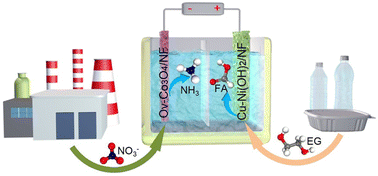
Paired electrolysis, which integrates a productive cathodic reaction, such as the nitrate reduction reaction (NO3−RR) with selective oxidation at the anode, offers an intriguing way to maximize both atomic and energy efficiency. However, in a conventional design, the NO3−RR is often coupled with the anodic oxygen evolution reaction, leading to substantial energy consumption while yielding low-value oxygen. Here, we report a hybrid electrolysis system that combines cathodic reduction of nitrate to ammonia and anodic oxidation of polyethylene-terephthalate-derived ethylene glycol (EG) to formic acid (FA), utilizing oxygen-vacancy-rich (OV) Co3O4 and Cu doped Ni(OH)2 as the cathode and anode, respectively. Remarkably, this paired electrolysis system demonstrates a faradaic efficiency (FE) of 92% for cathodic ammonia production and a FE of 99% for anodic FA production, while reducing the cell voltage by 0.54 V compared to the conventional NO3−RR system at the same current density of 100 mA cm−2. Experimental investigations combined with theoretical calculations reveal that the OV introduction effectively addresses the insufficient NO3− adsorption and hydrogenation on bare Co3O4. Additionally, Cu incorporation increases the Ni–O covalency, resulting in an improved EG adsorption ability. This work presents a promising way for waste management via paired electrolysis.