Vipul V. Betkekar, Mio Harachi, Keisuke Suzuki and Ken Ohmori
Org. Biomol. Chem., 2019,17, 9129-9134
DOI:
10.1039/C9OB01896D,
Communication
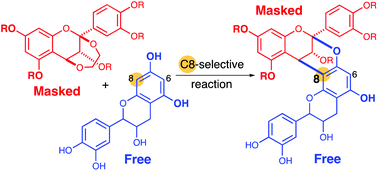
A synthesis method of doubly linked flavan dimers is reported via the acid-promoted annulation reaction using nascent catechins, (+)-catechin or (−)-epicatechin, as a dianionic partner and an ethylenedioxy-bridged flavan as a dicationic partner. Procyanidins A1 and A2 were synthesized. On the high regioselectivity of the annulation reactions, model experiments and computational studies were carried out.