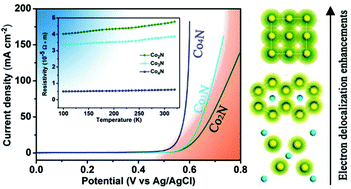
The development of highly-efficient, stable and cost-effective electrocatalysts for the oxygen evolution reaction (OER) is critical for a range of renewable-energy technologies, including metal–air batteries, fuel cells and water-splitting reactions. However, most of the well-developed electrocatalysts are semiconductors or insulators with poor conductivity, which has profoundly inhibited their overall OER efficiency. In this study, metallic cobalt nitrides (Co2N, Co3N and Co4N) arising from electron delocalization modulation have been investigated for OER electrocatalysts in alkaline solution for the first time. Benefiting from the synergistical engineering of the electrical conductivity and nitrogen content, the simple metallic Co4N catalyst without modifications exhibits a stable current density of 10 mA cm−2 at a small overpotential of 330 mV for OER with a Tafel slope as low as 58 mV dec−1 in alkaline medium, which is superior to most of the unmodified metal oxide electrocatalysts reported to date. Our finding introduces new possibilities for the design of highly active electrocatalysts using synergistical electrical conductivity regulation and composition modulation.