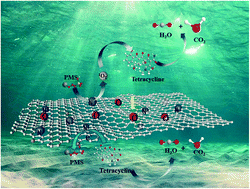
A new dual-decontamination strategy has been employed to synthesize graphitic carbon nanosheets (GCNs) from Reactive Red 2, a common chemical pollutant in textile wastewater, for efficient tetracycline (TC) degradation via nonradical oxidation processes. Detailed physical characterization indicates that the GCN possesses a layered and flat structure, high microporosity, and microscopic configuration of graphene-like nanosheets with abundant surface O-, N- and S-containing functional groups (including graphitic N and carbonyl groups). From the kinetic model, quenching experiments and mechanism studies, only 1O2 is detected in the GCN/PMS system, without SO4˙−, HO˙ or O2˙−. 1O2 contributed to only 17.8% of overall TC degradation. Surface-confined electron transfer appears to be the major oxidation pathway (82.2%) for TC degradation. Furthermore, full-scale biological assays verify that the biotoxicity of the TC degradation products is significantly reduced in comparison with that of the precursor. This work provides a new insight toward the conversion from a chemical pollutant into a metal-free carbon catalyst for wastewater treatment.