Revannath L. Sutar, Nikita Erochok and Stefan M. Huber
Org. Biomol. Chem., 2021,19, 770-774
DOI:
10.1039/D0OB02503H,
Communication
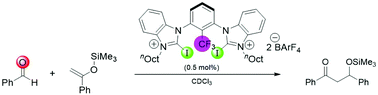
A series of cationic monodentate and bidentate iodo(benz)imidazolium-based halogen bond (XB) donors were employed as catalysts in a Mukaiyama aldol reaction. While 5 mol% of a monodentate variant showed noticeable activity, a syn-preorganized bidentate XB donor provided a strong performance even with 0.5 mol% loading. In contrast to the very active BArF4 salts, PF6 or OTf salts were either inactive or showed background reaction through Lewis base catalysis. Repetition experiments clearly ruled out a potential hidden catalysis by elemental iodine and demonstrated the stability of our catalyst over three consecutive cycles.