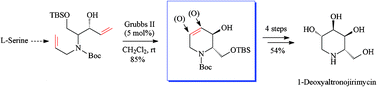
Examining advances in the class of natural and non-natural piperidine azasugars, important therapeutic agents and potent glycosidase inhibitors, this review looks at syntheses applying olefin metathesis as a highly efficient key step and gateway strategy for discovery of better iminosugar leads for treatment of widespread affections like viral and metabolic diseases. Amply illustrated is how ring-closing metathesis (RCM and RCEYM), promoted by commercial ruthenium alkylidene catalysts, manage to construct the common tetrahydropyridine core while cross-metathesis (CM), starting from this generic scaffold, provides general access to families of novel azasugars. Special consideration is given to high-profile iminosugar drugs of this class (1-deoxynojirimycin and congeners, adenophorine, fagomine, isofagomine and some of their N- and C-substituted analogues) stressing upon newest trends for enhancing biological activity and modulating previously unexploited targets in multispecific therapies.