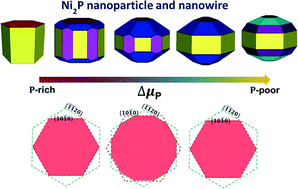
Knowledge of the equilibrium crystal shape and structure of the exposed surfaces of nickel phosphide (Ni2P) nanostructures is essential for understanding and control of their catalytic performance. Ab initio atomistic thermodynamics was used to investigate computationally the effects of the experimental conditions (temperature, pressure, and chemical potentials) on the relative stabilities of low-Miller index surfaces and on the equilibrium crystal morphology of Ni2P nanoparticles and nanowires. The P-covered (0001)-Ni3P2 (denoted as (0001)-A-P) surface was found to be the most stable surface at a considerably wide range of chemical potentials, whereas the (0001)-A, (10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 1)-Ni/P and (10
1)-Ni/P and (10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 2)-Ni/P surfaces are the thermodynamically most favored phases just in narrow chemical potential regions. The theoretical equilibrium shapes and structures of the Ni2P nanoparticles and nanowires were obtained based on the Wulff construction at various chemical potentials. The morphology of the surfaces of the Ni2P nanoparticles and nanowires does depend on the chemical potential; thus, it can be tailored for particular applications by a suitable choice of experimental conditions. The (0001), (10
2)-Ni/P surfaces are the thermodynamically most favored phases just in narrow chemical potential regions. The theoretical equilibrium shapes and structures of the Ni2P nanoparticles and nanowires were obtained based on the Wulff construction at various chemical potentials. The morphology of the surfaces of the Ni2P nanoparticles and nanowires does depend on the chemical potential; thus, it can be tailored for particular applications by a suitable choice of experimental conditions. The (0001), (10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0) and (10
0) and (10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 1) side facets dominate the nanoparticle surface in a wide range of chemical potentials but other side facets can also appear at particular ranges of chemical potentials. Results reported herein give new insight into the Ni2P nanoparticle morphology showing how it depends on the experimental conditions; this information can help to tailor the surface and shape of Ni2P nanoparticles for specific applications, e.g., in catalysis.
1) side facets dominate the nanoparticle surface in a wide range of chemical potentials but other side facets can also appear at particular ranges of chemical potentials. Results reported herein give new insight into the Ni2P nanoparticle morphology showing how it depends on the experimental conditions; this information can help to tailor the surface and shape of Ni2P nanoparticles for specific applications, e.g., in catalysis.