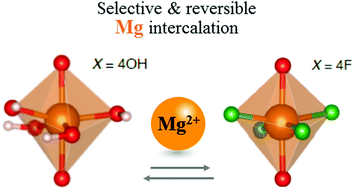
In anatase TiO2, substituting oxide anions with singly charged (F,OH) anions allows the controlled formation of cation vacancies, which act as reversible intercalation sites for Mg2+. We show that ion-transport (diffusion coefficients) and intercalation (reversible capacity) properties are controlled by two critical parameters: the vacancy concentration and the local anionic environment. Our results emphasise the complexity of this behaviour, and highlight the potential benefits of chemically controlling cationic-defects in electrode materials for rechargeable multivalent-ion batteries.