Qing-Feng Wu, Chao Zheng, Chun-Xiang Zhuo and Shu-Li You
Chem. Sci., 2016,7, 4453-4459
DOI:
10.1039/C6SC00176A,
Edge Article
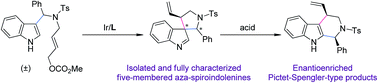
An Ir-catalyzed asymmetric synthesis of five-membered aza-spiroindolenines is achieved. Based on the detailed investigation of the reaction patterns of the aryl iminium migration, a one-pot asymmetric allylic dearomatization/migration sequence from racemic indole derivatives is realized, affording enantioenriched Pictet–Spengler-type products bearing an additional allylic stereogenic center adjacent to the C3 position of the indole core.