Quan-Quan Zhou, Fu-Dong Lu, Dan Liu, Liang-Qiu Lu and Wen-Jing Xiao
Org. Chem. Front., 2018,5, 3098-3102
DOI:
10.1039/C8QO00805A,
Research Article
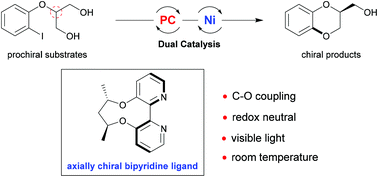
1,4-Benzodioxane widely exists as the core structure in many therapeutic agents and bioactive natural compounds. Hence, to access this kind of molecule, an enantioselective desymmetric C–O cross coupling reaction has been developed through dual visible light photoredox and nickel catalysis. Notably, the use of an axially chiral 2,2′-bipyridine ligand is the key to success. A series of chiral 1,4-benzodioxanes were afforded in high yields and moderate to good enantioselectivity under mild reaction conditions.