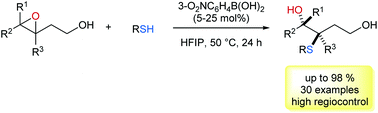Hongqing Yao, Jiawei Liu and Chuan Wang
Org. Biomol. Chem., 2019,17, 1901-1905
DOI:
10.1039/C8OB02141D,
Paper
In this protocol we described a boronic acid-catalysed C-3 selective ring opening of 3,4-epoxy alcohols with thiophenols and thiols as nucleophiles. This diastereo- and enantiospecific reaction provides an efficient entry to prepare a variety of hydroxyl sulfides. Through the directing effect of the hydroxyl group, nucleophilic attack on the C-3 position of the epoxide moiety is favoured. It can be rationalized in a proposed transition state, in which the boronic acid catalyst tethers both epoxides and S-nucleophiles.