Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Simultaneous enzymatic esterification and ester extraction in Pickering emulsions for the recovery of butanol from fermentation broth†
Yaoyao
Feng
a,
Pierre-Louis
Carrette
b,
Christine
Dalmazzone‡
a and
Etienne
Jourdier‡
 *a
*a
aIFP Energies nouvelles, 1 et 4 avenue de Bois-Préau, 92852 Rueil-Malmaison, France. E-mail: etienne.jourdier@ifpen.fr
bIFP Energies nouvelles, Rond-point de l'échangeur de Solaize, BP 3, 69360 Solaize, France
First published on 10th April 2025
Abstract
The recovery of biobutanol from highly diluted aqueous fermentation broth usually suffers from intensive energy consumption. In this study, we developed a Pickering emulsion system stabilized by silica nanoparticles for the rapid and efficient recovery of low concentrations of butanol (<20 g L−1) from fermentation broth in the form of esters. Each droplet in the emulsion system serves as a microreactor for enzymatic esterification of butanol in the water phase, and the ester product is spontaneously extracted to the oil phase, thereby promoting the esterification reaction. The system offers a significantly larger interfacial area and a 2–5 times improvement in reaction rate compared to the biphasic system. Under optimal conditions, the conversion and extraction of butanol from the fermentation broth into butyl butyrate achieved a yield of 79% in the presence of a Pickering emulsion. This study presents a sustainable and efficient approach for the recovery of biobased butanol.
Introduction
The depletion of oil reserves and rising prices over recent decades have drawn significant attention to the development of biofuels. Biobutanol has been considered as a promising alternative to fossil fuel due to its low volatility, high energy content and hydrophobic nature.1–3 The biobutanol can be produced at the industrial scale via procedures known as acetone–butanol–ethanol (ABE)4,5 or isopropanol–butanol–ethanol (IBE)6,7 fermentation from renewable resources. It is in fact known that the concentration of butanol in the fermentative production is limited to 20 g L−1 because of its severe toxicity on the microorganism. One of the most critical challenges in utilizing butanol produced by fermentation is the separation of water and other impurities present in the fermentation broth.8 To separate the butanol from dilute fermentation broths, conventional distillation has been used as the dominant purification method, but intensive energy is required and makes the process economically unfavorable.9,10 Several strategies have been proposed for the separation of butanol from fermentation broth, including liquid–liquid extraction,11–13 gas stripping,14,15 membrane filtration,16 pervaporation,17 and adsorption.18 One of the promising techniques for separation of butanol from fermentation broth is liquid–liquid extraction, because of its high selectivity, low energy consumption, mild operating temperature and easy scale-up. In this regard, several extractants have been studied for butanol extraction such as fatty ester,19,20 vegetable oil21,22 and primary alcohol.23 However, these extractions with high partition coefficient for butanol are most likely toxic to the producing microorganism. Zhang et al. used a mixture of aliphatic acid and oleyl alcohol as extractant to accumulate isopropanol and butanol because of the high biocompatibility and high distribution coefficient.24 The main drawback of using oleyl alcohol is its market price, combined with the high energy required for recovery, making the process economically unfavourable.Given the favourable selectivity and reactivity of butanol with acids, the esterification of butanol with an appropriate acid to produce a more hydrophobic ester presents a significant opportunity for the extraction and purification of butanol from fermentation broth.25,26 If desired, the purified ester can be obtained through subsequent distillation. If necessary, the ester can also be hydrolysed with simultaneous removal of acid and water to produce high-purity butanol. This approach highlights the economic potential of recovering butanol in the form of esters directly from the fermentation broth. van den Berg et al. disclosed that one-pot combination of butanol esterification and ester extraction offers a simplified method for the recovery and purification of the alcohol product in a fermentation medium.27 Esters are generated by the reaction of alcohols within the fermentation medium and carboxylic acids (such as fatty acids) in the presence of a catalyst. Specifically, the butyl butyrate produced in the extractant phase could be used as biofuel without further separation and purification steps, as it exhibits excellent properties as components of both gasoline and diesel due to its unique fuel characteristics.
Bearing these facts in mind, it is possible to perform the esterification of butanol to butyl butyrate in highly diluted fermentation broth simultaneously with the extraction of this ester by an organic solvent. The organic extractant and diluted fermentation broth form a biphasic mixture. The very high partition coefficient of the ester in the organic phase drives the reaction towards synthesis on the ester side even at relatively low concentration of butanol. Despite their benefits, conventional biphasic systems often suffer from high mass/heat transfer resistances because of the limited organic/aqueous interfacial area between the organic extractant and aqueous phase.28,29 To address this limitation, Pickering emulsion is a promising strategy to improve the efficiency of biphasic reactions. In the Pickering emulsion systems, solid nanoparticles serve as emulsifiers at the organic/aqueous interface, increasing the interfacial area at the nano- and microscales and reducing diffusion limitations in biphasic reactions.30–33 This system not only enhances mass transport by expanding the interfacial area but also simplifies emulsifier separation and recovery compared to the use of surfactant.
Regarding the catalyst, esterification reactions are classically carried out under mild conditions using lipase or esterase enzymes, in particular lipase B from Candida antarctica, or the lipase from Candida rugosa. To facilitate the natural reaction of hydrolysis of insoluble substrates, these enzymes are amphiphilic and have a mode of action at the interface between the aqueous and organic phases.34,35 Maximising the interface between the aqueous and organic phases is therefore of interest not only for ester extraction but also for enzymatic catalyst activation.
Herein, we report a simultaneous enzymatic esterification and ester extraction system based on a Pickering emulsion for recovering butanol in the form of esters from fermentation broth. The system utilizes silica nanoparticles of different hydrophobicity to prepare both oil-in-water (O/W) and water-in-oil (W/O) emulsions (Fig. 1). The physicochemical properties and stability of the obtained emulsions were determined. We first addressed the enzymatic esterification of butanol with a carboxylic acid in the presence of lipase to evaluate the impact of the experimental conditions such as pH effect, emulsion type, lipase concentration and alcohol/acid ratio on the catalytic performance. The catalytic system is versatile and can be applied to the esterification of various carboxylic acids. Furthermore, the separation performance was also investigated in the case of the real fermentation broth.
Experimental
Materials
AEROSIL® R972 and R816 are kind gifts from Evonik Industries AG (Rheinfelden, Germany). AEROSIL® R972 is prepared by treating hydrophilic fumed silica with dimethyldichlorosilane, while AEROSIL® R816 is produced by modifying hydrophilic fumed silica with hexadecylsilane. n-Dodecane (99%), hexadecane (99%) and heptane (99%) were supplied by Fisher Scientific. 1-Butanol (99%), butyric acid (99%), hexanoic acid (99%), palmitic acid (99%), linoleic acid (99%) and butyl palmitate (99%) were purchased from Sigma-Aldrich. Butyl butyrate (99%), isopropyl butyrate (99%), and butyl hexanoate (≥98%) were supplied by Fisher Scientific. Lipase B Candida antarctica solution (CaLB, enzyme activity ≥5000 LU g−1) was supplied from Sigma-Aldrich (ref. L3170).Methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 rpm for 2 min at room temperature.
500 rpm for 2 min at room temperature.
The type of emulsion was determined by observing the evolution of a drop of each emulsion when a volume of either oil or water was added (dilution test). Optical micrographs were carried out using an Olympus BX51 digital microscope equipped with a video camera. The emulsion was diluted in the continuous phase, and several images were captured from different locations of the emulsion droplets to represent the overall mean droplet size of the emulsion sample. The images were analyzed using ImageJ software (National Institutes of Health, USA) to measure the droplet diameter. The size distribution and average diameter of the droplets were determined by measuring the diameters of 150–200 individual droplets.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 rpm for 2 min, and then sealed and incubated at 40 °C for 6 h under magnetic stirring at 500 rpm. During the reaction, the reaction medium was sampled, the emulsion was broken by centrifugation, and the supernatant oil solution was filtered and collected.
500 rpm for 2 min, and then sealed and incubated at 40 °C for 6 h under magnetic stirring at 500 rpm. During the reaction, the reaction medium was sampled, the emulsion was broken by centrifugation, and the supernatant oil solution was filtered and collected.
The oil phase was analyzed using an Agilent 7890 GC equipped with a flame ionization detector (FID) detector and a HP-1 ms column (length 15 m, inner diameter 0.32 mm, film thickness 0.25 μm). Helium was used as carrier gas at a flow rate of 1.6 mL min−1. The column was gradually heated from 50 °C to 250 °C in a 10 min run. Mass balance errors were within 5% for all catalytic tests. The amount of ester and alcohol were quantified by interpolation of the corresponding calibration curves using hexadecane as internal standard. The yield of ester was calculated as follows with respect to the initial amount of butanol: Ester yield (t) = nEster(t)/n0Butanol ×100 (1) where n0Butanol refers to the moles number of butanol at time = 0, and nEster(t) is the moles number of the corresponding ester at time = t (h).
For control experiments, the catalytic reaction was performed at the same reaction conditions, but without the addition of emulsifiers. In a typical test, 6 mL water containing butanol (0.1 mol L−1) and butyric acid (0.1 mol L−1) and 6 mL dodecane were added to a glass vial, followed by the addition of 0.3% v/v fresh lipase solution (volume of lipase solution relative to the volume of aqueous phase). The mixture was sealed, stirred at 500 rpm and heated at 40 °C for 6 h. After reaction, the upper oil phase was collected and analyzed by GC system.
For blank experiments, the catalytic reaction was carried out at identical conditions without the addition of lipase.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio with butanol), 6 mL dodecane, 1.0 wt% silica nanoparticles (with respect to the total weight of the biphasic system) and 0.3% v/v fresh lipase solution (volume of lipase solution relative to the volume of aqueous phase). The final dispersion was emulsified using an Ultra-TURRAX at 13
1 molar ratio with butanol), 6 mL dodecane, 1.0 wt% silica nanoparticles (with respect to the total weight of the biphasic system) and 0.3% v/v fresh lipase solution (volume of lipase solution relative to the volume of aqueous phase). The final dispersion was emulsified using an Ultra-TURRAX at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 rpm for 2 min, and then sealed and stirred at 40 °C for 6 h at 500 rpm. After reaction, the supernatant oil solution was collected and analyzed by GC.
500 rpm for 2 min, and then sealed and stirred at 40 °C for 6 h at 500 rpm. After reaction, the supernatant oil solution was collected and analyzed by GC.
Results and discussion
Physicochemical properties of silica stabilized Pickering emulsions
As a first step in the development of our Pickering-assisted esterification and extraction methodology, we studied the preparation of W/O and O/W Pickering emulsions and characterized their stability. Hydrophobic R972 and hydrophilic R816 nanoparticles were chosen to prepare both kinds of emulsion and their emulsifying properties were explored for a 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
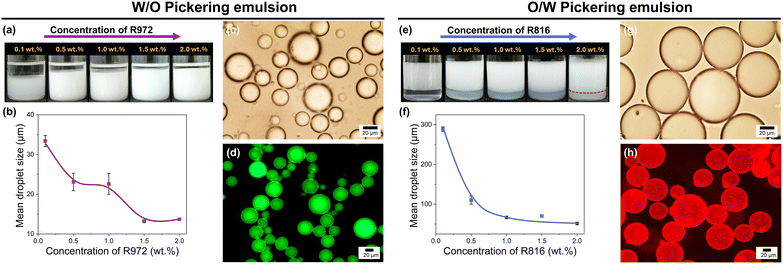
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 (v/v) water/dodecane biphasic system as a function of particle concentrations at room temperature (Fig. 2 and S1†). As expected, emulsions stabilized by R972 and R816 nanoparticles were successfully generated in all cases. The dilution tests performed on both types of emulsions suggested that R972 particles allowed the formation of a W/O emulsion, while R816 favoured the formation of an O/W emulsion. For the R972 nanoparticles, an excess of oil phase is always present regardless of particle concentration (probably due to the sedimentation of water droplets), whereas the R816 nanoparticles stabilized emulsion exhibits an excess of the water phase (probably due to the creaming of oil droplets). The droplet size of emulsions stabilized with R972 nanoparticles gradually decreases from 33 to 23 μm as the silica concentration increases from 0.1 wt% to 1.0 wt%. Further increasing the R972 concentration to 2.0 wt% results in a smaller droplet size, while the emulsion volume remains relatively unchanged. In parallel, the droplet size of the emulsion stabilized with R816 nanoparticles is 4–9 times larger compared to the average size of the emulsion stabilized by R972 nanoparticles (Fig. 2b and f). As the R816 nanoparticles concentration increases from 0.1 wt% to 1.0 wt%, the droplet size decreases significantly from 289 μm to 67 μm, which then remains nearly unchanged. To confirm the emulsion types, we separately labelled organic phase and water phase with Nile red and fluorescein isothiocyanate isomer I for fluorescence imaging. Fluorescence microscopy images revealed that the R972 nanoparticles formed a W/O emulsion (Fig. 2d), whereas the R816 nanoparticles led to the formation of an O/W emulsion (Fig. 2h), as expected from the initial dilution test.
50 (v/v) water/dodecane biphasic system as a function of particle concentrations at room temperature (Fig. 2 and S1†). As expected, emulsions stabilized by R972 and R816 nanoparticles were successfully generated in all cases. The dilution tests performed on both types of emulsions suggested that R972 particles allowed the formation of a W/O emulsion, while R816 favoured the formation of an O/W emulsion. For the R972 nanoparticles, an excess of oil phase is always present regardless of particle concentration (probably due to the sedimentation of water droplets), whereas the R816 nanoparticles stabilized emulsion exhibits an excess of the water phase (probably due to the creaming of oil droplets). The droplet size of emulsions stabilized with R972 nanoparticles gradually decreases from 33 to 23 μm as the silica concentration increases from 0.1 wt% to 1.0 wt%. Further increasing the R972 concentration to 2.0 wt% results in a smaller droplet size, while the emulsion volume remains relatively unchanged. In parallel, the droplet size of the emulsion stabilized with R816 nanoparticles is 4–9 times larger compared to the average size of the emulsion stabilized by R972 nanoparticles (Fig. 2b and f). As the R816 nanoparticles concentration increases from 0.1 wt% to 1.0 wt%, the droplet size decreases significantly from 289 μm to 67 μm, which then remains nearly unchanged. To confirm the emulsion types, we separately labelled organic phase and water phase with Nile red and fluorescein isothiocyanate isomer I for fluorescence imaging. Fluorescence microscopy images revealed that the R972 nanoparticles formed a W/O emulsion (Fig. 2d), whereas the R816 nanoparticles led to the formation of an O/W emulsion (Fig. 2h), as expected from the initial dilution test.
To better characterize the stability of the emulsion, we performed multiple light scattering measurements using Turbiscan® (Formulaction, France) to monitor real-time information on the destabilization processes (see ESI† Experimental section). The delta transmission (ΔT) and delta backscattering (ΔBS) profiles of the emulsion stabilized by both R972 and R816 nanoparticles are shown in Fig. S2(a and b).† For the emulsion stabilized by R972 nanoparticles (W/O emulsion), a sharp decline of ΔBS at the top of the sample with a concomitant increase of the ΔT signal suggests the gravity-induced migration of the water droplets. Moreover, the slight change of ΔBS in the middle portion of the emulsion can be explained as an increase in the droplet size due to either coalescence or flocculation. In the presence of R816 nanoparticles (O/W emulsion), the ΔBS value at the bottom layer of the emulsion drops sharply as the ΔT signal dramatically increases, which suggests that the bottom of the emulsion is clarified due to creaming. In addition, the oil droplets migrate through the continuous water phase from the bottom to the top of the samples, leading to a progressive concentration increase at the mid-height of the samples. This is characterized by an increase in the backscattering signal at the middle of the samples. These findings support that the layering process occurs immediately after emulsion preparation, and that emulsions can maintain long-term stability once equilibrium is reached.
Furthermore, we evaluated the emulsion stability of both R972 and R816 nanoparticles in the presence of substrates (0.1 mol L−1 butanol and butyric acid in the aqueous phase), as well as using fermentation broth as the aqueous phase (Fig. S2c–f†). The emulsions stabilized by R972 nanoparticles remain almost unchanged across different aqueous medium (Fig. S3†). In contrast, the ΔBS signals drop sharply throughout the entire height of the emulsion stabilized by R816 nanoparticles, indicating lower resistance to flocculation and/or partial coalescence. However, no appearance of supernatant oily phase is noted which would indicate the total coalescence of the oil drops, which proves that the emulsion is stable over 12 h regarding coalescence.
Catalytic performance
Considering the emulsion properties of the prepared system, we aim to utilize the enhanced interfacial contact between the hydrophilic reagents and hydrophobic extractants to improve the catalytic performance of simultaneous enzymatic esterification and ester extraction in the biphasic system. As a proof-of-concept, the enzymatic esterification of butanol (0.1 mol L−1) and butyric acid in a diluted aqueous solution was chosen as a model reaction to mimic a fermentation broth. The amount of butyl butyrate in the organic extractant phase was used to assess reaction efficiency. For comparison, control experiments were performed under the same reaction conditions, but in the conventional biphasic system.As expected, no yield of ester is observed for the blank experiment without lipase addition in both the Pickering emulsion and the biphasic system (Table 1 entries 1 and 2). With the addition of 0.3% v/v lipase in the biphasic system, the yield of ester is only 9% after 1 h. In contrast, the ester yield in the R972 nanoparticle stabilized W/O Pickering emulsion system is 5 times higher, reaching 47% after 1 h (Table 1 entries 3 and 4). This result is clearly attributed to the micro-structured reaction medium, which largely facilitates mass transfer and enzyme interaction. The water droplets act as “microreactor”, improving contact area with dodecane and boosting extraction efficiency. It should be noted that the thermodynamic equilibrium of the esterification reaction is very unfavourable in water due to the excess of water, and even more so with very diluted reactants. As a result, the extent of the reaction would be very limited in an aqueous phase alone. The very high partition coefficient of butyl butyrate in the organic extractant phase can “push” the esterification toward the ester synthesis side even at a highly diluted butanol concentration.
| Entry | Lipase | Emulsifier | Emulsion type | pH | Yield/% |
|---|---|---|---|---|---|
| Reaction conditions: 6 mL water (containing 0.1 mol L−1 butanol and 0.1 mol L−1 butyric acid), 6 mL dodecane, 1.0 wt% silica nanoparticles, 0.3% v/v CaLB solution (relative to the volume of aqueous phase), emulsification at 13500 rpm for 2 min, 40 °C for 1 h, 500 rpm. The pH of the original aqueous solution is 2.7 and can be adjusted to 4.0 or 5.0 using KOH solution. | |||||
| 1 | — | — | — | 2.7 | 0 |
| 2 | — | R972 | W/O | 2.7 | 0 |
| 3 | CaLB | — | — | 2.7 | 9 |
| 4 | CaLB | R972 | W/O | 2.7 | 47 |
| 5 | CaLB | — | — | 4.0 | 12 |
| 6 | CaLB | R972 | W/O | 4.0 | 42 |
| 7 | CaLB | R816 | O/W | 4.0 | 31 |
| 8 | CaLB | — | — | 5.0 | 5 |
| 9 | CaLB | R972 | W/O | 5.0 | 20 |
To gain insight into the effect of pH on esterification, we performed the catalytic test after adjusting the pH of the aqueous solution, as the pH of the reaction medium affects both the esterification equilibrium and lipase activity. By increasing pH of the aqueous solution from 2.7 to 4.0, the yield of esters decreases smoothly from 47% to 42%, whereas it slightly increases to 12% in the biphasic system (Table 1 entries 5 and 6). However, increasing the pH from 4.0 to 5.0 sharply reduces the ester yield from 42% to 20%, a similar trend also observed in the conventional biphasic system (Table 1 entries 8 and 9). This result is consistent with the fact that protonated butyric acid is less available when the pH is above the pKa of butyric acid (4.8), in favor of the non-reactive deprotonated form. From an esterification point of view, it is favorable to perform esterification at lower pH because only undissociated butyric acid will react with butanol in aqueous media. However, from a lipase activity point of view, very acidic pH should be used carefully since lipase could be denatured at low pH.36,37
The catalytic performance was also studied in the O/W Pickering emulsion stabilized by R816 nanoparticles. The ester yield is 31% after 1 h, which is lower than that obtained with W/O emulsion (Table 1 entry 7). This could be related to the physicochemical properties of the emulsion (Fig. S3†), because the average droplets size is larger, hence the interfacial area is smaller, or it could also indicate that the confinement of the biocatalyst within water droplets in a W/O emulsion enhances extraction efficiency compared to an O/W emulsion, where oil droplets recover ester from the continuous phase.38 Indeed, the stability of Pickering emulsions is not always easy to assess under actual reaction conditions, as substrates, products, and the reaction itself can influence emulsion stability.39 Analysis of the emulsion properties (Fig. S1–S3†) reveals that emulsions stabilized by R816 nanoparticles are more susceptible to the variations of aqueous solution properties compared to those stabilized by R972 nanoparticles, suggesting that emulsions stabilized by R816 nanoparticles are likely to exhibit lower catalytic performance.
Given the high catalytic performance of the emulsion system, the kinetic profiles were studied for both emulsion and biphasic system at a fixed pH and variable lipase concentrations (Fig. 3a and b). The ester yield increases steadily from 11% to 42% after 1 h when the lipase concentration is increased from 0.05 to 0.3% in the Pickering emulsion system. A similar trend is observed in the biphasic system, though the ester yield increased from 3% to 12% after 1 h. The emulsion system exhibits a much faster reaction rate than the biphasic system, with the ester yield nearly reaching equilibrium within 1 h in the Pickering emulsion system, whereas the biphasic system requires 6 h or more to reach a similar level. Notably, the ester yield achieved with 0.05% lipase concentration in the Pickering emulsion system is comparable to that of the biphasic system at 0.3% lipase concentration, highlighting the cost-effectiveness of the Pickering emulsion system.
We therefore investigated the effect of butanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) butyric acid ratio on the catalytic performance (Fig. 3c and d). Decreasing the alcohol/acid ratio from 1
butyric acid ratio on the catalytic performance (Fig. 3c and d). Decreasing the alcohol/acid ratio from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 substantially increases both the initial rate and the equilibrium yield of ester. After 1 h, butyl butyrate yield increases from 42% to 72% in the Pickering emulsion system, and from 12% to 25% in the biphasic system. The equilibrium yield in the Pickering emulsion system increases from 47% to 72% within 1 to 2 h, which is consistent with the enhanced availability of butyric acid. This trend is also observed in the biphasic system, although it takes 6 h to achieve yields comparable to the Pickering emulsion system. The kinetic profile was also measured for the O/W emulsion stabilized by R816 nanoparticles at butanol
3 substantially increases both the initial rate and the equilibrium yield of ester. After 1 h, butyl butyrate yield increases from 42% to 72% in the Pickering emulsion system, and from 12% to 25% in the biphasic system. The equilibrium yield in the Pickering emulsion system increases from 47% to 72% within 1 to 2 h, which is consistent with the enhanced availability of butyric acid. This trend is also observed in the biphasic system, although it takes 6 h to achieve yields comparable to the Pickering emulsion system. The kinetic profile was also measured for the O/W emulsion stabilized by R816 nanoparticles at butanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) butyric acid ratio of 1
butyric acid ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (Fig. 3e). The ester yield reaches 62% after 1 h, reaching almost equilibrium after 2 h. This kinetic rate is slightly lower than the one in W/O emulsion. Additional enzymatic esterification experiments were performed using either heptane or hexadecane as organic phase (Fig. 3f). In all cases, emulsions are generated successfully and show comparable catalytic performance, both in terms of initial rate and final equilibrium yield.
3 (Fig. 3e). The ester yield reaches 62% after 1 h, reaching almost equilibrium after 2 h. This kinetic rate is slightly lower than the one in W/O emulsion. Additional enzymatic esterification experiments were performed using either heptane or hexadecane as organic phase (Fig. 3f). In all cases, emulsions are generated successfully and show comparable catalytic performance, both in terms of initial rate and final equilibrium yield.
Based on the above results, it can be concluded that Pickering emulsions play a critical role in enhancing catalytic efficiency for the enzymatic esterification of butanol and butyric acid in the biphasic system. The formation of a Pickering emulsion allows the increase of the interface area between reactants and extractants at microscale, reducing the diffusion-related limitation, which allows maximal lipase activity by eliminating product-related slowdown, which ultimately speeds up the entire reaction process. Furthermore, solid emulsifiers are biologically compatible and environmentally friendly as they do not inactivate the enzyme and might simplify the separation and purification of the product.
Scope of substrates
To extend the generality of the system, the enzymatic esterification of butanol and other carboxylic acids was carried out at the same reaction conditions. For hexanoic acid, the yield of butyl hexanoate is 67% after 0.5 h and reaches equilibrium within 1 h in the W/O emulsion system. This is comparable to the yield in the O/W emulsion and 5 times higher than the yield after 1 h in the biphasic system (Fig. 4b). Similarly, the esterification of butanol with palmitic acid to form butyl palmitate shows a yield of 76% after 0.5 h in the W/O emulsion system, while the biphasic system reaches only 22% after 1 h (Fig. 4c). In the case of linoleic acid, no commercial butyl linoleate ester was available to prepare a standard, so the conversion of butanol was used to quantify the catalytic efficiency. The enzymatic esterification of linoleic acid shows 83% conversion of butanol after 0.5 h in the W/O emulsion system, whereas only 17% is obtained after 1 h in their biphasic counterpart (Fig. 4d).Surprisingly, in the O/W emulsion, both the ester yields of palmitic acid and linoleic acid are much lower compared to the biphasic system, with after 1 h only 13% butyl palmitate yield (Fig. 4c) and trace amounts of butanol conversion to butyl linoleate (Fig. 4d). This suggests that the O/W emulsion has a negative effect in these cases compared to the biphasic system. It is worth noting that both palmitic acid and linoleic acid can also act as surfactants. We hypothesize a kind of double mechanism of stabilization occurs in the Pickering emulsion: hydrophilic R816 silica nanoparticles adsorb at the interface from the aqueous phase, while palmitic acid or linoleic acid adsorb at the interface from the oil phase.40 In this case, the silica nanoparticle/surfactant complex forms a viscoelastic film at the water/oil interface, allowing the formation of droplets covered by tightly packed nanoparticle/surfactant complex monolayers or multilayers.41,42 This phenomenon could limit the diffusion between lipase and substrates, as the lipase is confined to the aqueous phase, resulting in unsatisfactory catalytic performance.
Overall, these results clearly demonstrate the versatility of enzymatic esterification and ester extraction in Pickering emulsion systems, which can be performed in both W/O and O/W emulsions, across various pH levels, organic phases, and acid co-substrates. The reduced yield observed in the specific case of using long-chain acids in O/W emulsion would require further work to understand the limitation and optimize reaction conditions.
Enzymatic esterification in fermentation broth
A real fermentation broth was then studied with enzymatic esterification and ester extraction in the Pickering emulsion under the aforementioned conditions.The concentrations of the components in the fermentation broth are presented in Table 2, indicating that the main components produced were butanol and isopropanol. Butanol accounted for 77% of the total alcohols produced by fermentation, with a concentration of 0.23 mol L−1 in the fermentation broth (see ESI† Experimental section). The original fermentation broth was used as raw material, along with the addition of extra butyric acid for enzymatic esterification process (Fig. 5). A biphasic system without the addition of emulsifiers was carried out at the same conditions as a control for comparison with the Pickering emulsion system.
| Component | Concentration | |
|---|---|---|
| (g L−1) | (mol L−1) | |
| Butanol | 17.04 | 0.23 |
| Isopropanol | 3.94 | 0.07 |
| Ethanol | 1.07 | 0.02 |
| Acetone | 0.05 | 0.00 |
| Lactic acid | 1.22 | 0.01 |
| Acetic acid | 1.16 | 0.02 |
| Butyric acid | 1.75 | 0.02 |
In the W/O emulsion system, the butyl butyrate yield from the fermentation broth reaches 41% and 63% after 1 h and 6 h, respectively (Fig. 5c). In contrast, the biphasic system shows only 12% yield of butyl butyrate after 1 h (Fig. 5a). To enhance the final ester yield, we investigated the effect of lower butanol/butyric acid ratio for the esterification performance. At a high butyric acid concentration (butanol/butyric acid ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), the butyl butyrate yield after 1 h is 63% in the W/O emulsion system (Fig. 5d), which is 3 times higher compared to the biphasic system (21%) (Fig. 5b). The equilibrium yield increases from 63% to 79% in the W/O emulsion system because of the increased availability of butyric acid.
3), the butyl butyrate yield after 1 h is 63% in the W/O emulsion system (Fig. 5d), which is 3 times higher compared to the biphasic system (21%) (Fig. 5b). The equilibrium yield increases from 63% to 79% in the W/O emulsion system because of the increased availability of butyric acid.
In addition to the W/O emulsion, we developed an O/W emulsion system for the enzymatic esterification of the fermentation broth (Fig. 5e). The kinetic profile of the O/W emulsion increases not as fast as the W/O emulsion, giving a yield of 31% of butyl butyrate after 1 h. At lower butanol/butyric acid ratio (Fig. 5f), the yields of butyl butyrate are 47% and 66% after 1 h and 6 h, respectively. The catalytic performance of the O/W emulsion system can be related to the previously observed lower interface area compared to the W/O emulsion in the same emulsification conditions (Fig. 2) which is also observed using this fermentation broth as the aqueous phase (Fig. S3†). Consistent with the case of a pure solution (Fig. 3e), we observe here that the droplet size is a key parameter for the performance of the system: the kinetics are faster when the droplets are smaller.
It is noteworthy that isopropyl butyrate can be observed during the esterification process, which is attributed to the presence of 0.07 mol L−1 isopropanol in the fermentation broth. Unlike the esterification behavior of butanol and butyric acid, the yield of isopropyl butyrate proceeds slowly in both biphasic and emulsion systems.43,44 The conversion of isopropanol into isopropyl butyrate in the presence of W/O emulsion is 6% after 6 h, and the yield is 5% after 6 h in biphasic system (Fig. 5a and c). A higher yield of isopropyl butyrate is also observed by increasing the availability of butyric acid, with yields of 15% and 10% after 6 h in the W/O Pickering emulsion and biphasic system, respectively (Fig. 5b and d). Likewise, the yield of isopropyl butyrate in the presence of O/W emulsion is only 5% (Fig. 5f), which is consistent with the reduced catalytic performance observed for butyl butyrate in this case.
The reaction was also performed in an artificial solution with the same butanol concentration as the fermentation broth (0.23 mol L−1 Fig. S4†). In W/O emulsions, the reaction kinetics for the artificial solution and the fermentation broth are almost identical at both 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 butanol/butyric acid ratios, reaching equilibrium yields of 65% and 84%, respectively (Fig. S4a and b†). In the same way in O/W emulsions, the kinetics using the artificial solution are very similar to those obtained with the fermentation broth, although slightly slower than in W/O emulsion as already discussed (Fig. S4c and d†). Therefore, no apparent effect of the presence of complex components in the fermentation broth on the butyl butyrate yield is observed. This demonstrates the robustness of the designed system for real-life applications such as processing a complex fermentation broth without any prior treatment other than solid–liquid separation.
3 butanol/butyric acid ratios, reaching equilibrium yields of 65% and 84%, respectively (Fig. S4a and b†). In the same way in O/W emulsions, the kinetics using the artificial solution are very similar to those obtained with the fermentation broth, although slightly slower than in W/O emulsion as already discussed (Fig. S4c and d†). Therefore, no apparent effect of the presence of complex components in the fermentation broth on the butyl butyrate yield is observed. This demonstrates the robustness of the designed system for real-life applications such as processing a complex fermentation broth without any prior treatment other than solid–liquid separation.
Conclusions
In conclusion, we developed a versatile Pickering emulsion system for the recovery of butanol from a fermentation broth in the form of ester. Both W/O and O/W Pickering emulsions with high stability were achieved in water/dodecane system by using two types of silica nanoparticles. The catalytic performance was influenced by the emulsion properties, with reaction rates 2–5 times higher in the emulsion system compared to the biphasic system, which allowed the equilibrium to be reached in less than 2 h in our setup. Notably, the Pickering emulsion system can be applied to the enzymatic esterification of butanol and various carboxylic acids, and using different oil phases. Furthermore, the methodology was applied to the extraction of butanol from an original and untreated fermentation broth, showing performances identical to the ones obtained using an artificial pure butanol solution. The results presented in this study pave the way for designing highly efficient tools to separate bio-based components.Data availability
All data supporting the results have been included in the main text or as part of the ESI.†Author contributions
YF: conceptualization, investigation, methodology, visualization, validation, writing – original draft; PLC: funding acquisition, project administration, conceptualization, supervision, writing – review & editing; CD: conceptualization, supervision, validation, visualization, writing – review & editing; EJ: conceptualization, supervision, validation, writing – review & editing.Conflicts of interest
IFP Energies nouvelles has filed a patent application on this methodology.Acknowledgements
This research was carried out within the ECOCHEM project, funded by the PEPR Spleen Research Program, as part of the France 2030 investment plan. IFPEN Clostridium Fermentation Team (Hélène Velly, Sandra Menir and Angelique Bisson) are acknowledged for providing the fermentation broth. The authors are grateful to SAFIC ALCAN (distributor of Evonik in France) for kind donate of AEROSIL® R972 and R816 samples. The authors would also like to thank Corinne Burnichon and Pascal Hayrault for their help in the experiment part.References
- X. Zhen, Y. Wang and D. Liu, Bio-butanol as a new generation of clean alternative fuel for SI (spark ignition) and CI (compression ignition) engines, Renewable Energy, 2020, 147, 2494–2521 CrossRef CAS.
- A. Pugazhendhi, T. Mathimani, S. Varjani, E. R. Rene, G. Kumar, S.-H. Kim, V. K. Ponnusamy and J.-J. Yoon, Biobutanol as a promising liquid fuel for the future-recent updates and perspectives, Fuel, 2019, 253, 637–646 CrossRef CAS.
- T. A. Ewing, N. Nouse, M. van Lint, J. van Haveren, J. Hugenholtz and D. S. van Es, Fermentation for the production of biobased chemicals in a circular economy: a perspective for the period 2022–2050, Green Chem., 2022, 24, 6373–6405 RSC.
- Y. Li, W. Tang, Y. Chen, J. Liu and F. L. Chia-fon, Potential of acetone-butanol-ethanol (ABE) as a biofuel, Fuel, 2019, 242, 673–686 CrossRef CAS.
- Y. Tashiro, T. Yoshida, T. Noguchi and K. Sonomoto, Recent advances and future prospects for increased butanol production by acetone-butanol-ethanol fermentation, Eng. Life Sci., 2013, 13, 432–445 CrossRef CAS.
- V. dos Santos, C. Ferreira, F. M. Filho, R. M. Filho and A. P. Mariano, Acetone-free biobutanol production: Past and recent advances in the Isopropanol-Butanol-Ethanol (IBE) fermentation, Bioresour. Technol., 2019, 287, 121425 CrossRef PubMed.
- H. M. de Gérando, F. Wasels, A. Bisson, B. Clement, F. Bidard, E. Jourdier, A. M. López-Contreras and N. L. Ferreira, Genome and transcriptome of the natural isopropanol producer Clostridium beijerinckii DSM6423, BMC Genomics, 2018, 19, 1–12 CrossRef PubMed.
- V. García, J. Päkkilä, H. Ojamo, E. Muurinen and R. L. Keiski, Challenges in biobutanol production: how to improve the efficiency?, Renewable Sustainable Energy Rev., 2011, 15, 964–980 CrossRef.
- V. Outram, C.-A. Lalander, J. G. M. Lee, E. T. Davis and A. P. Harvey, A comparison of the energy use of in situ product recovery techniques for the Acetone Butanol Ethanol fermentation, Bioresour. Technol., 2016, 220, 590–600 CrossRef CAS PubMed.
- K. Dudek, I. Valdez-Vazquez and J. Koop, Butanol recovery from synthetic fermentation broth by vacuum distillation in a rotating packed bed, Sep. Purif. Technol., 2022, 297, 121551 CrossRef CAS.
- A. M. Dumitrescu, I. Banu and G. Bumbac, Process modeling and simulation for butanol removing from fermentation broth by extraction with biodiesel, Renewable Energy, 2019, 131, 137–143 CrossRef CAS.
- W. J. Groot, H. S. Soedjak, P. B. Donck, R. van der Lans, K. C. am Luyben and J. M. Timmer, Butanol recovery from fermentations by liquid-liquid extraction and membrane solvent extraction, Bioprocess Eng., 1990, 5, 203–216 CrossRef CAS.
- P. Demling, M. von Campenhausen, C. Grütering, T. Tiso, A. Jupke and L. M. Blank, Selection of a recyclable in situ liquid–liquid extraction solvent for foam-free synthesis of rhamnolipids in a two-phase fermentation, Green Chem., 2020, 22, 8495–8510 RSC.
- T. de Vrije, M. Budde, H. van der Wal, P. am Claassen and A. M. López-Contreras, “In situ” removal of isopropanol, butanol and ethanol from fermentation broth by gas stripping, Bioresour. Technol., 2013, 137, 153–159 CrossRef CAS PubMed.
- D. Cai, Z. Dong, J. Han, H. Yu, Y. Wang, P. Qin, Z. Wang and T. Tan, Co-generation of bio-butanol and bio-lipids under a hybrid process, Green Chem., 2016, 18, 1377–1386 RSC.
- Q. Liu, B. Huang and A. Huang, Polydopamine-based superhydrophobic membranes for biofuel recovery, J. Mater. Chem. A, 2013, 1, 11970–11974 RSC.
- P. J. Hickey and C. S. Slater, The selective recovery of alcohols from fermentation broths by pervaporation, Sep. Purif. Methods, 1990, 19, 93–115 CrossRef CAS.
- C. Xue, F. Liu, M. Xu, I.-C. Tang, J. Zhao, F. Bai and S.-T. Yang, Butanol production in acetone-butanol-ethanol fermentation with in situ product recovery by adsorption, Bioresour. Technol., 2016, 219, 158–168 CrossRef CAS PubMed.
- R. D. Offeman, S. K. Stephenson, G. H. Robertson and W. J. Orts, Solvent extraction of ethanol from aqueous solutions using biobased oils, alcohols, and esters, J. Am. Oil Chem. Soc., 2006, 83, 153–157 CrossRef CAS.
- S. A. Survase, R. Zebroski, C. Bayuadri, Z. Wang, G. Adamos, G. Nagy and V. Pylkkanen, Membrane assisted continuous production of solvents with integrated solvent removal using liquid-liquid extraction, Bioresour. Technol., 2019, 280, 378–386 CrossRef CAS PubMed.
- E. Crabbe, C. Nolasco-Hipolito, G. Kobayashi, K. Sonomoto and A. Ishizaki, Biodiesel production from crude palm oil and evaluation of butanol extraction and fuel properties, Process Biochem., 2001, 37, 65–71 CrossRef CAS.
- L. Adhami, B. Griggs, P. Himebrook and K. Taconi, Liquid–liquid extraction of butanol from dilute aqueous solutions using soybean-derived biodiesel, J. Am. Oil Chem. Soc., 2009, 86, 1123–1128 CrossRef CAS.
- S. Tanaka, Y. Tashiro, G. Kobayashi, T. Ikegami, H. Negishi and K. Sakaki, Membrane-assisted extractive butanol fermentation by Clostridium saccharoperbutylacetonicum N1-4 with 1-dodecanol as the extractant, Bioresour. Technol., 2012, 116, 448–452 CrossRef CAS PubMed.
- S. Zhang, X. Huang, C. Qu, Y. Suo, Z. Liao and J. Wang, Extractive fermentation for enhanced isopropanol and n-butanol production with mixtures of water insoluble aliphatic acids and oleyl alcohol, Biochem. Eng. J., 2017, 117, 112–120 CrossRef CAS.
- C. Mutschler, J. Aparicio, I. Mokbel, M. Capron, P. Fongarland, M. Araque and C. Nikitine, Reactive Distillation of Glycolic Acid Using Heterogeneous Catalysts: Experimental Studies and Process Simulation, Front. Chem., 2022, 10, 909380 CrossRef CAS PubMed.
- X. Sun, Q. Wang, W. Zhao, H. Ma and K. Sakata, Extraction and purification of lactic acid from fermentation broth by esterification and hydrolysis method, Sep. Purif. Technol., 2006, 49, 43–48 CrossRef CAS.
- C. van den Berg, A. S. Heeres, L. A. M. van der Wielen and A. J. J. Straathof, Simultaneous clostridial fermentation, lipase-catalyzed esterification, and ester extraction to enrich diesel with butyl butyrate, Biotechnol. Bioeng., 2013, 110, 137–142 CrossRef CAS PubMed.
- A. M. B. Rodriguez and B. P. Binks, Catalysis in Pickering emulsions, Soft Matter, 2020, 16, 10221–10243 RSC.
- D. Dedovets, Q. Li, L. Leclercq, V. Nardello-Rataj, J. Leng, S. Zhao and M. Pera-Titus, Multiphase Microreactors Based on Liquid–Liquid and Gas–Liquid Dispersions Stabilized by Colloidal Catalytic Particles, Angew. Chem., Int. Ed., 2021, 2–25 Search PubMed.
- M. Pera-Titus, L. Leclercq, J.-M. Clacens, F. de Campo and V. Nardello-Rataj, Pickering interfacial catalysis for biphasic systems: from emulsion design to green reactions, Angew. Chem., Int. Ed., 2015, 54, 2006–2021 CrossRef CAS PubMed.
- Y. Feng, J.-F. Dechezelles, Q. d'Acremont, E. Courtade, V. de Waele, M. Pera-Titus and V. Nardello-Rataj, Light-driven Pickering interfacial catalysis for the oxidation of alkenes at near-room temperature, Green Chem., 2023, 25, 1417–1423 RSC.
- B. Yang, L. Leclercq, J.-M. Clacens and V. Nardello-Rataj, Acidic/amphiphilic silica nanoparticles: new eco-friendly Pickering interfacial catalysis for biodiesel production, Green Chem., 2017, 19, 4552–4562 RSC.
- L. Qi, Z. Luo and X. Lu, Modulation of starch nanoparticle surface characteristics for the facile construction of recyclable Pickering interfacial enzymatic catalysis, Green Chem., 2019, 21, 2412–2427 RSC.
- S. Arana-Peña, N. S. Rios, D. Carballares, L. R. Gonçalves and R. Fernandez-Lafuente, Immobilization of lipases via interfacial activation on hydrophobic supports: Production of biocatalysts libraries by altering the immobilization conditions, Catal. Today, 2021, 362, 130–140 CrossRef.
- E. Piacentini, R. Mazzei and L. Giorno, Comparison between lipase performance distributed at the o/w interface by membrane emulsification and by mechanical stirring, Membranes, 2021, 11, 1–14 CrossRef PubMed.
- C. H. Ng and K.-L. Yang, Lipase in biphasic alginate beads as a biocatalyst for esterification of butyric acid and butanol in aqueous media, Enzyme Microb. Technol., 2016, 82, 173–179 CrossRef CAS PubMed.
- A. Buthe, T. Recker, M. Heinemann, W. Hartmeier, J. Büchs and M. B. Ansorge-Schumacher, pH-optima in lipase-catalysed esterification, Biocatal. Biotransform., 2005, 23, 307–314 CrossRef CAS.
- A. M. B. Rodriguez, L. Schober, A. Hinzmann, H. Gröger and B. P. Binks, Effect of particle wettability and particle concentration on the enzymatic dehydration of n-octanaloxime in pickering emulsions, Angew. Chem., Int. Ed., 2021, 60, 1450–1457 CrossRef PubMed.
- F. Chang, C. M. Vis, W. Ciptonugroho and P. C. A. Bruijnincx, Recent developments in catalysis with Pickering Emulsions, Green Chem., 2021, 23, 2575–2594 RSC.
- E. Santini, E. Guzmán, M. Ferrari and L. Liggieri, Emulsions stabilized by the interaction of silica nanoparticles and palmitic acid at the water–hexane interface, Colloids Surf., A, 2014, 460, 333–341 CrossRef CAS.
- M. Zembyla, A. Lazidis, B. S. Murray and A. Sarkar, Water-in-oil Pickering emulsions stabilized by synergistic particle–particle interactions, Langmuir, 2019, 35, 13078–13089 CrossRef CAS PubMed.
- C. Huang, J. Forth, W. Wang, K. Hong, G. S. Smith, B. A. Helms and T. P. Russell, Bicontinuous structured liquids with sub-micrometre domains using nanoparticle surfactants, Nat. Nanotechnol., 2017, 12, 1060–1063 CrossRef CAS PubMed.
- M. D. Romero, J. M. Gómez, B. Díaz-Suelto and A. García-Sanz, Study of the influence of alcohols in the synthesis of short chain esters, Chem. Eng. Trans., 2011, 24, 37–42 Search PubMed.
- F. Molinari, R. Gandolfi and F. Aragozzini, Microbial catalyzed esterification of primary and secondary alcohols in organic solvent, Biotechnol. Tech., 1996, 10, 103–108 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4re00625a |
| ‡ Co-last authors. |
| This journal is © The Royal Society of Chemistry 2025 |