Construction of synergistic binding sites in a robust MOF for excellent C2H4 purification and C3H6 recovery performance†
Abstract
C2H4 purification and C3H6 recovery by physisorbents face a huge dilemma in simultaneously achieving good separation performance, easy scalability, economic feasibility, and great stability for industrial applications. Herein, we propose a strategy of building multiple affinities in robust Ni(bdc)(dabco)0.5 for highly effective one-step C2H4 purification from a C3H6/C2H4 mixture in the products of the MTO process and steam cracking of naphtha. Due to its nonpolar pores containing available O binding sites with suitable pore restriction and high BET surface area, Ni(bdc)(dabco)0.5 shows not only one of the highest C3H6 uptake (80.6 cm3(STP) g−1) at 0.1 bar and excellent C3H6 uptake (148.8 cm3(STP) g−1) at 1.0 bar but also top-level C3H6/C2H4 selectivity (10.7) and separation potential (115.3 cm3(STP) g−1) at 298 K and 1.0 bar. Meanwhile, its C3H6/C2H4 uptake ratio (10.9) exhibits a record value at 0.1 bar with a lower 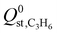 (26.7 kJ mol−1), breaking the trade-off with C3H6/C2H4 separation and setting a new benchmark. Theoretical simulation and in situ FT-IR unveiled that the nonpolar pores with rich O binding sites supplied stronger multiple supramolecular affinities for C3H6 over C2H4. Breakthrough tests proved its total separation performance for C3H6/C2H4 with good recyclability, offering one of the highest dynamic C3H6 uptake and C2H4 productivity (101.2 and 57.3 cm3 (STP) g−1, respectively). Additionally, the MOF is easy to synthesize on a gram-scale using cheap reagents. Ni(bdc)(dabco)0.5 fulfills the benchmark combination of good separation performance, great stability, good renewability, and easy scalability, which endows it with great prospect for industrial C2H4 purification.
(26.7 kJ mol−1), breaking the trade-off with C3H6/C2H4 separation and setting a new benchmark. Theoretical simulation and in situ FT-IR unveiled that the nonpolar pores with rich O binding sites supplied stronger multiple supramolecular affinities for C3H6 over C2H4. Breakthrough tests proved its total separation performance for C3H6/C2H4 with good recyclability, offering one of the highest dynamic C3H6 uptake and C2H4 productivity (101.2 and 57.3 cm3 (STP) g−1, respectively). Additionally, the MOF is easy to synthesize on a gram-scale using cheap reagents. Ni(bdc)(dabco)0.5 fulfills the benchmark combination of good separation performance, great stability, good renewability, and easy scalability, which endows it with great prospect for industrial C2H4 purification.

- This article is part of the themed collection: 2025 Inorganic Chemistry Frontiers HOT articles


 Please wait while we load your content...
Please wait while we load your content...