrossRef CAS.
- D. Li, Y. Pan, X. Wei and J. Lin, Chem. Eng. J., 2021, 420, 127673 CrossRef CAS.
- J. Qiang, L. Wang, T. Wang, Y. Yu, D. Deng, C. Wu, S. Liao and S. Li, Ceram. Int., 2022, 48, 17253–17260 CrossRef CAS.
- Y. Yu, C. Jiang, G. Chang, S. Liao and R. Li, J. Lumin., 2024, 270, 120532 CrossRef CAS.
- R. Zhang, H. Lin, Y. Yu, D. Chen, J. Xu and Y. Wang, Laser Photonics Rev., 2014, 8, 158–164 CAS.
- M. He, J. Jia, J. Zhao, X. Qiao, J. Du and X. Fan, Ceram. Int., 2021, 47, 2963–2980 CrossRef CAS.
- M. Xia, J. Luo, C. Chen, H. Liu and J. Tang, Adv. Opt. Mater., 2019, 7, 1900851 CrossRef CAS.
- B. Yang, F. Zheng, S. Mei, Z. Chen, Y. Xie, H. Dai, X. Wei, W. Zhang, F. Xie and J. Ju, Appl. Surf. Sci., 2020, 512, 145655 CrossRef CAS.
- Y. H. Kim, P. Arunkumar and W. B. Im, Ceram. Int., 2015, 41, 5200–5204 CrossRef CAS.
- Y. Zhang, X. Zhang, H. Zhang, J. Zhuang, C. Hu, Y. Liu, Z.-C. Wu, L. Ma, X. Wang and B. Lei, J. Colloid Interface Sci., 2019, 545, 195–199 CrossRef CAS PubMed.
- M. Li, X. Zhang, Y. Zheng, Y. Xu, H. Zhang, Y. Liu and B. Lei, Ceram. Int., 2020, 46, 21560–21568 CrossRef CAS.
- H. Chen, Z. Gao, P. Lv, L. Sun and C. Xu, J. Non-Cryst. Solids, 2022, 597, 121867 CrossRef CAS.
- H. Chen, T. Lin, F. Huang, S. Li, X. Tang and R. J. Xie, Adv. Opt. Mater., 2022, 10, 2200836 CrossRef CAS.
- M. Zhou, J. Sun, B. Zhang, Y. Hua, F. Huang, H. Ma, R. Ye and S. Xu, J. Eur. Ceram. Soc., 2023, 43, 3563–3571 CrossRef.
- Z. Wang, Z. Yang, N. Wang, Q. Zhou, J. Zhou, L. Ma, X. Wang, Y. Xu, M. G. Brik and M. D. Dramićanin, Adv. Opt. Mater., 2020, 8, 1901512 CrossRef.
- Y. Zhou, C. Yu, E. Song, Y. Wang, H. Ming, Z. Xia and Q. Zhang, Adv. Opt. Mater., 2020, 8, 2000976 CrossRef.
- J. Zhou, Y. Wang, Y. Chen, Y. Zhou, B. Milićević, L. Zhou, J. Yan, J. Shi, R. S. Liu and M. Wu, Angew. Chem., 2021, 133, 3986–3991 CrossRef.
| This journal is © The Royal Society of Chemistry 2025 |
