Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Stable lithium plating/stripping electrochemistry promoted by a MnO2 modified copper current collector for stable lithium metal anodes†
Bidhan
Pandit
 *a and
Chun
Huang
*abc
*a and
Chun
Huang
*abc
aDepartment of Materials, Imperial College London, South Kensington Campus, Exhibition Road, London SW7 2AZ, UK. E-mail: physics.bidhan@gmail.com; b.pandit@imperial.ac.uk; a.huang@imperial.ac.uk
bThe Faraday Institution, Quad One, Becquerel Ave, Harwell Campus, Didcot, OX11 0RA, UK
cResearch Complex at Harwell, Rutherford Appleton Laboratory, Didcot, OX11 0FA, UK
First published on 28th May 2025
Abstract
Due to lower redox potential and excellent theoretical capacity, lithium metal anodes are being explored for high-energy density next generation rechargeable batteries. The lithium metal anode is dragged out of use in practical applications because of its high reactivity and considerable volume expansion, which also cause an unstable solid electrolyte interface, severe side reactions, dendrite growth, electrode degradation, low coulombic efficiency, and even significant safety concerns. To establish a stable solid electrolyte interphase (SEI) and avoid dendrite issues, a variety of methods are being investigated. It is also shown that the initial Li nucleation, which determines the interface and reversibility of future cycles, may be aided by the deposition of a thin film of MnO2 on the current collector. By lowering the electrode's nucleation barrier, the lithiophilic properties of MnO2 may successfully promote smooth and homogeneous plating of Li on the Cu collector surface. Such a MnO2 nanorod structure enables effective electron conduction between the conductive substrate and lithiophilic layer, improves Li-ion transfer kinetics, and significantly minimizes the local current inhomogeneity. The structure effectively prevents dendritic growth and volume change as evidenced by its ability to retain a constant coulombic efficiency over an extended period of time of up to 186 h (2 mA cm−2). This technology has made it possible to demonstrate Li metal anodes with excellent coulombic efficiency, paving the way for steady, efficient, and long-cycle life Li metal batteries with less Li loading.
1. Introduction
The progress of inexpensive and sustainable energy storage technology is crucial given the world's insatiable desire for energy.1–3 Due to their performance in terms of safety during cyclic charge/discharge operations with intercalation reactions, lithium-ion batteries (LIBs) are now extensively employed in numerous applications, including portable devices, electric vehicles (EVs), and smart grids.4–7 However, consumers' current preference for and pursuit of mobility causes additional issues for LIBs with regard to high energy density demand.8–10 Due to its very low redox potential of −3.04 V versus SHE and high theoretical capacity (2061 mA h cm−3 and 3860 mA h g−1, respectively), lithium metal is the perfect choice as an anode for rechargeable batteries.11–14 The usage of lithium metal anodes in rechargeable batteries, such as Li metal batteries,15–17 Li–S batteries,18–20 and Li–air batteries21–23 has lately gained a lot of attention due to the urgent requirement for high energy density batteries.Li forms dendritic growth because inhomogeneous nuclei are produced by non-uniform current and Li+ flux.24,25 The absence of a host is what causes the morphology of the nucleus to be unstable. Li dendrites are more likely to penetrate through the separator when dendritic Li develops, resulting in battery short circuits, even explosion or combustion.26–28 Additionally, due to the spontaneous interaction of Li metal with Li salt anions and electrolyte solvents, a solid electrolyte interphase (SEI) layer develops on the metallic Li surface.29–31 The weak SEI will ultimately break down because of the frequent Li plating and stripping, which increases the exposure of Li metal to electrolyte and results in strong side reactions between electrolyte and Li in addition to poor coulombic efficiency (CE).32 The anode's volume also goes through a totally new process that results in significant volume variations throughout charge/discharge processes.33–35 Internal tension and the solid–liquid interface alter as a result of this transformation. Thermal runaway, internal short circuits, or even an explosion might ensue from the dendritic growth by heterogeneous Li deposition.36–38 As a result, Li metal batteries (LMBs) have struggled extremely, which has resulted in low CE, rapid cycle durations, and even safety issues.39,40 The SEI may continue to break and form as long as the deposition procedure is continuing. More dendritic Li is exposed to the electrolyte because of its increased surface area. It uses a lot of electrolytes as a result, in addition to working on the rapid development of Li dendrites and Li inventory. The use of lithium metal anode for commercial applications is limited by these issues.41,42
So far, lithium alloys,43–45 electrolyte additives,46–48 synthetic SEI films,49–51 3D Li hosts,52–54 and solid/gel electrolytes55–57 have all been used as ways to stop lithium dendrite growth and keep the electrode/electrolyte interface stable. However, the Li dendrite development is difficult to control well, and the additive will be consumed completely. The introduced protective layer may be damaged and peeled off from the electrode owing to the volumetric change during a cycle, and solid electrolytes have low ionic conductivity. It is thus recommended to choose an electrode with a broad surface area that is rich in channels and/or porosity in order to lessen volume expansion and scatter local current intensities for homogeneous Li deposition. Because of their ability to effectively block the dendrite due to the spatial impact, metal oxides with a range of structures, such as ZnO nanorod arrays,58 Cu2O nanoparticles,59 CuO nanosheets,60 Co3O4 nanoarrays61etc., are gaining a lot of attention as modified current collectors.
In this study, a lithium metal anode is stabilized on MnO2 nanorods that are deposited on a bare Cu current collector (MnO2@Cu) when using 1 M lithium bis-(trifluoromethane) sulfonimide (LiTFSI) in dioxolane/dimethoxyethane (DOL/DME) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) with a 2% addition of lithium nitrate (LiNO3) additive as the electrolyte. The selection of 1 M LiTFSI in DOL/DME (1
1, v/v) with a 2% addition of lithium nitrate (LiNO3) additive as the electrolyte. The selection of 1 M LiTFSI in DOL/DME (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) with 2% LiNO3 as the electrolyte is based on its well-documented efficacy in lithium–sulfur (Li–S) battery systems.62,63 The nature of the solution plays a more crucial role in Li–S batteries than ordinary Li-ion batteries, since it not only acts as an ionic conductor for mass transfer but also participates substantially in the conversion processes of both lithium and sulfur. The DOL/DME solvent combination assists in balancing polysulfide solubility, decreasing the shuttle effect, and enhancing cycle life.64,65 LiNO3 facilitates the establishment of a stable SEI on the lithium metal anode, limiting dendrite growth and side reactions. LiTFSI enables strong ionic conductivity while limiting reactivity with lithium metal compared to other lithium salts.66,67 This electrolyte solution allows steady long-term cycling, which is crucial for lithium–sulfur battery applications. The choice of electrolyte remains significant owing to its known compatibility with lithium metal anodes and its efficacy in tackling the primary difficulties of lithium–sulfur batteries. Moreover, the following benefits of the MnO2@Cu host make it feasible to properly control the Li deposition properties: (i) a large-surface-area porous conductive structure that effectively reduces the local current density, (ii) a porous structure that accommodates quick volume expansion at high current, (iii) a stable skeleton that buffers compressive stress in the course of long cycling, (iv) lithiophilic sites that homogenizes Li deposition and direct the distribution of crystal nuclei, and (v) the reversible reactions to efficiently remove nucleation barriers. In fact, the uniformly distributed MnO2 nanorods, after forming a stable SEI just after the plating step, increase the substrate's lithophilic properties, offer a significant number of lithium plating sites, direct homogeneous Li+ flux scattering, and speed up lithium transportation. This can control the following plating and stripping steps as well as dramatically decrease the nucleation barrier of lithium at a primary phase. As a consequence, MnO2@Cu shows an average CE of 84% over 100 cycles at a current density of 2 mA cm−2 with a fixed capacity of 2 mA h cm−2, greater than that of the cell with the Cu anode (35 cycles), demonstrating its encouraging potential for usage in real-world applications. Even at high areal capacity deposition, the compositional and structural advantages allow for a homogeneous and steady lithium plating on MnO2@Cu. MnO2@Cu performs as predicted, offering a high CE, dendrite-free lithium plating and stripping performance across 20 cycles at sequentially increasing current densities (2–5 mA cm−2). At extremely high current densities greater than 5 mA cm−2, MnO2@Cu cells significantly outperform conventional cells regarding steady cycling and rate capability performance.
1, v/v) with 2% LiNO3 as the electrolyte is based on its well-documented efficacy in lithium–sulfur (Li–S) battery systems.62,63 The nature of the solution plays a more crucial role in Li–S batteries than ordinary Li-ion batteries, since it not only acts as an ionic conductor for mass transfer but also participates substantially in the conversion processes of both lithium and sulfur. The DOL/DME solvent combination assists in balancing polysulfide solubility, decreasing the shuttle effect, and enhancing cycle life.64,65 LiNO3 facilitates the establishment of a stable SEI on the lithium metal anode, limiting dendrite growth and side reactions. LiTFSI enables strong ionic conductivity while limiting reactivity with lithium metal compared to other lithium salts.66,67 This electrolyte solution allows steady long-term cycling, which is crucial for lithium–sulfur battery applications. The choice of electrolyte remains significant owing to its known compatibility with lithium metal anodes and its efficacy in tackling the primary difficulties of lithium–sulfur batteries. Moreover, the following benefits of the MnO2@Cu host make it feasible to properly control the Li deposition properties: (i) a large-surface-area porous conductive structure that effectively reduces the local current density, (ii) a porous structure that accommodates quick volume expansion at high current, (iii) a stable skeleton that buffers compressive stress in the course of long cycling, (iv) lithiophilic sites that homogenizes Li deposition and direct the distribution of crystal nuclei, and (v) the reversible reactions to efficiently remove nucleation barriers. In fact, the uniformly distributed MnO2 nanorods, after forming a stable SEI just after the plating step, increase the substrate's lithophilic properties, offer a significant number of lithium plating sites, direct homogeneous Li+ flux scattering, and speed up lithium transportation. This can control the following plating and stripping steps as well as dramatically decrease the nucleation barrier of lithium at a primary phase. As a consequence, MnO2@Cu shows an average CE of 84% over 100 cycles at a current density of 2 mA cm−2 with a fixed capacity of 2 mA h cm−2, greater than that of the cell with the Cu anode (35 cycles), demonstrating its encouraging potential for usage in real-world applications. Even at high areal capacity deposition, the compositional and structural advantages allow for a homogeneous and steady lithium plating on MnO2@Cu. MnO2@Cu performs as predicted, offering a high CE, dendrite-free lithium plating and stripping performance across 20 cycles at sequentially increasing current densities (2–5 mA cm−2). At extremely high current densities greater than 5 mA cm−2, MnO2@Cu cells significantly outperform conventional cells regarding steady cycling and rate capability performance.
2. Experimental section
2.1. Synthesis of MnO2
Manganese(II) acetate as a manganese precursor was oxidized with ammonium persulfate for MnO2 preparation (Fig. 1), as per our previous report.68 For the necessary synthesis, 100 mL of sodium hydroxide was first developed and separated into two beakers; one for the manganese and other for the ammonium persulfate precursor. Then, they were mixed and placed in a Teflon-coated autoclave. The arrangement was maintained at 180 °C for 14 h. The resulting product was cleaned in water and ethanol, followed by a drying process for 12 hours at 60 °C. The final product was annealed at 350 °C in an oven for 4 h.Our method for preparing manganese dioxide involves two synthetic processes. The interaction between sodium hydroxide and the manganese precursor results in the formation of a manganese hydroxide compound:
| (CH3COO)2Mn + 3OH− → Mn(OH)3− + 2CH3COO− | (1) |
Taking into account the first step, the second one is associated with the reaction process involving persulfate anions and manganese hydroxide:
| Mn(OH)3− + S2O82− + OH− → MnO2 + 2SO42− + 2H2O | (2) |
The details about materials characterizations are as per Note S1.†
2.2. Electrochemistry measurements
In order to prepare the electrodes, poly(vinylidene fluoride) (PVdF) was used as a binder in the solvent 1-methyl-2-pyrrolidinone (NMP).69,70 A homogeneously mixed slurry comprising MnO2 (88%) and binder (12%) was tape cast onto copper foil, dried at ambient temperature for 12 hours, and then dried under vacuum at 80 °C for one day.71,72 The total area of the electrodes was 1.13 cm2.For the fabrication of 2032 coin-type cells with a Li metal sheet as reference and counter electrodes, electrochemical performance was analyzed. Half-cells were prepared by employing an electrolyte of 1 M LiTFSI in DOL/DME (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) with 2% LiNO3 additive. Note that each cell's electrolyte content was kept constant at 160 μl. A glovebox containing high-quality argon with less than 1 ppm oxygen and water was used to construct all of the coin cells. Glass fiber from Whatman was used as a separator. Then, tests for electrochemical analysis were conducted at several capacities and current densities with a fixed 0.5 V cutoff voltage.
1, v/v) with 2% LiNO3 additive. Note that each cell's electrolyte content was kept constant at 160 μl. A glovebox containing high-quality argon with less than 1 ppm oxygen and water was used to construct all of the coin cells. Glass fiber from Whatman was used as a separator. Then, tests for electrochemical analysis were conducted at several capacities and current densities with a fixed 0.5 V cutoff voltage.
3. Results and discussion
3.1. Structural and morphological analysis
Detailed structural and morphological analysis has been already reported in our previous article.73 However, significant characterizations and discussions have been emphasised here. The important XRD pattern associated with Rietveld refinement is shown in Fig. S1a,† utilizing a pseudo-Voigt work function, confirming the tetragonal structure of MnO2. Various crystallographic planes are identified by the distinct diffraction peaks. The profile coefficients and cross fragment cutoff were altered using the Rietveld method. The results show that the experimental pattern (dark circles) and selected model (red line) have a respectable agreement for the I4/m space group using our technique. The oxidation states of the synthesized material were examined using the XPS spectrum. The core-level standard XPS spectra of Mn 2p is displayed in Fig. S1b.† The Mn 2p spectrum is split into a doublet, with peaks at 642.5 and 654 eV and may be easily distinguished from 2p3/2 and 2p1/2, separately, according to Fig. S1b.† The manganese in MnO2 is believed to be in the +4 oxidation state since the limiting energy difference between the aforementioned Mn 2p doublet is around 11.5 eV.74The high-resolution transmission electron microscopy (HRTEM) image in Fig. S1c† demonstrates that the average diameter of as-prepared MnO2 nanorods is in the range of 10–30 nm. The selected area electron diffraction (SAED) pattern of the structure in Fig. S1d† again demonstrates the diffraction spots that are clearly identifiable as originating from the crystallographic planes of the structure.
3.2. Electrochemical behavior of Cu and MnO2@Cu current collectors
To investigate the electrochemical properties, the half cells of Li//Cu and Li//MnO2@Cu were constructed. The half-cells were also cycled at 2 mA cm−2 and a constant plating/stripping capacity of 2 mA h/cm2 for each cycle in order to understand how MnO2 on Cu affects the cycle stability (Fig. 2 and 3). The Li//MnO2@Cu battery offers more stable and long-term plating/stripping cycles than the Li//Cu cell. MnO2@Cu has a short circuit duration of 186 h, which suggests that 372 mA h cm−2 lithium may be plated on it (Fig. 3a). The voltage of bare Cu, in contrast, starts to quickly decline after 65 h, which may indicate a rapid cell short circuit (Fig. 2a).The CE, analyzed as the ratio of stripping to plating capacity, is a critical measure of the viability, applicability, and cyclability of lithium deposition.75Fig. 2b and 3b show the findings as CE vs. cycle number for cells at 2 mA cm−2 with the same 2 mA h cm−2 capacity. MnO2@Cu exhibited extended and stable cycles with a good CE of 84% up to 100 cycles, in contrast to pure Cu, which has an unstable CE and declines quickly after 35 cycles at 2 mA cm−2. An increase in irreversible side reactions, such as the weak SEI layer deterioration, is linked to the quick decay of Cu.76,77 More specifically, at 2 mA cm−2, the Li//MnO2@Cu cell displays stable CE for 186 h. Be aware that certain processes, such as quick Li+ intercalation and Mn–Li alloying into the MnO2 structure, may occur during the whole cycle and lower the associated CE of the MnO2@Cu anode, particularly during the first few cycles. However, due to the development of a steady SEI and expansion of Li metal without dendrites, the CE will continue to be remarkably stable in the cycles that follow. Long-term cycling performance and stable CE on modified current collectors are also explained by the plating of Li on the MnO2 nanostructure, which remains generally stable even after 100 cycles.78 The cyclic performance of the Li//Cu cell, however, is much shorter, lasting fewer than 65 h and 35 cycles, respectively.
Fig. 2c and 3c show the plating/stripping profiles of Cu and MnO2@Cu, respectively. The change in the sharp voltage tip and following steady voltage was compared in order to calculate the nucleation overpotential of lithium plating on bare Cu and MnO2@Cu current collectors.79,80 A lower polarization value for the Li//MnO2@Cu cell utilizing the MnO2@Cu electrode than the bare Cu electrode suggests the low Li+ and e− transportation resistance in the MnO2@Cu electrode during operation.81,82 The improved lithiophilic properties of the MnO2@Cu electrode are shown by the fact that its initial nucleation overpotential is lower than that of the bare Cu substrate.83,84 The lithiophilic coating, which also makes it easier for uniform Li deposition and increases the reversibility of lithium plating/stripping cycles, is what causes the lower Li nucleation overpotential on MnO2@Cu in comparison to the Cu host.85 The nucleation overpotential, which temporarily emerges during the early phases of Li deposition, is a reflection of the electrode surface's lithiophilicity.86 It varies from the mass-transfer overpotential, which is governed by Li+ ion mobility and current density and lasts throughout deposition. The nucleation overpotential is illustrated by the rapid voltage decrease in the first cycle, as shown in Fig. 3c.81,82 After a few cycles, the potential of lithium plating and stripping varies according to the number of cycles performed. They don't change for the next 35 h of testing. As the nucleation potential increases during this process, the areal energy for nucleation decreases.87
In Fig. 4, the sequential deposition of Li on MnO2@Cu is conceptually shown. Metallic Li is used to cover the MnO2 nanorods with a smooth layer of Li, and further Li deposition fills in the spaces between the nanorods to create a homogeneous Li metal anode free of Li dendrites. The deposited MnO2 thin film on the Cu current collector reacts with lithium in the first cycle before the metallic lithium phase is formed, hindering the formation of Li dendrites, according to a very insignificant nucleation overpotential value. At normal temperature, bare Cu has little to no solubility in lithium metal, which causes a substantial nucleation overpotential.88
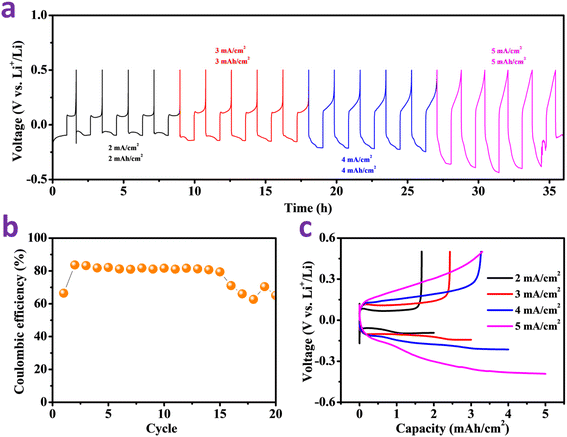
Fig. 5a and 6a show the voltage profiles of lithium plating/stripping in the case of two electrodes at various current densities (2–5 mA cm−2) and capacities (2–5 mA h cm−2). MnO2@Cu exhibits a stable discharge plateau between 2 and 5 mA cm−2 (Fig. 6a), but short-circuiting instigated by Li dendrites causes bare Cu's discharge plateau at 3 mA cm−2 to fluctuate (Fig. 5a). Actually, when the capacity increases from 2 to 5 mA h cm−2, the Li dendrites formation accelerates in the half-cells with bare Cu electrodes. The MnO2@Cu electrode nonetheless exhibits a consistent cycling reversibility under challenging testing conditions (plating capacity of 5 mA h cm−2). The electrode sustains 20 cycles even when a higher current density is used. However, due to irregular lithium nucleation and dendrite growth, the bare Cu collector exhibits significant fluctuations and short circuits as well.
Fig. 5b and 6b show the CE of lithium plating/stripping cycles associated with Cu and MnO2@Cu at different areal capacities and current densities. However, the CE of the Cu anode fluctuates randomly at the relatively higher areal capacity of 5 mA h cm−2, and the corresponding battery fails at the end of its cycling. All of the MnO2@Cu collectors show better CE values and cycling capabilities that last longer and are more stable at varying current densities. The MnO2@Cu anode's CE is capable of supporting Li areal capacities of 2 to 5 mA h cm−2. Bare Cu collectors could only cycle for a maximum of 20 cycles, whereas MnO2@C collectors could cycle for more than 20 cycles with high CE. It is noteworthy that the CE in our experiment exhibits stability despite a high current density and a high deposition capacity, indicating the exceptional electrochemical qualities of the MnO2@Cu collector. This results from the MnO2@Cu collector's favorable lithiophilic properties, which reduce the associated nucleation overpotential, lower the related deposition barrier, and constrain lithium dendrite growth throughout plating/stripping cycles. The MnO2@Cu current collectors, in contrast, also demonstrated clear benefits compared to Cu collectors. For bare Cu electrodes, a short circuit occurs at the fifth cycle.
The voltage profiles of the Cu and MnO2@Cu anodes are shown in Fig. 5c and 6c, respectively. MnO2@Cu's discharge profiles are stable during cycling. According to the outcomes of CE profiles, the stripping areal capacity of MnO2@Cu exhibits improved coincidence and doesn't visibly decline as the cycle number increases. The inability of the Cu electrodes to discharge at 5 mA cm−2 current density may be attributed to the rapid formation of lithium dendrites. Due to the large surface area of the MnO2 nanorods and their lithiophilic coating, MnO2@Cu has a low Li overpotential, as shown in Fig. 6c. The detailed rate performances (2–7 mA cm−2) have been added to the ESI (Fig. S2).†
It is obvious that the lithium plating/stripping properties of copper foil and MnO2@Cu are different. Since copper foil has a lower nucleation potential than MnO2@Cu, a large amount of nucleation capacity is needed before lithium can be plated on MnO2@Cu. These disparities are thought to be caused by variations in the surface characteristics of these two current collectors. Due to MnO2's porous structure, lithium may be easily plated on it, which results in the development of condensed lithium on its surface. Given that MnO2@Cu has a much stronger chemical affinity for metallic lithium, this may result in a lower nucleation overpotential for MnO2@Cu. In contrast, when using Cu foil, the solvated lithium ions inevitably plate and undergo exfoliation or pulverization. It is clear from the discussion above that the MnO2 nanorods on Cu current collectors made it easier to form an excellent SEI layer, homogenize ion flux scattering, improve Li+ conductivity, allow for uniform lithium deposition, and prevent lithium dendrite growth, which together enable the enhancement of plating/stripping performance. Table S1† compares the performance of lithium-ion batteries associated with various Cu-modified current collectors.
4. Conclusions
Here, we offer a straightforward, scalable technique for modifying the Cu collector using MnO2 nanorods. Strong lithiophilicity of MnO2 nanorods may influence the process of the lithium plating and prevent growth of Li dendrites, in addition to lowering the nucleation barrier by acting as nuclei to direct homogeneous lithium nucleation. MnO2 may provide a large number of active sites to promote Li nucleation. Strong ionic conductivity on the MnO2 protective layer and high electron conductivity inside nanorods may be used to coordinate the control of Li+ flux and electron distribution, leading to dendrite-free Li plating/stripping behavior. Additionally, the lithium metal stored in the space between nanorods can effectively reduce volume change during repeated charging and discharging. These benefits are made possible by MnO2 modified Cu current collectors, which also provide low overpotential and high CE. These advantages result in a simple, scalable process for manufacturing lithium metal anodes for industrial use, which in turn promotes the fabrication of safe Li-metal batteries.Data availability
Data are available upon request from the authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Bidhan Pandit acknowledges the Iberdrola Foundation under the Marie Skłodowska-Curie Grant Agreement No. 101034297. CH acknowledges funding from the ERC Starting Grant (UKRI Guarantee fund EP/Y009908/1), the Faraday Institution research programme grants FIRG060, FIRG082, and FIRG066, and Imperial College London UKRI Impact Acceleration Account EP/X52556X/1.References
- A. Dutta, S. Mitra, M. Basak and T. Banerjee, Energy Storage, 2023, 5, e339 CrossRef CAS.
- G. Sadeghi, Energy Storage Mater., 2022, 46, 192–222 CrossRef.
- B. Pandit, M. T. Sougrati, B. Fraisse and L. Monconduit, Nano Energy, 2022, 95, 107010 CrossRef CAS.
- X. Zhao, Z. Zhao-Karger, M. Fichtner and X. Shen, Angew. Chem., Int. Ed., 2020, 59, 5902–5949 CrossRef CAS PubMed.
- M. Abdel-Monem, O. Hegazy, N. Omar, K. Trad, P. Van den Bossche and J. Van Mierlo, in 2017 19th European Conference on Power Electronics and Applications (EPE’17 ECCE Europe), IEEE, 2017, vol. 2017-Janua, p. P.1-P.16 Search PubMed.
- M. A. Jamil, G. Ali, K. I. Khan, F. Jan Iftikhar, S. Zaman, S. F. Shaikh, B. Pandit, Q. Wali and S. A. Patil, J. Alloys Compd., 2022, 900, 163447 CrossRef CAS.
- J. M. Naranjo-Balseca, C. S. Martínez-Cisneros, B. Pandit, B. Levenfeld, J.-Y. Sanchez and A. Varez, J. Power Sources, 2025, 630, 236175 CrossRef CAS.
- Y. Tian, G. Zeng, A. Rutt, T. Shi, H. Kim, J. Wang, J. Koettgen, Y. Sun, B. Ouyang, T. Chen, Z. Lun, Z. Rong, K. Persson and G. Ceder, Chem. Rev., 2021, 121, 1623–1669 CrossRef CAS PubMed.
- J. W. Choi and D. Aurbach, Nat. Rev. Mater., 2016, 1, 16013 CrossRef CAS.
- V. Ahuja, S. Singh, R. Vengarathody and P. Senguttuvan, ECS Meet. Abstr., 2021, MA2021-01, pp. 254 Search PubMed.
- Y. Fan, H. Li, X. He, Y. Huang, C. Sun, T. Zhu, H. Liu, E. Huangzhang, F. Sun and J. Nan, ACS Appl. Energy Mater., 2022, 5, 10034–10044 CrossRef CAS.
- Y. Fan, H. Li, X. He, Y. Huang, C. Sun, T. Zhu, H. Liu, E. Huangzhang, F. Sun and J. Nan, ACS Appl. Energy Mater., 2022, 5, 10034–10044 CrossRef CAS.
- H. Zhang, R. Li, L. Chen, Y. Fan, H. Zhang, R. Zhang, L. Zheng, J. Zhang, S. Ding, Y. Wu, B. Ma, S. Zhang, T. Deng, L. Chen, Y. Shen and X. Fan, Angew. Chem., 2023, 135, e202218970 CrossRef.
- W. Jiang, S. Wang, Y. Wang, J. Hu, D. Yang and W. Zhang, Energy Technol., 2023, 2300053 CrossRef CAS.
- R. Li, Y. Fan, C. Zhao, A. Hu, B. Zhou, M. He, J. Chen, Z. Yan, Y. Pan and J. Long, Small Methods, 2022, 2201177 Search PubMed.
- S. Yuan, K. Ding, X. Zeng, D. Bin, Y. Zhang, P. Dong and Y. Wang, Adv. Mater., 2023, 35, 2206228 CrossRef CAS PubMed.
- D. Yan, H. Y. Yang and Y. Bai, Nano Res., 2023, 1–18 CAS.
- L. Zhou, D. L. Danilov, R. Eichel and P. H. L. Notten, Adv. Energy Mater., 2021, 11, 2001304 CrossRef CAS.
- Y. Gao, Q. Guo, Q. Zhang, Y. Cui and Z. Zheng, Adv. Energy Mater., 2021, 11, 2002580 CrossRef CAS.
- M. R. Al Khazraji, J. Wang and S. Wei, Energy Technol., 2023, 11, 2200944 CrossRef CAS.
- H.-F. Wang, X.-X. Wang, F. Li and J.-J. Xu, Small Sci., 2022, 2, 2200005 CrossRef CAS PubMed.
- L. Liu, H. Guo, L. Fu, S. Chou, S. Thiele, Y. Wu and J. Wang, Small, 2021, 17, 1903854 CrossRef CAS PubMed.
- X. Zou, Q. Lu, K. Liao and Z. Shao, Energy Storage Mater., 2022, 45, 869–902 CrossRef.
- N. Kim, H. Cha, S. Chae, T. Lee, Y. Lee, Y. Kim, J. Sung and J. Cho, Energy Environ. Sci., 2023, 16, 2505–2517 RSC.
- B. Pandit, S. R. Rondiya, S. F. Shaikh, M. Ubaidullah, R. Amaral, N. Y. Dzade, E. S. Goda, A. ul Hassan Sarwar Rana, H. Singh Gill and T. Ahmad, J. Colloid Interface Sci., 2023, 633, 886–896 CrossRef CAS PubMed.
- Q. Liu, R. Liu, Y. Cui, M. Zhou, J. Zeng, B. Zheng, S. Liu, Y. Zhu and D. Wu, Adv. Mater., 2022, 34, 2108437 CrossRef CAS PubMed.
- J. Deng, X. Yang and G. Zhang, Mater. Today Commun., 2022, 31, 103570 CrossRef CAS.
- Z. Deng, J. Gu, Y. Li, S. Li, J. Peng, X. Li, J. Luo, Y. Huang, C. Fang, Q. Li, J. Han, Y. Huang and Y. Zhao, Electrochim. Acta, 2019, 298, 121–126 CrossRef CAS.
- A. Wang, S. Kadam, H. Li, S. Shi and Y. Qi, npj Comput. Mater., 2018, 4, 15 CrossRef.
- H. Wu, H. Jia, C. Wang, J. Zhang and W. Xu, Adv. Energy Mater., 2021, 11, 2003092 CrossRef CAS.
- H. Adenusi, G. A. Chass, S. Passerini, K. V Tian and G. Chen, Adv. Energy Mater., 2023, 13, 2203307 CrossRef CAS.
- Q. Li, B. Quan, W. Li, J. Lu, J. Zheng, X. Yu, J. Li and H. Li, Nano Energy, 2018, 45, 463–470 CrossRef CAS.
- Y. Liu, D. Lin, Z. Liang, J. Zhao, K. Yan and Y. Cui, Nat. Commun., 2016, 7, 10992 CrossRef CAS PubMed.
- Y. Sun, C. Zhao, K. R. Adair, Y. Zhao, L. V. Goncharova, J. Liang, C. Wang, J. Li, R. Li, M. Cai, T.-K. Sham and X. Sun, Energy Environ. Sci., 2021, 14, 4085–4094 RSC.
- R. Cong, J.-Y. Choi, J.-B. Song, M. Jo, H. Lee and C.-S. Lee, Sci. Rep., 2021, 11, 1283 CrossRef CAS PubMed.
- X. Zhang, A. Wang, X. Liu and J. Luo, Acc. Chem. Res., 2019, 52, 3223–3232 CrossRef CAS PubMed.
- H. Liu, X. B. Cheng, Z. Jin, R. Zhang, G. Wang, L. Q. Chen, Q. B. Liu, J. Q. Huang and Q. Zhang, EnergyChem, 2019, 1, 100003 CrossRef.
- H. Liu, X.-B. Cheng, Z. Jin, R. Zhang, G. Wang, L.-Q. Chen, Q.-B. Liu, J.-Q. Huang and Q. Zhang, EnergyChem, 2019, 1, 100003 CrossRef.
- H. Liu, X. Chen, X. Cheng, B. Li, R. Zhang, B. Wang, X. Chen and Q. Zhang, Small Methods, 2019, 3, 1800354 CrossRef.
- J. Meng, F. Chu, J. Hu and C. Li, Adv. Funct. Mater., 2019, 29, 1902220 CrossRef.
- X. Guan, A. Wang, S. Liu, G. Li, F. Liang, Y.-W. Yang, X. Liu and J. Luo, Small, 2018, 14, 1801423 CrossRef PubMed.
- Y. Zhu, J. Xie, A. Pei, B. Liu, Y. Wu, D. Lin, J. Li, H. Wang, H. Chen, J. Xu, A. Yang, C.-L. Wu, H. Wang, W. Chen and Y. Cui, Nat. Commun., 2019, 10, 2067 CrossRef PubMed.
- T. Chen, W. Kong, P. Zhao, H. Lin, Y. Hu, R. Chen, W. Yan and Z. Jin, Chem. Mater., 2019, 31, 7565–7573 CrossRef CAS.
- B. Li, Y. Wang and S. Yang, Adv. Energy Mater., 2018, 8, 1702296 CrossRef.
- G. Wang, H. Wang, B. Zhong, L. Zhang and J. Zhang, in Electrochemical Energy, ed. J. Z. Pei Kang Shen, C.-Y. Wang, S. P. Jiang and X. Sun, CRC Press, Boca Raton, 1st edn, 2015, pp. 479–492 Search PubMed.
- H. Zhang, G. G. Eshetu, X. Judez, C. Li, L. M. Rodriguez-Martínez and M. Armand, Angew. Chem., Int. Ed., 2018, 57, 15002–15027 CrossRef CAS PubMed.
- L. Li, H. Dai and C. Wang, Nano Sel., 2021, 2, 16–36 CrossRef CAS.
- S. Li, Z. Luo, L. Li, J. Hu, G. Zou, H. Hou and X. Ji, Energy Storage Mater., 2020, 32, 306–319 CrossRef.
- D. Kang, M. Xiao and J. P. Lemmon, Batteries Supercaps, 2021, 4, 445–455 CrossRef CAS.
- U. S. Meda, L. Lal, S. M and P. Garg, J. Energy Storage, 2022, 47, 103564 CrossRef.
- H. Wang and Y. Tang, Chem. Res. Chin. Univ., 2020, 36, 402–409 CrossRef CAS.
- S. Ni, S. Tan, Q. An and L. Mai, J. Energy Chem., 2020, 44, 73–89 CrossRef.
- D. Li, B. Chen, H. Hu and W. Lai, Adv. Sustainable Syst., 2022, 6, 2200010 CrossRef CAS.
- T. Wei, J. Lu, M. Wang, C. Sun, Q. Zhang, S. Wang, Y. Zhou, D. Chen and Y.-Q. Lan, Chin. J. Chem., 2023, 41, 1861–1874 CrossRef CAS.
- C. S. Martinez-Cisneros, B. Pandit, B. Levenfeld, A. Varez and J.-Y. Sanchez, J. Power Sources, 2023, 559, 232644 CrossRef CAS.
- C. S. Martínez-Cisneros, B. Pandit, C. Antonelli, J. Y. Sanchez, B. Levenfeld and A. Varez, J. Eur. Ceram. Soc., 2021, 41, 7723–7733 CrossRef.
- J. M. Naranjo-Balseca, C. S. Martínez-Cisneros, B. Pandit and A. Várez, J. Eur. Ceram. Soc., 2023, 43, 4826–4836 CrossRef CAS.
- G. Wang, X. Xiong, P. Zou, X. Fu, Z. Lin, Y. Li, Y. Liu, C. Yang and M. Liu, Chem. Eng. J., 2019, 378, 122243 CrossRef CAS.
- Q. Zhang, J. Luan, Y. Tang, X. Ji, S. Wang and H. Wang, J. Mater. Chem. A, 2018, 6, 18444–18448 RSC.
- C. Zhang, W. Lv, G. Zhou, Z. Huang, Y. Zhang, R. Lyu, H. Wu, Q. Yun, F. Kang and Q.-H. Yang, Adv. Energy Mater., 2018, 8, 1703404 CrossRef.
- K. Tang, H. Gao, J. Xiao, M. Long, J. Chen, H. Liu and G. Wang, Chem. Eng. J., 2022, 436, 134698 CrossRef CAS.
- M. Barghamadi, A. S. Best, A. I. Bhatt, A. F. Hollenkamp, M. Musameh, R. J. Rees and T. Rüther, Energy Environ. Sci., 2014, 7, 3902–3920 RSC.
- L. F. Nazar, M. Cuisinier and Q. Pang, MRS Bull., 2014, 39, 436–442 CrossRef CAS.
- A. Rosenman, E. Markevich, G. Salitra, D. Aurbach, A. Garsuch and F. F. Chesneau, Adv. Energy Mater., 2015, 5, 1500212 CrossRef.
- S. S. Zhang, Electrochim. Acta, 2013, 97, 226–230 CrossRef CAS.
- S. Xiong, K. Xie, Y. Diao and X. Hong, Electrochim. Acta, 2012, 83, 78–86 CrossRef CAS.
- S. Xiong, K. Xie, Y. Diao and X. Hong, J. Power Sources, 2014, 246, 840–845 CrossRef CAS.
- B. Pandit, E. S. Goda, M. H. Abu Elella, A. ur Rehman, S. Eun Hong, S. R. Rondiya, P. Barkataki, S. F. Shaikh, A. M. Al-Enizi, S. M. El-Bahy and K. Ro Yoon, J. Energy Chem., 2022, 65, 116–126 CrossRef CAS.
- B. Pandit, M. Johansen, C. Susana Martínez-Cisneros, J. M. Naranjo-Balseca, B. Levenfeld, D. B. Ravnsbæk and A. Varez, Chem. Mater., 2024, 36, 2314–2324 CrossRef CAS PubMed.
- S. F. Shaikh, S. Aftab, B. Pandit, A. M. Al-Enizi, M. Ubaidullah, S. Ekar, S. Hussain, Y. B. Khollam, P. S. More and R. S. Mane, Dalton Trans., 2023, 52, 11481–11488 RSC.
- B. Pandit, M. Johansen, B. P. Andersen, C. S. Martínez-Cisneros, B. Levenfeld, D. B. Ravnsbæk and A. Varez, Chem. Eng. J., 2023, 472, 144509 CrossRef CAS.
- B. Pandit, B. Fraisse, L. Stievano, L. Monconduit and M. T. Sougrati, Electrochim. Acta, 2022, 409, 139997 CrossRef CAS.
- B. Pandit, S. R. Rondiya, N. Y. Dzade, S. F. Shaikh, N. Kumar, E. S. Goda, A. A. Al-Kahtani, R. S. Mane, S. Mathur and R. R. Salunkhe, ACS Appl. Mater. Interfaces, 2021, 13, 11433–11441 CrossRef CAS PubMed.
- Z.-H. Huang, Y. Song, D.-Y. Feng, Z. Sun, X. Sun and X.-X. Liu, ACS Nano, 2018, 12, 3557–3567 CrossRef CAS PubMed.
- L. Liu, Y.-X. Yin, J.-Y. Li, N.-W. Li, X.-X. Zeng, H. Ye, Y.-G. Guo and L.-J. Wan, Joule, 2017, 1, 563–575 CrossRef CAS.
- R. Pathak, K. Chen, A. Gurung, K. M. Reza, B. Bahrami, J. Pokharel, A. Baniya, W. He, F. Wu, Y. Zhou, K. Xu and Q. Qiao, Nat. Commun., 2020, 11, 93 CrossRef CAS PubMed.
- P. Xu, X. Hu, X. Liu, X. Lin, X. Fan, X. Cui, C. Sun, Q. Wu, X. Lian, R. Yuan, M. Zheng and Q. Dong, Energy Storage Mater., 2021, 38, 190–199 CrossRef.
- S. Cui, P. Zhai, W. Yang, Y. Wei, J. Xiao, L. Deng and Y. Gong, Small, 2020, 16, 1905620 CrossRef CAS PubMed.
- Z. Zhang, J. Wang, X. Yan, S. Zhang, W. Yang, Z. Zhuang and W.-Q. Han, Energy Storage Mater., 2020, 29, 332–340 CrossRef.
- C. Wei, H. Fei, Y. An, Y. Tao, J. Feng and Y. Qian, J. Mater. Chem. A, 2019, 7, 18861–18870 RSC.
- S. Chen, K. Tao, X. Chen, Y. Meng, M. Wang, J. Zhou, C. Chen, Y. Wang, K. Nam Hui, C. W. Bielawski and J. Geng, Chem. - Eur. J., 2021, 27, 15706–15715 CrossRef CAS PubMed.
- S.-S. Chi, Y. Liu, W.-L. Song, L.-Z. Fan and Q. Zhang, Adv. Funct. Mater., 2017, 27, 1700348 CrossRef.
- S. Wang, Y. Wang, Y. Song, J. Zhang, X. Jia, J. Yang, D. Shao, Y. Li, J. Liao and H. Song, Energy Storage Mater., 2022, 50, 505–513 CrossRef.
- Y. Sun, J. Zhou, H. Ji, J. Liu, T. Qian and C. Yan, ACS Appl. Mater. Interfaces, 2019, 11, 32008–32014 CrossRef CAS PubMed.
- M. Baek, J. Kim, K. Jeong, S. Yang, H. Kim, J. Lee, M. Kim, K. J. Kim and J. W. Choi, Nat. Commun., 2023, 14, 1296 CrossRef CAS PubMed.
- Y. Sun, J. Zhou, H. Ji, J. Liu, T. Qian and C. Yan, ACS Appl. Mater. Interfaces, 2019, 11, 32008–32014 CrossRef CAS PubMed.
- G. Yang, Y. Li, Y. Tong, J. Qiu, S. Liu, S. Zhang, Z. Guan, B. Xu, Z. Wang and L. Chen, Nano Lett., 2019, 19, 494–499 CrossRef CAS PubMed.
- C. Yang, Y. Yao, S. He, H. Xie, E. Hitz and L. Hu, Adv. Mater., 2017, 29, 1702714 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5se00181a |
| This journal is © The Royal Society of Chemistry 2025 |