Nyancy Halder, Mohandas Sangeetha, Dandamudi Usharani and Harapriya Rath
Org. Biomol. Chem., 2019,17, 6131-6135
DOI:
10.1039/C9OB01062A,
Communication
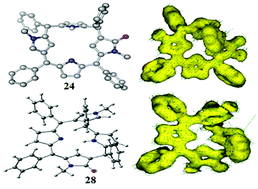
A concise and convenient synthetic methodology leading to an unambiguous isolation of two hitherto unknown highly stable single conformers of meso-aryl substituted unorthodox 5,10-porphodimethenes has been developed by the inclusion of N-methyl pyrrole units with α,β and β,β-linkages into the core of heterocyclic macrocycles.