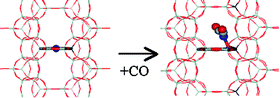
The preferred Cu+ sites and formation of mono-, di-, and tricarbonyl complexes in the Cu-FER were investigated at the periodic density functional theory level and by means of FTIR spectroscopy. The site-specificity of adsorption enthalpies of CO on Cu-FER and of vibrational frequencies of polycarbonyl complexes were investigated for various Cu+ sites in Cu-FER. Large changes in the Cu+ interaction with the zeolite framework were observed upon the formation of carbonyl complexes. The dicarbonyl complexes formed on Cu+ in the main channel or on the intersection of the main and perpendicular channels are stable and both, adsorption enthalpies and CO stretching frequencies are not site-specific. The fraction of Cu+ ions in the FER cage, that cannot form dicarbonyl can be determined from IR spectra (about 7% for the Cu-FER with Si/Al = 27.5 investigated here). The tricarbonyl complexes can be formed at the Cu+ ions located at the 8-member ring window at the intersection of main and perpendicular channel. The stability of tricarbonyl complexes is very low (ΔH°(0 K)
≥
−4 kJ mol−1).