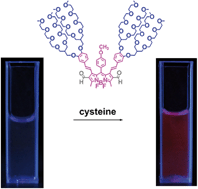
Shilei Zhu, Jingtuo Zhang, Giri Vegesna, Ashutosh Tiwari, Fen-Tair Luo, Matthias Zeller, Rudy Luck, Haihua Li, Sarah Green and Haiying Liu
RSC Adv., 2012,2, 404-407
DOI:
10.1039/C1RA00678A,
Communication