 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Water-mediated synthesis of hydrogen-bonded metal–organic frameworks†
Zongjing
Xiao
a,
Pengfei
Li
b,
Beibei
Sun
a,
Xinrui
Bao
a,
Lei
Gan
 a and
Huajun
Yang
a and
Huajun
Yang
 *a
*a
aJiangsu Key Laboratory of Biomedical Materials, College of Chemistry and Materials Science, Nanjing Normal University, Nanjing 210023, China. E-mail: huajunyang@nnu.edu.cn
bSchool of Environment, Nanjing Normal University, Nanjing 210023, China
First published on 23rd July 2025
Abstract
The integration of different types of frameworks into one would provide a unique opportunity for the development of crystalline frameworks that can combine the unique advantages of each. In this work, we successfully realize the integration of metal–organic frameworks (MOFs) and hydrogen-bonded organic frameworks (HOFs) through the synthesis of two hydrogen-bonded metal–organic frameworks, namely NNM-1(M) (M = Cu or Ni). The structures of NNM-1 are built on (4, 4)-squared coordinative metal–organic layers. Between the layers, two adjacent protonated carboxylic groups serve as both hydrogen donors and acceptors, leading to the formation of hydrogen-bonded pillars with R22(8) dimeric hydrogen bonding, the most common hydrogen-bonded motif in HOFs. It was found that water plays a vital role in the structural transformation from 3D MOFs to H-MOFs here. Under a low pressure of 0.1 bar, the material NNM-1(Cu) can adsorb 4.84 mmol g−1 of NH3, making it a promising adsorbent for ammonia capture.
1 Introduction
Over the past several decades, crystalline porous materials, including metal–organic frameworks (MOFs),1–4 covalent organic frameworks (COFs),5–7 hydrogen-bonded organic frameworks (HOFs),8,9 and also some traditional porous materials, have experienced explosive growth.10 Each type of framework clearly has its own advantages that some or all of the others do not have. For example, HOFs, constructed by weak hydrogen bonding, have excellent self-healing and regeneration properties.11 COFs usually exhibit ultra-stable structures that can survive in very harsh chemical environments.12 In comparison with HOFs and COFs, the existence of metals endows MOFs with greater potential in gas adsorption and separation,13 catalysis,14,15 electrochemistry,16–19 and so on.The integration of different types of frameworks into one should provide a unique opportunity for the development of crystalline frameworks that can combine the unique advantages of each. One such example is metal-covalent organic frameworks (or MCOFs),20 with the introduction of metal ions into COFs, which have emerged as a distinct class of porous materials with great potential in catalysis,21 optics,22 and so on.
We are interested in integrating MOFs and HOFs so that the new frameworks can inherit the advantages of both, such as the metal sites in MOFs and the excellent processability of HOFs. In fact, the coexistence of coordination bonds and hydrogen bonds in crystalline frameworks is not rare.23–25 However, in most scenarios, such frameworks would be simplified into a zero-dimensional structure based on coordination metal complexes or metal–organic cages without consideration of hydrogen bonding.26,27 In other words, the nature of the frameworks aligns with that of HOFs, which is also probably why such materials are commonly called M-HOFs.
In this work, we successfully realize the integration of metal–organic frameworks and hydrogen-bonded organic frameworks through the synthesis of two hydrogen-bonded metal–organic frameworks, namely NNM-1(M) (M = Cu or Ni). The structures of NNM-1 are built on (4, 4)-squared coordinative metal–organic layers with hydrogen-bonded pillars. Between the layers, two adjacent protonated carboxylic groups serve as both hydrogen donors and acceptors, leading to the formation of R22(8) dimeric hydrogen bonding, the most common hydrogen-bonded motif in HOFs.
2 Results and discussion
2.1 Synthesis and structural features
Rod-shaped crystals (NNM-1) suitable for single crystal X-ray diffraction (SCXRD) can be obtained by a solvothermal reaction of M(NO3)2·xH2O (M = Cu or Ni), 1,4-diazabicyclo[2.2.2]octane (DABCO), and bicyclo[2.2.2]octane-1,4-dicarboxylic acid (H2BODC) in the mixed solvents of DMF and H2O. The phase purity of both crystals was validated using their PXRD patterns (a drop of HBF4 was required for NNM-1(Cu) to improve the phase purity, Fig. S1 and S2†). NNM-1 crystallized in the orthorhombic system with the space group of Immm (No. 71, Table S1†). The asymmetric unit contains one quarter of metal, one eighth of DABCO, one quarter of HBODC−, and one eighth of BODC2−, which thus gives a formula of M2(DABCO)(HBODC)2(BODC) (Fig. S3 and S4†). It is worth mentioning that the ligand of H2BODC has two kinds of deprotonated forms in the final structures including the fully deprotonated one (BODC2−) and the partially deprotonated HBODC− with a protonated end. Two adjacent protonated carboxylic groups serve as both hydrogen donors and acceptors, leading to the formation of R22(8) dimeric hydrogen bonding, the most common hydrogen-bonded motif in HOFs (Fig. S5†).28The structure of NNM-1 can be viewed as a pillar-layered structure with unique hydrogen-bonded pillars. The secondary building unit (SBU) of NNM-1 is the prototypical paddle-wheel metal dimer. The two metals in the SBU connect two BODC2− and two DABCO to form (4, 4)-squared layers (Fig. 1b). The layers are further pillared by pairs of hydrogen-bonded HBODC− (Fig. 1c) to form pcu-type H-MOFs. The final NNM-1 possesses a double-interpenetrated structure composed of two evenly staggered and independent sets of frameworks (Fig. 1d and S6†).
The most striking structural feature of NNM-1 is the observation of the prototypical hydrogen-bonded motif as the linkage in a MOF structure. In fact, the coexistence of coordination bonds and hydrogen bonds in crystalline frameworks is not rare. However, in most scenarios, such frameworks would be simplified to a zero-dimensional structure based on coordination metal complexes or metal–organic cages without consideration of hydrogen bonding.29,30 In other words, the nature of the frameworks aligns with that of HOFs, which is also probably why such materials are commonly called M-HOFs (Scheme 1). NNM-1 is still two-dimensional and porous without considering hydrogen bonding, which still falls into the category of MOFs.
NNM-1 bears a close resemblance to the three-dimensional M2(BODC)2(DABCO) MOF. The transformation from M2(BODC)2(DABCO) to NNM-1 could be viewed as the replacement of one BODC2− ligand in one direction by a pair of HBODC− (Fig. 2). Alternatively, NNM-1 can also be treated as defective M2(BODC)2(DABCO) with missing-cluster defects.
 | ||
| Fig. 2 Comparison of the structures of (a) M2(BODC)2(DABCO) and (b) a single set of NNM-1; and (c) the reaction scheme for M2(BODC)2(DABCO) and NNM-1. | ||
It was found that water plays a vital role in the structural transformation from M2(BODC)2(DABCO) to NNM-1. For the synthesis of M2(BODC)2(DABCO), only DMF was used as the solvent.31–33 With the addition of water, the final product transformed to NNM-1, even with the same metal to ligand ratio (Fig. S7†). Such a synthetic rule was subsequently found to be applicable to a series of DMF/water solvents with different volume ratios and different reaction temperatures (Fig. S8–S11†). As shown in Fig. S10 and S11,† regardless of whether the reaction temperature is 100 °C or 120 °C, the product obtained is NNM-1 as long as the water is added, while the product is M2(BODC)2(DABCO) without the addition of water. A possible reason is that Cu2+/Ni2+, as a borderline Lewis acid (not too hard or too soft), has a relatively balanced affinity for water and all the ligands. Therefore, a large amount of water can compete with the ligands to coordinate with Cu2+/Ni2+, leading to missing-cluster defects in NNM-1.
We also directly put Cu2(BODC)2(DABCO) crystals and H2BODC ligands into the solvents (DMF and water with 1 drop of HBF4) and after aging for 2 days at 100 °C, the cell parameters obtained by single crystal diffraction tests are identical to those of NNM-1(Cu), and the PXRD pattern agrees well with that of NNM-1(Cu), which can sufficiently prove that the final product has transformed into NNM-1(Cu) (Fig. 3).
 | ||
| Fig. 3 The variation of PXRD patterns during the post-synthesis of NNM-1(Cu) with Cu2(BODC)2(DABCO) as the raw material. | ||
2.2 Stability
As can be seen from the TGA curves of NNM-1(Cu), the structure gradually collapses at around 300 °C and completely collapses after 400 °C (Fig. S12†). NNM-1(Ni) has much higher thermal stability, which does not degrade until almost 450 °C (Fig. S13†). The chemical stability of both materials was also tested by immersing them in aqueous solutions of different pH for 1 day. PXRD patterns indicated that both materials could maintain the crystallinity in solutions with pH from 3 to 11, which was further confirmed by CO2 adsorption with essentially unchanged CO2 uptake (Fig. S14–S17†).2.3 Porosity and NH3 adsorption isotherms
The guest-accessible volume was determined to be 167 Å3 per unit cell (∼8%) using the PLATON program. N2 adsorption isotherms at 77 K showed negligible uptake for both materials (Fig. S18 and S19†). The permanent porosity of the two MOFs was thus evaluated based on the CO2 adsorption isotherms collected at 195 K (Fig. S20 and S21†). The Brunauer–Emmett–Teller (BET) specific surface areas of NNM-1(Cu) and NNM-1(Ni) were determined to be 67.82 m2 g−1 and 178.98 m2 g−1, respectively.NH3 capture properties were investigated due to the combination of relatively high stability, ultra-microporosity, and high density of carboxylic acid sites for NNM-1. Although NNM-1(Cu) has a lower surface area, its NH3 uptake at 1 bar is up to 7.89 mmol g−1 at 298 K and 9.96 mmol g−1 at 273 K, significantly higher than that of NNM-1(Ni) (Fig. 4a and S22). In particular, when converted to volumetric uptake, the uptake at 298 K is as high as 237.4 mg cm−3, higher than that of FDU–HOF–3, KUF-1a and other carboxylic acid-based frameworks (Table S2†).34–36 Under a low pressure of 0.1 bar, the material NNM-1(Cu) can adsorb 4.21 mmol g−1 of NH3 in the first cycle, and after two cycles, the amount of ammonia adsorbed in the third cycle at 0.1 bar even increased, reaching 4.84 mmol g−1 (Fig. S23–S26†). Under 25 mbar, NNM-1(Cu) can still adsorb 2.81 mmol g−1. As shown in Fig. 4b, the maximum adsorption capacity of NNM-1(Cu) remains essentially unchanged after three cycles, which indicates that this material possesses high cycling stability.
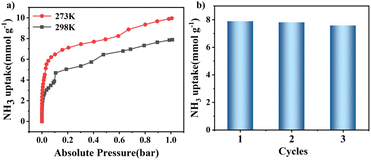 | ||
| Fig. 4 (a) NH3 adsorption isotherms of NNM-1(Cu) at 273 K and 298 K; (b) comparison of the maximum NH3 adsorption capacity of the three cycles of NNM-1(Cu) at 298 K. | ||
It could be reasonably proposed that NH3 uptake in NNM-1(Cu) occurs by chemisorption through breaking of the cupric carboxylate bond, as observed in Cu(cyhdc).37 Exposure of activated NNM-1(Cu) to ammonia solution also caused a color change from green to blue, confirming a significant change in the coordination environment of Cu2+ (Fig. S27†). The infrared spectrum (Fig. S28†) showed a distinct NH4+ vibration peak, indicating that NH3 reacted with –COOH in the structure of NNM-1 during the ammonia adsorption process. The PXRD patterns of NNM-1(Cu) after NH3 adsorption also showed a phase transformation with the main peaks shifting towards lower angles (Fig. S29 and S30†). Unfortunately, we failed to obtain the structure by single crystal XRD due to its low crystallinity.
In order to investigate the effect of NNM-1(Cu) on the removal of NH3 at low concentrations in air, we conducted breakthrough experiments for NH3 removal under dry conditions as well as at certain humidity (Fig. S31†) and obtained water adsorption curves for both materials (Fig. S32 and S33†). In the breakthrough test under dry conditions with a mixture of 1000 ppm ammonia in N2 through a fixed bed filled with NNM-1(Cu) at 298 K, a strong retention of ammonia was observed. As shown in the breakthrough curves displayed in Fig. S29,† the breakthrough time of NH3 under dry air conditions is measured to be 142 min g−1 for NNM-1(Cu) and the saturation NH3 uptake amount is found to be 5.45 mmol g−1. NNM-1(Cu) showed a significant decrease in the NH3 sorption capacity under humid conditions, indicating competitive adsorption between H2O and NH3 molecules of the active adsorption sites, even though the water adsorption capacity is relatively low in a wide pressure range.
3 Conclusions
In summary, this work reports the creation of a new family of materials with the integration of MOFs and HOFs into H-MOFs. H-MOFs here are different from previously reported M-HOFs, for which the frameworks would be simplified into zero-dimensional structures based on coordination metal complexes or metal–organic cages without consideration of hydrogen bonding. It was found that water played a vital role in the structure transformation from three-dimensional MOFs to H-MOFs. The water-mediated synthesis of NNM-1 offers a promising synthetic strategy with great potential for the development of new materials and applications.Data availability
The data that support the findings of this study are available in the ESI† of this article.Author contributions
H. Y. and L. G. conceived and designed the research. Z. X., P. L., B. S., and X. B. performed the synthesis and measurements. H. Y. and Z. X. wrote the paper. L. G. and H. Y. discussed the results and revised the paper. All authors have given approval to the final version of the manuscript.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
We acknowledge the financial support from the National Natural Science Foundation of China (Grant No. 22301138), Jiangsu Specially Appointed Professorship, and the startup funding from Nanjing Normal University.Notes and references
- O. M. Yaghi, G. Li and H. Li, Selective binding and removal of guests in a microporous metal–organic framework, Nature, 1995, 378, 703–706 CrossRef CAS.
- H. Li, M. Eddaoudi, M. O'Keeffe and O. M. Yaghi, Design and synthesis of an exceptionally stable and highly porous metal-organic framework, Nature, 1999, 402, 276–279 CrossRef CAS.
- H.-C. Zhou, J. R. Long and O. M. Yaghi, Introduction to Metal–Organic Frameworks, Chem. Rev., 2012, 112, 673–674 CrossRef CAS PubMed.
- A. Schoedel, M. Li, D. Li, M. O'Keeffe and O. M. Yaghi, Structures of Metal–Organic Frameworks with Rod Secondary Building Units, Chem. Rev., 2016, 116, 12466–12535 CrossRef CAS PubMed.
- A. P. Côté, A. I. Benin, N. W. Ockwig, M. O'Keeffe, A. J. Matzger and O. M. Yaghi, Porous, Crystalline, Covalent Organic Frameworks, Science, 2005, 310, 1166–1170 CrossRef PubMed.
- K. Geng, T. He, R. Liu, S. Dalapati, K. T. Tan, Z. Li, S. Tao, Y. Gong, Q. Jiang and D. Jiang, Covalent Organic Frameworks: Design, Synthesis, and Functions, Chem. Rev., 2020, 120, 8814–8933 CrossRef CAS PubMed.
- H. M. El-Kaderi, J. R. Hunt, J. L. Mendoza-Cortés, A. P. Côté, R. E. Taylor, M. O'Keeffe and O. M. Yaghi, Designed Synthesis of 3D Covalent Organic Frameworks, Science, 2007, 316, 268–272 CrossRef CAS PubMed.
- Y. He, S. Xiang and B. Chen, A Microporous Hydrogen-Bonded Organic Framework for Highly Selective C2H2/C2H4 Separation at Ambient Temperature, J. Am. Chem. Soc., 2011, 133, 14570–14573 CrossRef CAS PubMed.
- I. Hisaki, C. Xin, K. Takahashi and T. Nakamura, Designing Hydrogen-Bonded Organic Frameworks (HOFs) with Permanent Porosity, Angew. Chem., Int. Ed., 2019, 58, 11160–11170 CrossRef CAS PubMed.
- J. H. Song and D. W. Kang, Hazardous nitroaromatic explosives detection by emerging porous solid sensors, Coord. Chem. Rev., 2023, 492, 215279 CrossRef CAS.
- C. Chen, L. Shen, H. Lin, D. Zhao, B. Li and B. Chen, Hydrogen-bonded organic frameworks for membrane separation, Chem. Soc. Rev., 2024, 53, 2738–2760 RSC.
- Z. Zhang, X. Dong, J. Yin, Z. G. Li, X. Li, D. Zhang, T. Pan, Q. Lei, X. Liu, Y. Xie, F. Shui, J. Li, M. Yi, J. Yuan, Z. You, L. Zhang, J. Chang, H. Zhang, W. Li, Q. Fang, B. Li, X. H. Bu and Y. Han, Chemically Stable Guanidinium Covalent Organic Framework for the Efficient Capture of Low-Concentration Iodine at High Temperatures, J. Am. Chem. Soc., 2022, 144, 6821–6829 CrossRef CAS PubMed.
- I. Senkovska, V. Bon, A. Mosberger, Y. Wang and S. Kaskel, Adsorption and Separation by Flexible MOFs, Adv. Mater., 2025, e2414724 CrossRef.
- V. Virender, V. Pandey, G. Singh, P. K. Sharma, P. Bhatia, A. A. Solovev and B. Mohan, Hybrid Metal-Organic Frameworks (MOFs) for Various Catalysis Applications, Top. Curr. Chem., 2024, 383, 3 CrossRef PubMed.
- L. Cai, M. Khanpour, Q. Yin, Z.-Y. Wang, Z.-B. Fang, H.-X. Liu, Y. Hou, C. Liu, W. Deng and T.-F. Liu, Well-Defined Microenvironment in Metal–Organic Frameworks Enable Green, Benign, and Isolation-Free Catalytic Oxidation Reaction, CCS Chem., 2024, 1–10 Search PubMed.
- L. Ji, J. Wang, K. Wu and N. Yang, Tunable Electrochemistry of Electrosynthesized Copper Metal–Organic Frameworks, Adv. Funct. Mater., 2018, 28, 1706961 CrossRef.
- L. Yang, X. He and M. Dincă, Triphenylene-Bridged Trinuclear Complexes of Cu: Models for Spin Interactions in Two-Dimensional Electrically Conductive Metal–Organic Frameworks, J. Am. Chem. Soc., 2019, 141, 10475–10480 CrossRef CAS PubMed.
- L. Yang and M. Dincă, Redox Ladder of Ni3 Complexes with Closed-Shell, Mono-, and Diradical Triphenylene Units: Molecular Models for Conductive 2D MOFs, Angew. Chem., Int. Ed., 2021, 60, 23784–23789 CrossRef CAS PubMed.
- A.-A. Zhang, X. Cheng, X. He, W. Liu, S. Deng, R. Cao and T.-F. Liu, Harnessing Electrostatic Interactions for Enhanced Conductivity in Metal-Organic Frameworks, Research, 2021, 2021 Search PubMed.
- J. Dong, X. Han, Y. Liu, H. Li and Y. Cui, Metal-Covalent Organic Frameworks (MCOFs): A Bridge Between Metal-Organic Frameworks and Covalent Organic Frameworks, Angew Chem. Int. Ed. Engl., 2020, 59, 13722–13733 CrossRef CAS PubMed.
- W. K. Han, Y. Liu, J. D. Feng, X. Yan, H. Pang and Z. G. Gu, Engineering a molecular ruthenium catalyst into three-dimensional metal covalent organic frameworks for efficient water oxidation, Chem. Sci., 2023, 14, 11768–11774 RSC.
- T. Liu, Q. Tao, Y. Wang, R. Luo, J. Ma and J. Lei, Tailored Cis-Trans Isomeric Metal-Covalent Organic Frameworks for Coordination Configuration-Dependent Electrochemiluminescence, J. Am. Chem. Soc., 2024, 146, 18958–18966 CrossRef CAS PubMed.
- X. Y. Wang, S. Y. Liao, H. P. Huang, Q. F. Wang, Y. Y. Shi, P. L. Zhu, Y. G. Hu, R. Sun and Y. J. Wan, Enhancing the Chemical Stability of MXene Through Synergy of Hydrogen Bond and Coordination Bond in Aqueous Solution, Small Methods, 2023, 7, e2201694 CrossRef PubMed.
- M. Guadalupe Vasquez-Rios, G. Campillo-Alvarado and L. R. MacGillivray, Mechanochemical Mediated Coexistence of B<–N Coordination and Hydrogen Bonding, Angew Chem. Int. Ed. Engl., 2023, 62, e202308350 CrossRef CAS PubMed.
- Z. Yan, M. He, A. Hu, M. Liu, J. Chen, J. Liu, N. Chen, L. Cao, B. Li and J. Long, Manipulating hydrogen and coordination bond chemistry for reversible zinc metal anodes, J. Colloid Interface Sci., 2023, 650, 257–265 CrossRef CAS.
- H. Li, R. Chao, J. Liang, Z. Guo, B. Zhang and F. Guo, Metalo-Hydrogen-Bonded Organic Frameworks (MHOFs) Self-Assembled by Second-Sphere Coordination for Guest Exchange, Cryst. Growth Des., 2024, 24, 6719–6728 CrossRef CAS.
- J.-Y. Zhu, K. Kumari, S.-J. Chen, D. Shao, J. Yang, L. Shi, S. K. Singh and Y.-Z. Zhang, Magnetic and Porous Regulation in Cobalt(II) Hydrogen-Bonded Organic Framework via Supramolecular Isomerism, Cryst. Growth Des., 2024, 24, 3449–3457 CrossRef CAS.
- P. Li, M. R. Ryder and J. F. Stoddart, Hydrogen-Bonded Organic Frameworks: A Rising Class of Porous Molecular Materials, Acc. Mater. Res., 2020, 1, 77–87 CrossRef CAS.
- Z.-H. Zhu, H.-L. Wang, H.-H. Zou and F.-P. Liang, Metal hydrogen-bonded organic frameworks: structure and performance, Dalton Trans., 2020, 49, 10708–10723 RSC.
- B.-T. Liu, S.-H. Gong, X.-T. Jiang, Y. Zhang, R. Wang, Z. Chen, S. Zhang, K. O. Kirlikovali, T.-F. Liu, O. K. Farha and R. Cao, A solution processible single-crystal porous organic polymer, Nat. Synth., 2023, 2, 873–879 CrossRef CAS.
- Y. S. Su, E. S. Lamb, I. Liepuoniute, A. Chronister, A. L. Stanton, P. Guzman, S. Pérez-Estrada, T. Y. Chang, K. N. Houk, M. A. Garcia-Garibay and S. E. Brown, Dipolar order in an amphidynamic crystalline metal–organic framework through reorienting linkers, Nat. Chem., 2021, 13, 278–283 CrossRef CAS PubMed.
- K. Li, J. Lee, D. H. Olson, T. J. Emge, W. Bi, M. J. Eibling and J. Li, Unique gas and hydrocarbon adsorption in a highly porous metal-organic framework made of extended aliphatic ligands, Chem. Commun., 2008, 6123–6125 RSC.
- J. Zhou, K. Wen, T. Ke, J. Li, Y. Jin, J. Li, Z. Zhang, Z. Bao, Q. Ren and Q. Yang, Nonlinear 3D Ligand-Based Metal–Organic Framework for Thermodynamic–Kinetic Synergistic Splitting of Mono-/Dibranched Hexane Isomers, J. Am. Chem. Soc., 2024, 146, 16838–16847 CrossRef CAS PubMed.
- X. Song, Y. Wang, C. Wang, X. Gao, Y. Zhou, B. Chen and P. Li, Self-Healing Hydrogen-Bonded Organic Frameworks for Low-Concentration Ammonia Capture, J. Am. Chem. Soc., 2023, 146, 627–634 CrossRef PubMed.
- D. W. Kang, M. Kang, H. Kim, J. H. Choe, D. W. Kim, J. R. Park, W. R. Lee, D. Moon and C. S. Hong, A Hydrogen-Bonded Organic Framework (HOF) with Type-IV NH3 Adsorption Behavior, Angew. Chem., Int. Ed., 2019, 58, 16152–16155 CrossRef CAS PubMed.
- K. Ma, P. Li, J. H. Xin, Y. Chen, Z. Chen, S. Goswami, X. Liu, S. Kato, H. Chen, X. Zhang, J. Bai, M. C. Wasson, R. R. Maldonado, R. Q. Snurr and O. K. Farha, Ultrastable Mesoporous Hydrogen-Bonded Organic Framework-Based Fiber Composites toward Mustard Gas Detoxification, Cell Rep. Phys. Sci, 2020, 1, 100024 CrossRef.
- B. E. R. Snyder, A. B. Turkiewicz, H. Furukawa, M. V. Paley, E. O. Velasquez, M. N. Dods and J. R. Long, A ligand insertion mechanism for cooperative NH3 capture in metal–organic frameworks, Nature, 2023, 613, 287–291 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2433919 and 2433920. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5sc02337h |
| This journal is © The Royal Society of Chemistry 2025 |


